Abstract
Cannabinoids and their endogenous and synthetic analogs exert powerful hypotensive and cardiodepressor effects by complex mechanisms involving direct and indirect effects on myocardium and vasculature. On the one hand, endocannabinoids and cannabinoid receptors have been implicated in the hypotensive state associated with hemorrhagic, endotoxic and cardiogenic shock, and advanced liver cirrhosis. On the other hand, there is emerging evidence suggesting that the endocannabinergic system plays an important role in the cardiovascular regulation in hypertension. This review is aimed to discuss the in vivo hypotensive and cardiodepressant effects of cannabinoids mediated by cannabinoid and TRPV1 receptors, and focuses on the novel therapeutical strategies offered by targeting the endocannabinoid system in the treatment of hypertension.
Keywords: Hypertension, Cannabinoids, Hemodynamics, Cardiac contractility
1. Introduction
The biological effects of marijuana and its main psychoactive ingredient, Δ9-tetrahydrocannabinol (THC), are mediated by specific G protein-coupled cannabinoid (CB) receptors. To date, two such receptors have been identified: the CB1 receptor, which is highly expressed in the brain (Matsuda et al., 1990), but is also present in heart and vascular tissues (Gebremedhin et al., 1999; Liu et al., 2000; Bonz et al., 2003), and the CB2 receptor, expressed primarily by haematopoietic and immune cells (Munro et al., 1993). Arachidonoyl ethanolamide or anandamide and 2-arachidonoylglycerol, the natural ligands of these receptors, are lipid-like substances called endocannabinoids (reviewed by Mechoulam et al., 1998; Kunos et al., 2000). Apart from their well-known neurobehavioral and immunological actions, cannabinoids also elicit potent cardiovascular effects, such as profound hypotension (Lake et al., 1997a; Hillard, 2000; Kunos et al., 2002; Randall et al., 2002; Ralevic et al., 2002). Although several lines of evidence indicate that the cardiovascular depressive effects of cannabinoids are mediated by peripherally localized CB1 receptors, recent studies also suggest the existence of as yet undefined endothelial and cardiac receptor(s) that mediate certain endocannabinoid-induced cardiovascular effects (Begg et al., 2003; Járai et al., 1999; Ford et al., 2002; Ho and Hiley, 2003; Kunos et al., 2002; Offertáler et al., 2003; O’Sullivan et al., 2004), however, the discussion of the latter is beyond the main scope of this summary. It has been established that the endocannabinergic system plays a pivotal role in cardiovascular regulation under various pathophysiological conditions associated with hypotension including hemorrhagic (Wagner et al., 1997), endotoxic (Varga et al., 1998) and cardiogenic shock (Wagner et al., 2001a, 2003), and advanced liver cirrhosis (Bátkai et al., 2001; Ros et al., 2002). Additional evidence also implicates this system in the regulation of blood pressure (Lake et al., 1997b; Li et al., 2003; Bátkai et al., 2004a). The present review focuses on the in vivo hypotensive and cardiodepressant effects of cannabinoids and on the novel therapeutical strategies offered by targeting the endocannabinergic system in the treatment of hypertension.
2. Cardiovascular effects of cannabinoids in vivo, role of CB1 and TRPV1 receptors
The in vivo cardiovascular effects of cannabinoids are complex and may comprise direct effects on the myocardium (Bonz et al., 2003) and vasculature (Gebremedhin et al., 1999; Járai et al., 1999; Wagner et al., 2001b), as well as modulation of autonomic outflow in the central (Niederhoffer and Szabo, 2000) and the peripheral nervous systems (Ishac et al., 1996; Malinowska et al., 1997). CB1 receptors are present in the myocardium where they mediate negative inotropy (Bonz et al., 2003; Pacher et al., 2004) and also in the vasculature (Gebremedhin et al., 1999; Liu et al., 2000), where they lead to vasodilation (Gebremedhin et al., 1999), and both of these sites are implicated in the hypotensive effect of anandamide (Wagner et al., 2001b; Pacher et al., 2004). The cardiovascular depressor effects of anandamide are devoid of a centrally mediated component (Varga et al., 1996), although some synthetic cannabinoids can cause centrally mediated sympathoexcitatory effects (Niederhoffer and Szabo, 2000). Presynaptic CB1 are also present in sympathetic nerve terminals where their stimulation inhibits norepinephrine release (Ishac et al., 1996) contributing to the bradycardic effects of anandamide (Wagner et al., 2001b).
Intravenous administration of anandamide in anesthetized rats initiates a triphasic blood pressure response with a major prolonged hypotensive effect (phase III) preceded by a transient, vagally mediated fall in heart rate and blood pressure (phase I) followed by a brief, non-sympathetically mediated pressor response of unknown mechanism (phase II) (Varga et al., 1995). Capsazepine and ruthenium red, antagonists of the TRPV1 receptor, dose-dependently inhibit the phase I bradycardic response in anesthetized rats, without affecting the phase III hypotension, which was abolished by the cannabinoid CB1 receptor antagonist SR141716 (Malinowska et al., 2001) and was also absent in CB1 receptor knockout mice (Ledent et al., 1999; Járai et al., 1999).
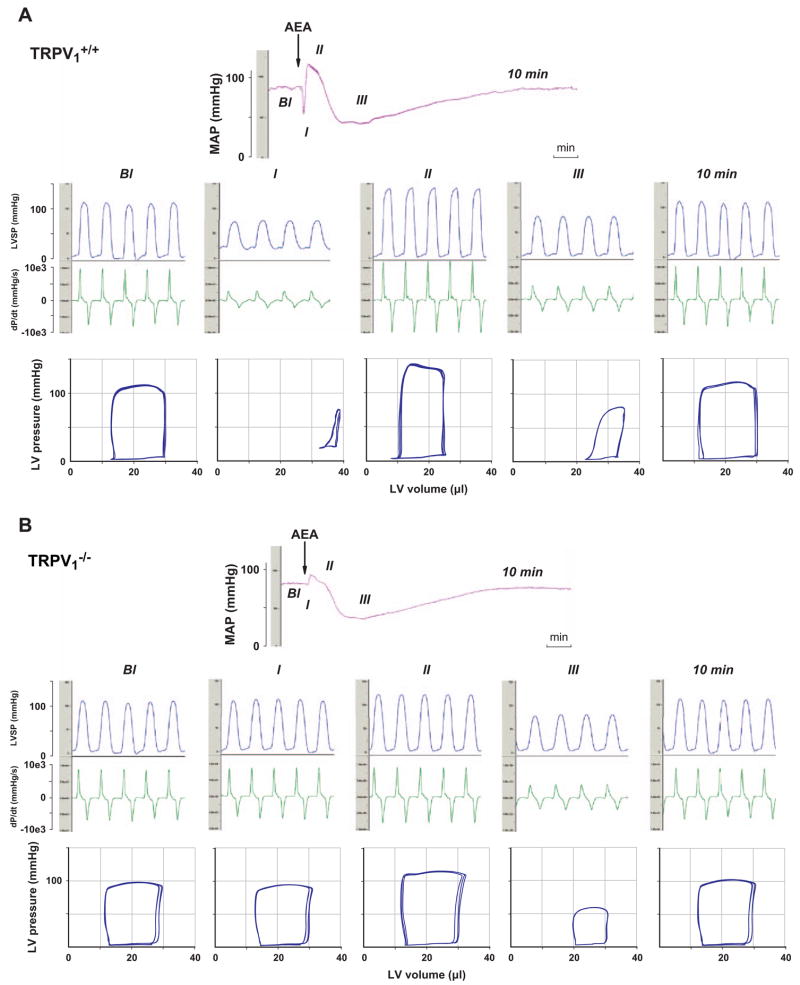
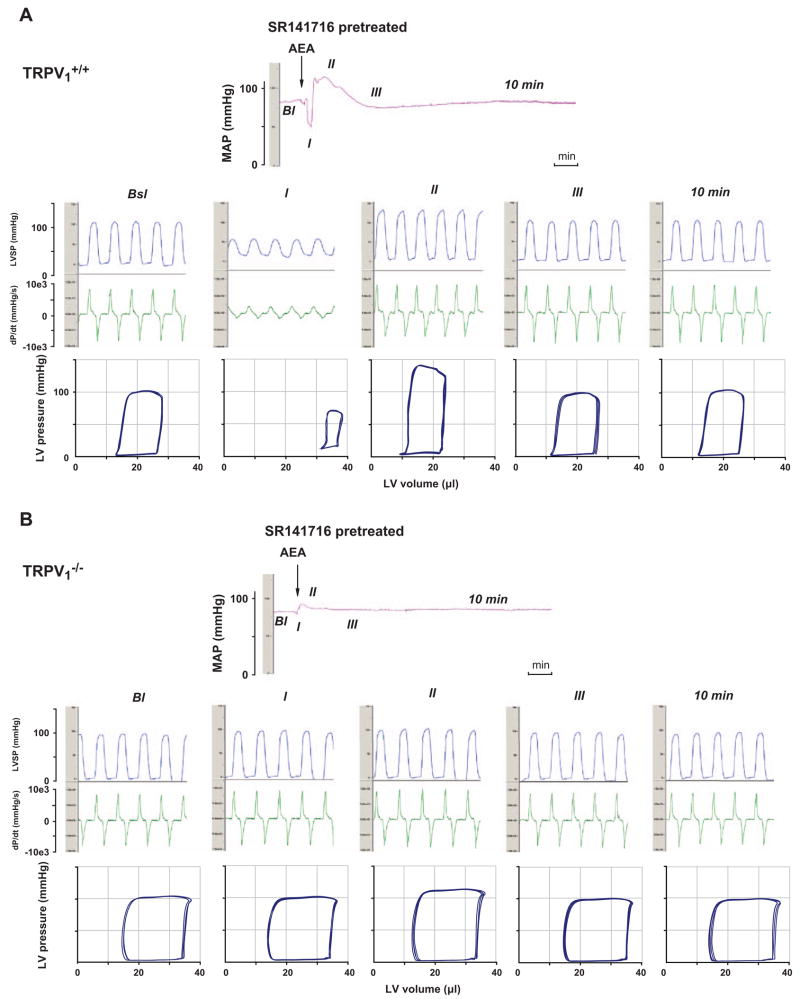
Anandamide also activates TRPV1 receptors on sensory nerve terminals, triggering the release of calcitonin gene-related peptide that elicits vasorelaxation in isolated blood vessels in vitro (Zygmunt et al., 1999), which raised the possibility that anandamide activation of TRPV1 receptors could contribute to its in vivo hypotensive effect. In a recent study (Pacher et al., 2004) we have tested this possibility by analyzing the detailed hemodynamic effects of anandamide in and mice. Similar to previous findings in the rat (Varga et al., 1995), bolus injections of anandamide (20 mg/kg i.v.) caused a triphasic effect in mice (Fig. 1A). The transient first phase lasted for a few seconds and was characterized by profound decreases in cardiac contractility and heart rate and an increase in total peripheral resistance (TPR), followed by a brief pressor response (phase II) associated with increased cardiac contractility. The third, prolonged hypotensive phase (phase III) was characterized by decreased cardiac contractility and TPR and it lasted up to 10 min (Fig. 1A). Pretreatment of the mice with a CB1 receptor antagonist, SR141716 (3 mg/kg i.v.), had no effect on the first and second phases of the response to anandamide, but completely prevented the subsequent hypotension and the associated decreases in TPR and cardiac contractility (Fig. 2A). In mice, the anandamide-induced initial component (phase I), present in littermates, was absent, and the phase II pressor response was also markedly reduced (Fig. 1B). In contrast, the subsequent prolonged hypotensive response accompanied by decreased cardiac contractility and TPR was similar to the responses observed in mice, and was similarly completely antagonized by pretreatment with SR141716 (Fig. 1B and 2B). In but not mice, capsaicin (10 and 100 μg/kg i.v.) evoked a brief blood pressure response similar to phase I and phase II responses to anandamide (Pacher et al., 2004).
Fig. 1.
Representative recordings of the effect of anandamide (20 mg/kg, i.v., AEA) on mean arterial pressure (MAP, top panel) and cardiac contractility (LVSP and dP/dt; middle panel) and pressure–volume relations (bottom panel) in anesthetized (A) and (B) mice. The five parts of the middle and bottom panels represent baseline conditions (Bl), phase I, phase II, and phase III of the anandamide response and conditions 10 min after injection (10 min). The arrows indicate the injection of the drug. Figure is reproduced with permission from Pacher et al. (2004).
Fig. 2.
Representative recordings of the effects of anandamide (20 mg/kg i.v., AEA) after pretreatment with SR141716 (3 mg/kg, i.v.) on mean arterial pressure (MAP, top panel) and cardiac contractility (LVSP and dP/dt; middle panel) and pressure–volume relationship (bottom panel) in anesthetized (A) and mice (B). The five parts of the middle and bottom panels represent the same five stages as described in the legend for Fig. 1. The arrows indicate the injection of the drugs. Figure is reproduced with permission from Pacher et al. (2004).
The above results clearly indicate that the prolonged hypotensive effect of anandamide involves a profound decrease in cardiac contractility in addition to a decrease in TPR, and these effects are mediated exclusively by CB1 and not receptors. The role of TRPV1 receptors is limited to the transient activation of the cardiogenic sympathetic reflex (Bezold–Jarisch reflex) by the very high initial plasma concentration of anandamide achieved after rapid bolus injections (Pacher et al., 2004).
3. Role of the endocannabinergic system in the hypotension associated with hemorrhagic, endotoxic, cardiogenic shock and advanced liver cirrhosis
The extreme, long-lasting, yet reversible, hypotension elicited by potent synthetic cannabinoids (Lake et al., 1997a) raised the question of whether endocannabinoids may contribute to the profound hypotension associated with various forms of shock. Indeed, as demonstrated by several studies over the last decade macrophage- and platelet-derived endocannabinoids contribute to the hypotension associated with hemorrhagic (Wagner et al., 1997), endotoxic (Varga et al., 1998; Liu et al., 2003; Bátkai et al., 2004; Wang et al., 2001) and cardiogenic shock (Wagner et al., 2001a, 2003). Furthermore, a similar mechanism seems to be involved in the vasodilated state associated with advanced liver cirrhosis (Bátkai et al., 2001; Ros et al., 2002), which is possibly secondary to the endotoxemia frequently found in patients with late-stage cirrhosis (Lumsden et al., 1988). Importantly, in all of the above-mentioned pathological conditions the hypotension could be reversed or inhibited by the CB1 antagonist SR141716. The involvement of CB1 receptors in many of these conditions was also suggested by the observation that circulating macrophages and platelets from endotoxemic or cirrhotic animals had elevated levels of endocannabinoids and, when isolated and injected into normal rats, these cells elicited SR141716-sensitive hypotension. In cirrhosis, the role of CB1 receptors was further supported by increase in CB1 receptor mRNA and binding sites in vascular endothelial cells from cirrhotic human livers (Bátkai et al., 2001).
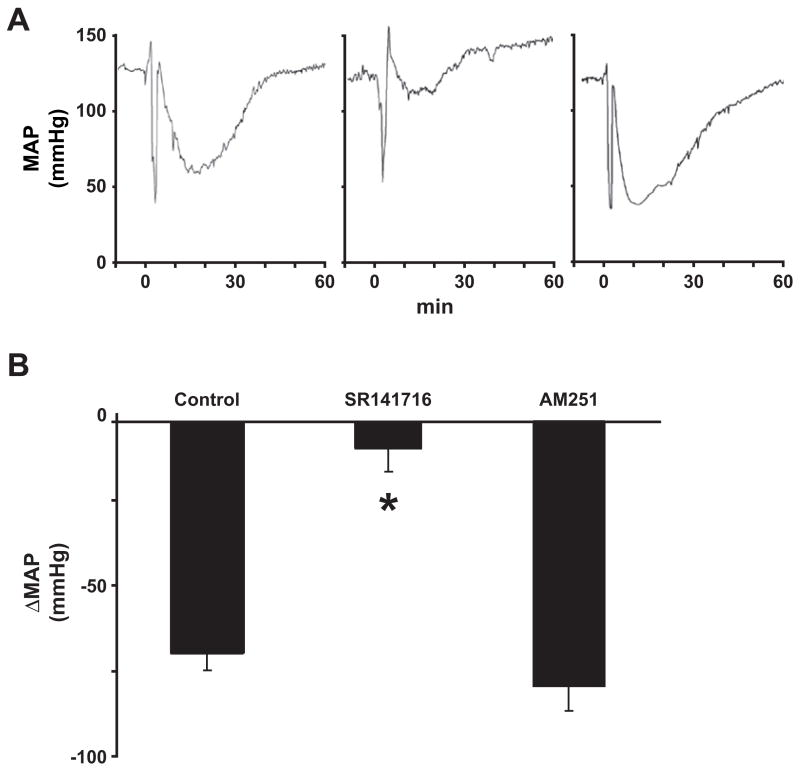
It should be emphasized, however, that SR141716 may also inhibit endothelial and/or myocardial receptor(s) distinct from conventional CB1 or CB2 (Járai et al., 1999; Ford et al., 2002; Ho and Hiley, 2003; O’Sullivan et al., 2004). Indeed, in a detailed hemodynamic study in anesthetized rats (Bátkai et al., 2004b), the acute hypotensive effect of LPS could be attributed to decreased cardiac contractility rather than decreased TPR, and it could be inhibited by SR141716, but not by AM251, an antagonist equipotent with SR141716 at CB1 receptors (Gatley et al., 1997) but lacking inhibitory potency at SR141716-sensitive endothelial and myocardial receptors (Fig. 3) (Ford et al., 2002; Ho and Hiley, 2003; O’Sullivan et al., 2004). In the same study we have demonstrated that LPS elicited similar, SR141716-sensitive hypotension in wild-type mice and in mice deficient in CB1 or both CB1 and CB2 receptors (Bátkai et al., 2004b). Taken together, these findings imply that receptors distinct from CB1 or CB2 are primarily responsible for the acute, SR141716-sensitive cardiodepressive effects of LPS, which are responsible for the hypotension. Anandamide is a ligand for such receptor(s) and LPS increases the synthesis of anandamide (Liu et al., 2003) in macrophages. The existence of additional putative endogenous ligands for such receptors needs to be explored.
Fig. 3.
LPS-induced hypotension is inhibited by SR141716 but not by AM251 in anesthetized rats. (A) Representative tracings of the effects of LPS on MAP in a rat pretreated with vehicle (left), SR141716 (3 mg/kg i.v.; center), and AM251 (3 mg/kg i.v.; right). (B) Mean ± SE ΔMAP from similar experiments from 4 to 5 animals. *Significant difference from values in vehicle + LPS-treated rats (P < 0.05). Figure is reproduced with permission from Bátkai et al. (2004b).
Treatment with the cannabinoid agonists THC or HU-210 improved endothelial function and increased survival in cardiogenic (Wagner et al., 2001a, 2003) and endotoxic shock (Varga et al., 1998), while blockade of CB1 receptors in hemorrhagic (Wagner et al., 1997) and cardiogenic shock (Wagner et al., 2001a, 2003) increased mortality, despite the increase in blood pressure. These results suggest that endocannabinoid-mediated cardiovascular effects appear to have survival value. The beneficial effects of cannabinoids could involve improvement of tissue oxygenation by counteracting the excessive sympathetic vasoconstriction triggered by hemorrhage or myocardial infarction and also potent anti-inflammatory effects (reviewed in Hiley and Ford, 2003, 2004; Walter and Stella, 2004).
4. Role of the endocannabinergic system in blood pressure regulation in hypertension
The intriguing possibility of using cannabinoid ligands as antihypertensive agents was first considered more than 30 years ago (Archer, 1974; Adams et al., 1977; Birmingham, 1973; Crawford and Merritt, 1979; Varma and Goldbaum, 1975; Zaugg and Kyncl, 1983). However, initial enthusiasm was tempered by the inability to separate the neurobehavioral and cardiovascular effects of cannabinergic ligands and also by a report of the development of rapid tolerance to the hypotensive and bradycardic effects of THC (Adams et al., 1976). Even though a subsequent study in spontaneously hypertensive rats (SHR) found no evidence for tolerance to the same effects during a 10-day treatment period (Kosersky, 1978), interest in this issue waned over the next 20 years.
The cloning of CB1 receptor in 1990, the introduction of selective CB receptor antagonists in the mid 1990s and the development of receptor knockout mice rekindled the interest in the cardiovascular regulatory effects of cannabinoids. Treatment of normotensive rats or mice with CB1 antagonists alone was found not to affect blood pressure (Lake et al., 1997a; Varga et al., 1995), and baseline blood pressure was similar in CB1 knockout mice and their wild-type littermates (Járai et al., 1999; Ledent et al., 1999). Further studies demonstrated that a relatively modest hypotensive effect of anandamide was present in anesthetized (Lake et al., 1997a; Varga et al., 1995) but not in conscious normotensive rats (Stein et al., 1996; Lake et al., 1997b), and a lack of significant hypotension following inhibition of anandamide transport (Calignano et al., 1997). All of these findings indicate the absence of an endocannabinergic ‘tone’ in the maintenance of normal blood pressure. In contrast, both anandamide (Lake et al., 1997b; Bátkai et al., 2004a) and THC (Kosersky, 1978) evoke larger and longer lasting hypotension in SHR than in normotensive rats, and the effect in the former does not depend on the absence or presence of anesthesia (Lake et al., 1997b). Consistent with these observations, THC inhalation was found to result in a greater and longer lasting decrease of arterial blood pressure in hypertensive as compared to normotensive individuals (Crawford and Merritt, 1979). Although the mechanisms behind this increased sensitivity require further clarification, it strongly suggests a role for the endocannabinergic system in cardiovascular regulation in hypertension.
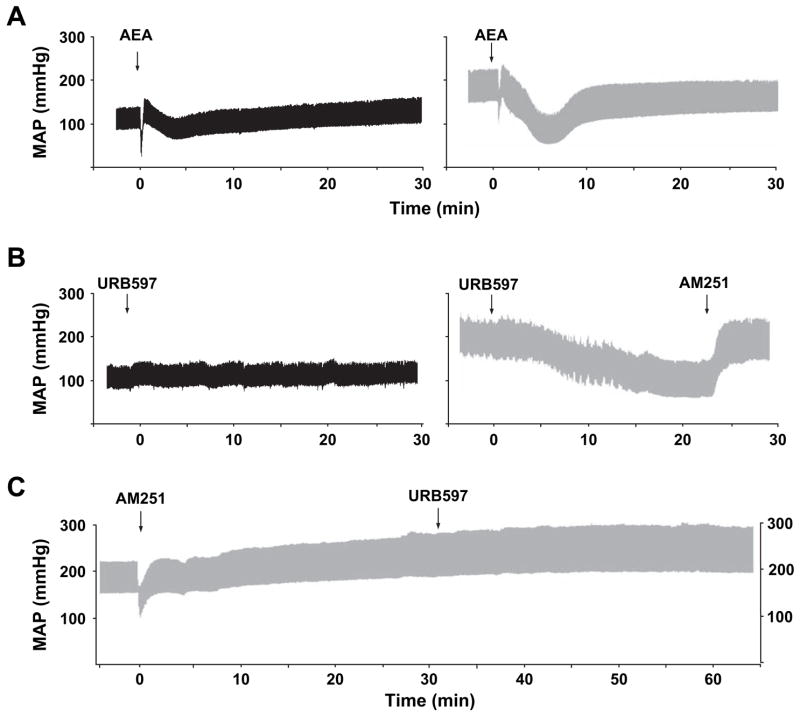
To explore this possibility, we employed a sophisticated pressure–volume (PV) analysis system (Pacher et al., 2003) to determine the hemodynamic effects of cannabinoid agonists and antagonists in three different models of experimental hypertension (Bátkai et al., 2004a). In pentobarbital-anesthetized normotensive Wistar Kyoto rats (WKY), i.v. injection of the CB1 antagonists SR141716 and AM251, as already mentioned above, had no effect on blood pressure and cardiac contractility. On the contrary, in SHR the same two antagonists, but not a CB2 antagonist, evoked marked and sustained further increase in blood pressure and cardiac contractility without changing the heart rate (Bátkai et al., 2004a). A similar pressor response to CB1 blockade was also evident in angiotensin II-induced and Dahl salt-sensitive hypertensive rats, but not in their corresponding normotensive controls (Bátkai et al., 2004a). The most likely explanation of these findings is the existence of an endocannabinergic tone in hypertension that limits the elevation of blood pressure and cardiac contractile performance through tonic activation of cardiac and probably vascular CB1. Indeed, we have found increased expression of cardiac and vascular CB1 in SHR as compared to their normotensive controls (Bátkai et al., 2004a). Consistent with previous results, anandamide induced more pronounced and prolonged hypotension in SHR but also in AII-induced hypertensive rats (Fig. 4A) than in the corresponding normotensive controls, and the effect of anandamide was primarily mediated by decreased cardiac contractility and only to a lesser extent by a reduction in TPR (Bátkai et al., 2004a). The effect of the synthetic CB agonist HU-210 was similarly potentiated in SHR compared to WKY rats. Importantly, the hypotensive and cardiodepressive effects of anandamide in hypertensive rats were fully prevented or reversed by CB1 antagonists, but were unaffected by capsazepine, an antagonist of TRPV1 receptor (Bátkai et al., 2004a).
Fig. 4.
Representative recordings of the effects of anandamide (10 mg/kg i.v., AEA; panel A) and the FAAH antagonist URB597 (10 mg/kg i.v.; panel B) on blood pressure in normotensive (left column) and in angiotensine II-induced hypertensive (right column) anesthetized rats, and reversal (panel B left) or prevention (panel C) of the hypotensive effects of URB597 in hypertensive rats by the CB1 antagonist AM251. The arrows indicate the injection of the drugs.
Next, we examined whether the effects of endogenous anandamide are similarly potentiated in hypertension, by testing the hemodynamic effects of a potent inhibitor of fatty acid amidohydrolase (FAAH), the enzyme responsible for the degradation of anandamide in vivo. The hemodynamic effects of the FAAH inhibitor, URB597 (10 mg/kg i.v.) (Kathuria et al., 2003), were remarkably similar to those of exogenous anandamide (Fig. 4B) (Bátkai et al., 2004a). In hypertensive rats, URB597 decreased arterial pressure to near-normotensive values by decreasing cardiac contractility and TPR, while in normotensive rats it had no detectable hemodynamic effects (Fig. 4B). URB597 raised the concentration of anandamide in the myocardium, and its hemodynamic effects were completely prevented (Fig. 4C) or reversed (Fig. 4B) by the CB1 antagonist AM251 but not by the TRPV1 antagonist capsazepine. This indicates that the action of URB597 is fully accounted for by the endocannabinoid-mediated stimulation of CB1. Treatment with the anandamide transport inhibitors AM404 (10 mg/kg i.v.) or OMDM-2 (5 mg/kg i.v.), which increase anandamide levels at the receptors by blocking its cellular uptake, also induced greater blood pressure reduction in hypertensive animals than in normotensive controls (Bátkai et al., 2004a).
CB1 receptors in the brain mediate the addictive psychological effects of marijuana, so treating a chronic disease with a drug that directly stimulates CB1 receptors would be socially unacceptable. Recent evidence indicates, however, that targeting the endocannabinoid system by preventing anandamide degradation may not have the same potential for abuse (Kathuria et al., 2003). Further studies are in progress to test the antihypertensive efficacy of FAAH inhibitors in awake hypertensive animals.
5. Conclusion
The endocannabinergic system plays an important cardiovascular regulatory role not only in pathophysiological conditions associated with excessive hypotension but also in hypertension. Thus, the pharmacological manipulation of this system may offer novel therapeutic approaches in a variety of cardiovascular disorders.
References
- Adams MD, Chait LD, Earnhardt JT. Tolerance to the cardiovascular effects of delta9-tetrahydrocannabinol in the rat. Br J Pharmacol. 1976;56(1):43–48. doi: 10.1111/j.1476-5381.1976.tb06957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD, Earnhardt JT, Martin BR, Harris LS, Dewey WL, Razdan RK. A cannabinoid with cardiovascular activity but no overt behavioral effects. Experientia. 1977;33:1204–1205. doi: 10.1007/BF01922330. [DOI] [PubMed] [Google Scholar]
- Archer RA. The cannabinoids: therapeutic potentials. Annu Rev Med Chem. 1974;9:253–259. doi: 10.1016/s0065-7743(08)61448-7. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbascheck R, Garcia N, Jr, Sanyal AJ, Kunos G. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Pacher P, Járai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol. 2004b;287(2):H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertáler L, Mackie K, Rudd A, Bukoski RD, Kunos G. Endocannabinoids acting at CB1 receptors regulate cardiovascular function in hypertension. Circulation. 2004a;110(14):1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Bátkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278(46):46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Birmingham MK. Reduction by 9-tetrahydrocannabinol in the blood pressure of hypertensive rats bearing regenerated adrenal glands. Br J Pharmacol. 1973;48(1):169–171. doi: 10.1111/j.1476-5381.1973.tb08236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonz A, Laser M, Kullmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Beltramo M, Makriyannis A, Piomelli D. Potentiation of anandamide hypotension by the transport inhibitor, AM404. Eur J Pharmacol. 1997;337(1):R1–R2. doi: 10.1016/s0014-2999(97)01297-1. [DOI] [PubMed] [Google Scholar]
- Crawford WJ, Merritt JC. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int J Clin Pharmacol Biopharm. 1979;17(5):191–196. [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:PL191–PL197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol. 1999;266:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- Hiley CR, Ford WR. Endocannabinoids as mediators in the heart: a potential target for therapy of remodelling after myocardial infarction? Br J Pharmacol. 2003;138(7):1183–1184. doi: 10.1038/sj.bjp.0705155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol Rev Camb Philos Soc. 2004;79(1):187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. Endocannabinoids and vascular function. J Pharmacol Exp Ther. 2000;294:27–32. [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kosersky DS. Antihypertensive effects of delta9-tetrahydrocannabinol. Arch Int Pharmacodyn Ther. 1978;233:76–81. [PubMed] [Google Scholar]
- Kunos G, Bátkai S, Offertáler L, Mo F, Liu J, Karcher J, Harvey-White J. The quest for a vascular endothelial cannabinoids receptor. Chem Phys Lipids. 2002;121:45–56. doi: 10.1016/s0009-3084(02)00145-7. [DOI] [PubMed] [Google Scholar]
- Kunos G, Járai Z, Bátkai S, Goparaju SK, Ishac EJ, Liu J, Wang L, Wagner JA. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997a;281:1030–1037. [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997b;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- Liu J, Bátkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Gao B, Kunos G. LPS induces anandamide synthesis in macrophages via CD14/MAPK/PI3K/NF-κB independently of platelet activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, Kunos G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346:835–840. [PMC free article] [PubMed] [Google Scholar]
- Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromatographic assay in portal, hepatic and peripheral blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Godlewski G, Boucher B, Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoids CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young CA, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabionoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J Pharmacol Exp Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA, Randall MD. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA) Br J Pharmacol. 2004;141(5):803–812. doi: 10.1038/sj.bjp.0705643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol (Lond) 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabo E, Ungvári Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Smart D. Cannabinoid modulation of sensory neurotransmission via cannabinoid and vanilloid receptors: roles in regulation of cardiovascular function. Life Sci. 2002;71:2577–2594. doi: 10.1016/s0024-3205(02)02086-6. [DOI] [PubMed] [Google Scholar]
- Randall MD, Harris D, Kendall DA, Ralevic V. Cardiovascular effects of cannabinoids. Pharmacol Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Ros J, Claria J, To-Figueras J, Planaguma A, Cejudo-Martin P, Fernandez-Varo G, Martin-Ruiz R, Arroyo V, Rivera F, Rodes J, Jimenez W. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br J Pharmacol. 1996;119:107–114. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Varma DR, Goldbaum D. Effect of delta9-tetrahydrocannabinol on experimental hypertension in rats. J Pharm Pharmacol. 1975;27(10):790–791. doi: 10.1111/j.2042-7158.1975.tb09406.x. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, Kunos G, Ertl G. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol. 2001a;38:2048–2054. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Hu K, Karcher J, Bauersachs J, Schafer A, Laser M, Han H, Ertl G. CB(1) cannabinoid receptor antagonism promotes remodeling and cannabinoid treatment prevents endothelial dysfunction and hypotension in rats with myocardial infarction. Br J Pharmacol. 2003;138(7):1251–1258. doi: 10.1038/sj.bjp.0705156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Járai Z, Bátkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB(1) receptors. Eur J Pharmacol. 2001b;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141(5):775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Ito Y, Hashiguchi T, Kitajima I, Yamakuchi M, Shimizu H, Matsuo S, Imaizumi H, Maruyama I. Simultaneous measurement of anandamide and 2-arachidonoylglycerol by polymyxin B-selective adsorption and subsequent high-performance liquid chromatography analysis: increase in endogenous cannabinoids in the sera of patients with endotoxic shock. Anal Biochem. 2001;294:73–82. doi: 10.1006/abio.2001.5015. [DOI] [PubMed] [Google Scholar]
- Zaugg HE, Kyncl J. New antihypertensive cannabinoids. J Med Chem. 1983;26(2):214–217. doi: 10.1021/jm00356a017. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang HH, Sorgard M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]