Abstract
Study Objectives:
Nonalcoholic fatty liver disease (NAFLD) is a disorder that often presents with elevated serum aminotransferase levels. Although it has classically been linked with the metabolic syndrome, recent studies suggest NAFLD may also be associated with obstructive sleep apnea (OSA). This study evaluates the association between serum aminotransferase levels and factors connected with: either the metabolic syndrome (elevated body mass index [BMI], lipid profile, blood pressure, fasting glucose), or with OSA severity (apnea hypopnea index, lowest oxygen saturation level, oxygen desaturation index, percent of time below 90% saturation [%T<90]).
Design:
Retrospective case series.
Patients and Setting:
109 adult patients with OSA at a university hospital general clinical research center.
Measurements and Results:
Markers of hypoxia (lowest oxygen saturation level and %T<90), correlated significantly with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (Pearson's r = −0.31 to −0.38, P <0.003), while apnea hypopnea index, body mass index, blood pressure, fasting glucose, triglyceride, and cholesterol levels did not. Hierarchical linear regression was then done to determine the best predictors of aminotransferase levels. Markers of metabolic syndrome were entered as one block and markers of sleep apnea as another. Regression analyses explained 16.3% of the variance in AST and 18.9% of the variance in ALT, with %T<90 playing the largest role.
Conclusions:
In patients with obstructive sleep apnea, serum aminotransferase levels are better predicted by markers of oxygen desaturation than by factors traditionally associated with the metabolic syndrome.
Citation:
Norman D; Bardwell WA; Arosemena F; Nelesen R; Mills PJ; Loredo JS; Lavine JE; Dimsdale JE. Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. SLEEP 2008;31(1):-121-126.
Keywords: Transaminases, fatty liver, obstructive sleep apnea, hypoxia, metabolic syndrome X
INTRODUCTION
NONALCOHOLIC FATTY LIVER DISEASE (NAFLD) REPRESENTS A SPECTRUM OF LIVER DISORDERS, RANGING FROM ISOLATED FATTY INFILTRATION OF the liver to steatohepatitis (fatty infiltration with accompanying inflammation), to end-stage cirrhosis. Definitive diagnosis of NAFLD requires liver biopsy; but the disease is often first suspected as an asymptomatic elevation in serum aminotransferase levels.1 Although its exact pathogenesis is unknown, NAFLD has been traditionally linked with the metabolic syndrome (“Syndrome X”),2 which consists of obesity, dyslipidemia, insulin resistance, and hypertension. Factors associated with the metabolic syndrome, such as blood pressure, BMI, and fasting glucose, cholesterol, and triglyceride levels, have been linked with elevated serum aminotransferase levels3 and ultrasonographic evidence4 of NAFLD. NAFLD is far more prevalent in obese individuals (57%–74%) than in the general population (10%–24%).5 In one case series of 42 patients with nonalcoholic steatohepatitis (NASH), 95% were obese, 75% were hyperlipidemic, and nearly half were hyperglycemic.6 Mechanistic theories of NAFLD pathogenesis often invoke insulin resistance and dyslipidemia in the setting of metabolic syndrome as key promoters of fatty-acid deposition in the liver.5 However, such theories do not fully explain NAFLD pathogenesis, as not all patients with NAFLD have metabolic syndrome. In fact, one case series reported that 42% (of 33) patients with NAFLD were nonobese and had normal glucose and lipid levels.7
Some authors have suggested that obstructive sleep apnea (OSA) may be another contributor to NAFLD development. OSA is a disorder that is characterized by repeated episodes of sleep disruption due to upper airway obstruction, with associated decrements in oxyhemoglobin saturation levels. Like NAFLD, OSA is more prevalent in, but not confined to obese individuals.8 OSA has also been linked to other features of the metabolic syndrome, including dyslipidemia,9 insulin resistance,10 and hypertension.10 Sing et al.11 found OSA symptoms were prevalent in 46% of 190 patients with aminotransferase levels, imaging, or biopsy findings suggestive of NAFLD. The prevalence of OSA symptoms tended to be even higher (63%) among the subset of patients whose liver biopsies showed more advanced disease.11 A case series of 163 patients undergoing evaluation for OSA showed that 20% had elevated liver enzymes; and that the presence of severe OSA (AHI>50) was a far stronger predictor of elevated liver enzymes (odds ratio [OR] 5.9) than was elevated BMI (OR 1.13).12 The presence of severe OSA was also associated with greater degrees of steatosis, necrosis and fibrosis on liver biopsy.12 Hypoxia from OSA has been associated with serum markers of liver fibrosis.13 Chin et al.14 found that 35% of 44 obese OSA patients had abnormal aminotransferase levels, and that AST levels rose at night in OSA patients prior to therapy. Furthermore, CPAP therapy reduced the nighttime rise in aminotransferase levels, both immediately and over 1 and 6 months of CPAP therapy.14
These studies demonstrate that, like the metabolic syndrome, OSA is common in patients with NAFLD and may play some role in its pathogenesis. However, the considerable overlap between the 3 disease entities (OSA, NAFLD, and the metabolic syndrome) makes it difficult to separate the contributors from the confounders of NAFLD pathogenesis.
We hypothesized that OSA severity, particularly nocturnal oxygen desaturation, is associated with markers of NAFLD. To evaluate this hypothesis, we examined components of the metabolic syndrome (BMI, blood pressure, fasting glucose, and lipid panels) as well as markers of OSA severity (AHI, oxygen desaturation index, nadir oxygen saturation, percentage of time spent with SpO2 < 90% [%T<90]) to see which factors correlate best with elevated aminotransferase levels, and to build a predictive model for aminotransferase levels in a cohort of patients with OSA.
METHODS
Subjects and Protocol
Data were gathered from databases of previous and ongoing studies examining the pathophysiology of the sympathetic nervous system in patients with OSA. Subjects were recruited through public advertisements that called for people with symptoms of heavy snoring, difficulty breathing during sleep, morning headaches, and/or excessive daytime sleepiness. Additional subjects were found through word-of-mouth referral from prior research participants. Men and women between 25 and 65 years of age who were free from major illnesses other than hypertension and who had a BMI between 19.5 and 50 kg/m2 were evaluated. All subjects provided written informed consent prior to study participation. The study protocol was approved by the University of California San Diego (UCSD) Human Research Protections Program. Subjects underwent screening history and physical examination at the time of recruitment. Anthropometric measures were obtained, and BMI was calculated. Blood pressure readings were obtained using a manual cuff while the patients were seated; readings were averaged across 3 measurements. Patients were subsequently admitted to the General Clinical Research Center, and underwent overnight polysomnography (described below), followed by a fasting venous blood draw the following morning for serum aminotransferase levels (AST and ALT), glucose, and lipid panel testing.
Polysomnography
Overnight polysomnography was recorded with a Grass Heritage polysomnogram (model PSG36-2, West Warwick, RI), which recorded the following parameters: electrocardiogram, central and occipital electroencephalogram, bilateral electro-oculogram, submental and anterior tibialis electomyogram, nasal airflow using a nasal cannula and pressure transducer, naso-oral airflow using a thermistor, and respiratory effort using chest and abdominal piezoelectric belts. Oxyhemoglobin saturation (SpO2) was monitored using a pulse oximeter (Biox 3740; Ohmeda: Louisville, Colorado). Sleep staging was scored according to the criteria of Rechtshaffen and Kales.15 Apneas were defined as decrements in airflow ≥ 90% from baseline for ≥ 10 seconds. Hypopneas were defined as decrements in airflow ≥ 50% but < 90% from baseline for ≥ 10 seconds. The number of apneas and hypopneas per hour of sleep were calculated to obtain the apnea-hypopnea index (AHI). Respiratory events were derived primarily from the nasal cannula-pressure transducer. The oxygen desaturation index (ODI) was defined as the total numbers of episodes of oxyhemoglobin desaturation ≥ 3% from the immediate baseline, ≥ 10 seconds but < 3 minutes, divided by the total sleep time.
Statistical Analysis
Data were analyzed using SPSS version 14.0 software (SPSS Inc., Chicago, IL). Pearson's correlation coefficients were calculated between serum aminotransferase values and glucose, total cholesterol, HDL, LDL, BMI, mean arterial pressure (MAP), age, mean nocturnal SpO2, lowest SpO2 level during the night, percent of time in bed with SpO2 <90% (%T<90), oxyhemoglobin desaturation index (ODI), and apnea hypopnea index (AHI). Correlations were corrected for multiple comparisons using the Bonferroni method. P <0.0036 was considered significant. Data were then analyzed using hierarchical linear regressions aimed at determining the extent to which variables associated with metabolic syndrome and with OSA predicted serum aminotransferase levels. Forced entry was used with key metabolic syndrome variables (glucose, total cholesterol, triglycerides, BMI, and MAP) as the first block, and OSA associated variables (AHI and %T<90) as the second block. To simplify our model, only one measure of cholesterol (total cholesterol) and of nocturnal oxyhemoglobin saturation (%T<90) were included in the hierarchical regression. Statistical significance in the regression model was defined as P <0.05.
RESULTS
Demographic data from the 109 subjects included in this study are reported in Table 1. On average, subjects were middle aged (mean ± SD age: 48.5 ± 9.5 y), mildly obese (BMI: 31.4 ± 5.4 kg/m2), and suffered from moderately severe OSA (AHI: 53.0 ± 37.5/h). Twenty-eight of the subjects were hypertensive (defined as mean of 3 blood pressure readings >140 mm Hg systolic or >90 mm Hg diastolic).
Table 1.
Demographics, Lab Values, and Polysomnographic Data
| Subject Characteristics | n | Mean | Standard Deviation |
|---|---|---|---|
| Age (years) | 109 | 48.5 | 9.5 |
| BMI (kg/m2) | 109 | 31.4 | 5.4 |
| Blood Pressure Variables (mmHg) | |||
| Systolic blood pressure | 109 | 129.4 | 15.8 |
| Diastolic blood pressure | 109 | 78.3 | 9.2 |
| Mean arterial blood pressure | 109 | 95.3 | 10.5 |
| Sleep Variables | |||
| Apnea hypopnea index (events/hr) | 109 | 53.0 | 37.5 |
| Average SpO2 (%) | 105 | 92.7 | 4.5 |
| Lowest SpO2 level (%) | 105 | 75.3 | 17.2 |
| Time spent with SpO2 < 90% (minutes) | 106 | 38.4 | 70.0 |
| Percent of time in bed with SpO2<90% | 106 | 9.9 | 17.4 |
| Oxygen Desaturation Index (events/hr) | 105 | 35.3 | 30.9 |
| Fasting Serum Levels | |||
| Glucose (mg/dL) | 107 | 94.5 | 12.6 |
| AST (IU/L) | 106 | 26.0 | 10.4 |
| ALT (IU/L) | 102 | 39.7 | 19.6 |
| Total Cholesterol (mg/dL) | 104 | 196.1 | 35.2 |
| Triglycerides (mg/dL) | 103 | 155.9 | 117.8 |
| HDL (mg/dL) | 104 | 40.3 | 11.0 |
| LDL (mg/dL) | 102 | 127.8 | 34.5 |
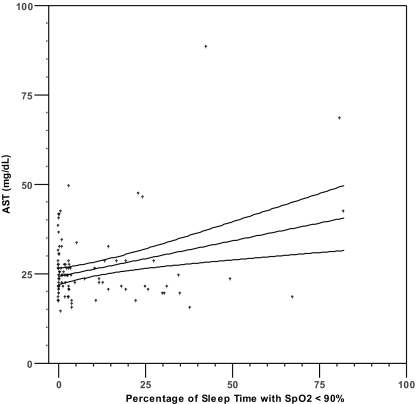
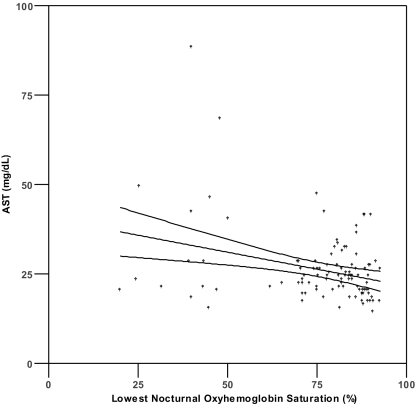
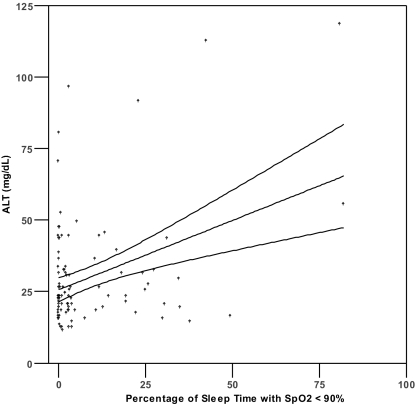
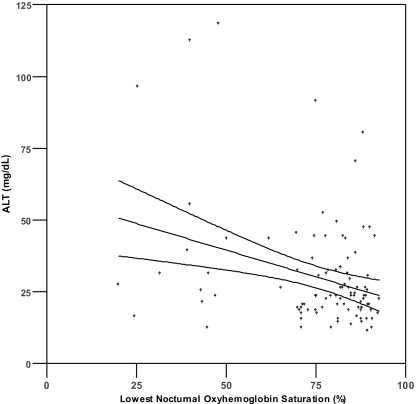
Pearson's correlation analysis (Table 2) demonstrated a significant correlation (P <0.0036) between serum aminotransferase levels and both lowest SpO2 level (r = − 0.307 to − 0.316) and %T<90 (r = 0.307 to 0.376) (Figure 1). No significant correlations were found between aminotransferase levels and serum glucose, total cholesterol, HDL, LDL, triglycerides, BMI, MAP, or AHI (r = −0.259 to 0.223, all P >0.003). Because %T<90 showed the strongest univariate correlations with ALT and AST, this variable was selected as the measure of oxyhemoglobin saturation for inclusion in the hierarchical linear regression analyses (see below). We also found a significant correlation existed between age and ALT (r = −0.340, P <0.001), but not AST (r = − 0.160, P = 0.102).
Table 2.
Pearson's Correlations for Various Markers of the Metabolic Syndrome and OSA with Serum Aminotransferase Levels.
| Pearson's r | ||
|---|---|---|
| AST |
ALT |
|
| Glucose | 0.093 | 0.158 |
| Triglycerides | 0.104 | 0.083 |
| Total Cholesterol | 0.092 | 0.040 |
| HDL | −0.020 | −0.089 |
| LDL | 0.031 | 0.048 |
| BMI | 0.087 | 0.140 |
| MAP | 0.138 | 0.122 |
| Age | −0.160 | −0.340* |
| Average SpO2 | −0.182 | −0.259 |
| Lowest SpO2 Level | −0.307* | −0.316* |
| Percent of Time in Bed with SpO2 <90% | 0.307* | 0.376* |
| Oxyhemoglobin Desaturation Index | 0.122 | 0.223 |
| Apnea Hypopnea Index | 0.104 | 0.153 |
(* Denotes Significance at P <0.0036)
Figure 1.
Scatterplots of AST or ALT and percentage of sleep time with SpO2 < 90% (1a, 1b) and lowest nocturnal oxyhemoglobin saturation (1c, 1d). Lines represent best fit by linear regression and 95% confidence intervals.
Hierarchical linear regression analyses (Tables 3 and 4) demonstrated that metabolic syndrome-associated variables did not significantly account for the variance in ALT (R2=0.062, P = 0.31) or AST (R2=0.060, P = 0.31). On the other hand, OSA-associated variables explained an additional 12.7% of the variance in ALT and 20.3% of the AST (significance of overall model and of change in F, all P '0.02). When the variables entered into the model were examined individually, none of the metabolic syndrome variables were significant individual predictors of either ALT (P = 0.14 to 0.72) or AST (P = 0.24 to 0.68). Of the OSA variables, only %T<90 was significant as an independent predictor of ALT or AST (both P ≤0.02).
Table 3.
Hierarchical Linear Regression Analysis, with Markers of the Metabolic Syndrome in the First Step, and Markers of OSA in the second Step, Used to Predict ALT
| ALT | B | S.E.B | T-score | P value |
|---|---|---|---|---|
| Step 1 | ||||
| GLU | 0.246 | 0.166 | 1.479 | 0.143 |
| TRI | 0.013 | 0.018 | 0.719 | 0.474 |
| CHOL | 0.021 | 0.057 | 0.362 | 0.718 |
| BMI | 0.330 | 0.410 | 0.806 | 0.422 |
| MAP | 0.164 | 0.198 | 0.832 | 0.407 |
| Step 2 | ||||
| GLU | 0.221 | 0.158 | 1.398 | 0.166 |
| TRI | 0.015 | 0.017 | 0.904 | 0.369 |
| CHOL | 0.026 | 0.053 | 0.491 | 0.625 |
| BMI | −0.235 | 0.428 | −0.548 | 0.585 |
| MAP | 0.155 | 0.186 | −0.836 | 0.405 |
| %of time <90% | 0.482 | 0.137 | 3.523 | 0.001 |
| AHI | 0.017 | 0.056 | 0.311 | 0.756 |
Step 1: 6.2% of variance, (F=1.205, df=5,91, P = 0.313)
Step 2: 18.9% of variance, (F=2.965, df=7,89, P = 0.008), sig of F change P = 0.002
Table 4.
Hierarchical Linear Regression Analysis, with Markers of the Metabolic Syndrome in the First Step, and Markers of OSA in the Second Step, Used to Predict AST
| AST | B | S.E. B | T-score | P value |
|---|---|---|---|---|
| Step 1 | ||||
| GLU | 0.102 | 0.087 | 1.172 | 0.244 |
| TRI | 0.009 | 0.009 | 0.984 | 0.328 |
| CHOL | 0.025 | 0.030 | 0.840 | 0.403 |
| BMI | 0.086 | 0.210 | 0.408 | 0.684 |
| MAP | 0.116 | 0.100 | 1.158 | 0.250 |
| Step 2 | ||||
| GLU | 0.084 | 0.084 | 1.003 | 0.318 |
| TRI | 0.011 | 0.009 | 1.258 | 0.211 |
| CHOL | 0.028 | 0.028 | 0.977 | 0.331 |
| BMI | −0.196 | 0.226 | −0.867 | 0.388 |
| MAP | 0.122 | 0.096 | 1.272 | 0.206 |
| %of time <90% | 0.224 | 0.069 | 3.255 | 0.002 |
| AHI | 0.001 | 0.030 | 0.037 | 0.971 |
Step 1:6.0 % of variance, (F=1.202, df=5,94, P = 0.314)
Step 2: 16.3% of variance, (F=2.559, df=7,92, P = 0.019), sig of F change P = 0.005
DISCUSSION
Although recent studies have suggested the existence of a link with OSA, NAFLD has traditionally been associated with the metabolic syndrome. Our results demonstrate that a marker of nocturnal hypoxia explained a greater amount of variance in serum aminotransferase levels than did markers of the metabolic syndrome in patients with moderately severe OSA. At the same time, our results showed that the apnea-hypopnea index was not significantly associated with the variance in serum aminotransferase levels. Thus, it appears that oxyhemoglobin desaturation, not episodic airflow limitation per se, is associated with markers of NAFLD.
A “two-hit hypothesis” for the development of NAFLD, suggested by Day and Saksena,16 postulates that insulin resistance acts as a “first hit” to result in hepatic steatosis, and that oxidative injury acts as a “second hit” to then promote hepatocyte inflammation and cell death. Our results may be consistent this hypothesis in two ways: repetitive hypoxia in OSA has been proposed as a mechanism for the development of insulin resistance17 (the first step) and as a cause of oxidative stress18 (the second step). Indeed, Tatsumi and Saibara13 compared a cohort of 83 (nonobese) patients with OSA with 41 age, sex, and BMI matched non-OSA controls. They found that levels of p-III-p, a serum marker of hepatic fibrosis, were independently associated with average nocturnal oxyhemoglobin saturation (SpO2), but not with AHI, BMI, or visceral fat. Mishra et al.19 similarly demonstrated in a sample of 46 patients who underwent bariatric surgery that mean nocturnal SpO2 was a significant predictor of pathologic grade of NAFLD activity severity, but that AHI was not.
Our results support the findings of the above studies, and suggest that liver injury associated with OSA is more directly associated with the severity of nocturnal hypoxemia than with the number of episodes of airflow limitation alone. This conclusion is further supported by animal experiments that have demonstrated that intermittent hypoxia increases liver triglyceride content in both lean20 and obese21 mice. Interestingly, we found that nadir SpO2 levels and %T<90 better predicted serum aminotransferase values than did mean SpO2 or ODI. These findings may suggest that mild sustained desaturation, and frequent small drops in SpO2 (for example, from 98% to 94%), while technically qualifying as “desaturation” under the 3% rule, do not contribute as much to liver injury as do the more pronounced intermittent drops in SpO2 to the 80%–90% range throughout the night. This finding would be in keeping with current theories that severe intermittent hypoxia contributes more to oxidative stress than does prolonged, moderate hypoxia.22
Our study has a number of limitations. Although we performed a careful history and physical examination on all of our subjects and excluded those with medical conditions other than OSA and hypertension, we did not specifically test them for viral hepatitis, hemochromatosis, or Wilson disease. Thus, we cannot exclude the possibility that occult liver diseases other than NAFLD existed. Another potential limitation is the use of fasting glucose levels as one of the markers of metabolic syndrome. Various other methods to assess insulin resistance have been used, including homeostatic model for assessment of insulin resistance, insulin sensitivity index, and insulin to glucose ratio. However, fasting glucose level remains one of the key diagnostic criteria for the metabolic syndrome in definitions from the World Health Organization,23 the American Heart Association,24 and National Heart, Lung, and Blood Institute.24 Another potential concern is that although we found age to be correlated with ALT, we did not include age in our regression formula. Our hierarchical linear regression model was constructed a priori, based on the hypothesis that markers of metabolic syndrome and sleep apnea would be significant predictors of the variance in aminotransferase levels. Review of the literature does reveal a few studies of the general population that describe an association between age and serum levels of aminotransferases (particularly ALT).25,26 However, when we repeated our analyses using a 3-step hierarchical linear regression with age as the first block, our main findings did not change. Again, %T<90 remained the only significant predictor of the variance in AST (T-score = 3.018, P = 0.003). As would be expected, age was a significant predictor of the variance in ALT (T-score in overall model = −3.378, P = 0.001). The inclusion of age did unmask a signal between fasting glucose levels and ALT (T-score = 2.051, P = 0.043), but %T<90 remained a stronger predictor of the variance in ALT (T-score = 3.208, P = 0.002). One final limitation in our study is that we did not have pathologic data available from liver biopsies, and instead used aminotransferase levels as a surrogate marker for the presence of NAFLD. While the latter method exhibits diminished sensitivity and specificity for NAFLD, it obviates the risks associated with liver biopsy, and has been employed previously by a number of studies to examine potential links between OSA and NAFLD.12–14 Furthermore, our results are consistent with findings obtained by Mishra and colleagues19 who found an association between degree of nocturnal hypoxemia and NAFLD histopathologic activity.
Our results do not suggest that OSA is the singular cause or even the most important contributor to NAFLD development. Our study focused on patients with known OSA, and there are likely a significant proportion of NAFLD patients who do not suffer from sleep apnea. Instead, we suspect that NAFLD represents a wide spectrum of disorders, with multifactorial etiologies, and that repetitive nocturnal hypoxia from OSA is one of a number of potential contributors to its pathogenesis. This study demonstrates that in patients with OSA, serum aminotransferase levels are more closely linked to markers of oxygen desaturation than to factors traditionally associated with the metabolic syndrome. To help establish causality, future studies should examine the effects of OSA therapy on NAFLD severity in patients who suffer from both disorders.
ACKNOWLEDGMENTS
Financial support provided by: HL44915, K23 HL04056, and RR00827 from the National Institutes of Health
This article does not involve off-label or investigational use of products.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Loredo has received equipment from Respironics for use in a research project. Dr. Dimsdale has received research support from Sepracor. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20:594–8. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Oh SY, Cho YK, Kang MS, et al. The association between increased alanine aminotransferase activity and metabolic factors in nonalcoholic fatty liver disease. Metab Clin Exp. 2006;55:1604–9. doi: 10.1016/j.metabol.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Jin Kim Y, Yong Jeon T, et al. Obesity is the only independent factor associated with ultrasound-diagnosed non-alcoholic fatty liver disease: a cross-sectional case-control study. Scand J Gastroenterol. 2006;41:566–72. doi: 10.1080/00365520500319591. [DOI] [PubMed] [Google Scholar]
- 5.Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50:171–80. doi: 10.1007/s10620-005-1267-z. [DOI] [PubMed] [Google Scholar]
- 6.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 7.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–9. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–11. [PubMed] [Google Scholar]
- 9.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175:190–5. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 10.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–5. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Pollock R, Uhanova J, Kryger M, Hawkins K, Minuk GY. Symptoms of obstructive sleep apnea in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2005;50:2338–43. doi: 10.1007/s10620-005-3058-y. [DOI] [PubMed] [Google Scholar]
- 12.Tanne F, Gagnadoux F, Chazouilleres O, et al. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290–6. doi: 10.1002/hep.20725. [DOI] [PubMed] [Google Scholar]
- 13.Tatsumi K, Saibara T. Effects of obstructive sleep apnea syndrome on hepatic steatosis and nonalcoholic steatohepatitis. Hepatol Res. 2005;33:100–04. doi: 10.1016/j.hepres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Chin K, Nakamura T, Takahashi K, et al. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med. 2003;114:370–6. doi: 10.1016/s0002-9343(02)01570-x. [DOI] [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. Manual of standardized techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service and Brain Research Institute; 1968. [Google Scholar]
- 16.Day CP, Saksena S. Non-alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol. 2002;17:S377–84. doi: 10.1046/j.1440-1746.17.s3.31.x. [DOI] [PubMed] [Google Scholar]
- 17.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40:1683–92. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra P, Weinstein M, Vithiananthan S, et al. Mean nocturnal oxygen saturation is predictive of non-alcoholic fatty liver disease (NAFLD) activity score (NAS) in patients with morbid obesity. Sleep Med. 2006;7(S2):S54. [Google Scholar]
- 20.Li J, Thone LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Grigoryev DN, Ye SQ, et al. Chronic intermittent hypoxia uprgulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99:1643–8. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem. 2004;385:217–21. doi: 10.1515/BC.2004.015. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Elinav E, Ben-Dov IZ, Ackerman E, et al. Correlation between serum alanine aminotransferase activity and age: an inverted U curve pattern. Am J Gastroenterol. 2005;100:2201–4. doi: 10.1111/j.1572-0241.2005.41822.x. [DOI] [PubMed] [Google Scholar]
- 26.Mohamadnejad M, Pourshams A, Malekzadeh R, et al. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J of Gastroenterol. 2003;9:2322–4. doi: 10.3748/wjg.v9.i10.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]