Abstract
Annexin V and Sytox Green are widely used markers to evaluate apoptosis in various cell types using flow cytometry and fluorescent microscopy. Recently, a novel fluoroprobe MitoSOX Red was introduced for selective detection of superoxide in the mitochondria of live cells and was validated for confocal microscopy and flow cytometry. This protocol describes simultaneous measurements of mitochondrial superoxide generation with apoptotic markers (Annexin V and Sytox Green) by both flow cytometry and confocal microscopy in endothelial cell lines. The advantages of the described flow cytometry method over other cell-based techniques are the tremendous speed (1–2 h), exquisite precision and the possibility of simultaneous quantitative measurements of mitochondrial superoxide generation and apoptotic (and other) markers, with maximal preservation of cellular functions. This method combined with fluorescent microscopy may be very useful to reveal important spatial–temporal changes in mitochondrial superoxide production and execution of programmed cell death in virtually any cell type.
INTRODUCTION
The discovery that the free radical superoxide anion is a biologically significant molecule has stimulated a remarkable momentum for scientific research in all the fields of biology and medicine1. Emerging recent evidence (based mostly on experiments derived from isolated mitochondria) suggests that under various pathological conditions (e.g., neurodegenerative and inflammatory disorders, diabetes, diabetic complications, cancer and aging) mitochondria may emerge as a significant site of superoxide and other reactive oxygen and nitrogen species generation, in addition to the cytosolic xanthine and NADPH oxidases2–5. The recognition of the crucial role of the mitochondria in the pathological generation of reactive oxygen species in disease rekindled significant interest in the development of methods to assess the mitochondrial superoxide generation, which was largely hindered by the lack of a sensitive and specific assay6–8. Hydroethidine (HE) has been widely used to detect intracellular superoxide anion. The oxidation of HE by superoxide leads to the fluorescent product 2-hydroxy-ethidium9,10, which is excitable at 480 nm wavelength, with emission maximum at 567 nm9,10. Recently, a novel fluoroprobe, a derivative of HE, MitoSOX Red was introduced for selective detection of superoxide in the mitochondria of live cells. The positive charge on the phosphonium group in MitoSOX Red selectively targets this cell-permeant HE derivative to mitochondria, where it accumulates as a function of mitochondrial membrane potential and exhibits fluorescence upon oxidation and subsequent binding to mitochondrial DNA7. In numerous recent studies, MitoSOX was validated with fluorescent/confocal microscopy7,11–19 and flow cytometry19–25 for selective detection of mitochondrial superoxide production in endothelial cells18,19,25, cardiomyocytes16,19,22,25, keratinocytes11, fibroblasts17, epithelial and lymphoid cells20,21,23,24, and neuronal cells7,12–15, to name just a few (see also Table 1 for more details), as well as in isolated vessels26.
TABLE 1.
Reported loading conditions with MitoSOX for various cell types.
| Cell type | Method used | MitoSOX loading conditions | Positive control | Negative control | Reference |
|---|---|---|---|---|---|
| Human keratinocytes | Fluorescence microscopy | 5 μM at 37 °C in the dark for 15 min, followed by two washes | 11 | ||
| Cultured motor neurons | Confocal microscopy | 0.1 μM at 37 °C for 15 min, followed by wash | 12 | ||
| Cultured cerebellar granule neurons | Confocal microscopy | 0.1 or 0.2 μM at 37 °C for30 min, followed by wash | Antimycin A | Cell-permeable SOD mimetic | 13 |
| Cultured oligodendrocytes | Confocal microscopy | 0.1 μM at 37 °C for 15 min, followed by wash | Antimycin A | Cell-permeable SOD mimetic | 7 |
| Hippocampal and cortical neurons | Fluorescent microscopy | 10 μM for 10 min at room temperature, followed by wash | Glucose deprivation and reoxygenation | Mitochondrial uncouplers | 14 |
| CHP212 human neuroblastoma cells | Confocal microscopy | 3 μM for 20 min, followed by wash | Rotenone, SOD mutations | SOD overexpression | 15 |
| Isolated mouse cardiomyocytes | Confocal microscopy | 5 μM at room temperature for 15 min | 16 | ||
| Human fibroblasts | Confocal microscopy | 3 μM at 37 °C for 20 min, followed by wash | 17 | ||
| PMVECs | Confocal microscopy | 1.25 μM at 37 °C for30 min, followed by wash | Bolus superoxide (X/XO) | 18 | |
| Ramos cells | Flow cytometry | 5 μM at 37 °C in the dark for 10 min, followed by wash | 20 | ||
| HL-60 cells | Flow cytometry | 5 μM at 37 °C in the dark for 30 min, followed by wash | Atractyloside | 21 | |
| HepG2 and H9C2 cells | Flow cytometry | 5 μM at 37 °C in the dark for 10 min | 22 | ||
| HCT-116 cells | Flow cytometry | 5 μM at 37 °C in the dark for 10 min, followed by wash | Deoxycholate | 23 | |
| Seg-1 cells, Barrett’s esophagus cells, HET-1A cells | Fluorescence microscopy and flow cytometry | 5 μM at 37 °C for 15 min | Acidic pH + bile acid | 24 | |
| H9C2 and HCAECs | Flow cytometry and confocal microscopy | 5 μM at 37 °C in the dark for 20–30 min, followed by two washes | Antimycin A paraquat, doxorubicin, high glucose | Cell-permeable SOD or SOD mimetics, mitochondrial uncouplers | 19, 25 |
| HCAECs and PMVECs | Flow cytometry and confocal microscopy | 5 μM at 37 °C in the dark for 30 min, followed by two washes | Antimycin A, doxorubicin | Cell-permeable SOD mimetics | Present study |
Abbreviations: CHP212, human neuroblastoma cells; HCAECs, human coronary artery endothelial cells; HCT-116 cells, colon carcinoma epithelial cells; HepG2 cells, human hepatoma cells; HET-1A, human esophageal epithelial cells; HL-60 cells, human promyelocytic leukemia cells; H9C2 cells, rat cardiac myocytes; PMVECs, pulmonary microvascular endothelial cells; Ramos cells, human Burkitt’s lymphoma cells; Seg-1 cells, human esophageal adenocarcinoma cells; SOD, superoxide dismutase; X, xanthine; XO, xanthine oxidase.
Annexin Vand Sytox Green are widely used markers to evaluate apoptosis in various cell types using confocal microscopy and/or flow cytometry27–29. In apoptotic cells, phosphatidylserine is externalized to the plasma membrane surface, which can be measured by flow cytometry and confocal microscopy using phosphatidylserine-binding protein Annexin V conjugated to fluorochromes18,28,29. In conjunction with the permeability probe Sytox Green dye (which works similarly to propidium iodide), a distinction can be made between dying cells with intact plasma membrane integrity and necrotic cells (Sytox Green is impermeant to live or apoptotic cells but stains dead cells with intense green fluorescence by binding to cellular nucleic acids27–29).
As mitochondria play a fundamental role in apoptosis, which can be triggered by increased reactive oxygen and nitrogen species, through mitochondrial membrane permeabilization and release of proapoptotic factors from the mitochondrial intermembrane space to the cytosol, there is considerable interest in cell biology to study the spatial–temporal aspects of changes in mitochondrial superoxide generation and execution of programmed cell death. We have developed a protocol that allows simultaneous measurements of mitochondrial superoxide generation with apoptotic markers (Annexin A5 and/or Sytox Green) by both flow cytometry and confocal microscopy. The advantages of the described flow cytometry method over other cell-based techniques are the tremendous speed, exquisite precision and the possibility of simultaneous quantitative measurements of mitochondrial superoxide generation with apoptosis (or other) markers, with maximal preservation of cellular functions. This method combined with fluorescent microscopy, with careful interpretation of its limitations, may be very useful to reveal important spatial–temporal changes in mitochondrial superoxide production and execution of programmed cell death in virtually any cell type. Although in this protocol we used endothelial cells as a model system to simultaneously study the mitochondrial superoxide production and apoptosis in live cells by flow cytometry and confocal microscopy, this method can easily be adapted for cell types in which the use of MitoSOX was already reported either by confocal microscopy or by flow cytometry (e.g., H9c2 cardiomyocytes19, isolated cardiomyocytes16, human coronary artery and mouse pulmonary microvascular endothelial cells (MPMVECs)18,19,25, motor12, hippocampal14, cortical14 and cerebellar13 neurons, oligodendrocytes7, neuroblastoma cells15, fibroblasts17, human Burkitt’s lymphoma20, human promyelocytic leukemia21, human hepatoma22, colon carcinoma epithelial23 and human esophageal adenocarcinoma cells24, and possibly any other cell types following proper optimization of the loading conditions) (see also Table 1 for reported examples). The use of proper positive controls (e.g., Antimycin A, doxorubicin (DOX), high glucose) and negative controls (e.g., superoxide dismutase mimetics, mitochondrial uncouplers) to validate mitochondrial superoxide generation with MitoSOX in a previously not reported cell type is very important.
MATERIALS
REAGENTS
Fluorescence-activated cell sorting (FACS) binding buffer; see REAGENT SETUP
Dimethyl sulfoxide (DMSO) (Hybri-Max; Sigma, cat. no. D2650)
12 × 75 mm round-bottom tubes (BD Falcon, cat. no. 352052)
Human coronary artery endothelial cells (HCAECs) (Cell Applications Inc., cat. no. 300-05a), passages 2–6, or other cells of interest, with optimized passage and culture conditions ▲ CRITICAL HCAECs were grown in cell culture dishes or in a 12-well plate coated with 0.2% (wt/vol) gelatin.
HCAECs growth medium (Cell Applications Inc., 212K-500) or an appropriate medium for growing cells of interest
MitoSOX Red (Molecular Probes, Invitrogen, cat. no. M36008) supplied in 50 μg vials; see REAGENT SETUP
Sytox Green nucleic acid stain (Molecular Probes, Invitrogen, cat. no. S7020); see REAGENT SETUP
Allophycocyanin (APC)-Annexin V (cat. no. A35110) and Alexa Fluor 647-Annexin V (cat. no. A23204; Molecular Probes, Invitrogen) (use directly from supplied stock). Store at 4 °C ▲ CRITICAL Protect material from long-term exposure to light; it may be exposed to light for short periods of time.
Antimycin A (Sigma, cat. no. A8674); see REAGENT SETUP
DOX (Sigma, cat. no. 44583); see REAGENT SETUP
DETANONOate (cat. no. 82120); see REAGENT SETUP
SOD-PEG (cat. no. S9549); see REAGENT SETUP
MPMVECs
RPMI (Invitrogen) medium supplemented with 10% FBS, essential amino acids, endothelial cell growth supplement and antibiotics
DMEM (Invitrogen) supplemented with 10% FBS, nonessential amino acids, endothelial cell growth supplement and antibiotics
Extracellular medium (ECM); see REAGENT SETUP
Endothelial cell growth supplement (Upstate Biotech)
Experimental imaging solution (ECM containing 0.25% BSA) containing sulfinpyrazone (Sigma)
Annexin binding buffer (10 mM HEPES, 140 mM NaCl and 2.5 mM CaCl2, pH 7.4)
pEYFP-Mito (Clontech, BD Biosciences, cat. no. 6115-1)
Basic Nucleofector kit for primary mammalian endothelial cells (Amaxa Biosystems, cat. no. VPI-1001)
EQUIPMENT
Tissue culture equipment
4 and −20 °C refrigerators
Incubator (37 °C, 5% CO2 (vol/vol))
Centrifuge
Flow cytometer (FACSCalibur) (488 nm argon laser and 635 nm Red diode or 633 nm HeNe laser line, for excitation)
Amaxa Nucleofector Device (Amaxa Biosystems)
Appropriate imaging system, for example, Bio-Rad Radiance 2000 imaging system (Carl Zeiss MicroImaging) equipped with a Kr/Ar ion laser source and red diode laser source with excitation at 488, 568 and 638 nm
Temperature-controlled stage (PDMI-2, open perfusion micro-incubator; Harvard Apparatus)
Flow JO Version 8.5.2 (Three Stars Inc.)
REAGENT SETUP
FACS binding buffer Hank’s buffered salt solution (GIBCO, Invitrogen) containing 5 mM calcium chloride and magnesium chloride with 1% wt/vol BSA (cell culture tested; Sigma)
MitoSOX Red Store at −20 °C. Dissolve in 13.2 μl DMSO for 5 mM(1,000×) stock just before (<15 min) the experiment. Prepare in the dark and cover with a foil. ! CAUTION Potentially carcinogenic; use nitrile gloves.
Sytox Green nucleic acid stain Supplied as a 5 mM solution in DMSO. Prepare working stock at 1 μM in FACS binding buffer. Store at −20 °C. ! CAUTION Potentially harmful; use nitrile gloves.
Antimycin A Prepare a 10 mM stock in ethyl alcohol. Store at −20 °C. ! CAUTION Highly toxic; use gloves and prepare in a biohazard hood.
DOX Dissolve 1.16 mg in 2 ml water to make 1 mM stock. Store at −20 °C in aliquots. ! CAUTION Toxin and potentially carcinogenic; use gloves.
DETANONOate Dissolve 1.22 mg of DETANONOate in 250 μl water to make 30mM solution (1,000× stock). Prepare just before use. ! CAUTION Potentially hazardous; use gloves.
SOD-PEG Dissolve as 50 U μl−1 stock. Use 500 U per 2 ml media per well. ! CAUTION Potentially skin irritant and hazardous.
ECM 121 mM NaCl, 5 mM NaHCO3, 10 mM Na-HEPES, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2 mM CaCl2, 10 mM glucose and 2.0% dextran, pH 7.4; all obtained from Sigma.
PROCEDURE
-
Mitochondrial superoxide production can be measured by flow cytometry (see option A) or by confocal microscopy (see option B).
-
Measurement of apoptosis and mitochondrial superoxide by flow cytometry
-
Grow cells to 80% confluence and add fresh media before the experiment.
▲ CRITICAL STEP Do not grow the cells for more than 48 h in gelatin-coated plates or dishes.
? TROUBLESHOOTING
If looking at a chronic model where analyses include apoptosis marker, treat cells with PBS or DOX at 5 μM for 16 h in a CO2 incubator at 37 °C in 12-well plates. Otherwise, leave cells untreated.
-
Add MitoSOX Red to give a final concentration of 5 μM and incubate cells for 30 min at 37 °C to allow loading of MitoSOX Red. Keep the cells covered with the foil to prevent light exposure.
? TROUBLESHOOTING
Trypsinize off cells for 5 min or less and wash two times with Hank’s buffered salt solution containing calcium and magnesium.
Resuspend cells at 1–2 × 107 cells ml−1. Place cells in a sterile FACS tube at a concentration of 5–10× 106 cells per 100 μl. Dilute samples to a final volume of 500 μl with FACS binding buffer.
If the cells have not already been treated with an apoptosis-inducing agent, treat them with PBS (control) or 5 or 20 μM Antimycin A (as a mitochondrial superoxide generator) and run samples as described in Step (viii) at 0- to 40-min time points.
-
If you wish to stain apoptotic and dead cells, add 1 μl Sytox Green and 5 μl APC-Annexin V, and incubate for 15 min at room temperature (25 °C) before analysis.
■ PAUSE POINT Store cells at 4 °C for a maximum of 30 min until ready to run samples on a flow cytometer.
-
Run samples on the flow cytometer with 488 nm excitation to measure oxidized MitoSOX Red in the FL2 and FL3 channels. Collect at least 5,000 events in each sample. If you have also stained with Sytox Green and Annexin V, also collect data from the FSC (forward scatter), SSC (side scatter) and FL1 and FL4 channels. Collect at least 10,000 events.
▲ CRITICAL STEP Individual control samples stained with each of the dyes separately are needed for instrument setup.
▲ CRITICAL STEP Keep samples cold during acquisition on the cytometer. Run samples as soon as possible, at the latest within 30 min of the addition of dyes. Compensation may be necessary for the FL2 channel from the FL3 channel in analog flow cytometer.
-
Perform data analysis. Plot cells for FSC and SSC. Cell debris with low FSC and SSC should be excluded from analyses. Then, plot cells for APC-Annexin V (FL4) and Sytox Green (FL1), followed by plotting each population as a histogram of mean intensity (FL2, MitoSOX). We use Flow JO Version 8.5.2.
? TROUBLESHOOTING
-
-
Visualization of apoptosis and mitochondrial superoxide by confocal microscopy
Transfect cells with pEYFP-Mito. We use MPMVECs and transfect with 2 μg ml−1 pEYFP-Mito via electroporation (Program S-05) using the Basic Nucleofector kit for primary mammalian endothelial cells. Culture cells in RPMI medium supplemented with 10% FBS, essential amino acids, endothelial cell growth supplement and antibiotics overnight.
Replace medium with complete culture medium consisting of DMEM supplemented with 10% FBS, nonessential amino acids, endothelial cell growth supplement and antibiotics. Following transfection, plate cells at low density to facilitate colony growth.
Select colonies positive for Mito-eGFP and passage to increase the number of GFP-positive cells and plate on 0.2% gelatin-coated coverslips.
Load MPMVECs adherent to 0.2% gelatin-coated 25-mm-diameter glass coverslips with the mitochondrial superoxide (O2 •−)-sensitive fluorescent dye MitoSOX Red (10 μM) in supplemented ECM medium at 37 °C for 30 min.
If you also wish to stain with Annexin V, following MitoSOX Red loading, suspend cells in Annexin binding buffer and incubate with the apoptosis marker Annexin V Alexa Fluor 647 conjugate for 15 min at room temperature. Add 25 μl of the Annexin V conjugate to each 500-μl portion of cell suspension.
After dye loading, wash the cells and resuspend in the experimental imaging solution (ECM containing 0.25% BSA) containing sulfinpyrazone to minimize dye loss. Place the cells on a temperature-controlled stage of an appropriate imaging system. We use the Bio-Rad Radiance 2000 imaging system equipped with a Kr/Ar ion laser source with excitation at 488 and 568 nm for Mito-eGFP and MitoSOX Red, respectively, using a Nikon TE3000 inverted microscope with a × 60 oil objective. Record Mito-eGFP and MitoSOX Red fluorescence every 15 s. If you also wish to detect Annexin V, excite at 638 nm. Record Mito-eGFP, MitoSOX Red and Annexin V fluorescence as soon as possible following cell staining. We analyze images using Metamorph or Spectralyzer, custom software.
Add Antimycin A (2 μM) following 2 min of baseline recording. Obtain tracings representative of the mean cellular response by mitochondrial masking of Mito-eGFP and MitoSOX Red fluorescence (Metamorph or Spectralyzer, custom software).
-
TIMING
The total amount of time necessary for flow cytometry is about 1–2 h, but may vary, largely depending on the experimental design. The procedure consists of 30 min of MitoSOX Red loading, 15 min for processing the cells, 15 min for Sytox Green/Annexin V-APC binding or Antimycin A treatment, and 15 min for flow cytometry measurement, including control dye standardization. For confocal microscopy, MitoSOX loading requires 30–40 min and Annexin V-APC 15 min. Also considering the warm-up time for the microscope (1 h), acclimatization of cells and image capture, depending on the number of preparations and captured images, the process may take up to 2–8 h.
? TROUBLESHOOTING
Step 1A(i): Endothelial cells should not be allowed to grow longer than 48 h before the experiments in plate or dish, because that will prolong trypsinization time, which may interfere with Annexin V binding.
Step 1A(iii): MitoSOX Red loading is dependent on the mitochondrial membrane potential, and any reagents interfering with it should be avoided. Depending on the cell type, the time for MitoSOX Red loading and optimal MitoSOX Red loading concentrations may vary significantly (see also Table 1), and should be optimized individually. Addition of nitric oxide synthase inhibitors may improve the MitoSOX Red intensity in certain cell types in which nitric oxide may form peroxynitrite fast from the mitochondrial superoxide to reduce the MitoSOX Red oxidation.
Step 1A(ix): We observed strong nuclear staining of the MitoSOX Red in some instances due to loss of either mitochondrial structure or membrane potential in dying cells, a limitation that we explain in detail in the next section. For proper comparison among different groups, it is important to analyze multiple markers, including early apoptosis marker Annexin V and cell death dye Sytox Green.
ANTICIPATED RESULTS
Simultaneous measurement of mitochondrial superoxide generation with apoptotic markers by flow cytometry allows simple, quick and quantitative detection of these markers, with maximal preservation of cellular functions. Importantly, this method also reveals important limitations of the use of MitoSOX Red alone for flow cytometry, without simultaneous measurements of cell death markers, under conditions when a high rate of cell death is expected (see Fig. 1 and below). This method combined with fluorescent microscopy may be very useful to reveal important spatial–temporal changes in mitochondrial superoxide production and execution of programmed cell death in virtually any cell type. Below we describe some experiments we have performed using this protocol, as an example of the results that can be obtained.
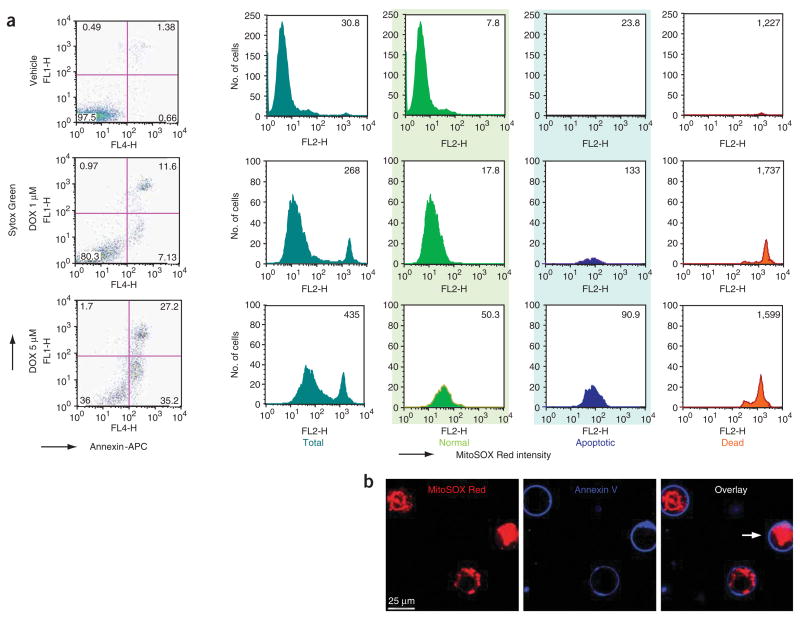
Figure 1.
Simultaneous determination of apoptotic markers and mitochondrial superoxide formation by flow cytometry and confocal microscopy following DOX treatment. (a) Dose-dependent changes in the number of apoptotic (Annexin V positive) and necrotic (Annexin V and Sytox Green positive) endothelial cells and MitoSOX fluorescence in total (dark green), normal (light green), apoptotic (blue) and dead cells (orange) following DOX treatment for 16 h. (b) Simultaneous live imaging of endothelial mitochondrial superoxide production and apoptosis following DOX treatment. All the three cells shown are Annexin V positive (blue rings). In two early apoptotic cells, MitoSOX signal shows mitochondrial pattern, whereas in one late apoptotic/dead cell (marked with an arrow), there is a strong nuclear but no mitochondrial staining pattern. Note that detailed analysis by flow cytometry of various cell populations also revealed very strong oxidized MitoSOX fluorescence in dead cells (orange histograms), because of the release of the fluorophore from the mitochondria and nuclear binding of MitoSOX Red in dead cells (Fig. 1b, marked cell). Therefore, the dead cells should be excluded from the analysis of the mitochondrial superoxide generation by flow cytometry.
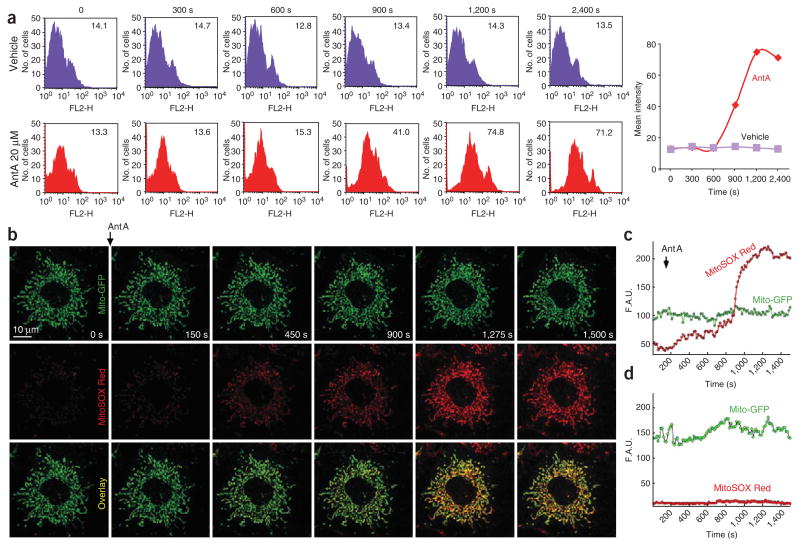
First, we measured acute mitochondrial superoxide formation by flow cytometry in HCAECs using only MitoSOX Red under a condition when apoptosis is not significant. Antimycin A rapidly increased mitochondrial superoxide generation in HCAECs measured by both flow cytometry (Fig. 2a) and confocal microscopy (Fig. 2b–d). Oxidized MitoSOX Red (red color) was nicely colocalized with GFP (green color) targeted to the mitochondria (Fig. 2b, colocalization is shown in yellow in overlaid images).
Figure 2.
Determination of mitochondrial superoxide production measured by flow cytometry and confocal microscopy using MitoSOX. (a) Complex III inhibitor Antimycin A (AntA, 20 μM) time-dependently increases mitochondrial-derived superoxide generation measured by flow cytometry. There is no significant change in the mean fluorescence intensity of vehicle-treated cells (upper row, purple histograms and trace), whereas AntA shifts histograms to the right, thereby increasing the mean fluorescence intensity of MitoSOX (lower row, red histograms and trace). For each histogram, the x axis shows % cells and the y axis shows the FL2 channel (Mitosox Red). (b) Time-lapse images of Mito-GFP-transfected MPMVECs incubated with the mitochondrial O2•−-sensitive fluorescent dye MitoSOX Red (10 μM) before and after the addition of the mitochondrial complex III inhibitor AntA (2 μM). As shown, the representative cell expresses Mito-GFP (green fluorescence) and has very low MitoSOX fluorescence (red color) before stimulation. AntA triggers time-dependent increase in mitochondrial superoxide formation (increasing red fluorescence, which is colocalized with green Mito-GFP fluorescence; see yellow color in overlaid images). (c) Representative tracings of Mito-GFP and MitoSOX Red fluorescence in response to AntA (2 μM) showing increased MitoSOX Red fluorescence in stimulated cells. (d) MitoSOX Red fluorescence is unchanged in unstimulated cells. F.A.U., fluorescence arbitary unit.
Next, we simultaneously measured mitochondrial apoptotic markers and mitochondrial superoxide formation by flow cytomery in HCAECs using the chemotherapeutic agent DOX, which is known to trigger both a marked increase in mitochondrial superoxide production and cell death (both apoptotic and necrotic). The data in Figure 1a represent the distribution of nonapoptotic, apoptotic and dead cells by Annexin V-APC and Sytox Green staining and histogram analysis of mean intensity of oxidized MitoSOX Red in HCAECs treated with DOX for 16 h. Cells were separated into three groups: nonapoptotic normal cells (light green), early apoptotic cells (blue) and dead cells (orange) (Fig. 1b). As shown in Figure 1a,b, DOX induced a dose-dependent increase in the number of apoptotic and necrotic endothelial cells, as well as in the intensity of MitoSOX fluorescence. However, detailed analysis by flow cytometry of various cell populations, complemented with confocal microscopy, revealed that in dead (orange histograms) cells the oxidized MitoSOX gives very strong fluorescence (Fig. 1a, orange), because of the release of the fluorophore from the mitochondria and binding to nuclear DNA (Fig. 1b, marked cell). Therefore, it is extremely important to exclude the dead cells from the analysis of the mitochondrial superoxide generation (especially if a large number of such cells is expected during the experiment), because the total fluorescence intensity of oxidized MitoSOX Red measured by flow cytometry may be misleading.
The specificity of mitochondrial superoxide production measured by MitoSOX Red in normal cells could be verified by SOD-PEG (a cell-permeable superoxide dismutase that scavenges superoxide; 1,000 U per 2 ml) and DETANONOate (30 μM; a nitric oxide donor that promptly reacts with superoxide to form peroxynitrite) pretreatment, which markedly reduced the intensity of MitoSOX at the FL2 channel (data not shown); furthermore, it could be verified by the use of the superoxide generator system as described7,18,19. The specificity of the superoxide detection by confocal microscopy can further be enhanced by using the dual-wavelength excitation (at 396 and 510 nm) method proposed by Beckman and co-workers7. (The latter method is not feasible for flow cytometry at present, because most flow cytometers cannot use the proposed second low wavelength.)
Acknowledgments
This research was supported by the Intramural Research Program of NIH/NIAAA (to P.P.). M.M. was supported by AHA (0530087N) and NCRR (ISIORR022511-01A1) grants. B.J.H. was supported by a fellowship from the Hypertension Association. We are indebted to Professors Joseph S. Beckman and Balaraman Kalyanaraman for reading the protocol and for valuable comments.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 2.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 3.Csiszar A, Pacher P, Kaley G, Ungvari Z. Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol. 2005;3:285–291. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Robinson KM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soh N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal Bioanal Chem. 2006;386:532–543. doi: 10.1007/s00216-006-0366-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rezvani HR, et al. Hypoxia-inducible factor-1alpha, a key factor in the keratinocyte response to UVB exposure. J Biol Chem. 2007;282:16413–16422. doi: 10.1074/jbc.M611397200. [DOI] [PubMed] [Google Scholar]
- 12.Pehar M, et al. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- 14.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman MC, Oberley LW, Flanagan SW. Mutant SOD1-induced neuronal toxicity is mediated by increased mitochondrial superoxide levels. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04502.x. [DOI] [PubMed] [Google Scholar]
- 16.Fauconnier J, et al. Effects of palmitate on Ca2+ handling in adult control and ob/ob cardiomyocytes: impact of mitochondrial reactive oxygen species. Diabetes. 2007;56:1136–1142. doi: 10.2337/db06-0739. [DOI] [PubMed] [Google Scholar]
- 17.Iuso A, et al. Dysfunctions of cellular oxidative metabolism in patients with mutations in the NDUFS1 and NDUFS4 genes of complex I. J Biol Chem. 2006;281:10374–10380. doi: 10.1074/jbc.M513387200. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358:203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookes PS, et al. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid and its derivatives elicit human lymphoid cell apoptosis through a novel pathway involving the unregulated mitochondrial permeability transition pore. Cancer Res. 2007;67:1793–1802. doi: 10.1158/0008-5472.CAN-06-2678. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Soboloff J, Zhu Z, Berger SA. Inhibition of Ca2+ influx is required for mitochondrial reactive oxygen species-induced endoplasmic reticulum Ca2+ depletion and cell death in leukemia cells. Mol Pharmacol. 2006;70:1424–1434. doi: 10.1124/mol.106.024323. [DOI] [PubMed] [Google Scholar]
- 22.Lund KC, Peterson LL, Wallace KB. The absence of a universal mechanismof mitochondrial toxicity by nucleoside analogs. Antimicrob Agents Chemother. 2007 doi: 10.1128/AAC.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne CM, et al. Deoxycholate induces mitochondrial oxidative stress and activates NF-{kappa}B through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28:215–222. doi: 10.1093/carcin/bgl139. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak K, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut. 2007;56:763–771. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajesh M, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293:H610–H619. doi: 10.1152/ajpheart.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ungvari Z, et al. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 27.Poot M, Gibson LL, Singer VL. Detection of apoptosis in live cells by MitoTracker red CMXRos and SYTO dye flow cytometry. Cytometry. 1997;27:358–364. [PubMed] [Google Scholar]
- 28.van Genderen H, et al. In vitro measurement of cell death with the annexin A5 affinity assay. Nat Protoc. 2006;1:363–367. doi: 10.1038/nprot.2006.55. [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhyay P, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]