SUMMARY
Klf4 (formerly GKLF) is a zinc-finger transcription factor expressed in the epithelia of the skin, lungs, gastrointestinal tract and several other organs. In vitro studies have suggested that Klf4 plays an important role in cell proliferation and/or differentiation. Mice homozygous for a null mutation in Klf4 die within 15 hours of birth and show selective perturbation of late-stage differentiation structures in the epidermis, but the function of Klf4 in the gastrointestinal tract has not been investigated. To address this issue, we have generated Klf4−/− mice by homologous recombination in embryonic stem cells. In this study, we provide the first in vivo evidence that Klf4 is a goblet cell-specific differentiation factor in the colon. Klf4−/− mice exhibit normal cell proliferation and cell death rates in the colon on postnatal day 1. However, Klf4−/− mice demonstrate a 90% decrease in the number of goblet cells in the colon, show abnormal expression of the goblet cell-specific marker Muc2 by in situ hybridization, have abnormal staining of the colonic epithelium with Alcian Blue for acidic mucins, and lack normal goblet cell morphology by ultrastructural analysis. All other epithelial cell types are present in the colon of Klf4−/− mice. In summary, Klf4 plays a crucial role in colonic epithelial cell differentiation in vivo.
Keywords: Klf4, Goblet cell, Cell differentiation, Colon, Mouse
INTRODUCTION
The mammalian gastrointestinal tract undergoes continuous self-renewal throughout life (Stappenbeck et al., 1998). Stem cells, specialized epithelial cells characterized by the ability for self-maintenance, provide the basis for ongoing cell replacement. These cells divide asymmetrically, producing one stem cell, which remains multipotent and undifferentiated, and one daughter cell, which is committed to differentiation (Bach et al., 2000). Committed daughter cells undergo several additional rounds of cell division while migrating from the proliferative to the differentiated compartment. Upon differentiation, cells lose the ability to divide and eventually die. The mature gastrointestinal system therefore comprises, at all times, both undifferentiated, pluripotent stem cells and differentiated, functional epithelial cells.
In the colon, stem cells are located at the base of the crypts, with proliferation occurring in the lower third of these epithelial folds. Epithelial cells migrate in ordered cohorts towards the lumenal surface, differentiating to form one of three cell types: colonocytes, goblet cells and enteroendocrine cells. At the lumenal surface, cells enter a death program characterized by senescence and/or apoptosis, and are sloughed into the lumen (Potten, 1997). More than 80% of all epithelial tissue in the colon comprises colonocytes (Chang and Leblond, 1971). Colonocytes have both absorptive and secretory functions, absorbing water, sodium and short-chain fatty acids while secreting bicarbonate and potassium. Goblet cells, meanwhile, elaborate mucins, trefoil proteins and other factors that help protect the intestinal mucosa from injury and facilitate tissue repair (Deplancke and Gaskins, 2001). In the adult mouse descending colon, cells of this lineage form about 16% of the total crypt cell population (Karam, 1999). Enteroendocrine cells, the least common of the colonic epithelial cells, secrete various gastrointestinal hormones. Enteroendocrine cells normally represent less than 1% of colonic epithelial cells (Tsubouchi and Leblond, 1979). The three cell lineages of the colon are first evident at the endoderm-intestinal transition, which occurs on embryonic day 15–16 in mice (Traber, 1999). The epithelium of the colon continues to mature after birth, with the expression of certain genes delayed until the suckling-weaning transition around postnatal day 17 (Traber and Wu, 1995; Traber, 1999).
Cell proliferation, differentiation, and death in the gastrointestinal tract are under tight genetic control. In the intestine, complex signals mediate cellular differentiation programs, but up to now these signals have not been well understood. The basic helix-loop-helix (bHLH) protein BETA2 has been suggested to control terminal differentiation of secretin-producing enteroendocrine cells (Mutoh et al., 1998). Hes1 is a general repressor of bHLH factor-controlled endodermal endocrine cell differentiation in the stomach and proximal small intestine, as Hes1-null mice show excessive differentiation of multiple enteroendocrine cell types, as well as more intestinal goblet cells and fewer enterocytes (Jensen et al., 2000). A constitutively active form of the Rho GTPase Rac1 induces precocious differentiation of Paneth cells and enterocytes in the small intestine, without affecting cell division (Stappenbeck and Gordon, 2000). Recently, loss of the bHLH-factor Math1 (Atoh1 – Mouse Genome Informatics) was shown to deplete intestinal goblet, enteroendocrine and Paneth cells, but not enterocytes (Yang et al., 2001). Goblet, enteroendocrine and Paneth cells may arise from a progenitor that expresses Math1, while enterocytes arise from a progenitor that is Math1 independent. Taken together, these results suggest a hierarchical regulation of intestinal epithelial cell differentiation. Still, much of the complex regulatory network of differentiation remains to be understood.
The roles of the epithelial cells of the intestine have been studied in vitro and in vivo. Specific markers, such as Tff3 and Muc2 for intestinal goblet cells, have been identified, and targeted ablation of Paneth cells and goblet cells using transgenic mice has yielded insight into the function of these different cell types (Garabedian et al., 1997; Itoh et al., 1999a; 1999b; van Klinken et al., 1999). Mice deficient in Muc2, the most abundant murine intestinal mucin, display aberrant intestinal crypt morphology, with altered cell maturation and migration (Velcich et al., 2002). Muc2-null mice develop small intestinal adenomas, which progress to invasive adenocarcinomas, and rectal tumors, linking a specific product of intestinal goblet cells to the suppression of cancer. While keratinocyte growth factor, which induces proliferation of epithelial cells throughout the gastrointestinal tract, promotes goblet cell differentiation in vitro (Housley et al., 1994; Iwakiri and Podolsky, 2001a), the identification of differentiation factors specific for the goblet cell lineage in vivo has remained elusive.
Klf4 (formerly GKLF) is a zinc-finger transcription factor expressed in the epithelia of the skin, the lungs, the gastrointestinal tract and several other organs (Garrett-Sinha et al., 1996; Shields et al., 1996; Ton-That et al., 1997). In vitro studies have suggested a role for Klf4 in cell proliferation and/or differentiation (Bieker, 2001). In cultured fibroblasts, Klf4 mRNA is found at high levels in growth-arrested cells and is nearly undetectable in cells that are in the exponential phase of proliferation (Shields et al., 1996). Moreover, DNA synthesis is inhibited in fibroblasts transfected with a Klf4-expressing plasmid construct (Shields et al., 1996). Constitutive overexpression of Klf4 in a human colonic adenocarcinoma cell line (HT-29) decreases [3H]thymidine incorporation, whereas suppression of the Klf4 gene with antisense cDNA increases DNA synthesis (Shie et al., 2000). In the human colon cancer cell line RKO, inducible expression of Klf4 leads to a block at the G1/S transition phase of the cell cycle (Chen et al., 2001). In esophageal squamous epithelial cells, Klf4 transcriptionally activates the promoters for the late differentiation genes keratin 4 and Epstein-Barr virus ED-L2 (Jenkins et al., 1998).
In the embryo, expression of Klf4 mRNA is seen in the epithelium of the colon by embryonic day 15.5 (Garrett-Sinha et al., 1996). In the adult mouse, Klf4 mRNA localizes to the upper region of the crypt epithelium, a region that consists of cells that have undergone growth arrest and begun to differentiate into mature colonocytes (Shields et al., 1996). Mice homozygous for a null mutation in Klf4 are born at the expected Mendelian ratio but die within 15 hours of birth and show selective perturbation of the late-stage differentiation structures of the epidermis (Segre et al., 1999). The early lethality of Klf4-null mice has been attributed to the loss of skin barrier function as no defects in any organ other than skin were noted. To date, nothing has been reported about the function of Klf4 in gastrointestinal epithelial proliferation and differentiation in vivo.
In this study, we provide the first in vivo evidence that Klf4 is a goblet cell-specific differentiation factor in the colon. Using gene targeting, we demonstrate that Klf4 is required for the terminal differentiation of colonic goblet cells. This role of Klf4 in the colon is specific to the goblet cell lineage, as both colonocytes and enteroendocrine cells appear to undergo normal maturation.
MATERIALS AND METHODS
Generation of Klf4 mutant mice
A λ phage clone containing the murine Klf4 gene was isolated from a mouse 129SvEv library, and a targeting vector was designed to flank exons 2 and 3 of the Klf4 gene with loxP sites in introns 1 and 3 as follows. A neomycin resistance/thymidine kinase cassette flanked by two loxP sites was inserted into the HindIII site in intron 1. An oligonucleotide containing a loxP site was synthesized and ligated into the XbaI site in intron 3, destroying the original XbaI site. The targeting construct was then introduced into TL1 embryonic stem (ES) cells (Labosky et al., 1997), and the G418 resistant ES clones screened for homologous recombinants using a 400 bp ScaI-XhoI fragment located 5′ to the λ phage genomic clone and lacking repetitive sequences. ES clones containing the targeted Klf4 gene were selected and transiently transfected with a Cre-recombinase expression plasmid (Gu et al., 1994). ES cells with excision of the neomycin resistance/thymidine kinase cassette were then selected by culture in the presence of gancyclovir. These cells were subsequently screened by Southern blot analysis and PCR for clones in which the sequences between the 5′ most and the middle Cre-recombinase were deleted, resulting in a functionally wild type Klf4 gene flanked by loxP sites.
ES cells from a floxed Klf4 clone were injected into blastocysts derived from C57BL/6 mice. Blastocysts were transferred to pseudopregnant females and chimeric offspring were identified by the presence of agouti hair. Chimeric males were mated to C57BL/6 females to obtain ES cell-derived offspring. Mice heterozygous for the floxed Klf4 allele were mated with mice expressing Cre under the control of the CMV promoter to generate the null allele (White et al., 1997). The CMV-Cre transgene was then bred out, and heterozygous mutants (Klf4+/−) were mated inter se to generate homozygous mutants (Klf4−/−). Mice studied were on a mixed genetic background.
Embryos and mice were genotyped by PCR using three primers: Klf4 exon1, CTGGGCCCCCACATTAATGAG; Klf4 exon2, TCAGACTGTACCGACAGTCGC; and Klf4 intron3, TTTTGTCGGTCTTGCCGAGAC. PCR reactions were carried out for 32 cycles (95°C for 40 seconds; 60°C for 40 seconds; 72°C for 60 seconds) in a buffer containing 1.5 mM MgCl2. The wild-type allele produced a band of 172 bp, the floxed allele a band of 296 bp and the null allele a band of 425 bp.
Histology and cell counting
Mice were sacrificed at postnatal day 1 and colons were removed and fixed in 4% paraformaldehyde, embedded in paraffin, and 5 μm sections were applied to Probe-on Plus™ slides (Fisher). Sections were deparaffinized in xylene, then rehydrated in ethanol and stained with either Hematoxylin and Eosin or Alcian Blue and nuclear fast red as previously described (Kaestner et al., 1997). For Hematoxylin and Eosin staining, sections were incubated in Hematoxylin for 2.5 minutes and Eosin for 15 seconds. For Alcian Blue staining, sections were incubated in 1% Alcian Blue in 3% acetic acid, pH 2.5, for 30 minutes and 0.1% nuclear Fast Red for 10 seconds. After staining, sections were dehydrated in ethanol, cleared with xylene and mounted with Cytoseal™ 60 (Stephens Scientific). Images were captured on a Nikon Microphot FX microscope. The ratio of goblet cells to total epithelial cells was obtained by counting, in a blinded fashion, total epithelial cells and Alcian Blue-positive cells with goblet morphology from ten high-powered fields of well-oriented colonic cross-sections (Itoh et al., 1999a).
Electron microscopy
Mice were sacrificed at postnatal day 1, and tissues were removed and fixed overnight in 2% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.4, at 4°C. Sections were then prepared for electron microscopy using a standard protocol (Smith et al., 1985). Briefly, tissues were washed with 0.1M sodium cacodylate, post-fixed with 2% osmium tetroxide in 0.1M sodium cacodylate and washed at 4°C. Tissues were stained with 2% aqueous uranyl acetate, dehydrated in graded alcohol and embedded in Embed 812 resin (Electron Microscopy Sciences). Sections 7×10−8 m thick were cut using a Leica Ultracut S microtome and mounted on 200 mesh thin bar copper grids, stained in 7% aqueous uranyl acetate, and counterstained in bismuth subnitrite. Digital Images were collected on a JEOL JEM 1010 equipped with a Hamamatsu CCD camera and AMT 12-HR imaging software.
In situ hybridization
Tissues were fixed in Bouin’s fixative (for Klf4) or 4% PFA (for Muc2), embedded in paraffin, and 5 μm sections were applied to Probe-on Plus™ slides (Fisher). Sections were deparaffinized in xylene, then rehydrated in ethanol. Digoxigenin-labeled sense and antisense probes were transcribed in vitro from the following plasmids: a 220 bp fragment of the mouse Klf4 full-length cDNA (GenBank Accession Number, U20344) containing the nucleotides 1360–1574 cloned into the pBluescript SK (Stratagene) and a 357 bp fragment of the mouse Muc2 partial cDNA (GenBank Accession Number, AJ010437) containing the nucleotides 337 to 694 cloned into pCRII (Invitrogen). In situ hybridization was performed as described by Wilkinson (Wilkinson, 1992). Anti-digoxigenin antibody (Roche Diagnostics) was used at a dilution of 1:2000. The slides were developed overnight using NBT/BCIP substrate (Roche Diagnostics), counterstained with 0.1% nuclear Fast Red for 5 seconds or less, dehydrated and mounted with Cytoseal™ 60 (Stephens Scientific). Images were captured on a Nikon Microphot FX microscope.
Immunohistochemistry, cell proliferation and cell death assays
Mice on postnatal day 1 were injected 1 hour before sacrifice with BrdU labeling reagent (Zymed) as per manufacturer’s protocol. Tissues were removed and fixed in 4% paraformaldehyde, embedded in paraffin and 5 μm sections were applied to Probe-on Plus™ slides (Fisher). The primary antibodies used were mouse monoclonal anti-BrdU (1:500, Roche Diagnostics) and rabbit anti-chromogranin A (1:3000, Diasorin). Apototic cells were detected using the In Situ Death Detection Kit (Roche Diagnostics) as per manufacturer’s protocol. Anti-BrdU antibody was detected with biotinylated goat anti-mouse secondary antibody (1:200, Vector), alkaline phosphatase-labeled avidin-biotin complex (Biostain Super ABC, Biomeda) and NBT/BCIP substrate (Roche Diagnostics). Anti-chromogranin antibody was detected with biotinylated goat anti-rabbit secondary antibody (1:200, Vector), peroxidase labeled avidin-biotin complex (Vectastain ABC Kit, Vector) and DAB substrate-chromogen solution (Vector).
After wax removal and rehydration, slides were immersed in 0.01M citric acid buffer (pH 6.0) and microwaved to boil for 7 minutes, cooled, and washed twice in phosphate-buffered saline (PBS). Slides for chromogranin detection were incubated for 15 minutes in 1.5% hydrogen peroxide and washed twice in PBS prior to proceeding. Nonspecific binding of avidin-biotin was blocked (Avidin/Biotin Blocking Kit, Vector), and slides were washed once with PBS, then incubated for 15 minutes at room temperature with Protein Blocking Agent (Immunotech). Primary antibody diluted with 0.1% bovine serum albumin, 0.2% Triton X-100 in PBS (PBT) was applied and the tissue incubated overnight at 4°C in a moist chamber. After washing with PBS, secondary antibody diluted with PBT was applied and the tissue incubated for 40 minutes at 37°C in a moist chamber. The slides were developed using the avidin-biotin complex and substrate as per the manufacturer’s protocols, counterstained with Hematoxylin for 2.5 minutes or 0.1% nuclear fast red for 10 seconds, dehydrated and mounted with Cytoseal™ 60 (Stephens Scientific). Images were captured on a Nikon Microphot FX microscope.
RESULTS
Gene targeting of the Klf4 gene
To investigate the role of the zinc-finger transcription factor Klf4 in gastrointestinal proliferation and differentiation in vivo, we generated mice lacking a functional product of this gene by homologous recombination in mouse embryonic stem cells. The open-reading frame of the Klf4 gene encodes a polypeptide of 483 amino acids with a predicted molecular weight of 53 kDa. The entire polypeptide sequence of Klf4 with the exception of the first amino acid is encoded by exons 2, 3 and 4 (Mahatan et al., 1999). Deletion of exons 2 and 3 produces a frameshift mutation in exon 4. Concerned that the loss of Klf4 might produce early lethality, we employed the Cre/loxP system to generate both Klf4-null mice and Klf4 floxed mice, which could be used for conditional knockout experiments (Nagy, 2000). We chose to flank exons 2 and 3 with loxP sites, in order to generate our Klf4 mutant mice. This targeting strategy is depicted in Fig. 1A. ES cells were screened by Southern blot using a 400 bp probe external to the sequence of the targeting vector.
Fig. 1.
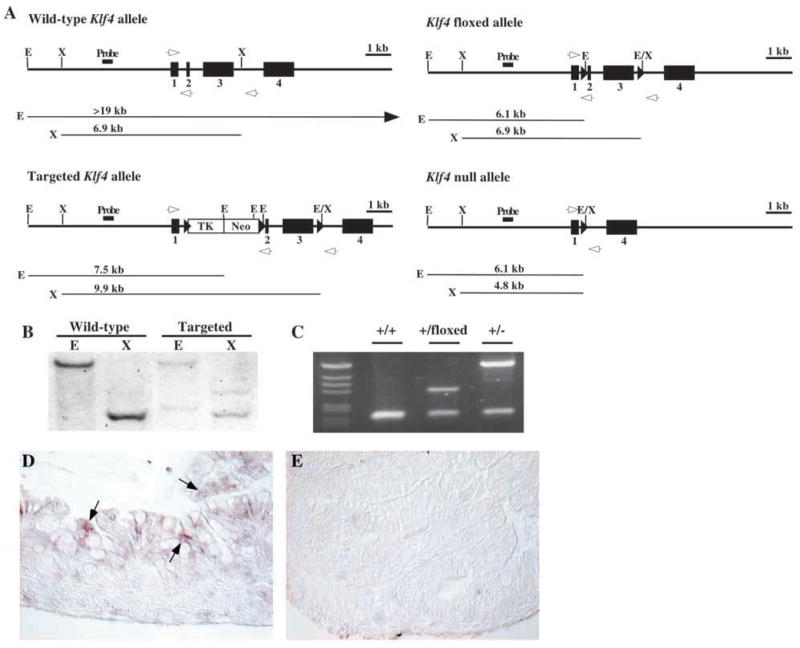
Targeting strategy and in situ hybridization for Klf4. (A) The four alleles used in the gene targeting of Klf4 are shown with predicted band sizes of restriction fragments used in Southern blot analysis. Deletion of exons 2 and 3 produces a frame shift in exon 4. Exon 1 encodes only the first amino acid of the Klf4 protein. A 400 bp fragment external to the targeting vector was used as the probe for Southern blot. White arrows depict PCR primer locations. (B) Southern blot showing the wild-type and targeted Klf4 bands. (C) PCR screening for Klf4 revealed 172 bp wild-type, 296 bp floxed and 425 bp null bands. (D,E) In situ hybridization for Klf4 in colon from postnatal day 1 mice. (D) Wild-type mice demonstrated expression of Klf4 mRNA (arrows) throughout the epithelium. (E) Klf4−/− mice showed no expression of Klf4 mRNA, confirming the successful deletion of Klf4. E, EcoRI; X, XbaI.
Of 345 stably transfected ES cell clones, five had undergone homologous recombination to yield the targeted Klf4 allele. Southern blot analysis of a wild-type and targeted clone is shown in Fig. 1B. Targeted clones were then transiently transfected with a Cre-expressing plasmid to yield the floxed Klf4 allele containing loxP sites in introns 1 and 3. Germline chimeras and mice heterozygous for the floxed Klf4 allele were obtained. Mice heterozygous for the floxed Klf4 allele were then crossed with a CMV-Cre transgenic mouse line to generate Klf4-null mice (White et al., 1997). These mice were then bred to remove the CMV-Cre transgene. Screening for mice carrying Klf4 floxed and Klf4 null alleles was performed by PCR (Fig. 1C).
The general appearance of the mice homozygous for a null mutation in Klf4 (Klf4−/−) has been previously noted (Segre et al., 1999). Our results were consistent with the prior report. Klf4−/− mice were born at approximately normal Mendelian frequency and were initially indistinguishable from wild-type littermates. However, all Klf4−/− mice died on postnatal day 1. No differences were observed between heterozygous and wild-type animals in overall development, histology, growth characteristics or gene expression.
In situ hybridization for Klf4 was performed on colon of wild-type and Klf4−/− mice on postnatal day 1. In Fig. 1D, wild-type mice demonstrated epithelial-specific expression of Klf4 mRNA in the colon (arrows). In the colon of adult mice, Klf4 expression is confined to the upper third of the crypt epithelium (Shields et al., 1996). In newborn mice, Klf4 expression was detected diffusely throughout the epithelium. Klf4−/− mice showed no expression of Klf4 mRNA in the colon, confirming the successful deletion of Klf4 (Fig. 1E).
Cell proliferation and cell death are normal in the colon of Klf4−/− mice
As Klf4 has been suggested to be a negative regulator of cell proliferation in vitro (Chen et al., 2001; Shie et al., 2000; Shields et al., 1996), we investigated the role of Klf4 in cell proliferation in vivo using immunohistochemistry for bromodeoxyuridine (BrdU). BrdU injected into the peritoneum reaches the gastrointestinal epithelium within minutes and is incorporated into the DNA of cells in S phase. For our experiments, wild-type and mutant mice were injected with BrdU and sacrificed 1 hour after injection. Staining for BrdU revealed no difference in the number of labeled cells in the colonic epithelium of wild-type mice (Fig. 2A) versus Klf4−/−mice (Fig. 2B). Moreover, no spatial differences in BrdU staining were observed. Thus, Klf4−/− mice on postnatal day 1 showed no change in the rate of cell proliferation in the colon. Wild-type and Klf4−/− mice also showed no changes in the number or location of epithelial cells undergoing apoptosis in the colon (data not shown).
Fig. 2.
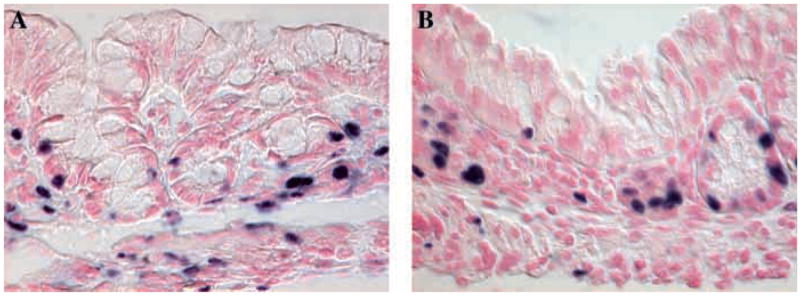
(A,B) Immunohistochemistry for BrdU, indicating dividing cells in the colonic epithelium of postnatal day 1 mice. Klf4−/−mice (B) were no different from wild-type (A) littermates in rates of cell proliferation in the colonic epithelium.
Klf4−/− mice show abnormal differentiation of goblet cells but not colonocytes or enteroendocrine cells in the colon
Homozygous deletion of Klf4 has been shown to affect late-stage differentiation in the skin (Segre et al., 1999). As Klf4 is highly expressed in the epithelial cells of the colon as well as the skin, we postulated that the loss of functional Klf4 might impact colonic differentiation as well. As the Klf4−/− mice die within 15 hours of birth, we examined these animals on postnatal day 1, the latest stage of intestinal differentiation before their death. Because the epithelium of the colon undergoes a continual and rapid renewal, both undifferentiated proliferative cells and fully differentiated epithelial cells are normally represented in the murine postnatal day 1 colon.
The colonic mucosa of the Klf4−/− mice on postnatal day 1 was abnormal when compared with wild-type littermates (Fig. 3A,B). The colons of Klf4−/− mice appeared, in general, to have subtle changes in epithelial contour. The most marked change in the Klf4−/− mice was a dramatic decrease in the number of mature goblet cells. Goblet cells are specialized epithelial cells that elaborate mucins, trefoil proteins and other factors that help protect the intestinal mucosa from injury and facilitate tissue repair (Deplancke and Gaskins, 2001). Cells of this lineage normally undergo a specific differentiation program, first forming pre-goblet cells, also called oligomucous cells, that are characterized by a few mucous granules, occasionally grouped into a narrow theca (Karam, 1999). Pre-goblet cells are generally confined to the colonic crypt base or mid-crypt, with some regional variation. Mature goblet cells form from the pre-goblet cells within 1–2 days and are recognized by the large and numerous mucous granules that cause the thecae to swell, giving the typical goblet appearance (Chang and Leblond, 1971; Chang and Nadler, 1975).
Fig. 3. Differentiation of goblet cells but not colonocytes and enteroendocrine cells was abnormal in Klf4−/− mice. (A,B) Hematoxylin and Eosin staining of the colon from postnatal day 1 mice.
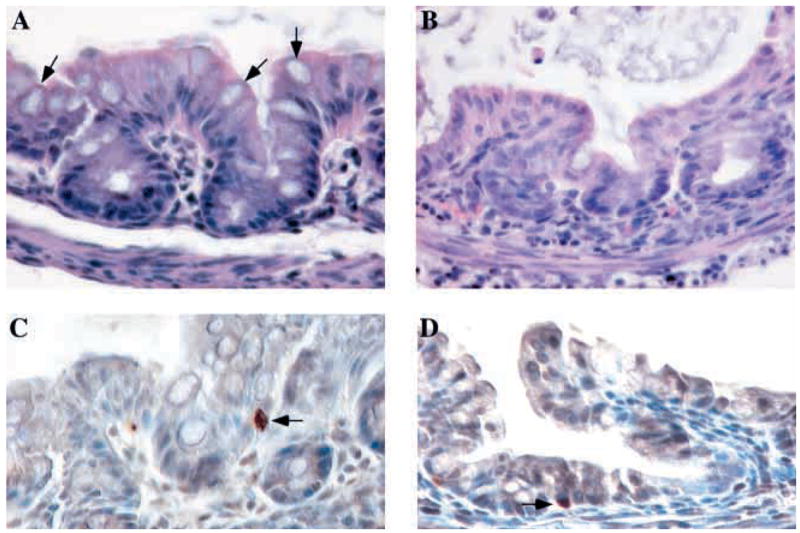
(A) The colonic epithelium of wild-type mice showed a normal contour and numerous goblet cells (arrows) along the crypts and surface epithelium.
(B) By contrast, goblet cells were nearly absent from Klf4−/− epithelium, and mutant mice appeared to have subtle changes in epithelial contour. Colonocytes appeared grossly normal.
(C,D) Immunohistochemistry for chromogranin A, a marker of enteroendocrine cells, in colon from postnatal day 1 mice. Enteroendocrine cells (arrows) were identified in both wild-type (C) and Klf4−/−mice (D). In both cases, enteroendocrine cells were seen at typically low numbers, with only one to two enteroendocrine cells per transverse section.
While wild-type mice (Fig. 3A) showed numerous goblet cells (arrows) throughout the crypts and surface epithelium, goblet cells were nearly absent in the Klf4−/− mice (Fig. 3B). By contrast, the morphology of colonocytes was grossly normal in the colon of Klf4−/− mice. To investigate whether the Klf4 null phenotype was specific for goblet cells, we examined the differentiation of enteroendocrine cells in the colon. Using immunohistochemistry for chromogranin A, a marker of enteroendocrine cells, we identified these cells (arrows) in the colon of both wild-type (Fig. 3C) and Klf4−/− mice (Fig. 3D). In both cases, enteroendocrine cells were seen at typically low numbers, with only one to two enteroendocrine cells per cross-section of the colon. Thus, among the three epithelial cell lineages in the colon, only differentiation of goblet cells appeared to be altered in Klf4−/− mice.
To further characterize the reduction of goblet cells in the colon of Klf4−/− mice, we performed Alcian Blue staining. Alcian Blue detects acidic mucins, which are normally restricted to pre-goblet and goblet cells in the colon. At low power, staining of wild-type colons revealed numerous Alcian Blue-positive cells (Fig. 4A). By contrast, very few Alcian Blue-positive cells were seen in colons of the Klf4−/− mice, indicating that goblet cell differentiation was dramatically impaired (Fig. 4B). Moreover, at higher power, Alcian Blue staining was seen only in goblet cells (arrows) of wild-type colons (Fig. 4C). By contrast, Klf4−/− colons displayed numerous epithelial cells with small amounts of Alcian Blue staining material (arrows) but no normal goblet cell morphology (Fig. 4D). This staining pattern in the Klf4−/− mice could represent either ectopic mucin expression or the presence of numerous pre-goblet cells throughout the epithelium. Periodic Acid Schiff (PAS) staining, which detects both neutral and acidic mucins, confirmed the results of the Alcian Blue staining (data not shown), demonstrating that the alterations in colonic goblet cells were not due to changes in expression of acidic mucins alone.
Fig. 4.
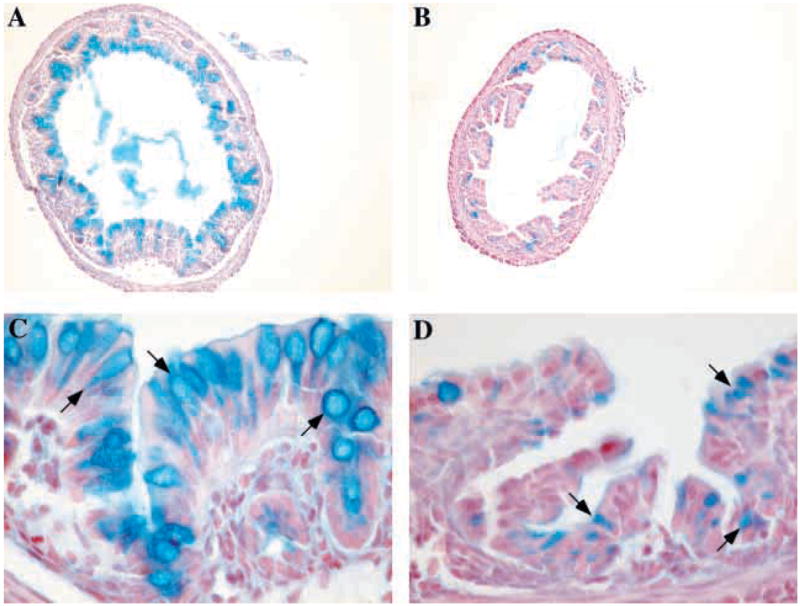
Klf4−/− mice showed a dramatic decrease in the number of goblet cells in the colon. (A–D) Alcian Blue staining for acidic mucins in the colon of postnatal day 1 mice. At low power, wild-type mice (A) had numerous Alcian Blue-positive cells, but very few Alcian Blue positive cells were seen in the Klf4−/−mice (B). At higher power, Alcian Blue staining was seen only in the goblet cells (arrows) of wild-type mice (C), while Klf4−/− mice (D) displayed numerous epithelial cells with small amounts of Alcian Blue staining material (arrows) but no normal goblet cell morphology.
In order to quantify the reduction in colonic goblet cell numbers in the Klf4−/− mice, we counted goblet cells as well as total epithelial cells in the colons of Klf4−/− mice and wild-type littermates. Only those epithelial cells with both Alcian Blue staining and mature goblet cell morphology were counted. In wild-type colons, 20% of epithelial cells were mature goblet cells; this fraction was reduced to 2% in Klf4−/−mice. Thus, Klf4−/− mice showed a 90% decrease in the ratio of mature goblet cells to total epithelial cells compared with wild-type littermates (P<1×10−8). The total number of epithelial cells was not significantly altered, and there was an increase in the number of cells having the morphological appearance of colonocytes. As there are no specific markers for colonocytes at this stage (Falk et al., 1994), it was unclear whether these cells represented mature colonocytes or immature cells of the goblet cell lineage.
Epithelial cells in Klf4−/− mice do not show the normal goblet cell morphology by ultrastructural analysis
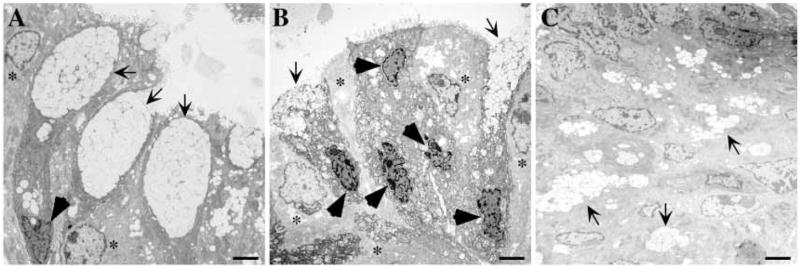
As the number and appearance of colonic goblet cells was abnormal in Klf4−/− mice, we performed electron microscopy to better define the ultrastructure of goblet cells. In the wild-type colon, cells with normal goblet cell morphology were seen (Fig. 5A, arrows). The nucleus of the goblet cell (Fig. 5A, dark arrowhead) is typically dark, dense and squeezed at the base of the goblet (Karam, 1999). The protein components of the intestinal mucins are synthesized by the rough endoplasmic reticulum and passed to the Golgi apparatus where they are combined with carbohydrate and packaged into membrane-bound secretory vacuoles (Burkitt et al., 1993). The apical portion of the cell was comprised largely of these secretory vacuoles. Normal colonocytes (Fig. 5B, *) are interspersed. In the colons from Klf4−/− mice, the normal goblet cell morphology was not seen. Several abnormally shaped or immature cells of the goblet lineage (arrows) emptied their contents into the lumen, and adjacent to these cells were several cells with densely packed nuclei (Fig. 5B, dark arrowheads), suggesting a goblet cell lineage, but lacking the typical secretory vacuoles and normal goblet cell shape. Adjacent to these cells, several colonocytes (Fig. 5B, *), recognized by their paler cytoplasm and less condensed nuclei, appeared normal. The colons from the Klf4−/− mice also revealed small clusters of secretory vacuoles scattered throughout the epithelium (Fig. 5C, arrows). The spatial orientation of these mucigen granules was reminiscent of the pattern of Alcian Blue staining described above in Klf4−/− mice (Fig. 4D).
Fig. 5.
Electron microscopy revealed abnormal ultrastructural morphology in the colonic epithelium of Klf4−/− mice. (A) Colonic epithelium from wild-type mice on postnatal day 1 showed the normal goblet cell morphology (arrows). The nucleus (black arrowhead) was densely packed and located at the base of the cell, while the apical portion of the cell was comprised largely of membrane-bound secretory vacuoles containing mucigen. Normal colonocytes (*) were interspersed. (B,C) Colonic epithelium from Klf4−/− mice on postnatal day 1. (B) Several abnormally shaped or immature cells of the goblet lineage (arrows) were seen emptying their contents into the lumen. Adjacent to these cells, several cells had densely packed nuclei (black arrowheads) suggestive of a goblet cell lineage but lacked the typical secretory vacuoles and normal goblet cell shape. Colonocytes (*), recognized by their paler cytoplasm and less condensed nuclei, appeared normal. (C) The colonic epithelium of the Klf4−/− mouse also demonstrated small clusters of secretory vacuoles (arrows) scattered throughout the epithelium. Scale bars: 2 μm.
Altered expression of the goblet cell marker Muc2 in Klf4−/− mice
To further characterize epithelial differentiation in the colon of the wild-type and Klf4−/− mice, we performed in situ hybridization using the goblet cell lineage marker Muc2. Epithelial mucins are large molecular weight glycoproteins which play cytoprotective roles in many organs (Deplancke and Gaskins, 2001; Strous and Dekker, 1992). Muc2 is an epithelial mucin expressed in the intestine, with particularly abundant expression in the colon (van Klinken et al., 1999). Muc2 appears to be the most prominent colonic mucin in the mouse and throughout the intestine is expressed exclusively in pre-goblet and goblet cells.
In situ hybridization for Muc2 in wild-type mice on postnatal day 1 revealed a goblet-cell specific expression pattern (Fig. 6A). Abundant expression of Muc2 mRNA (Fig. 6A, arrows) was seen adjacent to the apical mucigen granules. Staining was prominent only in surface epithelial cells. Klf4−/− mice, however, showed expression of Muc2 mRNA in the epithelium, but this expression was not confined to cells with mature goblet morphology (Fig. 6B). Patchy expression was seen in cells throughout the epithelium (Fig. 6B, arrows). The Muc2 mRNA expression in the Klf4−/− mice invoked the abnormal pattern of Alcian Blue staining and the mucigen granules detected on electron microscopy.
Fig. 6.
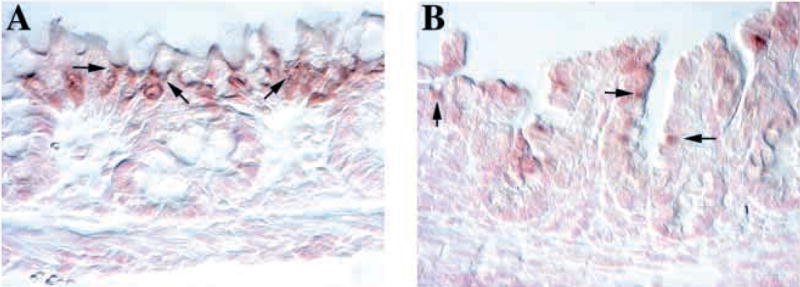
Altered expression of the goblet cell marker Muc2 in Klf4−/− mice. (A,B) In situ hybridization for Muc2 in the colon at postnatal day 1. (A) Wild-type mice showed a goblet-cell specific expression pattern of Muc2 mRNA (arrows) with abundant expression adjacent to the apical mucigen granules. Staining was prominent only in surface epithelial cells. (B) Klf4−/− mice had expression of Muc2 mRNA (arrows) in the epithelium, but this expression was not confined to cells with goblet morphology. Patchy expression was seen in cells throughout the epithelium.
DISCUSSION
In this study, we have identified the epithelial zinc-finger protein Klf4 as a goblet cell specific differentiation factor. While Klf4 has been implicated in growth arrest and downregulation of cell proliferation in vitro (Chen et al., 2001; Shie et al., 2000; Shields et al., 1996), loss of Klf4 is not associated with changes in colonic epithelial cell proliferation and apoptosis in postnatal day 1 mice. These results in the colon are consistent with the findings previously reported in the skin of Klf4−/− mice (Segre et al., 1999). In both cases, loss of functional Klf4 has a dramatic effect on epithelial cell differentiation, while no changes in proliferation rates are observed.
We confined our studies to the colon as the goblet cell phenotype of the Klf4−/− mice appears to be minimal in the small intestine. This is not surprising as Klf4 is more highly expressed in colon than small intestine (Shields et al., 1996). Moreover, while levels of Klf4 transcript approach adult levels in the colon by postnatal day 4, they are markedly reduced relative to the adult in the small intestine until at least postnatal day 12 (Ton-That et al., 1997). Notably, targeted ablation of goblet cells using a murine Tff3 promoter driving expression of an attenuated diptheria toxin produces a significant reduction in the number of goblet cells in the colon (~60%) but not the small intestine, suggesting distinct goblet cell regulatory programs in each organ (Itoh et al., 1999a).
In the colon, Klf4 deletion alters the terminal differentiation program of goblet cells. Numbers of mature goblet cells relative to total epithelial cells are reduced by 90% in Klf4−/−mice compared with littermate controls. This reduction is even greater than that reported by targeted ablation of intestinal goblet cells with attenuated diptheria toxin (Itoh et al., 1999a), demonstrating that goblet cell differentiation in the colon of Klf4−/− mice is severely perturbed. Numerous colonic epithelial cells in the Klf4−/− mice contain small amounts of acidic mucins shown by Alcian Blue staining or show expression of the goblet cell specific marker Muc2 by in situ hybridization, but lack normal goblet cell morphology by both histology and ultrastructural analyses. These cells probably represent pre-goblet cells, immature cells of the goblet cell lineage. It is unlikely that they represent colonocytes with ectopic mucin expression as these cells have ultrastructural features characteristic of a goblet cell lineage. Furthermore, this explanation would provide no basis for the decrease in goblet cell numbers. In addition, as epithelial cell differentiation in mice begins at least 5 days earlier and differentiation of colonocytes and enteroendocrine cells is unaffected, the abnormal terminal differentiation of goblet cells in Klf4−/− mice is not likely to be due solely to a developmental delay.
Several important questions remain to be answered. If Klf4 is essential for terminal differentiation of goblet cells in the colon, why are rare goblet cells observed in the Klf4−/− mice? As Klf4−/− mice die universally on postnatal day 1, what is the impact of the loss of Klf4 in the colon at later stages of development? By what mechanism does Klf4 alter the goblet cell differentiation program? Several explanations exist for the first question. Our experiments are performed on mice of a mixed genetic background, and regional differences in gene expression may be seen within the colon and throughout the gastrointestinal tract (Clatworthy and Subramanian, 2001), leading to phenotypic variability. Nonetheless, the goblet cells seen in the Klf4−/− mice are smaller and less elongated than their wild-type counterparts, suggesting that even in these cells, differentiation is perturbed. Most of these cells may, in fact, represent pre-goblet cells. In the Klf4−/− mice, cells in the goblet lineage appear to be immature with failure to complete the goblet cell differentiation program. Yet Klf4 mRNA is not expressed in a goblet cell-specific manner in the colon, and the cells in Klf4−/− mice do undergo cell fate specification and begin a goblet cell differentiation program. Thus, Klf4 is not crucial for initiation of goblet cell differentiation but rather for the terminal differentiation of goblet cells.
The importance of Klf4 later in life can be addressed through the generation of an intestine-specific knockout of Klf4. Klf4−/−mice were analyzed at the latest possible stage of intestinal development, as all animals die by postnatal day 1. As all three colonic cell lineages are present in the murine colon by embryonic day 15–16, the role of Klf4 on cellular differentiation has been addressed by this study. However, many other effects cannot be determined in the Klf4-null mice. Targeted ablation of goblet cells results in a paradoxical reduction in susceptibility to colonic injury (Itoh et al., 1999a). By contrast, goblet cell depletion is seen in inflammatory bowel disease and loss of Muc2 in mice leads to the development of small intestinal and rectal tumors (Chutkan, 2001; Velcich et al., 2002). Owing to the premature death of the Klf4−/− mice, the functional role of the reduction in goblet cell numbers on colonic epithelial injury and repair cannot be fully assessed. Future studies will use our Klf4loxP allele to address these questions.
While Klf4 does not directly impact cellular proliferation in the colon on postnatal day 1, it is possible that Klf4 deletion may result in tumor formation later in life. In humans, Klf4 mRNA levels are significantly decreased in the dysplastic epithelium of the colon, including adenomatous polyps and cancer (Shie et al., 2000). Klf4 is also downregulated in intestinal adenomas from the APCMin mouse, a model of intestinal neoplasia, and colonic adenomas of patients with familial adenomatous polyposis (FAP) (Dang et al., 2000). Individuals with FAP carry a germline mutation in the adenomatous polyposis coli (APC) gene. Many mouse models of intestinal tumorigenesis, including APCMin, do not develop evidence of epithelial hyperproliferation until at least several months of age (Donehower et al., 1992; Martin-Caballero et al., 2001; Shoemaker et al., 1997). Thus the role of Klf4 in the adult colonic epithelium remains to be explored.
The molecular mechanisms by which Klf4 regulates the terminal differentiation of goblet cells also remain to be elucidated. The phenotype of Muc2-deficient mice, which lack terminally differentiated goblet cells, as assessed by Alcian Blue staining, is reminiscent of the phenotype of the Klf4−/−mice (Velcich et al., 2002). The expression of Tff3 in some intestinal cells that lack mature goblet cell morphology from the Muc2−/− mice demonstrates that the goblet cell differentiation pathway is not completely ablated. Thus, it is possible that Klf4 directly or indirectly controls Muc2 expression, thereby producing the phenotype observed in Klf4−/− mice. Alternatively, Klf4 could control a whole set of genes required for the terminal differentiation of goblet cells. We cannot differentiate among these possibilities here, as Tff3 expression is not seen in the murine colon until postnatal day 3 (Mashimo et al., 1995), and no other markers exist at present for the terminal differentiation of goblet cells in newborn mice.
Some evidence suggests a mechanism by which Klf4 may regulate the goblet cell differentiation program. The promoter region of Klf4 contains binding sites for the Cdx2 protein (Mahatan et al., 1999), which has been found to regulate sucrase-isomaltase and lactase, markers of intestinal enterocyte differentiation (Fang et al., 2000; Suh et al., 1994). Forced expression of Cdx2 results in induction of enterocyte differentiation, and heterozygous deletion of Cdx2 results in colorectal cancer in mice (Chawengsaksophak et al., 1997; Suh and Traber, 1996). As a positive regulator of differentiation and a negative regulator of proliferation expressed in the intestinal epithelium, Cdx2 is thus a good candidate to direct Klf4-mediated goblet cell differentiation. In fact, expression of Klf4 in the human colon cancer cell line RKO is dependent upon Cdx2 (Dang et al., 2001).
Recently, Podolsky and colleagues recognized a goblet cell silencer inhibitor element (GCSI) on DNA and identified a GCSI binding protein (GCSI-BP), which is induced by keratinocyte growth factor and promotes goblet cell differentiation in vitro (Iwakiri and Podolsky, 2001a; Iwakiri and Podolsky, 2001b). Thus, one can postulate a pathway whereby Cdx2 binds Klf4, which can then transactivate GCSI-BP and/or other genes crucial for terminal differentiation of colonic goblet cells. Investigation of this regulatory relationship will require the cloning of the GCSI-BP, which has not yet been reported.
Klf4 can now be put in the context of the emerging model of hierarchical regulation of intestinal epithelial cell differentiation. Math1 and Hes1 may act in opposing manners to direct cell fate decisions between the absorptive enterocytes and the other three cell lineages (Jensen et al., 2000; Yang et al., 2001). Rac1 induces differentiation of both enterocytes and Paneth cells in the small intestine (Stappenbeck and Gordon, 2000). The Notch signaling pathway and several bHLH proteins are crucial for enteroendocrine cell fate decisions (Jensen et al., 2000; Mutoh et al., 1998). In the colon, Klf4 is required for the terminal differentiation of goblet cells, which appears to place it downstream of Math1.
In conclusion, we provide here the first evidence that Klf4 is a goblet cell-specific differentiation factor in the colon. We demonstrate that Klf4−/− mice display a marked decrease in the number of goblet cells in the colon, altered expression of the goblet cell marker Muc2 and abnormal goblet cell morphology. These effects are specific to the goblet cell lineage, as both colonocytes and enteroendocrine cells appear to undergo normal maturation. No alterations are seen in cell proliferation or cell death. Thus, Klf4 plays a crucial role in colonic epithelial cell differentiation in vivo.
Acknowledgments
We thank Drs Anil Rustgi and Doug Epstein for critical reading of the manuscript. We would like to acknowledge support from the Center for Molecular Studies in Digestive and Liver Disease at the University of Pennsylvania (P30 DK50306). We also acknowledge the work of the Morphology Core Facility, the Molecular Biology Core Facility, the Biomedical Imaging Core Facility and the Transgenic and Chimeric Mouse Facility at the University of Pennsylvania. This work was supported by NIH NIDDK RO1 DK53839 and an Industry Research Scholar Award from the American Digestive Health Foundation (to K. H. K.) and by NIH NIDDK RO1 DK52230 and NIH NCI RO1 CA84197 (to V. W. Y.). J. P. K. was supported by NIH NIDDK KO8 DK02809-01. N. P. was supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada (BP-220106-1999).
References
- Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis. 2000;21:469–476. doi: 10.1093/carcin/21.3.469. [DOI] [PubMed] [Google Scholar]
- Bieker JJ. Krüppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- Burkitt HG, Young B, Heath JW. Wheater’s Functional Histology: A Text and Color Atlas. New York: Churchill Livingstone; 1993. [Google Scholar]
- Chang WW, Leblond CP. Renewal of the epithelium in the descending colon of the mouse. I Presence of three cell populations: vacuolated-columnar, mucous and argentaffin. Am J Anat. 1971;131:73–99. doi: 10.1002/aja.1001310105. [DOI] [PubMed] [Google Scholar]
- Chang WW, Nadler NJ. Renewal of the epithelium in the descending colon of the mouse. IV Cell population kinetics of vacuolated-columnar and mucous cells. Am J Anat. 1975;144:39–56. doi: 10.1002/aja.1001440104. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, Sun R, Yang VW. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkan RK. Inflammatory bowel disease. Prim Care. 2001;28:539–556. vi. doi: 10.1016/s0095-4543(05)70052-x. [DOI] [PubMed] [Google Scholar]
- Clatworthy JP, Subramanian V. Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech Dev. 2001;101:3–9. doi: 10.1016/s0925-4773(00)00557-8. [DOI] [PubMed] [Google Scholar]
- Dang DT, Bachman KE, Mahatan CS, Dang LH, Giardiello FM, Yang VW. Decreased expression of the gut-enriched Krüppel-like factor gene in intestinal adenomas of multiple intestinal neoplasia mice and in colonic adenomas of familial adenomatous polyposis patients. FEBS Lett. 2000;476:203–207. doi: 10.1016/s0014-5793(00)01727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW. Expression of the gut-enriched Krüppel-like factor (Krüppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on Cdx2. Oncogene. 2001;20:4884–4890. doi: 10.1038/sj.onc.1204645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Falk P, Roth KA, Gordon JI. Lectins are sensitive tools for defining the differentiation programs of mouse gut epithelial cell lineages. Am J Physiol. 1994;266:G987–G1003. doi: 10.1152/ajpgi.1994.266.6.G987. [DOI] [PubMed] [Google Scholar]
- Fang R, Santiago NA, Olds LC, Sibley E. The homeodomain protein Cdx2 regulates lactase gene promoter activity during enterocyte differentiation. Gastroenterology. 2000;118:115–127. doi: 10.1016/s0016-5085(00)70420-3. [DOI] [PubMed] [Google Scholar]
- Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272:23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Housley RM, Morris CF, Boyle W, Ring B, Biltz R, Tarpley JE, Aukerman SL, Devine PL, Whitehead RH, Pierce GF. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Beck PL, Inoue N, Xavier R, Podolsky DK. A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest. 1999a;104:1539–1547. doi: 10.1172/JCI6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Inoue N, Podolsky DK. Goblet-cell-specific transcription of mouse intestinal trefoil factor gene results from collaboration of complex series of positive and negative regulatory elements. Biochem J. 1999b;341:461–472. [PMC free article] [PubMed] [Google Scholar]
- Iwakiri D, Podolsky DK. Keratinocyte growth factor promotes goblet cell differentiation through regulation of goblet cell silencer inhibitor. Gastroenterology. 2001a;120:1372–1380. doi: 10.1053/gast.2001.24029. [DOI] [PubMed] [Google Scholar]
- Iwakiri D, Podolsky DK. A silencer inhibitor confers specific expression of intestinal trefoil factor in goblet-like cell lines. Am J Physiol Gastrointest Liver Physiol. 2001b;280:G1114–G1123. doi: 10.1152/ajpgi.2001.280.6.G1114. [DOI] [PubMed] [Google Scholar]
- Jenkins TD, Opitz OG, Okano J, Rustgi AK. Transactivation of the human keratin 4 and Epstein-Barr virus ED-L2 promoters by gut-enriched Krüppel-like factor. J Biol Chem. 1998;273:10747–10754. doi: 10.1074/jbc.273.17.10747. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Silberg DG, Traber PG, Schütz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11:1583–1595. doi: 10.1101/gad.11.12.1583. [DOI] [PubMed] [Google Scholar]
- Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–D298. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- Labosky PA, Winnier GE, Jetton TL, Hargett L, Ryan AK, Rosenfeld MG, Parlow AF, Hogan BL. The winged helix gene, Mfh3, is required for normal development of the diencephalon and midbrain, postnatal growth and the milk-ejection reflex. Development. 1997;124:1263–1274. doi: 10.1242/dev.124.7.1263. [DOI] [PubMed] [Google Scholar]
- Mahatan CS, Kaestner KH, Geiman DE, Yang VW. Characterization of the structure and regulation of the murine gene encoding gut-enriched Krüppel-like factor (Krüppel-like factor 4) Nucleic Acids Res. 1999;27:4562–4569. doi: 10.1093/nar/27.23.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–6238. [PubMed] [Google Scholar]
- Mashimo H, Podolsky DK, Fishman MC. Structure and expression of murine intestinal trefoil factor: high evolutionary conservation and postnatal expression. Biochem Biophys Res Commun. 1995;210:31–37. doi: 10.1006/bbrc.1995.1623. [DOI] [PubMed] [Google Scholar]
- Mutoh H, Naya FJ, Tsai MJ, Leiter AB. The basic helix-loop-helix protein BETA2 interacts with p300 to coordinate differentiation of secretin-expressing enteroendocrine cells. Genes Dev. 1998;12:820–830. doi: 10.1101/gad.12.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Potten CS. Epithelial cell growth and differentiation. II Intestinal apoptosis. Am J Physiol. 1997;273:G253–G257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Shie JL, Chen ZY, O’Brien MJ, Pestell RG, Lee ME, Tseng CC. Role of gut-enriched Krüppel-like factor in colonic cell growth and differentiation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G806–G814. doi: 10.1152/ajpgi.2000.279.4.G806. [DOI] [PubMed] [Google Scholar]
- Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker AR, Gould KA, Luongo C, Moser AR, Dove WF. Studies of neoplasia in the Min mouse. Biochim Biophys Acta. 1997;1332:F25–F48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- Smith RM, Cobb MH, Rosen OM, Jarett L. Ultrastructural analysis of the organization and distribution of insulin receptors on the surface of 3T3-L1 adipocytes: rapid microaggregation and migration of occupied receptors. J Cell Physiol. 1985;123:167–179. doi: 10.1002/jcp.1041230204. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Gordon JI. Rac1 mutations produce aberrant epithelial differentiation in the developing and adult mouse small intestine. Development. 2000;127:2629–2642. doi: 10.1242/dev.127.12.2629. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Wong MH, Saam JR, Mysorekar IU, Gordon JI. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr Opin Cell Biol. 1998;10:702–709. doi: 10.1016/s0955-0674(98)80110-5. [DOI] [PubMed] [Google Scholar]
- Strous GJ, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994;14:7340–7351. doi: 10.1128/mcb.14.11.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton-That H, Kaestner KH, Shields JM, Mahatanankoon CS, Yang VW. Expression of the gut-enriched Krüppel-like factor gene during development and intestinal tumorigenesis. FEBS Lett. 1997;419:239–243. doi: 10.1016/s0014-5793(97)01465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber PG. Transcriptional regulation in intestinal development. Implications for colorectal cancer. Adv Exp Med Biol. 1999;470:1–14. doi: 10.1007/978-1-4615-4149-3_1. [DOI] [PubMed] [Google Scholar]
- Traber PG, Wu GD. Intestinal development and differentiation. In: Rustgi AK, editor. Gastrointestinal Cancers: Biology, Diagnosis, and Therapy. Philadelphia: Lippencott-Raven; 1995. pp. 21–33. [Google Scholar]
- Tsubouchi S, Leblond CP. Migration and turnover of enteroendocrine and caveolated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of [3H]-thymidine into mice. Am J Anat. 1979;156:431–451. doi: 10.1002/aja.1001560403. [DOI] [PubMed] [Google Scholar]
- van Klinken BJ, Einerhand AW, Duits LA, Makkink MK, Tytgat KM, Renes IB, Verburg M, Buller HA, Dekker J. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol. 1999;276:G115–G124. doi: 10.1152/ajpgi.1999.276.1.G115. [DOI] [PubMed] [Google Scholar]
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- White JK, Auerbach W, Duyao MP, Vonsattel JP, Gusella JF, Joyner AL, MacDonald ME. Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nat Genet. 1997;17:404–410. doi: 10.1038/ng1297-404. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. In Situ Hybridization: A Practical Approach. New York: Oxford University Press; 1992. [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]