Abstract
Background
Familial adenomatous polyposis is caused by a germ-line mutation in the adenomatous polyposis coli gene and is characterized by the development of hundreds of colorectal adenomas and, eventually, colorectal cancer. Nonsteroidal antiinflammatory drugs can cause regression of adenomas, but whether they can prevent adenomas is unknown.
Methods
We conducted a randomized, double-blind, placebo-controlled study of 41 young subjects (age range, 8 to 25 years) who were genotypically affected with familial adenomatous polyposis but phenotypically unaffected. The subjects received either 75 or 150 mg of sulindac orally twice a day or identical-appearing placebo tablets for 48 months. The number and size of new adenomas and side effects of therapy were evaluated every four months for four years, and the levels of five major prostaglandins were serially measured in biopsy specimens of normal-appearing colorectal mucosa.
Results
After four years of treatment, the average rate of compliance exceeded 76 percent in the sulindac group, and mucosal prostaglandin levels were lower in this group than in the placebo group. During the course of the study, adenomas developed in 9 of 21 subjects (43 percent) in the sulindac group and 11 of 20 subjects in the placebo group (55 percent) (P=0.54). There were no significant differences in the mean number (P=0.69) or size (P=0.17) of polyps between the groups. Sulindac did not slow the development of adenomas, according to an evaluation involving linear longitudinal methods.
Conclusions
Standard doses of sulindac did not prevent the development of adenomas in subjects with familial adenomatous polyposis.
Familial adenomatous polyposis is an autosomal dominant syndrome caused by a germ-line mutation of the adenomatous polyposis coli (APC) gene located at chromosome 5q21.1–4 The disorder is characterized by the development of hundreds of colorectal adenomas during adolescence.5 Colorectal cancer will develop in nearly all affected persons by the sixth decade of life if prophylactic colectomy is not performed.5
Regression of established adenomatous polyps in patients with familial adenomatous polyposis who received sulindac, a nonsteroidal antiinflammatory drug (NSAID), was described in case reports in 19836 and 1989.7 We and others have confirmed this observation in randomized studies of sulindac8–10 or celecoxib, a selective inhibitor of cyclooxygenase-2.11 These results led us to evaluate the ability of sulindac to prevent adenomas in subjects with the genetic abnormality of familial adenomatous polyposis who were phenotypically normal. We also measured tissue prostaglandin levels in colorectal mucosa because this is a reliable means of monitoring the local effect of NSAIDs in patients with familial adenomatous polyposis.12–14
METHODS
Study Population
The study was conducted from September 1993 to July 2001. Subjects were identified and recruited from the Johns Hopkins Polyposis Registry. Written informed consent was obtained from all subjects or their parents, and assent was obtained from subjects under 18 years of age. The protocol was approved by the Johns Hopkins Joint Committee on Clinical Investigation (the institutional review board).
The genotypic and phenotypic status of all potential subjects was assessed to determine their eligibility for the trial. All potential subjects and their parents (in the case of minors) received genetic counseling before undergoing genetic testing for APC gene mutations.15 Eligible subjects were older than eight years of age and had a disease-causing mutation of the APC gene but had no endoscopically detectable colorectal adenomatous polyps and no history of colonic surgery.
The following were reasons for exclusion from the study: use of an NSAID or aspirin for more than one week in the three months preceding the study, unwillingness to discontinue taking NSAIDs, absence of the use of effective birth control in girls and young women of childbearing age, pregnancy, a white-cell count of less than 4000 per cubic millimeter, a platelet count of less than 100,000 per cubic millimeter, a blood urea nitrogen level of more than 25 mg per deciliter (8.9 mmol per liter), a serum creatinine level of more than 1.5 mg per deciliter (132.6 μmol per liter), a history of peptic ulcer disease or gastrointestinal hemorrhage, a history of cancer, active bacterial infection, use of dimethyl sulfoxide, a history of aspirin allergy, or a body weight of less than 20 kg.
Study Design
The sponsor generously supplied both sulindac and placebo but was not otherwise involved in the design or conduct of the study. Data were held by the principal investigator.
Forty-one eligible subjects entered this double-blind, placebo-controlled trial. They were randomly assigned to receive sulindac orally twice a day for four years or identical-appearing placebo tablets. The sulindac dose was calculated on the basis of body weight and adjusted according to changes in weight during the course of the study. The 11 subjects in the sulindac group who weighed 20 to 44 kg at the beginning of the study received 75 mg of sulindac orally twice a day, and the 10 who weighed more than 44 kg took 150 mg of sulindac twice a day. By the end of the study, all but three subjects were receiving the higher dose. Compliance with treatment was assessed by means of pill counts, review of subjects’ diaries, and telephone calls every other week.
The development of rectosigmoid adenomatous polyps was assessed by sigmoidoscopy with an Olympus flexible video sigmoidoscope. One investigator, who did not review the records of previous examinations, made all the assessments. Evaluations were performed before treatment with sulindac or placebo was begun (month 0) and every 4 months after treatment was initiated, for a total of 48 months. At each examination, the endoscopist counted the total number of polyps in the circumference of the colorectum from 20 cm to the anal verge, and the examination was recorded on videotape. The diameter of up to five polyps just distal to 20 cm was measured in millimeters with a graduated scale passed through the biopsy channel of the sigmoidoscope. These measurements were averaged to determine the mean size of each subject’s polyps.
Evaluation of Safety
Adverse effects were monitored by means of telephone interviews every two to four weeks and at each four-month visit. A complete blood count was obtained and levels of glucose, blood urea nitrogen, serum creatinine, serum electrolytes, and bilirubin were measured at each visit. Adverse events were graded in accordance with the Common Toxicity Criteria of the National Cancer Institute.16 On this scale, a score of 0 indicates no adverse effects and a score of 5 life-threatening effects.
Measurement of Prostaglandin Levels
Biopsy specimens of the rectal mucosa were obtained before the initiation of treatment (month 0), at four months, and at one, two, three, and four years with standard biopsy forceps through a flexible sigmoidoscope. Tissue specimens were obtained from the normal-appearing mucosa 20 cm from the anal verge, snap-frozen in liquid nitrogen, and stored at −70°C until further analysis. Specimens were coded to disguise the subjects’ treatment assignment, and the levels of prostaglandin D2, prostaglandin E2, prostaglandin F2α, thromboxane B2, and 6-keto-prostaglandin F1α, the principal metabolite of prostacyclin, were measured by gas chromatography–mass spectrometry as described previously.12,13 The level of each prostaglandin was determined on the basis of the inclusion of known quantities of deuterated prostaglandins as internal standards. All levels of prostaglandin were adjusted for the quantity of protein in the sample.
Statistical Analysis
The primary outcome variables were the number and the size of polyps in the sulindac and placebo groups at 48 months or at the time of withdrawal from the study. Student’s t-test was used to compare the two groups according to the intention-to-treat principle. The sample size was calculated to provide the study with 80 percent power to detect a difference of 1 SD in the number of polyps between groups (a two-sided alpha of 0.05).
To determine whether the treatment assignment was associated with the outcome and predictor variables, we constructed random-effects linear longitudinal models.17 These models allowed us to compare the treatment groups while adjusting for the number and the size of polyps in the same patient over time. In contrast, the t-test compares mean values at a fixed point in time. In addition, the longitudinal model assesses the simultaneous effects of treatment group, time, and noncompliance with the assigned therapy. We assumed that the longitudinal effects within subjects were random, thus essentially devising a time trend for each subject. To fit the model, the raw data had to be transformed, which made the estimated coefficient difficult to interpret clinically. Therefore, we have provided estimates of the difference between groups at the end of treatment. We fitted two series of random-effects linear longitudinal models for each of the two outcomes (the number and the size of polyps); the covariates were time (in months), an indicator of early withdrawal from the study, and the treatment group. Interactions between covariates were evaluated and retained in the model if they were significant. All analyses were performed with the use of Stata software version 6.0.18
Secondary end points were the occurrence of polyps, the histologic features of polyps (tubular, tubulovillous, or villous), and the side effects of sulindac. We used Fisher’s exact test to determine whether these variables were associated with the treatment assignment.
We used Student’s t-test to compare the differences in the mean percent change in prostaglandin levels from base line in the sulindac group and the placebo group and in subjects in the sulindac group in whom polyps developed and those in the sulindac group who were free of polyps. The mean percent change was calculated as the mean of prostaglandin levels at four months and one, two, three, and four years divided by the base-line prostaglandin level.
All P values were two-sided. We also used nonparametric tests in the place of t-tests and confirmed the results.
RESULTS
Demographic Characteristics
All 41 eligible subjects had an APC gene mutation, as did their parents with familial adenomatous polyposis. Of these 41 subjects, 21 were randomly assigned to receive sulindac and 20 to receive placebo. There were no significant differences in demographic characteristics between the two groups (Table 1). By the end of the study, five subjects in the sulindac group had been withdrawn. Three were withdrawn because of an increasing number of polyps, and they were referred for surgical consultation. One had persistent neutropenia, and one was unable to make scheduled visits. Of the 20 subjects in the placebo group, 6 were withdrawn: 4 because of an increasing number of polyps (they were referred for surgical consultation), and 2 because they were unable to make scheduled visits.
Table 1.
Demographic Characteristics of the Subjects at Base Line.*
| Characteristic | Sulindac Group (N=21) | Placebo Group (N=20) |
|---|---|---|
| Age — yr | ||
| Mean | 12.9±5.1 | 15.8±8.7 |
| Range | 8–25 | 8–25 |
| Sex — no. (%) | ||
| Female | 16 (76) | 11 (55) |
| Male | 5 (24) | 9 (45) |
| Height — cm | 151.6±13.0 | 153.8±12.7 |
| Weight — kg | 47.4±17.2 | 56.3±26.6 |
Plus–minus values are means ±SD. There were no significant differences between the groups.
Compliance and Adverse Events
The mean (±SD) rate of compliance with treatment was 86.9±7.5 percent among patients in the sulindac group and 81.7±10.4 percent among patients in the placebo group. All subjects in the sulindac group took more than 76 percent of the scheduled doses of medication.
Treatment with sulindac for a four-year period was well tolerated. Few adverse events were reported, and 93 percent of these were minimal (grade 1) or mild (grade 2) (Table 2). Only one subject was withdrawn from the study because of possible drug-induced persistent neutropenia. The incidence of any adverse event did not differ significantly between the sulindac group and the placebo group.
Table 2.
Incidence and Severity of Adverse Events.*
| Adverse Event | Sulindac Group (N=21) | Placebo Group (N=20) |
|---|---|---|
| no. (%) | ||
| Grade 2 leukopenia | 1 (5)† | 0 |
| Dermatologic | ||
| Grade 2 photosensitivity | 0 | 1 (5) |
| Rash | ||
| Grade 1 | 1 (5)‡ | |
| Grade 2 | 1 (5)§ | |
| Grade 2 urticaria | 1 (5) | 0 |
| Gastrointestinal | ||
| Grade 1 diarrhea | 1 (5)¶ | 0 |
| Grade 2 vomiting | 1 (5) | 1 (5) |
| Hemorrhagic | ||
| Grade 1 epistaxis | 1 (5) | 0 |
| Grade 2 hematuria | 1 (5)|| | 0 |
| Grade 2 vaginal bleeding | 1 (5)** | 0 |
| Grade 2 hyperbilirubinemia†† | 1 (5) | 1 (5) |
| Grade 4 sensory neuropathy | 0 | 1 (5) |
| Grade 2 blurred vision | 0 | 1 (5) |
| Pain | ||
| Abdominal pain | ||
| Grade 1 | 0 | 2 (10) |
| Grade 3 | 0 | 1 (5) |
| Grade 4 | 1 (5)‡‡ | 0 |
| Grade 1 earache | 1 (5) | 0 |
| Grade 2 headache | 1 (5) | 1 (5) |
| Grade 2 myalgia | 0 | 1 (5) |
| Influenza-like syndrome§§ | ||
| Grade 1 | 6 (29) | 3 (15) |
| Grade 2 | 4 (19) | 5 (25) |
All adverse events are reported. A grade of 1 indicates minimal adverse effects, a grade of 2 mild effects, a grade of 3 moderate effects, and a grade of 4 severe effects.
A hematologic workup revealed no clear cause.
The rash was associated with a viral infection.
The rash was associated with mild ileus.
The diarrhea was due to lactose intolerance.
The hematuria was due to a urinary tract infection.
The subject’s sister and mother had a similar history.
Hyperbilirubinemia was due to Gilbert’s disease.
The abdominal pain was due to acute cholecystitis. The subject had a family history of cholecystitis at a young age.
The syndrome was characterized by fever and myalgia, with or without nausea, vomiting, diarrhea, headache, and abdominal cramps.
Efficacy
The number of subjects in whom one or more adenomas developed during the study did not differ significantly between the groups (Table 3). By the end of the study, adenomas had developed in 9 of the 21 subjects in the sulindac group (43 percent) and 11 of the 20 subjects in the placebo group (55 percent) (P=0.54). The groups did not differ significantly with respect to the number of subjects with multiple adenomas, large adenomas, or advanced adenomas (tubulovillous or villous adenomas) (Table 3).
Table 3.
Characteristics of Adenomatous Polyps at the End of Treatment.*
| Characteristic | Sulindac Group (N=21) | Placebo Group (N=20) |
|---|---|---|
| no. (%) | ||
| No. of adenomas | ||
| 0 | 12 (57) | 9 (45) |
| ⩾1 | 9 (43)† | 11 (55) |
| 1–10 | 3 (14) | 6 (30) |
| ⩾11 | 6 (29)‡ | 5 (25)§ |
| Large adenomas (⩾2.5 mm) | 4 (19) | 7 (35)¶ |
| Histologic type of adenoma | ||
| Tubular | 9 (43) | 11 (55)|| |
| Tubulovillous or villous | 0 | 0 |
Fisher’s exact test was used to calculate the P values.
P=0.54 for the comparison with subjects who were free of polyps.
P=1.00 for the comparison with subjects in the sulindac group in whom 1 to 10 polyps developed.
P=0.23 for the comparison with subjects in the placebo group in whom 1 to 10 polyps developed.
P=0.31 for the comparison with the sulindac group.
P=0.54 for the comparison with the sulindac group.
Among the patients who received treatment for 40 months or more, there were no significant differences between the groups in the mean number or size of polyps (Table 4). According to the intention-to-treat analysis, the overall difference in the number of polyps between the sulindac group and the placebo group was 0.52 (95 percent confidence interval, −0.29 to 2.73; P=0.27). Similarly, the overall difference in the size of polyps was 0.24 (95 percent confidence interval, −0.11 to 0.75; P=0.21).
Table 4.
Mean Number and Size of Adenomatous Polyps among Subjects Who Were Treated for at Least 40 Months.
| Variable | Sulindac Group (N=18) | Placebo Group (N=16) | P Value* |
|---|---|---|---|
| No. of adenomas | |||
| Mean ±SD | 5.9±8.9 | 7.5±15.5 | 0.69 |
| Range | 0–33 | 0–68 | |
| Size of adenomas (mm) | |||
| Mean ±SD | 0.70±1.0 | 1.2±1.3 | 0.17 |
| Range | 0–2.6 | 0–3.4 |
The t-test was used to calculate P values.
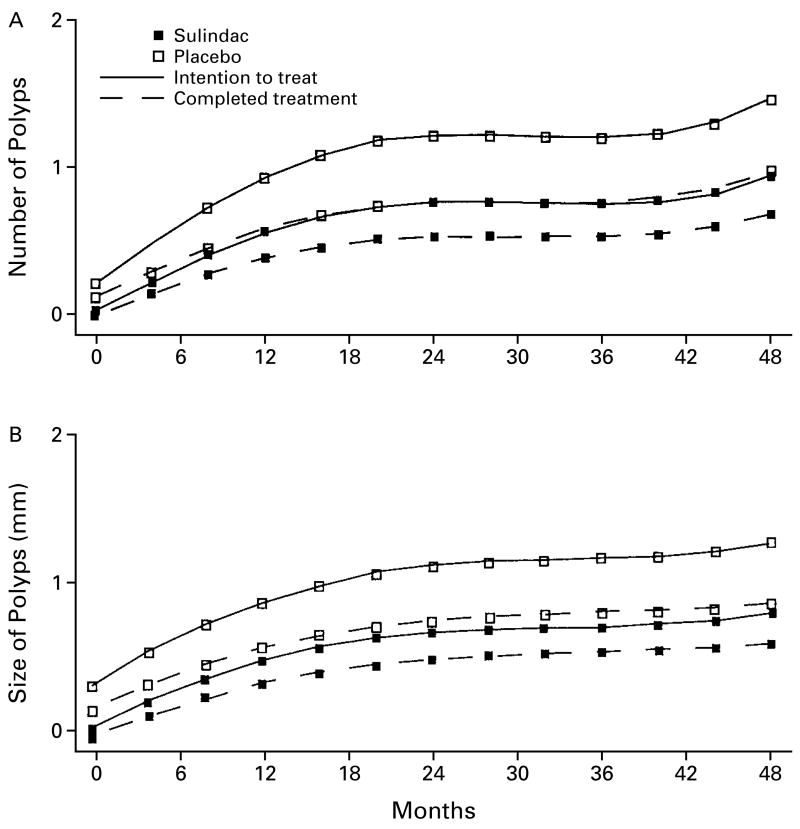
Random-effects linear longitudinal analysis revealed that treatment with sulindac did not influence the number of polyps (β=0.08, P=0.23) or the size of polyps (β=0.06, P=0.13) (Fig. 1).
Figure 1. Random-Effects Linear Longitudinal Analysis of the Effect of Sulindac on the Number (Panel A) and Size (Panel B) of Polyps, According to the Intention to Treat and to the Treatment Actually Received.
All subjects were included in the intention-to-treat analysis, and all subjects who completed the study (16 in the sulindac group and 14 in the placebo group) were included in the analysis according to the treatment received. There were no significant differences between groups in the number or size of polyps in either analysis.
Prostaglandin Levels
There was no significant difference between the sulindac and placebo groups in the base-line levels of prostaglandins (data not shown). After treatment, levels of prostaglandins D2, E2, and F2α and thromboxane B2 were significantly lower in the sulindac group than in the control group (Table 5), providing additional evidence of compliance with treatment.
Table 5.
Mean Percent Changes from Base Line in Prostaglandin Levels in Colorectal Mucosa.*
| Prostaglandin | Sulindac Group (N=21) | Placebo Group (N=20) | P Value† |
|---|---|---|---|
| percent change from base line | |||
| Prostaglandin D2 | 69.4±29.2 | 209.6±169.5 | <0.001 |
| Prostaglandin E2 | 80.8±53.5 | 233.3±226.8 | 0.006 |
| Prostaglandin F2α | 90.5±50.5 | 203.8±154.8 | 0.004 |
| Thromboxane B2 | 94.0±85.0 | 245.7±198.5 | 0.004 |
| 6-Keto-prostaglandin F1α | 110.9±94.5 | 208.4±202.7 | 0.06 |
Plus–minus values are means ±SD. Base-line values were 100 percent.
The t-test was used to calculate P values.
DISCUSSION
In this randomized, double-blind, placebo-controlled study, standard doses of sulindac did not prevent polyps in subjects who were genotypically affected with familial adenomatous polyposis but who were phenotypically unaffected initially. All subjects were carriers of APC gene mutations known to cause familial adenomatous polyposis in their parents, and there were no significant differences in base-line characteristics between the sulindac and placebo groups.
Compliance with treatment was excellent in the sulindac group. In addition, prostaglandin levels in the colorectal mucosa were significantly lower among subjects in this group than among those in the placebo group, verifying compliance with treatment. Although the amounts of sulindac we used are similar to those that have been shown to cause regression of established adenomas and reduce local prostaglandin levels, higher doses might be appropriate if another trial is planned.8–10
Evidence that sulindac has a short-lived effect on established polyps in patients with familial adenomatous polyposis has been reported. We showed that the rate of regression of adenomas was greater after six months of sulindac treatment than after nine months,8 and in some patients who had undergone ileorectal anastomosis, long-term use of sulindac resulted in the development of resistance to this medication.19,20 Moreover, colorectal cancer has developed in the rectal segment in at least three patients with familial adenomatous polyposis during maintenance therapy with sulindac.14,21,22
The lack of efficacy of primary chemoprevention could have been due to resistance to sulindac. Notably, combination treatment was more effective than sulindac alone in preventing adenomas in a murine model of familial adenomatous polyposis.23 The use of multiple drugs for both primary chemoprevention and the regression of adenomas in patients with familial adenomatous polyposis and those with hereditary nonpolyposis colorectal cancer deserves further evaluation.
In summary, our results do not provide support for the use of NSAIDs such as sulindac for the primary treatment of familial adenomatous polyposis. Prophylactic colectomy remains the treatment of choice to prevent colorectal cancer in patients with this disorder.
Acknowledgments
Supported in part by the Clayton Fund, the John G. Rangos, Sr., Charitable Foundation, and Merck and by grants (CA 53801, CA 63721, and P50 CA 62924-04) from the National Institutes of Health.
Footnotes
We are indebted to Ms. Linda Welch, Ms. Lilly Chua, Ms. Marianne Villani, Ms. Kathy Romans, and Dr. Mary C. Corretti for technical support.
References
- 1.Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 2.Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 gene in FAP and colorectal cancer patients. Science. 1991;253:665–9. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 3.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 4.Joslyn G, Carlson M, Thliveris A, et al. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:600–13. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 5.Bussey HJR. Familial polyposis coli: family studies, histopathology, differential diagnosis, and results of treatment. Baltimore: Johns Hopkins University Press; 1975. [Google Scholar]
- 6.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–7. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 7.Waddell WR, Ganser GF, Cerise EJ, Loughry RW. Sulindac for polyposis of the colon. Am J Surg. 1989;157:175–9. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- 8.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 9.Nugent KP, Farmer KCR, Spigelman AD, Williams CB, Phillips RKS. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–9. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 10.Labayle D, Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–9. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 11.Steinbach G, Lynch PM, Phillips RKS, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 12.Yang VW, Shields JM, Hamilton SR, et al. Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res. 1998;58:1750–3. [PubMed] [Google Scholar]
- 13.Giardiello FM, Spannhake EW, DuBois RN, et al. Prostaglandin levels in human colorectal mucosa: effects of sulindac in patients with familial adenomatous polyposis. Dig Dis Sci. 1988;43:311–6. doi: 10.1023/a:1018898120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang VW, Geiman DE, Hubbard WC, et al. Tissue prostanoids as biomarkers for chemoprevention of colorectal neoplasia: correlation between prostanoid synthesis and clinical response in familial adenomatous polyposis. Prostaglandins Other Lipid Mediat. 2000;60:83–96. doi: 10.1016/s0090-6980(99)00054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardiello FM, Brensinger JD, Petersen GM, et al. The use and interpretation of commercial APC gene testing for familial adenomatous polyposis. N Engl J Med. 1997;336:823–7. doi: 10.1056/NEJM199703203361202. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Therapy Evaluation Program. Bethesda, Md: National Cancer Institute; Mar, 1998. [Accessed February 4, 2002]. Common toxicity criteria. http://www.fda.gov.cder/cancer/oncrefto.htm. [Google Scholar]
- 17.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford, England: Oxford University Press; 1994. [Google Scholar]
- 18.Stata statistical software, release 6.0. College Station, Tex: Stata; 1999. [Google Scholar]
- 19.Tonelli F, Valanzano R, Messerini L, Ficari F. Long-term treatment with sulindac in familial adenomatous polyposis: is there an actual efficacy in prevention of rectal cancer? J Surg Oncol. 2000;74:15–20. doi: 10.1002/1096-9098(200005)74:1<15::aid-jso4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Correa MR, Hylind LM, Romans KE, Bookes SV, Giardiello F. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2001;120(Suppl):A252–A253. doi: 10.1053/gast.2002.31890. abstract. [DOI] [PubMed] [Google Scholar]
- 21.Niv Y, Fraser GM. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology. 1994;107:854–7. doi: 10.1016/0016-5085(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 22.Thorson AG, Lynch HT, Smyrk TC. Rectal cancer in FAP patient after sulindac. Lancet. 1994;343:180. doi: 10.1016/s0140-6736(94)90974-1. [DOI] [PubMed] [Google Scholar]
- 23.Torrance CJ, Jackson PE, Montgomery E, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–8. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]