Abstract
Background & Aims
Familial adenomatous polyposis because of germline mutation of the adenomatous polyposis coli gene is characterized by development of colorectal adenomas and, ultimately, colorectal cancer. The usefulness of colorectal mucosal compounds to predict the effect on adenoma development of primary chemoprevention with the nonsteroidal anti-inflammatory drug sulindac was evaluated.
Methods
A randomized, double-blind, placebo-controlled study of 41 subjects genotypically affected with familial adenomatous polyposis but phenotypically unaffected was conducted. Patients received either sulindac or placebo for 48 months, and development of new adenomas was evaluated. The levels of 5 prostanoids, ornithine decarboxylase, and polyamines were measured serially in normal-appearing rectal mucosa.
Results
There were no statistically significant differences between treatment groups in baseline levels of prostanoids, ornithine decarboxylase, or polyamines. At conclusion of the study, 4 of 5 prostaglandin levels were statistically significantly lower in the sulindac group than in the placebo group. Among the subset of patients taking sulindac, 3 of 5 prostaglandin levels were statistically significantly lower in patients who were polyp free than in those who developed polyps. By contrast, there were no statistically significant differences in ornithine decarboxylase or polyamines between treatment groups or in those on sulindac who were polyp free compared with those who developed polyps.
Conclusions
Colorectal mucosal prostaglandin levels, but not ornithine decarboxylase or polyamines, may be valuable biomarkers to assess appropriate drug dosage and medication compliance in patients undergoing primary chemoprevention therapy with sulindac. Reduction of mucosal prostaglandin levels may be necessary to achieve chemopreventive benefit from this agent.
Familial adenomatous polyposis is an autosomal dominant syndrome caused by germline mutation of the adenomatous polyposis coli gene located at chromosome 5q21.1–4 This disorder is characterized by the development of hundreds of colorectal adenomas in adolescence.5 Nearly all affected individuals will develop colorectal cancer by the sixth decade of life if prophylactic colectomy is not performed.5 Regression of established adenomatous polyps in patients with familial adenomatous polyposis has been demonstrated in case reports6 and randomized controlled studies with sulindac, a nonsteroidal anti-inflammatory drug,7–9 and with celecoxib, a selective inhibitor of cyclooxygenase 2.10
Prostaglandins and tromboxanes are the primary cellular metabolites from the action of the cyclooxygenases on arachidonic acid. Levels of prostanoids such as prostaglandin E2 (PGE2) are increased in neoplastic colonic tissues compared with normal mucosa in both humans and experimental animals.11–14 In addition, several recent studies have shown that reduction in mucosal prostanoids levels because of sulindac treatment in FAP patients is accompanied by regression of colonic adenomas.15–17 These findings suggest that prostanoids may play an important pathophysiologic role in the process of colorectal carcinogenesis.
The polyamines (putrescine, spermidine, and spermine) are compounds required for cellular differentiation and proliferation.18 Ornithine decarboxylase (ODC) is the first and is a rate-limiting enzyme in the polyamine biosynthetic pathway.18 In murine models of colorectal tumorigenesis, carcinogens induce ODC activity in colonic mucosa, and inhibitors of ODC suppress cancer development.19–23 In human beings, ODC and polyamine levels are elevated in neoplasms compared with normal-appearing adjoining mucosa.24–33 In addition, these compounds are elevated in the normal mucosa of gene carriers of familial adenomatous polyposis (FAP) before the development of adenomas, suggesting a role for these compounds in tumorigenesis in this disorder.
The ability of prostanoids, ODC, and polyamines to serve as biomarkers in chemoprevention trials remains unclear. Therefore, we evaluated the usefulness of these compounds to discriminate the development of neoplasia in a previous randomized, double-blind, placebo-controlled study of subjects genotypically affected with FAP but phenotypically unaffected; these patients received either sulindac or placebo and were followed for the development of colorectal polyps.
Materials and Methods
Study Population
The clinical trial design and results were reported previously.34 This study was conducted from September 1993 to July 2001. Forty-one subjects were recruited from The Johns Hopkins Hereditary Colorectal Cancer Registry. Written informed consent was obtained from all subjects or their parents, and assent was obtained from subjects under 18 years of age. Subjects were genotypically affected (had a disease-causing mutation of the APC gene) but were initially phenotypically unaffected with no endoscopically detectable polyps. These individuals were entered into a double-blind placebo-controlled trial and were randomized by the central hospital pharmacy to receive sulindac 75 to 150 mg orally twice a day for 4 years or identical-appearing placebo tablets. There were no significant differences in demographic characteristics between the sulindac- and placebo-treated subjects. The development of rectosigmoid polyps was assessed at baseline and then at 4-month intervals for 4 years by sigmoidoscopy with an Olympus flexible video sigmoidoscope by methods previously described.7,34 In brief, one investigator, who did not review records of previous examinations, made all the assessments. At each examination, the endoscopist counted the total number of polyps in the circumference of the colorectum from 20 cm to the anal verge, and the examination was recorded on videotape. The diameter of up to 5 polyps just distal to 20 cm was measured in millimeters with a graduated scale passed through the sigmoidoscope biopsy channel. The protocol was approved by the Johns Hopkins Joint Committee on Clinical Investigation (institutional review board).
Specimen Procurement
Mucosal prostaglandin levels, ODC activity, and poly-amine levels were measured in mucosal biopsy specimens taken with standard biopsy forceps during flexible sigmoidoscopic examination performed between 1 and 5 pm. Rectal mucosal biopsy specimens were collected before the initiation of treatment (time 0); at 4 months; and at 1, 2, 3, and 4 years. All subjects were prepared for the endoscopic procedure with clear liquid diet and oral cathartic solution. Enemas, which could influence mucosal biochemistry, were not given. In each patient, 10 rectal mucosal biopsy specimens were taken from flat mucosa 10 –12 cm from the anal dentate line. Two biopsy specimens for each assay were snap frozen in liquid nitrogen and stored at −70°C until laboratory analysis. In addition, 2 biopsy specimens were placed in formalin for histopathologic examination.
Measurement of Prostanoids Levels
Specimens were analyzed for prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), thromboxane B2 (TXB2), and 6 keto prostaglandin F1α (6KF1α), the principal prostaglandin I2 metabolite.
The processing of biopsy specimens for prostaglandin levels was previously described.13,17 Briefly, specimens were thawed on ice and manually homogenized in a glass microhomogenizer (Kontes Scientific Glassware, Vineland, NJ) in 50 μL HBSS (138 mmol/L NaCl, 5 mmol/L KCl, 4 mmol/L HaHCO3, 5.6 mmol/L d-glucose, 0.3 mmol/L Na2HP4, and 0.3 mmol/L KH2PO4) containing 1 mmol/L CaCl2 and transferred to a microcentrifuge tube. An additional 60 μL of the same solution was used to rinse the homogenizer and then added to the initial homogenate. The combined solution was sonicated for 20 seconds with a Fisher Scientific Model 550 Sonic Dismembrator with a microtip probe. After sonication, tissue debris was removed by microcentrifugation at 12,000g for 15 seconds. Ten microliters of the supernatant was removed for determination of protein concentrations using the BCA method (Pierce, Rockford, IL). The remaining supernatant was divided into 25-μL aliquots, which were then incubated at 37°C for 30 minutes.
At the completion of the reaction, 25 μL deuterated prostaglandin standards and 125 μL acetone were added to each reaction, and the combined solution was dried under a steady stream of nitrogen gas. As soon as the specimen was dried, 25 μL 2% O-methoxylamine HCl in pyridine was added to each sample. The samples were stored at −20°C until quantification by gas chromatography-mass spectrometry.
Prostaglandin levels were determined with a Fijnnagan MAT SSQ771 gas chromatograph-mass spectrometer (Fijnnagan MAT, San Jose, CA). The level of each prostanoid was determined based on the inclusion of known quantities of deuterated prostanoids as internal standards. All prostanoid levels were normalized to the amount of protein in the sample.
ODC and Polyamine Measurements
ODC activity in biopsy specimens was assayed according to the published methods of Seely and Pegg,35 as previously utilized in our laboratory.36 Enzyme determination was performed on replicates of cytosolic extracts. The polyamine contents of perchloric acid extracts of biopsy specimens were determined by reverse-phase, high-performance liquid chromatography with precolumn dansylation as previously reported from our laboratory36 as described by Kabra et al.37 1,7-Diaminoheptane was used as the internal standard. Proteins were quantitated by the methods of Bradford.38
Statistical Analysis
The primary clinical outcome variables in the trial were the number and size of polyps in the sulindac and placebo groups at 48 months or at the time of withdrawal from the study. Student t test was used to compare the groups according to the intention-to-treat principle. The sample size was calculated to provide the study with 80% power to detect a difference of 1 SD in the number of polyps between groups (a 2-sided α of 0.05).
Differences in mean percentage of baseline prostaglandin levels (mean average of prostaglandin levels at the 4-month and at the 1-, 2-, 3-, and 4-year time points divided by baseline prostaglandin level) were compared by Student t test in the sulindac and placebo groups and in the subset of patients administered sulindac who developed polyps and those who were polyp free. Polyp-free patients were those who remained free of colorectal adenomas during the duration of the study as assessed by serial sigmoidoscopic examinations to 20 cm. The same statistical analysis was used in assessing differences in ODC activity and polyamine mucosal levels. For all analyses, probability of P < 0.05, a 2-sided test was considered statistically significant.
Results
Efficacy
The results of the clinical trial and the demographics of the study groups were reported previously.34 The incidence of any adverse event did not differ significantly between the sulindac and placebo group. Sulindac had no effect on adenoma development or characteristics. Nine of 21 (43.0%) patients in the sulindac group and 11 of 20 (55.0%) patients in the placebo group developed adenomas (P = 0.54). There were no statistically significant differences in mean number (sulindac group, 5.9 ± 8.9; placebo group 7.9 ± 15.5; P = 0.69) and size (sulindac group, 0.7 ± 1.0 mm; placebo group, 1.2 ± 1.3 mm; P = 0.17) of polyps between groups. Sulindac did not slow progression of adenoma growth as evaluated by linear longitudinal methods.34
Prostaglandin Levels
No statistically significant differences in baseline (time 0) levels of prostanoids were noted between the sulindac and placebo group (P = 0.82–1.0) (Table 1). Over the 4-year study, in the sulindac group, all prostaglandin levels except 6KF1α were below baseline, in contrast to the placebo group in which all levels were elevated above baseline (Table 2). Specifically, 4 of the 5 measured prostaglandin levels were statistically significantly lower in patients taking sulindac than in the placebo group.
Table 1.
Baseline Prostaglandin Levels in Rectal Mucosa of the Sulindac Group Compared With the Placebo Group
| Prostaglandin levels (ng/mg protein) (± SD)
|
|||
|---|---|---|---|
| Prostaglandin | Sulindac group (n = 21) | Placebo group (n = 20) | P valuea |
| PGD2 | 0.68 ± 0.47 | 0.67 ± 0.44 | 0.94 |
| PGE2 | 0.76 ± 0.51 | 0.74 ± 0.63 | 0.91 |
| PGF2α | 0.67 ± 0.39 | 0.71 ± 0.77 | 0.83 |
| TXB2 | 0.47 ± 0.33 | 0.47 ± 0.34 | 1.0 |
| 6KF1α | 0.17 ± 0.13 | 0.17 ± 0.15 | 1.0 |
Calculated by t test.
Table 2.
Change in Posttreatment Prostaglandin Levels in Rectal Mucosa of Patients Taking Sulindac Compared With the Placebo Group
| Mean percentage of baseline value (± SD)
|
|||
|---|---|---|---|
| Prostaglandin | Sulindac group (n = 21) | Placebo group (n = 20) | P valuea |
| PGD2 | 69.4 ± 29.2 | 209.6 ± 169.5 | <0.001 |
| PGE2 | 80.8 ± 53.5 | 233.3 ± 226.8 | 0.006 |
| PGF2α | 90.5 ± 50.5 | 203.8 ± 154.8 | 0.004 |
| TXB2 | 94.0 ± 85.0 | 245.7 ± 198.5 | 0.004 |
| 6KF1α | 110.9 ± 94.5 | 208.4 ± 202.7 | 0.06 |
Calculated by t test.
In the sulindac group, 12 patients remained polyp free and 9 developed polyps over the observation period of the protocol. Among this subset of patients taking sulindac, in those remaining polyp free, 3 of 5 prostaglandin levels (PGD2 , TXB2 , 6KF1α) were significantly lower and prostaglandins PGE2 and PGF2α trended lower than in those administered sulindac who developed polyps (Table 3).
Table 3.
Change in Posttreatment Prostaglandin Levels in Rectal Mucosa of Patients Taking Sulindac Who Remained Polyp Free Compared With Patients on Sulindac Who Developed Polyps
| Mean percentage of baseline value (± SD)
|
|||
|---|---|---|---|
| Prostaglandin | Polyp-free group (n =11) | Group with polyps (n =10) | P valuea |
| PGD2 | 58.4 ± 21.0 | 86.6 ± 81.2 | 0.023 |
| PGE2 | 66.1 ± 23.1 | 102.4 ± 72.5 | 0.117 |
| PGF2α | 76.5 ± 29.3 | 115.2 ± 65.5 | 0.082 |
| TXB2 | 63.5 ± 28.0 | 138.1 ± 112.3 | 0.038 |
| 6KF1α | 70.2 ± 30.2 | 170.7 ± 150.5 | 0.035 |
Calculated by t test.
Serial mean mucosal prostanoid levels for each of 4 patient groups (sulindac patients remaining polyp free, sulindac patients developing polyps, placebo patients remaining polyp-free, placebo patients developing poltaglandins (prostaglandin D2 [PGD2], prostaglandin E2 [PGE2], prostayps) are shown in Figure 1. All serial group mean time points for sulindac patients polyp free remained below baseline levels with the exception of 1 time point for 6KF1α. In comparison, other group means were above baseline for most serial measurements.
Figure 1.
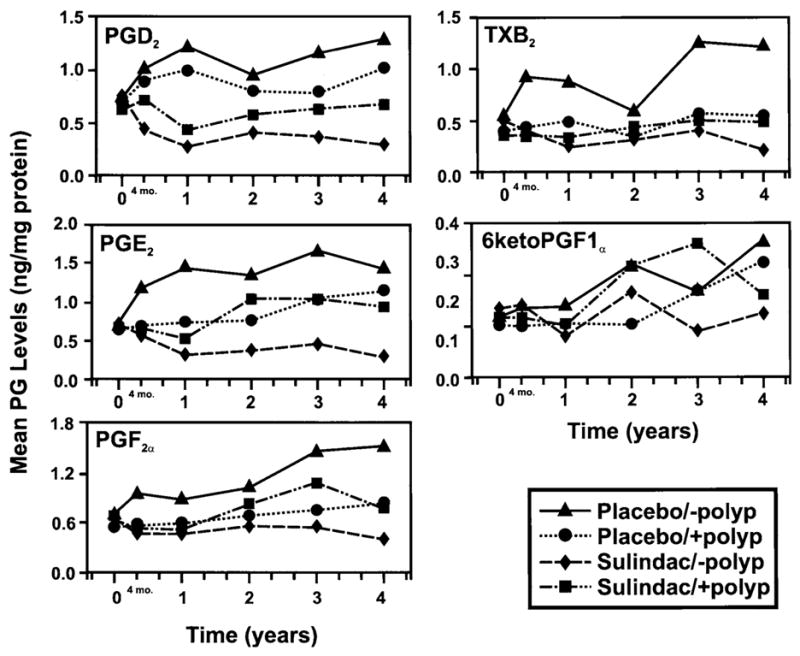
Serial mean prostaglandin levels (ng/mg/protein) in each of 4 patient groups: sulindac patients polyp free (◆), sulindac patients who developed polyps (■), placebo patients who are polyp free (▲), and placebo patients who developed polyps (●), for each of 5 prosglandin F2α [PGF2α], thromboxane B2 [TXB2], and 6 keto prostaglandin F1α [6KetoPGF1α]).
The baseline and 1-year mucosal prostanoid values for individual patients in the sulindac group who were polyp free or who developed polyps are shown in Figure 2. The 1-year prostanoid level was statistically lower than baseline in the patients who remained polyp free compared with patients who developed polyps for all prostanoids.
Figure 2.

Individual patient prostaglandin levels (ng/mg protein) at baseline (time 0) and 1 year for patients in the sulindac group who were polyp free (−) and those who developed polyps (+) by study end for each of 5 prostaglandins (prostaglandin D2 [PGD2], prostaglandin E2 [PGE2], prostaglandin F2α [PGF2α], thromboxane B2 [TXB2], and 6 keto prostaglandin F1α [6KetoPGF1α]). *Indicates P < 0.005 and †indicates P < 0.05 when 1 year mean values are compared with baseline.
ODC Level
No statistically significant differences in baseline (time 0) levels of ODC, putrescine, spermidine, or spermine were noted between the sulindac and placebo group (P = 0.15–0.85) (Table 4). There were no statistically significant differences noted in ODC or polyamines between the sulindac and placebo group over the 4 years of the protocol (P = 0.15–0.85) (Table 5). Among patients taking sulindac, there were no statistically significant differences in ODC activity, spermidine, and spermine content between those developing polyps and those remaining polyp free over the duration of the study.
Table 4.
Baseline ODC Activities and Polyamine Levels in Rectal Mucosa of the Sulindac Group Compared With the Placebo Group
| Parameter | Sulindac group (n =21) | Placebo group (n =20) | P valuea |
|---|---|---|---|
| ODC activityb | 444.7 ± 242.7 | 387.5 ± 275.1 | 0.38 |
| Putrescinec | 3.71 ± 1.81 | 3.80 ± 1.30 | 0.55 |
| Spermidine | 6.81 ± 3.73 | 5.78 ± 3.70 | 0.50 |
| Spermine | 18.37 ± 9.1 | 14.93 ± 10.8 | 0.40 |
Calculated by t test.
ODC activities are expressed as pmol CO2 per mg soluble protein per hour.
Polyamine contents are expressed in nmol of polyamine per mg soluble protein.
Table 5.
Change in Posttreatment ODC Activities and Polyamine Levels in Rectal Mucosa of the Sulindac Group Compared With the Placebo Group
| Mean percentage of baseline value (± SD)
|
|||
|---|---|---|---|
| Parameter | Sulindac group (n =21) | Placebo group (n =20) | P valuea |
| ODC activityb | 127 ± 0.09 | 214 ± 1.71 | 0.15 |
| Putrescinec | 133 ± 0.62 | 121 ± 0.74 | 0.67 |
| Spermidine | 124 ± 0.51 | 127 ± 0.48 | 0.85 |
| Spermine | 112 ± 0.44 | 129 ± 0.49 | 0.36 |
Calculated by t test.
ODC activities are expressed as pmol CO2 per mg soluble protein per hour.
Polyamine contents are expressed in nmol of polyamine per mg soluble protein.
Discussion
Elevation of prostanoid and COX-2 synthesis in the precancerous mucosa of FAP patients compared with control individuals has been noted by our group and others.13,39,40 Also, the concentration of prostanoids in adenomas increases with the size of the adenomas.13 The elevation in our 4-year study of mucosal prostanoid levels above baseline in the placebo group of FAP patients who were initially phenotypically negative further supports the role of prostanoids in progression of colorectal neoplasia. This is the first observation, to our knowledge, of the natural history of these compounds in human colorectal mucosa during the development of neoplasia.
In this study of markers in the setting of primary chemoprevention in FAP, prostaglandin levels in rectal mucosa were statistically significantly lower in patients who were administered sulindac compared with those taking placebo. Further examination of the differences between sulindac and placebo groups revealed that prostaglandin levels of patients taking sulindac fell below baseline (time 0) levels. This finding is consistent with our previous data that sulindac blunts mucosal levels of prostanoids17 and also verifies in the present trial that patients were compliant with sulindac administration.
In our study, sulindac given at usual doses had no clinically or statistically significant primary chemopreventive effect against the development of adenomas in patients genotypically affected but phenotypically unaffected with FAP. However, evaluation of the sulindac administered subset revealed significant differences in some prostaglandin levels between patients who remained polyp free and those who developed polyps during the clinical trial. As previously reported, prostaglandin levels may be good biomarkers of sulindac-mediated adenoma regression.41 Although the prescribed amounts (mg−1 · kg −1 · d) of sulindac utilized in this investigation were similar to those shown to regress established adenomas and reduce prostaglandin levels, greater drug doses may be necessary to blunt prostaglandin levels and achieve primary chemoprevention.7–9 Differences in mucosal prostaglandin levels between those with polyps and those without may represent differences in metabolism of sulindac because of genetic heterogeneity, modifier genes, or gut flora.
Previously, there was optimism for the utility of ODC and polyamines as biomarkers in chemoprevention studies with NSAIDs.33 These compounds were elevated in the flat mucosa of FAP patients compared with controls and in colorectal cancer compared with adjoining mucosa in those with sporadic colorectal cancer.33 However, in our study, ODC activity and polyamine levels were not influenced by the administration of sulindac. Moreover, in the placebo group, there was no increase in these markers from baseline with the development of polyposis, in contrast to the increase over baseline of prostanoid levels. A similar lack of association with carcinogenesis has been noted by others.42 In sporadic colorectal cancer, polyamine content did not correlate with Dukes stage, tumor size, or tumor site.42 Although ODC and polyamines were not useful markers in this study, they may be helpful in trials of other agents with different mechanisms of action.
Colorectal mucosal prostaglandin levels appear to be useful markers of medication compliance and drug effectiveness in chemoprevention protocols utilizing sulindac and possibly other NSAIDs. Also, prostaglandin levels appear to fulfill some of the requirements of a surrogate biomarker for colorectal adenoma as outlined by Einspahr et al.43 Prostaglandin levels are differentially expressed in normal and adenomatous tissue; correlate with adenoma development and regression; are readily accessible from colorectal mucosa for serial measurement; have a straightforward and highly sensitive assay; and despite intersubject variability in the levels of prostanoids, the assay is valid when a subject’s level of prostanoid is compared with his/her baseline levels. Also, prostanoid levels can be modified by chemopreventive agents.
However, several limitations exist in the use of these compounds as markers in primary chemopreventive trials. Importantly, colorectal cancer, not adenoma, is the true end point of interest in chemopreventive studies. Adenomas themselves are merely intermediate biomarkers of cancer risk, and even concordance between adenoma development and cancer development is less than complete. Moreover, significant correlations between changes in mucosal prostanoid levels and development of adenomas existed only in the sulindac group and not the placebo group. This limits the utility of these markers because they fail to distinguish clinical outcome among the control group.
In summary, prostaglandin levels appear to be useful markers to assess appropriate drug dosage and medication compliance in studies of NSAID chemoprevention. In future primary chemoprevention protocols, attention to prostaglandin mucosal levels in the experimental design should be considered, and greater reduction of prostaglandin levels may be necessary to obtain a chemopreventive effect with sulindac.
Acknowledgments
The authors thank Linda Welch, Lilly Chua, Marianne Villani, and Dr. Mary C. Corretti for technical support.
Supported in part by The John G. Rangos, Sr, Charitable Foundation; The Clayton Fund; Merck and Company; and NIH grants CA 53801, 63721, 51085, and P50 CA 93-16.
Abbreviations in this paper
- FAP
familial adenomatous polyposis
- ODC
ornithine decarboxylase
References
- 1.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 2.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Babba S, Hedge P. Mutations of chromosome 5q21 gene in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 3.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the Familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 4.Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, Groden J, Stevens J, Spirio L, Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:600–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 5.Bussey HJR. Familial polyposis coli. Family studies, histopathology, differential diagnosis, and results of treatment. Baltimore, Maryland: Johns Hopkins University Press; 1975. [Google Scholar]
- 6.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 7.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJA. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 8.Nugent KP, Farmer KCR, Spigelman AD, Williams CB, Phillips RKS. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 9.Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 10.Steinbach G, Lynch PM, Phillips RKS, Wallace MD, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su L, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 11.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 12.Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandins E2. Gut. 1994;35:675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang VW, Shields JM, Hamilton SR, Spannhake EW, Hubbard WC, Hylind LM, Robinson CR, Giardiello FM. Size-dependent increase in prostanoid adenomas of patients with familial adenomatous polyposis. Cancer Res. 1998;58:1750–1753. [PubMed] [Google Scholar]
- 14.Yamaguchi A, Ishida T, Nishimura G, Katoh M, Miyazaki I. Investigation of colonic prostaglandin in carcinogenesis in the rat colon. Dis Colon Rectum. 1991;34:572–576. doi: 10.1007/BF02049897. [DOI] [PubMed] [Google Scholar]
- 15.Nugent KP, Farmer KCR, Spigelman AD, Williams CB, Phillips RKS. Randomized controlled clinical trial of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 16.Winde G, Schmid KW, Schlegel W, Fischer R, Osswald H, Bunte H. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment. Dis Colon Rectum. 1995;38:813–830. doi: 10.1007/BF02049838. [DOI] [PubMed] [Google Scholar]
- 17.Giardiello FM, Spannhake EW, DuBois RN, Hylind LM, Robinson CR, Hubbard WC, Hamilton SR, Yang VW. Prostaglandin levels in human colorectal mucosa: effects of sulindac in patients with familial adenomatous polyposis. Dig Dis Sci. 1998;43:311–316. doi: 10.1023/a:1018898120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peg AD, McCann PP. Polyamine metabolism and function. Am J Physiol. 1982;243:C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- 19.Bull AW, Nigro ND, Golembieski WA, Crissman JD, Marnett LJ. In vivo stimulation of DNA synthesis and induction of ornithine decarboxylase in rat colon by fatty acid hydroperoxides, autooxidation products of unsaturated fatty acids. Cancer Res. 1984;44:4924–4928. [PubMed] [Google Scholar]
- 20.Luk GD, Hamilton SR, Yang P, Smith JA, O’Ceallaigh D, Mc-Avinchey D, Hyland J. Kinetic changes in mucosal ornithine activity during azoxymethane-induced colonic carcinogenesis in the rat. Cancer Res. 1986;46:4449–4452. [PubMed] [Google Scholar]
- 21.Symiyoshi H, Baer AR, Wargovich MJ. Heterogenicity of ornithine decarboxylase during mouse colon carcinogenesis and human colon tumors. Cancer Res. 1991;51:2069–2072. [PubMed] [Google Scholar]
- 22.Luk GD, Zhang SZ, Hamilton SR. Effects of timing of administration and dose of difluoromethylornithine on rat colonic carcinogenesis. J Natl Cancer Inst. 1989;81:421–427. doi: 10.1093/jnci/81.6.421. [DOI] [PubMed] [Google Scholar]
- 23.Rao CR, Tokumo K, Rigotty J, Zang E, Kelloff G, Reddy BS. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, α-difluoromethylornithine, 16 α-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Res. 1991;51:4528–4534. [PubMed] [Google Scholar]
- 24.Hixson LJ, Garewal GS, McGee DL, Sloan D, Fennerty MB, Sampliner RE, Gerner EW. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol Biomarkers Prev. 1993;2:369–374. [PubMed] [Google Scholar]
- 25.Porter CW, Herrera-Ornelas L, Pera P, Petrelli NF, Mittelman A. Polyamine biosynthetic activity in normal and neoplastic human colorectal tissue. Cancer. 1987;60:1275–1281. doi: 10.1002/1097-0142(19870915)60:6<1275::aid-cncr2820600619>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Nishioka K, Grossie VB, Chang T, Ajani JA, Ota DM. Colorectal ornithine decarboxylase activity in human mucosa and tumors: elevation of enzymatic activity in distal mucosa. J Surg Oncol. 1991;47:117–120. doi: 10.1002/jso.2930470211. [DOI] [PubMed] [Google Scholar]
- 27.LaMuraglia GM, Lacaine F, Malt R. A high ornithine decarboxylase activity and polyamine levels in human colorectal neoplasia. Ann Surg. 1986;204:89–93. doi: 10.1097/00000658-198607000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozhin J, Wilson PS, Bull AW, Nigro ND. Ornithine decarboxylase activity in rat and human colon. Cancer Res. 1984;44:3226–3230. [PubMed] [Google Scholar]
- 29.Lawson MJ, White LM, Coyle P, Butler RN, Roberts-Thomson IC, Conyers AJ. An assessment of proliferative and enzyme activity in transitional mucosa adjacent to colonic cancer. Cancer. 1989;64:1061–1066. doi: 10.1002/1097-0142(19890901)64:5<1061::aid-cncr2820640517>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Koo HB, Sigurdson ER, Daly JM, Berenson M, Groshen S, De-Cosse JJ. Ornithine decarboxylase levels in the rectal mucosa of patients with colonic neoplasia. J Surg Oncol. 1988;38:240–243. doi: 10.1002/jso.2930380407. [DOI] [PubMed] [Google Scholar]
- 31.Upp JR, Saydjari R, Townsend CM, Singh P, Barranco SC, Thompson JC. Polyamine levels and gastrin receptors in colon cancers. Ann Surg. 1988;207:662–668. doi: 10.1097/00000658-198806000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luk GD, Baylin SB. Ornithine decarboxylase as a biological marker in familial colonic polyposis. N Engl J Med. 1984;311:80–83. doi: 10.1056/NEJM198407123110202. [DOI] [PubMed] [Google Scholar]
- 33.Giardiello FM, Hamilton SR, Hylind LM, Yang VW, Tamez P, Casero RA. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57:199–201. [PubMed] [Google Scholar]
- 34.Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, Piantadosi S, Garrett E, Geiman D, Hubbard W, Offerhaus GJA, Hamilton SR. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1046–1049. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seely JE, Pegg AD. Ornithine decarboxylase (mouse kidney) Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]
- 36.Casero RA, Ervin SJ, Celano P, Baylin SB, Bergeron RJ. Differential response to treatment with Bis(ethyl) polyamine analogues between human small cell lung cancer and undifferentiated large cell lung carcinoma in culture. Cancer Res. 1989;49:639 –643. [PubMed] [Google Scholar]
- 37.Kabra PM, Lee HK, Lubich WP, Marton LJ. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reverse phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine, and tissues. Chromotog Biomed Appl. 1986;380:19 –32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- 38.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248 –254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Takafuji V, Lublin D, Lynch K, Roche JK. Mucosal prostanoid receptors and synthesis in familial adenomatous polyposis. Histochem Cell Biol. 2001;116:171–181. doi: 10.1007/s004180100287. [DOI] [PubMed] [Google Scholar]
- 40.Khan KN, Masferrer JL, Woerner BM, Soslow R, Koki AT. Enhanced cyclooxygenase-2 expression in sporadic and familial adenomatous polyposis of the human colon. Scand J Gastroenterology. 2001;36:865–869. doi: 10.1080/003655201750313405. [DOI] [PubMed] [Google Scholar]
- 41.Yang VW, Geiman DE, Hubbard WC, Spannhake EW, Hylind LM, Hamilton SR, Giardiello FM. Tissue prostanoids as biomarkers for chemoprevention of colorectal neoplasia: correlation between prostanoid synthesis and clinical response in familial adenomatous polyposis. Prostaglandins Other Lipid Mediat. 2000;60:83–96. doi: 10.1016/s0090-6980(99)00054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kingsnorth AN, Lumsden AB, Wallace HM. Polyamines in colorectal cancer. Br J Surg. 1984;71:791–794. doi: 10.1002/bjs.1800711019. [DOI] [PubMed] [Google Scholar]
- 43.Einspahr JG, Alberts DS, Gapstur SM, Bostick RM, Emerson SS, Gerner EW. Surrogate end-point biomarkers as measures of colon cancer risk and their use in cancer chemoprevention trials. Cancer Epidemiol Biomarkers Prev. 1997;6:37–48. [PubMed] [Google Scholar]


