Abstract
Objective
This study analyzed men and women separately by age at hospital diagnosis of psychotic disorder or schizophrenia and by maternal or paternal disease after taking several possible confounders into account.
Methods
The Multigeneration Register, in which all men and women born in Sweden from 1932 onwards are registered together with their parents, was linked to hospital data. This yielded 21,199 male and 19,029 female cases of psychotic disorders in addition to 12,799 paternal and 23,021 maternal cases of psychotic disorders (including schizophrenia). Standardized incidence ratios (SIRs) were calculated as the ratio of observed and expected number of cases among men and women with mothers and/or fathers affected by psychotic disorders or schizophrenia, compared with men and women whose mothers and/or fathers were not affected by psychotic disorders or schizophrenia.
Results
The overall significant SIRs among men and women with a mother, father or both parents hospitalized for psychotic disorder varied between 2.86 and 20.30. Maternal transmission of psychotic disorder was stronger than paternal, and the highest SIRs were found in the youngest age groups. Similar results were found when the subgroup schizophrenia was analyzed separately. Maternal or paternal schizophrenia implied higher risks for the offspring than maternal or paternal psychotic disorders.
Conclusions
Hereditary factors have a strong influence on the onset of psychotic disorders and schizophrenia. Young people and individuals with both parents affected by these diseases need special attention as their SIRs were particularly increased.
Keywords: Familial risk, Genetics, Hereditary factors, Psychotic disorders
1. Introduction
Psychotic disorders are severe and relatively common neuropsychiatric disorders characterized by mental dysfunction across multiple domains of the brain. This occurs in about 1% of the world’s general population. Although the exact etiology of the disorder is not understood, family, twin and adoption studies have suggested that genetic factors play a major role in the transmission of psychotic disorders. In a Danish cohort study, a 3.22-fold risk of psychotic disorders was observed among first-degree relatives (Laursen et al., 2005). From twin studies, the heritability of psychotic disorders was estimated to be over 80% (Cannon et al., 1998; Cardno et al., 1999). Previous studies also indicate that first-degree relatives of people with schizophrenia have a higher risk of psychotic disorders than first-degree relatives of control persons without psychotic disorders (2–9% and 0–1%, respectively) (Shih et al., 2004). Results from family and epidemiological studies have led to the suggestion that early-onset psychotic disorders may be more strongly genetically influenced (Alda et al., 1996; Kendler and MacLean, 1990; Pulver et al., 1990; Waddington and Youssef, 1996). These findings have not been reported consistently (Maier et al., 1993; Sham et al., 1994). The influence of genetic factors has been reported to have a significantly higher effect in women than men in some studies (Maier et al., 1993) but not in others (Albus and Maier, 1995; Cannon et al., 1998; Laursen et al., 2005). Consequently, there is a need for further research into the genetic etiology of psychotic disorders and schizophrenia with specific attention paid to age and sex effects.
The patterns of inheritance of psychotic disorders are probably complex. Environmental factors are also important in the development of psychotic disorders, which is based on recent research of the association between psychotic disorders and cannabis use, childhood trauma and urbanicity (Cougnard et al., 2007). A study of Finnish adoptees found evidence for a genotype–environment interaction in schizophrenia-spectrum disorders (Tienari et al., 2004).
For the future success of gene identification efforts, it is important that the familial risks are characterized in detail. It has been suggested that “age of onset is the single most important characteristic of schizophrenia that could yield clues to its origin” (DeLisi, 1992). We examined age-specific familial risks for psychotic disorders and schizophrenia using the nationwide Swedish MigMed Research Database. The novel contribution of this study is partly its approach; it was based on a nationwide register of all hospitalized cases in Sweden between 1987 and 2004, which yielded a substantial number of cases in two generations. Second, the use of hospitalized cases eliminated potential self-report and recall bias. Swedish data on medically diagnosed psychotic disorders were obtained from register sources with virtually complete coverage. In addition, the Swedish Multigeneration Register is a well-validated source that has been found to be reliable in the study of many familial diseases (Hemminki et al., 2005; Hemminki et al., 2006; Sundquist et al., 2004). This study examined age-specific familial risks among men and women with a mother and/or father with psychotic disorder or schizophrenia after adjustment for several confounders.
2. Materials and methods
2.1. MigMed research database
Data used in this study were retrieved from the MigMed database, located at the Center for Family and Community Medicine at the Karolinska Institute in Stockholm. MigMed is a single, comprehensive database that has been constructed using several national Swedish data registers, including but not limited to the Total Population Register, the Multigeneration Register, and the Swedish Hospital Discharge Register (1987–2004) (Rosen and Hakulinen, 2005; Statistics Sweden, 2005; The National Board of Health and Welfare). Information from the various registers in the database is linked at the individual level via the 10-digit national registration number assigned to each person in Sweden for his or her lifetime. Prior to inclusion in the MigMed database, national registration numbers were replaced by serial numbers to ensure the anonymity of all individuals.
Since the database contains information from the Multigeneration Register, it is possible to link more than 9 million index persons (persons born in or after 1932 and registered in Sweden at any time since 1961) with their biological parents, i.e. more than 3.2 million families. The latest version of the Multigeneration Register, which has been incorporated in the MigMed database, includes supplementary data from church records on index persons domiciled in Sweden between 1947 and 1961.
2.2. Outcome variable
Hospitalization for psychotic disorders was retrieved from hospital discharge records reported according to the 9th (1987–1996) and 10th (1997–2004) versions of the International Classification of Diseases. The following ICD codes were included for psychotic disorders, i.e. schizophrenia, schizotypal and delusional disorders: ICD-9: 295 (schizophrenic disorders), 297 (paranoid states), and 298 (other nonorganic psychoses); ICD-10: F20 (schizophrenia), F21 (schizotypal disorder), F22 (persistent delusional disorders), F23 (acute and transient psychotic disorders), F24 (induced delusional disorder), F25 (schizoaffective disorders), F28 (other nonorganic psychotic disorders), and F29 (unspecified nonorganic psychosis). The subgroup schizophrenia was analyzed separately (ICD-9: 295 and ICD-10: F20 and F21).
2.3. Individual variables
Gender: men and women.
Age at diagnosis was categorized in 5-year groups and the groups were merged as necessary.
Psychotic disorders in father/mother was dichotomized into Yes and No.
Immigrant status was categorized in the following groups: Sweden, Finland, Western countries, Southern Europe, countries in the former Soviet bloc, Eritrea/Ethiopia/Somalia, other African countries, Latin America, Middle Eastern countries, other Asian countries and all other countries. Immigrant status was included because it is associated with psychotic disorders according to previous research (Sundquist et al., 2004; Zolkowska et al., 2001).
Employment status was categorized as employed or unemployed.
Educational attainment was classified as completion of compulsory school or less (<=9 years), practical high school or some theoretical high school (10–11 years), or theoretical high school and/or college (>=12 years).
Family income was calculated as empirical quartiles based on the income distribution (Sundquist et al., 2004).
Education, income and employment were used as measures of socioeconomic status, which is associated with psychotic disorders (Sundquist et al., 2004).
Geographic region was divided into large cities (cities with a population of more than 200,000, i.e., Stockholm, Gothenburg, and Malmö), Southern Sweden, and Northern Sweden. Geographic region was included as an individual variable to adjust for possible differences between geographic regions in Sweden with regard to hospital admissions for psychotic disorders.
Time period was included in order to adjust for possible differences in hospitalization rates over time.
Number of children was included as a possible confounder because parents of numerous offspring will have a higher number of affected offspring, leading to increased risk estimates.
2.4. Statistical analysis
Person-years were calculated from the start of follow-up on January 1, 1987, until hospital diagnosis, death, emigration, or the end of the study on December 31, 2004. Age-standardized incidence ratios were calculated for the whole follow-up period, divided into six 3-year periods (1987–1989–1990–1992–1993–1995–1996–1998–1999–2001, and 2002–2004). Standardized incidence ratios (SIRs) were calculated as the ratio of the observed to the expected number of cases (Rothman and Greenland, 1998). The expected number of cases was calculated for age (in 5-year groups), gender, time period and the other individual variables. The expected number of cases was based on the number of cases among men and women whose mothers or fathers were not affected by psychotic disorders or schizophrenia, i.e. the reference group. Familial risks were calculated for men and women with mothers or fathers affected by psychotic disorders or schizophrenia compared with men and women whose mothers or fathers were not affected by psychotic disorders or schizophrenia. This means that if the SIR is 2.0, the risk is twice as high compared with the reference group. Each offspring was analyzed separately. Confidence intervals (95% CI) were calculated assuming a Poisson distribution. Statistical trend tests (χ2 tests) were used to examine risk by age at diagnosis. χ2 tests were also used to analyze possible differences in the transmission of parental schizophrenia and parental psychotic disorders. Finally, we analyzed possible maternal and paternal differences in the transmission of psychotic disorders and schizophrenia. The results of the χ2 tests are shown in Table 4.
Table 4.
Statistical trend test of risk by age at diagnosis and relative risk of psychotic disorders and schizophrenia in men and women by parental history
| Men
|
Women
|
|||||
|---|---|---|---|---|---|---|
| Parental history
|
Parental history
|
|||||
| Father | Mother | Both parents | Father | Mother | Both parents | |
| Age at diagnosis a | ||||||
| Psychotic | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| Schizophrenia | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| Schizophrenia versus psychotic disorders b | p<0.001 | p<0.001 | p=0.203 (ns) | p=0.003 | p<0.001 | p=0.961 (ns) |
| Mother versus father b | ||||||
| Psychotic disorders | p=0.006 | p<0.001 | ||||
| Schizophrenia | p=0.250 (ns) | p=0.030 | ||||
| Both parents versus father b | ||||||
| Psychotic disorders | p<0.001 | p<0.001 | ||||
| Schizophrenia | p=0.001 | p=0.064 (ns) | ||||
ns=non significant, p<0.05 is considered significant.
χ2 trend test over all age groups.
Relative risks were tested with the probability of chi-squared distribution based on the statistical test χ2 with 1 degree of freedom.
2.5. Ethical considerations
This study was approved by the Ethics Committee of the Karolinska Institute, Stockholm, Sweden.
3. Results
The database covered the years 1987 to 2004 in the Swedish Hospital Discharge Register and included 21,199 male cases and 19,029 female cases of psychotic disorders in addition to 12,799 paternal and 23,021 maternal cases of psychotic disorders (Table 1). The mean age at diagnosis was 53.8 and 54.8 years for fathers and mothers, respectively, and 35.9 and 38.4 years for men (sons) and women (daughters), respectively. The mean age at diagnosis of psychotic disorder was 31.3 and 34.3 years for sons and daughters, respectively, when a parent was affected with psychotic disorders. The overall hospitalization rates of psychotic disorders were 37.5/100,000 and 35.2/100,000 for men and women, respectively (Fig. 1). The rates increased with increasing age up to the age of 30–35 years and then decreased in the older age groups. Female rates tended to exceed male rates after the age of 40 years.
Table 1.
Number of cases of psychotic disorders in men and women and parents
| Men
|
Women
|
Fathers
|
Mothers
|
|||||
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | No. | % | No. | % | No. | % | No. | % |
| <20 | 1001 | 4.7 | 888 | 4.7 | 87 | 0.7 | 156 | 0.7 |
| 20–29 | 6048 | 28.5 | 4110 | 21.6 | 1044 | 8.2 | 1889 | 8.2 |
| 30–39 | 6414 | 30.3 | 5219 | 27.4 | 2364 | 18.5 | 3861 | 16.8 |
| 40–49 | 4865 | 22.9 | 4976 | 26.1 | 2726 | 21.3 | 4280 | 18.6 |
| 50–59 | 2350 | 11.1 | 3096 | 16.3 | 1777 | 13.9 | 3573 | 15.5 |
| 60–69 | 504 | 2.4 | 717 | 3.8 | 1402 | 11.0 | 2918 | 12.7 |
| 70–79 | 17 | 0.1 | 23 | 0.1 | 1720 | 13.4 | 3361 | 14.6 |
| 80– | 1679 | 13.1 | 2983 | 13.0 | ||||
| All | 21199 | 100.0 | 19029 | 100.0 | 12799 | 100.0 | 23021 | 100.0 |
Fig. 1.
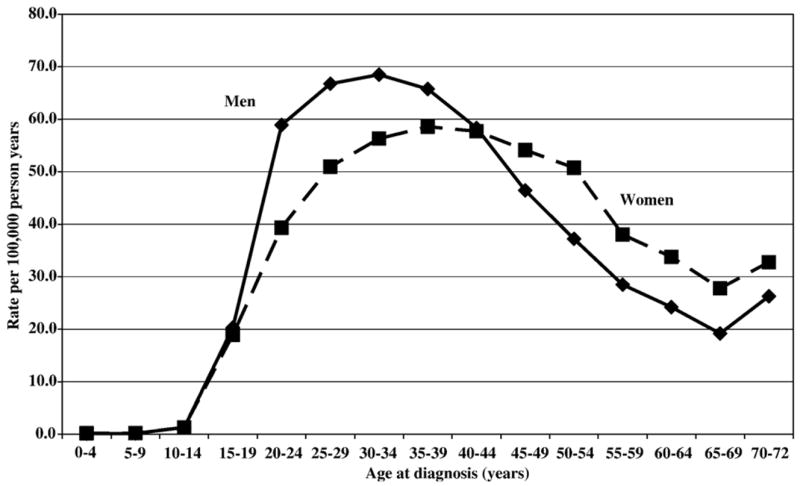
Age-specific incidence rates of psychotic disorders in men and women aged 0 to 72 years.
Familial risks were calculated separately for psychotic disorders in men and women according to the father’s and mother’s history of psychotic disorders (Table 2). Hospitalization for psychotic disorder is referred to below as “psychotic disorder.” All models were adjusted for all the explanatory variables simultaneously, i.e. immigrant status, employment status, education, income, geographic region, time period, and number of children. The overall SIRs were similar for men and women with a father with psychotic disorder (3.16 [95% CI 2.79–3.55] for men and 2.86, [95% CI 2.52–3.24] for women). This was also the case for men and women with a mother with psychotic disorder (SIR =3.84 [95% CI 3.55–4.15] for men and 3.79 [95% CI 3.49–4.11] for women). For both men and women, maternal transmission of psychotic disorders was stronger than paternal transmission. The highest overall SIRs were found among men and women with both parents hospitalized for psychotic disorders: SIRs were 18.63 (95% CI 11.63–28.00) for men and 20.30 (95% CI 11.57–33.05) for women. An apparent gradient was found for both men and women so that with increasing age the SIRs decreased. The highest SIRs were found in the youngest age groups among men and women with both parents affected with psychotic disorders. For those younger than 20 years, the SIR was 73.76 (95% CI 29.24–152.84) for men and 63.97 (95% CI 23.02–140.15) for women with both parents affected with psychotic disorders. However, only 7 male cases and 6 female cases were observed in this particular group.
Table 2.
SIRs for psychotic disorders in offspring of fathers/mothers with psychotic disorders
| Age at diagnosis (years) | Father with psychotic disorder
|
Mother with psychotic disorder
|
Both parents with psychotic disorder
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | ||||
| Men | ||||||||||||
| <20 | 37 | 8.53 | 6.00 | 11.77 | 70 | 12.66 | 9.87 | 16.00 | 7 | 73.76 | 29.24 | 152.84 |
| 20–29 | 121 | 4.86 | 4.03 | 5.81 | 246 | 5.85 | 5.14 | 6.63 | 14 | 38.64 | 21.05 | 65.01 |
| 30–39 | 54 | 1.98 | 1.49 | 2.58 | 183 | 3.36 | 2.89 | 3.89 | 1 | 2.55 | 0.00 | 14.63 |
| 40–49 | 46 | 2.28 | 1.67 | 3.04 | 103 | 2.59 | 2.11 | 3.14 | 1 | 3.13 | 0.00 | 17.93 |
| 50–59 | 12 | 1.53 | 0.79 | 2.68 | 36 | 1.56 | 1.09 | 2.17 | 0 | |||
| >=60 | 2 | 1.25 | 0.12 | 4.61 | 9 | 2.53 | 1.15 | 4.82 | 0 | |||
| All | 272 | 3.16 | 2.79 | 3.55 | 647 | 3.84 | 3.55 | 4.15 | 23 | 18.63 | 11.80 | 28.00 |
| Women | ||||||||||||
| <20 | 29 | 8.59 | 5.74 | 12.34 | 42 | 7.78 | 5.60 | 10.52 | 6 | 63.97 | 23.02 | 140.15 |
| 20–29 | 87 | 5.05 | 4.05 | 6.23 | 165 | 5.63 | 4.80 | 6.55 | 4 | 18.40 | 4.79 | 47.58 |
| 30–39 | 73 | 2.75 | 2.15 | 3.45 | 183 | 4.11 | 3.54 | 4.75 | 4 | 14.14 | 3.68 | 36.56 |
| 40–49 | 39 | 1.68 | 1.20 | 2.30 | 140 | 3.32 | 2.79 | 3.92 | 1 | 10.34 | 0.00 | 59.28 |
| 50–59 | 15 | 1.23 | 0.69 | 2.04 | 48 | 1.95 | 1.44 | 2.59 | 1 | 12.24 | 0.00 | 70.16 |
| >=60 | 5 | 1.21 | 0.38 | 2.85 | 14 | 1.37 | 0.75 | 2.31 | 0 | |||
| All | 248 | 2.86 | 2.52 | 3.24 | 592 | 3.79 | 3.49 | 4.11 | 16 | 20.30 | 11.57 | 33.05 |
O = observed number of cases; SIR = standardized incidence ratio; CI = confidence interval.
Bold type indicates statistical significance: 95% CI does not include 1.00.
Models adjusted for immigrant status, employment status, education, income, geographic region, time period, and number of children.
Table 3 shows the SIRs for schizophrenia in men and women by parental schizophrenia. Hospitalization for schizophrenia is referred to as “schizophrenia.” The overall SIRs were similar for men and women with a father with schizophrenia (5.97 [95% CI 4.26–8.13] for men and 5.45, [95% CI 3.41–8.26] for women). This was also the case for men and women with a mother with schizophrenia (7.34 [95% CI 6.14–8.71] for men and 9.01 [95% CI 7.35–10.94] for women). For both men and women, maternal transmission of schizophrenia tended to be stronger than paternal transmission. The highest SIRs were found among men and women with both parents affected with schizophrenia. However, only 2 cases were found among men (SIR=46.19, 95% CI 4.35–169.87) and 2 cases among women (SIR=19.57, 95% CI 1.84–71.97) in this particular group.
Table 3.
SIRs for schizophrenia in offspring of fathers/mothers with schizophrenia
| Age at diagnosis (years) | Father with schizophrenia
|
Mother with schizophrenia
|
Both parents with schizophrenia
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | ||||
| Men | ||||||||||||
| <20 | 3 | 7.99 | 1.51 | 23.66 | 8 | 29.39 | 12.55 | 58.19 | 0 | |||
| 20–29 | 23 | 10.72 | 6.79 | 16.12 | 44 | 10.27 | 7.46 | 13.79 | 2 | 71.17 | 6.71 | 261.75 |
| 30–39 | 7 | 2.59 | 1.03 | 5.37 | 52 | 5.96 | 4.45 | 7.82 | 0 | |||
| 40–49 | 4 | 3.37 | 0.88 | 8.72 | 21 | 5.55 | 3.43 | 8.50 | 0 | |||
| 50–59 | 2 | 7.40 | 0.70 | 27.21 | 6 | 7.12 | 2.56 | 15.59 | 0 | |||
| >=60 | 1 | 36.63 | 0.01 | 209.97 | 1 | 12.42 | 0.00 | 71.21 | 0 | |||
| All | 40 | 5.97 | 4.26 | 8.13 | 132 | 7.34 | 6.14 | 8.71 | 2 | 46.19 | 4.35 | 169.87 |
| Women | ||||||||||||
| <20 | 1 | 4.48 | 0.00 | 25.69 | 7 | 28.91 | 11.46 | 59.91 | 0 | |||
| 20–29 | 10 | 6.28 | 2.99 | 11.58 | 28 | 8.81 | 5.85 | 12.75 | 1 | 66.23 | 0.03 | 379.62 |
| 30–39 | 6 | 4.14 | 1.49 | 9.06 | 35 | 9.76 | 6.79 | 13.59 | 1 | 14.04 | 0.01 | 80.51 |
| 40–49 | 5 | 8.54 | 2.70 | 20.10 | 24 | 9.17 | 5.87 | 13.67 | 0 | |||
| 50–59 | 0 | 7 | 8.48 | 3.36 | 17.58 | 0 | ||||||
| >=60 | 0 | 1 | 1.14 | 0.00 | 6.56 | 0 | ||||||
| All | 22 | 5.45 | 3.41 | 8.26 | 102 | 9.01 | 7.35 | 10.94 | 2 | 19.57 | 1.84 | 71.97 |
O = observed number of cases; SIR = standardized incidence ratio; CI = confidence interval.
Bold type indicates statistical significance: 95% CI does not include 1.00.
Models adjusted for immigrant status, employment status, education, income, geographic region, time period, and number of children.
Table 4 shows trend tests of risk by age at diagnosis and differences in the transmission of parental schizophrenia and parental psychotic disorders. It also shows differences in transmission from mothers, both parents, or fathers (reference group). In all subgroups, there was a significant trend by age; lower age implied an increased risk of parental transmission of both psychotic disorders and schizophrenia (p < 0.05). Maternal or paternal schizophrenia implied higher risks for the offspring than maternal or paternal psychotic disorders. This was the case for both men and women. For psychotic disorders, maternal transmission was stronger than paternal transmission in both men and women. For schizophrenia, maternal transmission was stronger than paternal transmission only among women. Having two parents with psychotic disorder or schizophrenia implied a higher risk of disease transmission than having only a father with the disease, with the exception of the combination women with both parents with schizophrenia.
4. Discussion
The present study provided evidence of strong familial risks of psychotic disorders and schizophrenia in the entire Swedish population. The risk magnitudes in offspring were in line with earlier publications on familial psychotic disorders and schizophrenia (Albus and Maier, 1995; Cannon et al., 1998; Cardno et al., 1999; Kendler and MacLean, 1990; Laursen et al., 2005; Shih et al., 2004). The present study adds further knowledge to previous research because of its approach; it was based on all hospitalized cases in Sweden between 1987 and 2004, which yielded 21,199 male and 19,029 female cases of psychotic disorders in addition to 12,799 paternal and 23,021 maternal cases of psychotic disorders (including schizophrenia). This approach eliminated potential recall bias and allowed us to analyze men and women separately by age at hospital diagnosis of psychotic disorder or schizophrenia and by maternal or paternal disease after taking several possible confounders into account.
This study has a number of other strengths. For example, the study population represented the entire population of Sweden. Because of the national registration number assigned to each individual in Sweden, it was possible to track the records of every person for the whole follow-up period and calculate exact risk-time. The unique Swedish Population Registers are almost entirely complete with very few missing data. In addition, the data in the Swedish Hospital Discharge Register are nearly 100% complete. In 2001, the main diagnosis was missing in only 0.9% of the hospitalized cases and the national registration number was missing in 0.4% of all hospitalizations (Rosen and Hakulinen, 2005). The multigenerational part of the MigMed database includes information about index persons born in Sweden from 1932 and onwards and domiciled in Sweden at any time between 1947 and 2001 and their parents.
Another strength of our study was that we were able to present gender-specific familial risk estimates. We found no significant differences in the transmission of psychotic disorders between men and women, which is consistent with prior evidence (Albus and Maier, 1995; Cannon et al., 1998; Laursen et al., 2005). However, we found that maternal transmission is stronger than paternal transmission for both men and women and that maternal or paternal schizophrenia implied higher risk for the offspring than maternal or paternal psychotic disorders. In addition, the risk of psychotic disorders or schizophrenia was especially high for men and women with both parents affected by psychotic disorders or schizophrenia.
Because of the large number of cases, we were able to describe age-specific familial risks. In the offspring of affected parents, the highest risks were found in the youngest age groups. Previous studies have suggested that the etiology of psychotic disorders in childhood and adolescence may differ from that of adult onset psychotic disorders and that early-onset psychotic disorders may be more strongly genetically influenced than adult-onset psychotic disorders (Alda et al., 1996; Kendler and MacLean, 1990; Pulver et al., 1990; Waddington and Youssef, 1996). In a family history study, it was shown that a positive history of psychotic disorders in first-degree relatives predicted the risk of early-onset psychotic disorders in subjects under 17 years of age (Pulver et al., 1990).
The mechanisms of transmission of psychotic disorders and schizophrenia to offspring include genetic factors, which have been shown in several studies Albus ( and Maier, 1995; Cannon et al., 1998; Cardno et al., 1999; Laursen et al., 2005; Shih et al., 2004). However, there is also evidence of environmental risk factors in the development of psychotic disorders (Cougnard et al., 2007). For example, a study from the Netherlands found that victimisation in childhood may be associated with adult psychosis (Lataster et al., 2006). Other studies have found evidence that the outcome of developmental expression of psychosis is worse for adolescents growing up in an urban environment (Spauwen et al., 2006), and that urbanicity may shift a relatively large section of the adolescent population along a continuum of expression of psychosis (Spauwen et al., 2004). Some early risk factors associated with schizophrenia are prenatal exposure to viral infections (Takei et al., 1995) and poor nutrition (Brown et al., 1995). It is possible that some of these environmental risk factors may be more common psychotic families.
An important limitation of our study is the lack of information on environmental risk factors for psychotic disorders. However, we were able to adjust for socio-economic status and geographical region, i.e. variables that to some extent are related to environmental factors. Another limitation is that the mean age at diagnosis was higher among the parents than among the studied men and women because the Swedish Hospital Discharge Register began recording complete data on all discharges in Sweden only in 1986. However, we studied differential transmission of psychotic disorders risk from mothers and fathers, which implies that this possible bias affected the mothers and fathers to an almost equal extent. Furthermore, we were unable to test for the validity of the diagnoses because our data were based on the entire population. However, we only used main diagnoses psychotic disorders recorded in the hospital registers, i.e., all patients were hospitalized mainly for psychotic disorders, which increase the possibility that the diagnoses for psychotic disorders are valid.
5. Conclusion
Hereditary factors have a strong influence on the onset of psychotic disorders and schizophrenia. Young people and individuals with both parents affected by these diseases need special attention as their risks were particularly increased. Further progress in the understanding of the heritable basis of psychotic disorders and schizophrenia requires coordinated efforts in genetic epidemiology and molecular genetics.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-H271084-1), the Swedish Research Council (K2004-21X-11651-09A and K2005-27X-15428-01A), and the Swedish Council for Working Life and Social Research (2001-2373).
Role of funding source
The funding sources had no further role in study design, collection, analysis and interpretation of data, writing of the report, or in the decision to submit the manuscript for publication.
Footnotes
Contributors
XL and JK conceived the idea for the study. XL and JK designed the study. XL performed the statistical analysis. XL and JK drafted the manuscript. All the authors revised the manuscript.
Conflict of interest
There are no conflicts of interest.
References
- Albus M, Maier W. Lack of gender differences in age at onset in familial schizophrenia. Schizophr Res. 1995;18(1):51–57. doi: 10.1016/0920-9964(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Alda M, Ahrens B, Lit W, Dvorakova M, Labelle A, Zvolsky P, et al. Age of onset in familial and sporadic schizophrenia. Acta Psychiatr Scand. 1996;93(6):447–450. doi: 10.1111/j.1600-0447.1996.tb10676.x. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry. 1995;166(5):601 –606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry. 1998;55(1):67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry. 1999;56(2):162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- Cougnard A, Marcelis M, Myin-Germeys I, De Graaf R, Vollebergh W, Krabbendam L, et al. Does normal developmental expression of psychosis combine with environmental risk to cause persistence of psychosis? A psychosis proneness-persistence model. Psychol Med. 2007;37(4):513–527. doi: 10.1017/S0033291706009731. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. The significance of age of onset for schizophrenia. Schizophr Bull. 1992;18(2):209–215. doi: 10.1093/schbul/18.2.209. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Li X, Johansson S, Sundquist K, Sundquist J. Familial risks for migraine and other headaches among siblings based on hospitalisations in Sweden. Neurogenetics. 2005;6:217–224. doi: 10.1007/s10048-005-0019-8. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Li X, Sundquist K. Familial risks for main neurological diseases in siblings based on hospitalizations in Sweden. Twin Res Hum Genet. 2006;9:580–586. doi: 10.1375/183242706778024991. [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean CJ. Estimating familial effects on age at onset and liability to schizophrenia. I Results of a large sample family study. Genet Epidemiol. 1990;7(6):409–417. doi: 10.1002/gepi.1370070603. [DOI] [PubMed] [Google Scholar]
- Lataster T, van Os J, Drukker M, Henquet C, Feron F, Gunther N, et al. Childhood victimisation and developmental expression of non-clinical delusional ideation and hallucinatory experiences: victimisation and non-clinical psychotic experiences. Soc Psychiatry Psychiatr Epidemiol. 2006;41(6):423–428. doi: 10.1007/s00127-006-0060-4. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Labouriau R, Licht RW, Bertelsen A, Munk-Olsen T, Mortensen PB. Family history of psychiatric illness as a risk factor for schizoaffective disorder: a Danish register-based cohort study. Arch Gen Psychiatry. 2005;62(8):841–848. doi: 10.1001/archpsyc.62.8.841. [DOI] [PubMed] [Google Scholar]
- Maier W, Lichtermann D, Minges J, Heun R, Hallmayer J. The impact of gender and age at onset on the familial aggregation of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1993;242(5):279–285. doi: 10.1007/BF02190387. [DOI] [PubMed] [Google Scholar]
- Pulver AE, Brown CH, Wolyniec P, McGrath J, Tam D, Adler L, et al. Schizophrenia: age at onset, gender and familial risk. Acta Psychiatr Scand. 1990;82(5):344–351. doi: 10.1111/j.1600-0447.1990.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Rosen M, Hakulinen T. Use of disease registers. In: Ahrens W, Pigeot I, editors. Handbook of epidemiology. Springer; Verlag, Berlin: 2005. [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. 2. Lippincott; Raven, Philadelphia: 1998. [Google Scholar]
- Sham PC, Jones P, Russell A, Gilvarry K, Bebbington P, Lewis S, et al. Age at onset, sex, and familial psychiatric morbidity in schizophrenia. Camberwell Collaborative Psychosis Study. Br J Psychiatry. 1994;165(4):466 –473. doi: 10.1192/bjp.165.4.466. [DOI] [PubMed] [Google Scholar]
- Shih RA, Belmonte PL, Zandi PP. A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. Int Rev Psychiatry. 2004;16(4):260–283. doi: 10.1080/09540260400014401. [DOI] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Does urbanicity shift the population expression of psychosis? J Psychiatr Res. 2004;38(6):613–618. doi: 10.1016/j.jpsychires.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Evidence that the outcome of developmental expression of psychosis is worse for adolescents growing up in an urban environment. Psychol Med. 2006;36(3):407–415. doi: 10.1017/S0033291705006902. [DOI] [PubMed] [Google Scholar]
- Statistics Sweden, 2005. The Swedish Multi Generation Register (1960–1990). http://www.scb.se/templates/Standard____22842.asp. (In Swedish: Registret över totalbefolkningen/RTB).
- Sundquist K, Frank G, Sundquist J. Urbanisation and incidence of psychosis and depression: follow-up study of 4.4 million women and men in Sweden. Br J Psychiatry. 2004;184:293–298. doi: 10.1192/bjp.184.4.293. [DOI] [PubMed] [Google Scholar]
- Takei N, Sham PC, O’Callaghan E, Glover G, Murray RM. Schizophrenia: increased risk associated with winter and city birth—a case-control study in 12 regions within England and Wales. J Epidemiol Community Health. 1995;49(1):106–107. doi: 10.1136/jech.49.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Board of Health and Welfare, 2004. The Swedish Hospital Discharge Register and the Cause of Death Register (1961–2001). http://www.sos.se/epc/english/ParEng.htm.
- Tienari P, Wynne LC, Sorri A, Lahti I, Laksy K, Moring J, et al. Genotype–environment interaction in schizophrenia-spectrum disorder. Long-term follow-up study of Finnish adoptees. Br J Psychiatry. 2004;184:216–222. doi: 10.1192/bjp.184.3.216. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA. Familial-genetic and reproductive epidemiology of schizophrenia in rural Ireland: age at onset, familial morbid risk and parental fertility. Acta Psychiatr Scand. 1996;93(1):62–68. doi: 10.1111/j.1600-0447.1996.tb10620.x. [DOI] [PubMed] [Google Scholar]
- Zolkowska K, Cantor-Graae E, McNeil TF. Increased rates of psychosis among immigrants to Sweden: is migration a risk factor for psychosis? Psychol Med. 2001;31(4):669–678. doi: 10.1017/s0033291701003786. [DOI] [PubMed] [Google Scholar]


