Abstract
Study Objectives:
Serotonin (5-HT) has long been implicated in the control of sleep and wakefulness. This study evaluated the hypnotic efficacy of the 5-HT6 antagonist RO4368554 (RO) and the 5-HT2A receptor antagonist MDL100907 (MDL) relative to zolpidem.
Design:
A randomized, repeated-measures design was utilized in which Wistar rats received intraperitoneal injections of RO (1.0, 3.0, and 10 mg/kg), MDL (0.1, 1.0 and 3.0 mg/kg), zolpidem (10 mg/kg), or vehicle in the middle of the dark (active) period. Electroencephalogram, electromyogram, body temperature (Tb) and locomotor activity were analyzed for 6 hours after injection.
Measurements and Results:
RO, MDL, and zolpidem all produced significant increases in sleep and decreases in waking, compared with vehicle control. All 3 doses of MDL produced more consolidated sleep, increased non-rapid eye movement sleep (NREM) sleep, and increased electroencephalographic delta power during NREM sleep. The highest dose of RO (10.0 mg/kg) produced significant increases in sleep and decreases in waking during hour 2 following dosing. These increases in sleep duration were associated with greater delta power during NREM sleep. ZO Zolpidem induced sleep with the shortest latency and significantly increased NREM sleep and delta power but also suppressed rapid eye movement sleep sleep; in contrast, neither RO nor MDL affected rapid eye movement sleep. Whereas RO did not affect Tb, both zolpidem and MDL reduced Tb relative to vehicle-injected controls.
Conclusions:
These results support a role for 5-HT2A receptor modulation in NREM sleep and suggest a previously unrecognized role for 5-HT6 receptors in sleep-wake regulation.
Citation:
Morairty SR; Hedley L; Flores J; Martin R; Kilduff TS. Selective 5HT2A and 5HT6 receptor antagonists promote sleep in rats. SLEEP 2008;31(1):34-44.
Keywords: Serotonin, zolpidem, sleep consolidation, EEG delta power, telemetry
INTRODUCTION
SEROTONIN (5-HYDROXYTRYPTAMINE, 5-HT) IS KNOWN TO INFLUENCE SLEEP AND WAKEFULNESS1,2; HOWEVER, THE SPECIFIC 5-HT RECEPTOR SUBTYPES that subserve these effects remain to be fully elucidated. The 5-HT2A receptor subtype has been the focus of both preclinical and clinical study, resulting in a growing body of data to support a role for 5-HT2A antagonists in the treatment of insomnia. Interest in the 5HT2 receptor initially arose from the observations that ritanserin increased slow-wave sleep (SWS) in humans3 and animal subjects.4 Various agents that nonselectively inhibit 5-HT2A receptors, including the antipsychotics ziprasidone and risperidone, have since been shown to affect rapid eye movement (REM)sleep, non-REM (NREM) sleep, and SWS in psychiatric patients and normal volunteers.5, 6 However, attribution of these clinical outcomes to 5-HT2A receptor blockade has been problematic due to the limited pharmacologic selectivity of these agents. Indeed, a comparison of the effects of the more 5-HT2A-selective antagonists ketanserin and ritanserin on SWS in healthy volunteers found a possible role for both 5-HT2A and 5-HT2C receptor inhibition on clinical outcome.7 In rats, selective 5-HT2A receptor antagonists enhance SWS and delta power during NREM sleep and decrease the number of awakenings without significant effect on REM sleep.8–10
Studies in transgenic mice, in which specific 5-HT receptor subtypes or related genes have been eliminated, provide additional support for a role of 5-HT in sleep regulation.11 From such studies, the 5-HT1A,12 5-HT1B,13 and 5-HT714 receptors, as well as monoamine oxidase A and serotonin transporters,15 have been implicated in the control of REM sleep. Studies of 5-HT2A and 5-HT2C16, 17 receptor knockout mice have supported roles for these receptors in SWS. However, the influence of genetic ablation of these receptors on the expression levels of, or effects on, other receptors remains a possible confound in these studies. Indeed, although acute inhibition of 5-HT2A receptors by the antagonist MDL100907 induces an increase in NREM sleep in wild-type mice, chronic absence of receptor function in 5-HT2A−/− mice results in a tendency toward less NREM sleep.16 In addition, responses to 5-HT2B and 5-HT2C ligands differ between the 5-HT2A−/− mice and control strains.
To date, no investigations into the role of 5-HT6 receptors in sleep have been reported. However, a role for these receptors in sleep and wake may be anticipated based on their association with brain regions known to be important in the regulation of sleep and wake, such as the hypothalamus, thalamus, and striatum.18,19 Evidence supports the hypotheses that 5-HT6 receptors may modulate GABAergic and cholinergic neurotransmission,18,20,21 two neurotransmitter systems widely known to play roles in sleep-wake regulation. Taken together, these findings suggest that 5-HT6 receptors could influence the regulation of sleep and wakefulness. Consequently, we investigated the effect of 2 ligands, RO4368554 (3-benzenesulfonyl-7-(4-methyl-piperazin-1-yl)-1H-indole) and MDL100907 ((+/−) 2,3dimethoxyphenyl-1-[2-(4-piperidine)-methanol), that selectively inhibit either 5-HT6 or 5-HT2A receptors, respectively,20, 22 on sleep and wake and associated physiologic parameters during the active phase of the rodent circadian cycle. The effects of these compounds were compared with zolpidem, a hypnotic medication that acts as an agonist at the type I benzodiazepine (ω1) binding site on the GABAA receptor. Our results support 5-HT2A receptor involvement in NREM sleep but also suggest a previously unrecognized role for 5-HT6 receptors in sleep-wake regulation.
MATERIALS AND METHODS
Animal Recording and Surgical Procedures
Animals were housed in a temperature-controlled recording room under a reverse 12/12 light/dark cycle (lights on at 1700) and had food and water available ad libitum. Room temperature (24oC ± 2oC), humidity (50% ± 20% relative humidity), and lighting conditions were monitored continuously via computer. All experimental procedures involving animals were approved by SRI International's Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Nine male Wistar rats (300 ± 25 g; Charles River, Wilmington, MA) were implanted with chronic recording devices for continuous recordings of electroencephalography (EEG), electromyography (EMG), core body temperature (Tb), and locomotor activity (LMA) via telemetry. With the animals under isoflurane anesthesia (1%–4%), the fur was shaved from the top of the head and from the midabdominal region. After the skin was disinfected with Betadine and alcohol, a dorsal midline incision on top of the head and a midventral incision through the peritoneum was made along the linea alba. Sterile miniature transmitters (F40-EET, Data Sciences Inc., St Paul, MN) were inserted through this incision and sewn to the musculature with a single stitch of silk suture (4-0). Four biopotential leads from the transmitters were inserted subcutaneously to the neck and head region. The abdominal musculature was then closed with absorbable suture (Vicryl 3-0), and the peritoneum was closed with silk suture (4-0). Furacin ointment was applied to the sutured incision. The temporalis muscle was then retracted, and the skull was cauterized and thoroughly cleaned with a 2% hydrogen peroxide solution. Holes were drilled through the skull bilaterally at −5.0 mm AP from bregma and 2.0 mm ML. The two biopotential leads that were used as EEG electrodes were inserted into the holes and affixed to the skull with dental acrylic. The two biopotential leads that were used as EMG electrodes were sutured into the neck musculature. The incision was closed with suture (silk 4-0), and antibiotics were administered topically. Pain was relieved postoperatively by intramuscular injection of a long-lasting analgesic (buprenorphine). After surgery, animals were placed in a clean cage and observed until they were ambulatory.
EEG, EMG, Tb, and LMA were recorded via telemetry using DQ ART 3.1 software (Data Sciences Inc.). After a minimum of 2 weeks of recovery from surgery, animals were acclimated to the handling procedures and were given a mock dosing 3 days before the first experimental day. After data collection was completed, expert scorers who were blinded to experimental treatment classified each 10-second epoch as either wake, NREM sleep, or REM sleep by examining the EEG and EMG recordings visually using Sleep Sign software (Kissei Comtec, Irvine CA).
Experimental Design
A repeated-measures design was employed in which each rat received 8 separate intraperitoneal doses in random order with a minimum of 3 days between doses. The dosing conditions included 3 doses of RO4368554 (RO; 1.0, 3.0, and 10.0 mg/kg), 3 doses of MDL100907 (MDL; 0.1, 1.0 and 3.0 mg/kg), 1 dose of zolpidem (10.0 mg/kg), and a vehicle control administered in a counterbalanced random design. The doses of RO were based on previously published ex vivo binding data for this molecule (IC50 = 7.8 mg/kg intraperitoneal) and corroborated by behavior studies in rat cognition models.20, 21 The doses of MDL were based on doses previously reported to reverse 5-HT2A agonist-mediated head-twitch effects (minimum effective dose range 0.003–0.1 mg/kg when administered subcutaneously23, 24). Because MDL is rapidly metabolized in rats (plasma t1/2 = 71 min; t1/2 for clearance from brain extracellular fluid = 50 min25), doses were selected to allow occupancy of the 5-HT2A receptor for several hours after the dose, as used previously.26 The zolpidem dose used has been employed in other sleep studies in the rat.27 Dosing solutions were made fresh each experimental day. The vehicle was 95% physiologic saline and 5% ethanol; the dose volume was 2 mL/kg. The dosing procedure began approximately 6 hours after lights off at Zeitgeber time 19 (ZT19, where ZT12 is lights off) and was typically completed within 15 minutes.
Data Analyses
EEG and EMG data, scored in 10-second epochs as wake, NREM sleep, or REM sleep were analyzed and expressed as time spent in each state per hour. Sleep latency for each rat was calculated from the time of drug injection to the first 6 continuous 10-second epochs scored as sleep. To determine whether any of the treatments affected the consolidation of behavioral states, the duration and number of bouts for each state were calculated in hourly bins. A “bout” consisted of a minimum of 2 consecutive 10-second epochs of a given state and ended with any single state-change epoch. When a bout extended across the end of an hour into the beginning of the next hour, it was credited to the hour in which the majority of the bout occurred. The EEG spectra during NREM sleep were analyzed offline with a fast Fourier transform algorithm (Sleep Sign software, Kissei Comtec, Irvine CA) on all epochs without visually detectable artifact. EEG delta power (0.5–3.5 Hz) within NREM sleep was analyzed in hourly bins. For each animal, delta power was normalized to the average delta power in NREM sleep during the entire 6-hour recording after vehicle injection. Hourly averages of Tb and LMA were analyzed for the 6-hour period starting with the hour of dosing; Tb data were calculated as the change from the average Tb during the 6 hours immediately prior to dosing.
Sleep-latency data were analyzed using 1-way repeated-measures analysis of variance; all other data were analyzed using 2-way repeated-measures analysis of variance. Because we predicted both a treatment effect and an effect that changed (i.e., decreased) over time, we analyzed the treatment effect (factor A), time (factor B), and the time × treatment effects within each rat. When analysis of variance indicated statistical significance, Fisher LSD t-tests were performed to determine which groups differed.
RESULTS
Wakefulness, NREM Sleep, and REM Sleep Amounts
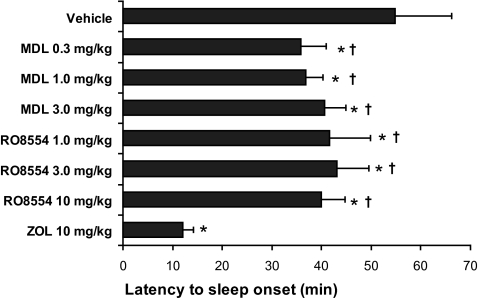
Zolpidem, MDL, and RO all significantly decreased wakefulness but with different time courses of onset and duration. Figure 1 presents the mean latency to the first continuous six 10-second epochs of sleep following drug administration. Latency to sleep onset was significantly reduced relative to vehicle following all doses of MDL, RO, and zolpidem. However, sleep-latency values for both MDL and RO were longer than that of zolpidem (10 mg/kg).
Figure 1.
Mean (± SEM) latency to the first 6 continuous epochs of sleep following drug administration. Latency to sleep onset was both significantly shorter than vehicle and longer than zolpidem (ZOL) following all doses of MDL and RO8554. Latency to sleep onset was shorter following ZOL, compared with all other conditions. *Different from vehicle P < 0.05; †Different from ZOL P < 0.05.
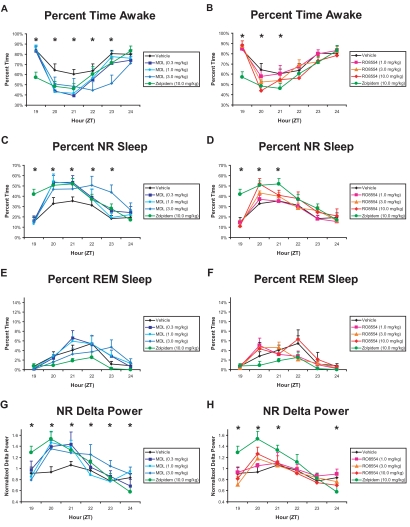
Differential effects of these compounds on sleep latency were also reflected in the hourly distribution of wake and NREM sleep following drug administration. After zolpidem treatment, wakefulness was significantly reduced relative to vehicle from ZT19 to ZT21 (Figure 2A and 2B). In contrast, MDL (0.3 and 1.0 mg/kg) reduced wakefulness relative to vehicle from ZT20-21, and MDL 3.0 mg/kg reduced wakefulness from ZT22-23 (Figure 2A). MDL, 3.0 mg/kg, also produced less wake time from ZT22-23 than did zolpidem. The highest dose of RO (10.0 mg/kg) reduced wakefulness compared with vehicle during ZT20 (Figure 2B).
Figure 2.
Effect of MDL100907 (graphs on left) and RO4368554 (graphs on right) on behavioral state measures for the 6 hours after treatment. A and B: Hourly percentage of time spent in wakefulness. C and D: Hourly percentage of time spent in non-rapid eye movement (NR) sleep. E and F: Hourly percentage of time spent in rapid eye movement (REM) sleep. G and H: Average hourly delta power during NR sleep, normalized relative to within-animal vehicle response. The values for the zolpidem (ZOL) standard (10 mg/kg) and vehicle are presented in all graphs for comparison. Data are mean ± SEM of average response binned over every hour for 8 animals. *P < 0.05 during that hour for the comparisons defined below; ZT refers Zeitgeber time (lights off at ZT12). Panel A: Three doses of MDL vs ZOL and vehicle. ZT19: ZOL < all other conditions. ZT20: ZOL and MDL 0.3 and 1.0 mg/kg < vehicle. ZT21: ZOL and MDL 0.3 and 1.0 mg/kg < vehicle. ZT22: MDL 3.0 mg/kg < ZOL and vehicle. ZT23: MDL 3.0 mg/kg < all other conditions. Panel B: Three doses of RO vs ZOL and vehicle. ZT19: ZOL < all other conditions. ZT20: ZOL and RO 10.0 mg/kg < vehicle. ZT21: ZOL < vehicle and RO 1.0 mg/kg. Panel C: Three doses of MDL vs ZOL and vehicle. ZT19: ZOL > all other conditions. ZT20: All drug conditions > vehicle. ZT21: ZOL, MDL 0.3 and 1.0 mg/kg > vehicle. ZT22: MDL 3.0 mg/kg > ZOL and vehicle. ZT23: MDL 3.0 mg/kg > all other conditions. Panel D: Three doses of RO vs ZOL and vehicle. ZT19: ZOL > all other conditions. ZT20: ZOL and RO 10.0 mg/kg > vehicle. ZT21: ZOL > vehicle. Panel G: Three doses of MDL vs ZOL and vehicle. ZT19: ZOL > all other conditions. ZT20: All drug conditions > vehicle. ZT21: MDL 0.3 and 1.0 mg/kg > vehicle. ZT22: MDL 3.0 mg/kg > all other conditions except ZOL. ZT23: MDL 3.0 mg/kg > vehicle and MDL 0.3 mg/kg. ZT24: MDL 1.0 and 3.0 mg/kg > ZOL Panel H: Three doses of RO vs ZOL and vehicle. ZT19: ZOL > all other conditions. ZT20: RO 10 mg/kg and ZOL > vehicle; ZOL > RO 1.0 and 3.0 mg/kg. ZT21: ZOL > RO 3.0 mg/kg. ZT24: RO 1.0 mg/kg > ZOL.
NREM sleep was promoted by all 3 compounds tested. Zolpidem produced significant increases in NREM sleep compared to vehicle from ZT19-21 (Figure 2C and 2D). During ZT19, this effect was significant relative to all conditions, consistent with the data in Figure 1. Following administration of MDL, 0.3 and 1.0 mg/kg, NREM sleep was significantly increased, compared with vehicle, from ZT20-21 (Figure 2C), reflecting the slower onset than that of zolpidem, which is also evident in Figure 1. The highest dose of MDL (3.0 mg/kg) produced significant increases in NREM sleep from ZT20-23 compared with vehicle; the effects at ZT22-23 were also greater than those of zolpidem. RO, 10.0 mg/kg, produced significant increases in NREM sleep during ZT20, compared with vehicle (Figure 2D). No significant differences in average hourly REM sleep amounts were found with any drug treatment (Figures 2E and 2F).
The accumulation of waking, NREM sleep, and REM sleep over the 6-hour recording showed significant drug-dependent effects. Cumulative wakefulness for the entire 6-hour recording was significantly reduced relative to vehicle for all 3 doses of MDL and for RO 10 mg/kg (Table 1). Cumulative wakefulness following zolpidem was also significantly less than vehicle and was less than all 3 doses of RO. Cumulative NREM sleep increased significantly relative to vehicle for all 3 doses of MDL and for RO 10 mg/kg. Cumulative NREM sleep following zolpidem was significantly greater than vehicle, all 3 doses of RO, and MDL 1.0 mg/kg.
Table 1.
Cumulative Amounts of Wake and NREM and REM Sleep for 6 Hours Following Treatment with MDL100907, RO4368554, Zolpidem, and Vehicle
| State | Vehicle | MDL100907, mg/kg |
RO4368554, mg/kg |
Zolpidem, mg/kg | ||||
|---|---|---|---|---|---|---|---|---|
| 0.3 | 1.0 | 3.0 | 1.0 | 3.0 | 10.0 | 10 | ||
| Wake | 260.0 ± 5.8 | 220.5 ± 10.5a | 228.9 ± 11.3a | 210.2 ± 8.3a | 260.5 ± 7.2b | 250.8 ± 10.1b | 236.8 ± 12.8ab | 220.1 ± 9.5a |
| NREM | 91.5 ± 5.7 | 127.5 ± 9.0a | 119.2 ± 9.3ab | 138.7 ± 7.0a | 90.2 ± 5.8b | 100.1 ± 8.9b | 112.4 ± 10.5ab | 136.3 ± 9.3a |
| REM | 8.5 ± 1.7 | 11.0 ± 1.7b | 11.2 ± 3.7b | 9.9 ± 2.2b | 7.9 ± 2.1b | 8.4 ± 1.9b | 10.4 ± 2.7b | 3.8 ± 0.9a |
Data are presented as mean number of minutes of cumulative sleep ± SEM. NREM refers to non-rapid eye movement sleep; REM, rapid eye movement sleep.
Different from vehicle P < 0.05
Different from zolpidem P < 0.05
Although average hourly REM sleep was not significantly different, as evident in Figures 2E and 2F, the cumulative amount of REM sleep did show some significant differences (Table 1). The primary result was for zolpidem to decrease cumulative REM sleep, compared with all other conditions. Cumulative amounts of REM sleep did not differ for any dose of MDL or RO when compared with vehicle.
Spectral Analysis of the EEG
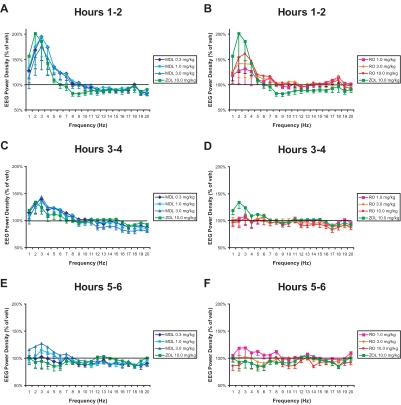
Figure 3 presents results of spectral analysis of the EEG (0-20 Hz) during NREM sleep in 2-hour bins for each of the drug treatments relative to vehicle treatment during the same time period. Among the drugs tested, zolpidem (10 mg/kg) had the greatest effect on EEG delta power during the first 2 hours after treatment, but it also reduced spectral power in the 8- to 11-Hz range (Figure 3A and 3B). These effects were greatly diminished during hours 3 to 4 after treatment (Figure 3C and 3D). During hours 5 to 6, EEG power density in the delta range of zolpidem-treated animals was below that observed after vehicle treatment (Figure 3E and 3F). MDL increased EEG power density in the 1- to 7-Hz range during the first 2 hours after treatment but did not cause the suppression in the 8- to 11-Hz range seen following zolpidem. As with zolpidem, the effects were greatly diminished during hours 3 to 4 after treatment (Figure 3C) and were absent during hours 5 to 6 (Figure 3E). RO produced a smaller but dose-dependent increase in EEG power density in the 1- to 4-Hz range during the first 2 hours following treatment (Figure 3B). By hours 3 to 4 after treatment, EEG spectra had returned to levels similar to those of vehicle (Figure 3D and 3F).
Figure 3.
Electroencephalogram power density in the 1- to 20-Hz range during non-rapid eye movement sleep produced by MDL100907 (graphs on left) and RO4368554 (graphs on right) and zolpidem (ZOL) during 3 consecutive 2-h time periods. The data points for each 1-Hz bin are presented as the percentage difference from the corresponding vehicle condition. Data are mean ± SEM of average response binned over every hour for 8 animals.
As suggested by Figure 3, delta power during NREM sleep was increased by all 3 compounds tested. Zolpidem increased NREM sleep delta power during ZT19 to ZT20 relative to vehicle; at ZT19, this effect was also significant relative to all other conditions (Figure 2G and 2H). MDL 0.3 and 1.0 mg/kg significantly increased NREM sleep delta power, compared with vehicle, from ZT20 to ZT21 (Figure 2G), the same time period during which these doses increased NREM sleep. MDL 3.0 mg/kg produced the longest lasting change: NREM sleep delta power increased during ZT20 compared with vehicle, during ZT22 compared with all other conditions except zolpidem, during ZT23 compared with vehicle, and during ZT24 compared with zolpidem (Figure 2G). RO, 10.0 mg/kg, increased NREM sleep delta during ZT20 compared with vehicle (Figure 2H), the same time period during which it increased NREM sleep. RO 1.0 mg/kg increased NREM sleep delta power during ZT24 compared with zolpidem. Because neither RO 1.0 mg/kg nor MDL 3.0 mg/kg increased delta power relative to vehicle during ZT24, the increased delta power with these treatments relative to zolpidem is likely due to suppression of delta power by zolpidem during this time period, as noted above.
Bout Duration and Number of Bouts
Measures of sleep-wake consolidation were also significantly affected by all 3 compounds. Although wake bout duration did not show statistically significant differences, the number of wake bouts did (Table 2). Zolpidem significantly increased the number of both waking and NREM sleep bouts during ZT19 compared with vehicle (Tables 2 and 3), indicating that the increased NREM sleep observed during this hour (Figure 2C and 2D) was highly fragmented. On the other hand, zolpidem also produced significantly fewer wake and NREM sleep bouts during ZT20 and ZT22 compared with vehicle, indicating that the significant increase in NREM sleep observed during ZT20 was a result of longer, more consolidated, NREM sleep bouts. Indeed, zolpidem significantly increased NREM sleep bout duration by 250% relative to vehicle treatment during ZT20 (Table 3). All doses of MDL produced fewer wake bouts from ZT20 to ZT22 than did vehicle, suggestive of consolidated NREM sleep bouts, and fewer wake bouts than zolpidem during both ZT19 and ZT21. Indeed, Table 3 documents that NREM sleep bout duration was significantly longer from ZT20 to ZT22 in all MDL doses relative to vehicle treatment; the effect of the 3.0 mg/kg dose lasted through ZT23. RO, 10.0 mg/kg, produced a significant reduction in the number of wake bouts compared with vehicle only during ZT20, also suggesting more consolidated NREM sleep during this hour. Table 3 confirms that RO, 10.0 mg/kg, more than doubled NREM sleep bout duration during ZT20. RO 1.0 mg/kg decreased the number of NREM sleep bouts relative to vehicle at ZT20, but the trend toward longer NREM sleep bout duration was only significant for the RO 10.0 mg/kg dose (Table 3).
Table 2.
Duration of Wake Time and Number of Wake Bouts Observed Per Hour Following Treatment with MDL100907, RO4368554, Zolpidem, and Vehicle
| ZT | Vehicle | MDL100907, mg/kg |
RO4368554, mg/kg |
Zolpidem, mg/kg | ||||
|---|---|---|---|---|---|---|---|---|
| 0.3 | 1.0 | 3.0 | 1.0 | 3.0 | 10.0 | 10.0 | ||
| Wake bout duration | ||||||||
| 19 | 6.79 ± 2.36 | 5.18 ± 1.58 | 5.60 ± 1.12 | 4.91 ± 1.45 | 4.06 ± 0.80 | 6.41 ± 4.48 | 7.44 ± 2.73 | 1.77 ± 0.15 |
| 20 | 3.53 ± 1.43 | 2.50 ± 0.56 | 4.42 ± 1.63 | 5.35 ± 1.72 | 3.20 ± 1.20 | 1.38 ± 0.15 | 2.29 ± 0.57 | 2.61 ± 0.35 |
| 21 | 2.14 ± 0.29 | 3.09 ± 1.03 | 2.00 ± 0.40 | 2.99 ± 0.61 | 3.16 ± 0.91 | 2.94 ± 0.63 | 4.51 ± 2.05 | 2.09 ± 0.50 |
| 22 | 2.87 ± 0.81 | 3.47 ± 1.05 | 12.89 ± 9.69 | 2.75 ± 0.70 | 4.71 ± 1.70 | 4.93 ± 1.77 | 4.15 ± 2.38 | 3.50 ± 0.71 |
| 23 | 5.02 ± 1.08 | 4.24 ± 0.50 | 7.64 ± 2.23 | 2.35 ± 0.44 | 4.13 ± 1.06 | 3.14 ± 0.44 | 4.30 ± 1.77 | 4.23 ± 0.89 |
| 24 | 4.82 ± 0.83 | 11.29 ± 6.93 | 4.96 ± 0.91 | 4.39 ± 0.94 | 10.28 ± 3.87 | 11.96 ± 4.31 | 17.38 ± 7.24 | 16.01 ± 7.08 |
| Number of bouts | ||||||||
| 19 | 7.00 ± 1.65 | 5.22 ± 1.06b | 8.78 ± 1.14b | 7.44 ± 1.43b | 9.44 ± 1.30b | 8.33 ± 2.48b | 6.22 ± 1.08b | 17.44 ± 2.03a |
| 20 | 19.11 ± 2.47 | 10.00 ± 1.39a | 10.33 ± 1.55a | 8.56 ± 1.60a | 14.33 ± 1.67 | 16.89 ± 1.03 | 14.11 ± 1.44a | 13.11 ± 1.21a |
| 21 | 18.78 ± 2.26 | 9.44 ± 0.82ab | 10.67 ± 0.88ab | 12.22 ± 1.54a | 17.11 ± 2.38 | 16.67 ± 1.91 | 14.00 ± 2.24 | 16.22 ± 2.20 |
| 22 | 18.67 ± 2.06 | 12.44 ± 1.31a | 12.33 ± 2.22a | 11.44 ± 1.59a | 16.22 ± 2.70 | 15.33 ± 3.14 | 17.44 ± 2.51 | 12.78 ± 1.47a |
| 23 | 14.78 ± 2.46 | 12.33 ± 1.42 | 10.67 ± 1.54 | 12.56 ± 1.17 | 13.56 ± 1.97 | 18.22 ± 3.12 | 14.78 ± 2.23 | 14.56 ± 2.24 |
| 24 | 10.67 ± 1.13 | 12.44 ± 2.01 | 11.00 ± 1.32 | 12.00 ± 1.21 | 9.78 ± 1.90 | 11.33 ± 2.63 | 12.33 ± 3.03 | 11.11 ± 2.66 |
Data are expressed as mean ± SEM duration of waking, in minutes, and mean number (± SEM) of bouts of waking. ZT refers to Zeitgeber time.
Different from vehicle P < 0.05
Different from zolpidem P < 0.05
Table 3.
Duration of NREM Sleep and Number of NREM Bouts Observed per Hour Following Treatment with MDL100907, RO4368554, Zolpidem, and Vehicle
| ZT | Vehicle | MDL100907, mg/kg |
RO4368554, mg/kg |
Zolpidem, mg/kg | ||||
|---|---|---|---|---|---|---|---|---|
| 0.3 | 1.0 | 3.0 | 1.0 | 3.0 | 10.0 | 10.0 | ||
| NREM bout duration | ||||||||
| 19 | 1.05 ± 0.20 | 1.81 ± 0.62 | 0.87 ± 0.18 | 0.78 ± 0.10 | 0.93 ± 0.19 | 0.60 ± 0.18 | 0.84 ± 0.20 | 1.63 ± 0.31 |
| 20 | 1.01 ± 0.14 | 3.49 ± 0.58ab | 3.63 ± 0.68ab | 4.61 ± 1.08ab | 1.81 ± 0.33 | 1.57 ± 0.13b | 2.29 ± 0.31a | 2.50 ± 0.41a |
| 21 | 1.26 ± 0.22 | 3.46 ± 0.54ab | 2.93 ± 0.27a | 2.67 ± 0.47a | 1.26 ± 0.21 | 1.55 ± 0.15 | 1.87 ± 0.17 | 2.11 ± 0.42 |
| 22 | 1.01 ± 0.09 | 1.94 ± 0.38a | 1.72 ± 0.41 | 2.91 ± 0.47ab | 1.14 ± 0.16 | 1.32 ± 0.22 | 1.32 ± 0.13 | 1.88 ± 0.33 |
| 23 | 0.82 ± 0.08 | 1.30 ± 0.13 | 0.93 ± 0.15 | 2.55 ± 0.59ab | 0.82 ± 0.11 | 0.96 ± 0.15 | 1.01 ± 0.09 | 1.19 ± 0.19 |
| 24 | 1.09 ± 0.21 | 1.01 ± 0.22 | 1.49 ± 0.28 | 1.38 ± 0.30 | 1.07 ± 0.15 | 0.92 ± 0.14 | 0.76 ± 0.17 | 0.84 ± 0.15 |
| Number of bouts | ||||||||
| 19 | 7.33 ± 1.64 | 5.78 ± 1.15b | 8.67 ± 0.85b | 8.67 ± 1.81b | 10.22 ± 1.27b | 8.22 ± 2.43b | 6.00 ± 1.17b | 17.67 ± 2.07a |
| 20 | 18.89 ± 2.38 | 10.22 ± 1.37a | 9.56 ± 1.51a | 9.11 ± 1.57a | 13.67 ± 1.86a | 17.11 ± 0.92 | 14.44 ± 1.43 | 13.56 ± 1.31a |
| 21 | 19.11 ± 2.25 | 9.78 ± 0.81ab | 11.22 ± 0.89a | 11.78 ± 1.59a | 17.33 ± 2.49 | 16.56 ± 1.94 | 14.33 ± 2.32 | 16.22 ± 2.31 |
| 22 | 18.56 ± 2.25 | 12.33 ± 1.24a | 12.11 ± 2.21a | 11.44 ± 1.83a | 16.11 ± 2.84 | 15.44 ± 3.18 | 17.22 ± 2.50 | 12.67 ± 1.38a |
| 23 | 14.78 ± 2.48 | 12.11 ± 1.33 | 11.44 ± 2.21 | 12.33 ± 1.15 | 13.22 ± 1.71 | 17.33 ± 2.58 | 15.00 ± 2.24 | 14.56 ± 2.27 |
| 24 | 10.44 ± 1.23 | 13.11 ± 2.71 | 10.33 ± 1.36 | 11.89 ± 1.36 | 8.67 ± 2.01 | 11.00 ± 2.49 | 12.00 ± 3.09 | 10.67 ± 2.87 |
Data are expressed as mean ± SEM duration of non-rapid eye movement sleep (NREM), in minutes, and mean number (± SEM) of bouts of NREM sleep. ZT refers to Zeitgeber time.
Different from vehicle P < 0.05
Different from zolpidem P < 0.05
Although the hourly percentage of time spent in REM sleep did not differ (Figure 2E and 2F), drug treatment had some effects on REM bout duration. Zolpidem greatly reduced REM bout duration relative to vehicle and all drug treatments at ZT20 (Table 4), which likely was a major factor in the reduced cumulative REM sleep (Table 1). MDL 0.3 and 1.0 mg/kg increased REM bout duration relative to vehicle at ZT21 but did not affect the number of REM bouts. RO had no effect on REM bout duration or the number of bouts relative to vehicle.
Table 4.
Duration of REM Sleep and Number of REM Bouts Observed Per Hour Following Treatment with MDL100907, RO4368554, Zolpidem, and Vehicle
| ZT | Vehicle | MDL100907, mg/kg |
RO4368554, mg/kg |
Zolpidem, mg/kg | ||||
|---|---|---|---|---|---|---|---|---|
| 0.3 | 1.0 | 3.0 | 1.0 | 3.0 | 10.0 | 10.0 | ||
| REM Bout Duration | ||||||||
| 19 | 0.32 ± 0.16 | 0.06 ± 0.06 | 0.19 ± 0.10 | 0.02 ± 0.02 | 0.28 ± 0.21 | 0.06 ± 0.06 | 0.30 ± 0.16 | 0.18 ± 0.08 |
| 20 | 0.67 ± 0.16 | 0.96 ± 0.24b | 1.06 ± 0.24b | 0.47 ± 0.19 | 0.71 ± 0.20b | 0.90 ± 0.16b | 0.83 ± 0.19b | 0.13 ± 0.10a |
| 21 | 0.83 ± 0.20 | 1.36 ± 0.20ab | 1.40 ± 0.25ab | 0.80 ± 0.22b | 0.56 ± 0.18 | 0.83 ± 0.16 | 0.77 ± 0.16 | 0.42 ± 0.09 |
| 22 | 0.94 ± 0.18 | 0.96 ± 0.22b | 0.80 ± 0.20 | 1.00 ± 0.28b | 0.53 ± 0.15 | 0.57 ± 0.24 | 0.87 ± 0.18 | 0.53 ± 0.14 |
| 23 | 0.25 ± 0.08 | 0.64 ± 0.26b | 0.92 ± 0.31ab | 0.69 ± 0.15b | 0.21 ± 0.14 | 0.44 ± 0.21 | 0.24 ± 0.14 | 0.16 ± 0.11 |
| 24 | 0.32 ± 0.20 | 0.33 ± 0.21 | 0.47 ± 0.24 | 0.54 ± 0.31b | 0.07 ± 0.04 | 0.04 ± 0.04 | 0.19 ± 0.10 | 0.02 ± 0.02 |
| Number of REM Bouts | ||||||||
| 19 | 0.44 ± 0.24 | 0.11 ± 0.11 | 0.33 ± 0.17 | 0.11 ± 0.11 | 0.22 ± 0.15 | 0.22 ± 0.22 | 0.56 ± 0.34 | 0.78 ± 0.36 |
| 20 | 2.22 ± 0.70 | 1.22 ± 0.28 | 1.33 ± 0.29 | 1.22 ± 0.55 | 2.44 ± 0.69 | 2.78 ± 0.70 | 2.11 ± 0.56 | 0.56 ± 0.44 |
| 21 | 2.78 ± 0.66 | 3.22 ± 0.62 | 2.78 ± 0.78 | 1.89 ± 0.56 | 2.78 ± 0.83 | 3.67 ± 0.73 | 2.89 ± 0.79 | 2.22 ± 0.46 |
| 22 | 3.56 ± 1.25 | 2.89 ± 0.79 | 3.11 ± 0.86 | 2.00 ± 0.50 | 2.33 ± 0.78 | 1.44 ± 0.50 | 3.89 ± 1.12 | 1.89 ± 0.65 |
| 23 | 1.44 ± 0.85 | 1.33 ± 0.55 | 1.11 ± 0.48 | 2.56 ± 0.69 | 0.67 ± 0.37 | 0.78 ± 0.32 | 1.44 ± 0.82 | 0.44 ± 0.34 |
| 24 | 0.44 ± 0.24 | 0.44 ± 0.24 | 0.78 ± 0.36 | 1.11 ± 0.51 | 0.44 ± 0.24 | 0.56 ± 0.56 | 0.44 ± 0.24 | 0.11 ± 0.11 |
Data are expressed as mean ± SEM duration of rapid eye movement sleep (REM), in minutes, and mean number (± SEM) of bouts of REM. ZT refers to Zeitgeber time.
Different from vehicle P < 0.05
Different from zolpidem P < 0.05
Tb and LMA
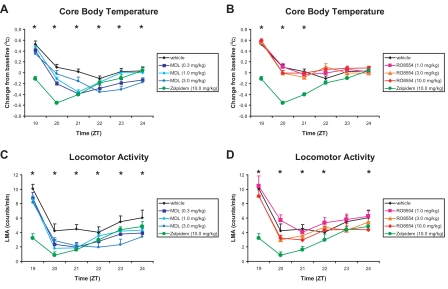
Although RO had no significant effect on Tb when compared with vehicle treatment, both MDL and zolpidem significantly decreased Tb (Figures 4A and 4B). After administration of zolpidem, Tb was significantly lower from ZT19 to ZT21 than after administration of vehicle treatment and lower relative to all drug treatments during ZT19 to ZT20. MDL 0.3 mg/kg produced significant decreases in Tb compared with vehicle from ZT20 to ZT24, whereas MDL 1.0 mg/kg significantly reduced Tb from ZT20 to ZT21, and the highest dose (3.0 mg/kg) of MDL had a delayed effect, producing significantly lower Tb compared with vehicle only during ZT23. LMA was significantly decreased by zolpidem and MDL, but only modest effects were seen following administration of RO (Figures 4C and 4D). Zolpidem significantly reduced LMA compared with vehicle from ZT19 to ZT21 and compared with all other treatments during ZT19. MDL 0.3 mg/kg significantly reduced LMA compared with vehicle from ZT20 to ZT22. MDL 1.0 mg/kg significantly decreased LMA compared with vehicle from ZT20 to ZT21. MDL 3.0 mg/kg significantly reduced LMA compared with vehicle from ZT21 to ZT24. RO had no significant effect on LMA when compared with vehicle treatment. The suppressive effect of zolpidem on LMA was also significant when compared with all doses of RO from ZT19 to ZT20; the suppressive effects of zolpidem relative to RO 1.0 mg/kg extended throughout the recording period.
Figure 4.
Effect of MDL100907 (graphs on left) and RO4368554 (graphs on right) on physiologic and behavioral parameters for the 6 hours after treatment. A: Effect of MDL100907 on core body temperature (Tb). B: Effect of RO4368554 on Tb. C: Effect of MDL100907 on locomotor activity (LMA). D: Effect of RO4368554 on LMA. The values for the zolpidem (ZOL) standard (10 mg/kg) and vehicle are presented in all graphs for comparison. Data are mean ± SEM of average response binned over every hour for 8 animals. *P < 0.05 during that hour for the comparisons defined below; ZT refers to Zeitgeber time (lights off at ZT12). Panel A: Tb following MDL vs ZOL and vehicle. ZT19: ZOL < all other conditions. ZT20: ZOL < all other conditions; MDL 0.3 and 1.0 mg/kg < vehicle. ZT21: ZOL, MDL 0.3 and 1.0 mg/kg < vehicle and MDL 3.0 mg/kg. ZT22: MDL 0.3 mg/kg < vehicle. ZT23: MDL 0.3 and 3.0 mg/kg < vehicle. ZT24: MDL 0.3 mg/kg < vehicle. Panel B: Three Tb following RO vs ZOL and vehicle. ZT19: ZOL < all other conditions. ZT20: ZOL < all other conditions. ZT21: ZOL < all other conditions. Panel C: LMA following MDL vs ZOL and vehicle. ZT19: ZOL < all other conditions. ZT20: ZOL < vehicle and MDL 3.0 mg/kg; MDL 0.3 and 1.0 mg/kg < vehicle. ZT21: all other conditions < vehicle. ZT22: MDL 3.0 mg/kg < vehicle. ZT23: MDL 3.0 mg/kg < all other conditions; MDL 0.3 mg/kg < vehicle. ZT24: MDL 0.3 and 3.0 mg/kg < vehicle. Panel D: LMA following RO vs ZOL and vehicle. ZT19: ZOL < all other conditions. ZT20: ZOL < all other conditions; RO 0.3 and 10.0 mg/kg < RO 1.0 mg/kg. ZT21: ZOL< vehicle and RO 1.0 and 3.0 mg/kg. ZT22: ZOL < RO 1.0 mg/kg. ZT24: RO 10.0 mg/kg < RO 1.0 mg/kg.
DISCUSSION
The present results confirm previous reports of the effects of 5-HT2A antagonists on sleep consolidation in rats8–10, 16 and extend the understanding of the role of this receptor in sleep using a specific ligand, MDL100907, with clinical potential for the treatment of insomnia. Furthermore, the results indicate a previously unreported role of 5-HT6 receptors in sleep.
Each of the 3 compounds used in the present study reduced wakefulness and increased NREM sleep but with different time courses and magnitudes. Although all 3 doses of MDL reduced the latency to sleep (Figure 1), this effect was not as strong as that of zolpidem; the reduction in wakefulness (Figure 2A) and increase in NREM sleep (Figure 2C) evoked by MDL were not evident until the second hour after treatment (ZT20). This increase in NREM sleep was characterized by increased EEG delta power during ZT20 to ZT21 (until ZT23 for the 3 mg/kg dose; Figure 2G). The increase in NREM sleep resulted from both an increase in the NREM sleep bout duration and a reduction in the number of both wake and NREM sleep bouts from ZT20 to ZT22 at all 3 doses (Table 3), indicating consolidated NREM sleep.
In contrast with MDL and zolpidem, RO4368554 had relatively modest effects on sleep, wakefulness, and other physiologic and behavioral parameters measured. The primary effects were a decrease in wakefulness (Figure 2B) and increase in NREM sleep (Figure 2D), specifically during ZT20 with the highest dose examined (10 mg/kg). The increase in NREM sleep was accompanied by increased EEG delta power (Figure 2H) and an increased NREM sleep bout duration (Table 3) and a reduction in the number of wake bouts (Table 2) during this hour. These factors combined to result in a significant reduction in the cumulative time awake beginning at ZT21, which persisted until the end of recording at ZT24, as well as a concomitant increase in the cumulative amount of NREM sleep during this same period without any effect on REM sleep (Table 1). However, in contrast with zolpidem and MDL, RO did not significantly reduce either LMA or Tb. Since a reduction in Tb usually occurs during sleep, the absence of a change in Tb may have contributed to the limited effects of RO on sleep.
Zolpidem produced the expected results, including a significantly reduced the latency to sleep (Figure 1), reduced wakefulness (Figures 2A and 2B), increased NREM sleep (Figures 2C and 2D), decreased cumulative REM sleep (Table 1), and decreased LMA (Figures 4C and 4D).27–29 The increase in NREM sleep was accompanied by an increase in EEG delta power during ZT19 to ZT20 (Figures 2G and 2F) and a reduction in Tb from ZT19 to ZT21 (Figures 4A and 4B). Despite the reduced latency and increased amount of NREM sleep during ZT19, however, the number of both wake and NREM sleep bouts significantly increased, indicating that sleep was fragmented during this hour. This is in contrast with ZT20 during which the number of wake and NREM sleep bouts decreased and the duration of NREM sleep bouts increased, indicating that sleep was more consolidated during this hour. To our knowledge, an initial period of increased fragmentation following zolpidem administration has not been previously reported in rats. Also to our knowledge, investigations of sleep bout duration or bout number have not been reported following administration of zolpidem at this ZT. However, it is important to note that the data reported in hour 1 include sleep-wake data during the dosing procedure. The arousing effects of handling, combined with the presence of humans in the room for 15 minutes during the dosing procedure, would tend to oppose the otherwise rapid-onset soporific effects of zolpidem. For all conditions other than zolpidem treatment, the arousal due to handling and dosing combined with absence of a rapid soporific effect resulted in an initial consolidation of wakefulness. Therefore, we believe the initial fragmentation observed following zolpidem is likely to be an artifact of the experimental procedure combined with how the data were analyzed (i.e., inclusion of data from the start of the hour during which dosing procedure occurred).
The doses of MDL100907 employed in this study were selected to render near maximal receptor occupancy, based on previously reported in vivo studies.23,24 Doses were selected to exceed the reported minimum effective dose in order to generate prolonged receptor occupancy over the 6 hours of data capture while allowing for its short plasma half-life in rats (71 minutes25). One possible confound with the use of MDL100907 is rapid formation of metabolites. In particular, rapid increases in concentration of the primary metabolite MDL105725, itself a 5-HT2A antagonist, have been reported in plasma and extracellular fluid, although MDL100907 does not appear to be metabolized to MDL105725 in brain.25 Nonetheless, given the reported selectivity of this molecule and its rapid clearance, it is likely that the effects of MDL100907 observed in this study, including those of its metabolites, predominantly arise from interaction with the 5-HT2A receptor. Indeed, the findings reported herein are in good agreement with findings reported with other chemically distinct 5-HT2A receptor antagonists.9,16
The novel finding that RO4368554 increases NREM sleep likely arises from selective inhibition of 5-HT6 receptors. Over the dose range used in this study, it is likely that RO4368554 acts selectively at 5-HT6 receptors where it binds with a greater than 100-fold selectivity over other monoamine receptor subtypes (−log M pKi = 9.4 at 5-HT6 and 7.1 at 5-HT2A). Several studies have determined that 3 mg/kg of RO4368554 is an effective minimum dose in rat cognition models known to be sensitive to 5-HT6 antagonists.20,21 However, much higher concentrations than administered in this study could affect other receptor systems.
The strongest effects of RO4368554 and MDL100907 were observed on NREM sleep, particularly increases in NREM sleep and EEG delta power. These findings suggest a role for these 2 receptor subtypes in sleep quality and consolidation and may indicate a clinical role for these classes of agents in treating sleep maintenance insomnia. In comparison, zolpidem, a sedating GABAA-active benzodiazepine, strongly altered both REM and NREM sleep parameters. Taken together, these data indicate that the roles for the 5-HT2A and 5-HT6 receptor subtypes in sleep are distinct from those established for the sedating benzodiazepine GABAA ligands, which is consistent with the known biochemistry.
Because this study was conducted during the latter half of the active phase of the rat's sleep-wake cycle, it was expected that aspects of sleep promotion and consolidation can be explored because sleep and wakefulness are more fragmented during this phase of the circadian cycle. The data showed that RO4368554 (10 mg/kg intraperitoneal), MDL100907 (0.3, 1 and 3 mg/kg intraperitoneal) and zolpidem (10 mg/kg intraperitoneal) all significantly decreased the percentage of time awake in the active phase of the rodent circadian cycle (hours ZT20–ZT21) relative to vehicle. Under the conditions tested here, the data indicate that the 5-HT2A and 5-HT6 antagonists, as well as zolpidem, have hypnotic effects in rats during the active phase. In comparison, studies in healthy volunteers suggest that, although benzodiazepine GABAA modulators have a profound impact on induction of daytime sleepiness,30 5-HT2A antagonists have virtually no effect on psychomotor or cognitive function, although a modest increase in reaction time is reported.31 Emerging clinical data with 5-HT6 antagonists also do not report sedation among the observed adverse events.32 In addition, preclinical data with RO4368554 tested at the same time points, doses, and routes of administration described herein demonstrate a procognitive effect in rats,20, 22 which would tend to argue against a disruptive sedative effect of this molecule. Similarly, 5-HT2A antagonists have been found to be nondisruptive in cognition models in rats and procognitive in primates.26, 33 Failure of these 5-HT2A and 5-HT6 antagonists to disrupt cognitive and performance measures may be related to the slow onset of soporific activity of these compounds, evident in Figures 1 and 2, relative to the rapid onset of zolpidem. Indeed, in a clinical study to determine the efficacy of the 5-HT2A antagonist SR 46439B (eplivanserin) on sleep parameters, subjects were instructed to take the medication 3 hours in advance of bedtime.34 This slow onset of activity suggests that 5-HT2A antagonists may be more effective for sleep maintenance insomnia than for sleep onset insomnia.
The outcomes for acute antagonism of both 5-HT6 and 5-HT2A were qualitatively similar; quantitative differences in duration of effect or size of change may reflect differences in receptor occupancy and ligand pharmacokinetics. It is not clear whether this indicates a commonality in the signaling pathways of these 2 receptor subtypes. Because these 2 receptor subtypes are differentially localized in rodent brain,18, 35 the similarity of the effects on sleep may be coincidental. Nonetheless, some overlap in receptor-mediated signaling is known to exist between these 2 receptor subtypes, particularly relating to indirect control of glutamatergic signaling. Synaptic glutamate levels are elevated following systemic administration of 5-HT6 antagonists, and 5-HT2A antagonists potentiate NMDA currents in rat prefrontal cortical neurons and increase NMDA-dependent long-term potentiation in rat (CA1) hippocampus.36, 37 However, these roles and functions are likely to be region or condition specific, as other publications suggest that 5-HT2A agonists both facilitate and inhibit NMDA-mediated responses in rat prefrontal cortex slice preparations in a manner that is reversed by 5-HT2A antagonism.38 Furthermore, SWS and REM sleep are generally associated with decreased glutamate levels,39 and antagonism of NMDA receptors increases delta power in NREM sleep in rats.40
Elucidation of the mechanism by which 5-HT6 receptors influence sleep and wakefulness will certainly require an understanding of the cellular localization of these receptors. Although little is known about what specific cell types colocalize with 5-HT6 receptors, it is interesting that they are found in the hypothalamus, a brain region known to have wake-promoting and sleep-active cell types. It is possible to speculate that the effects of RO4368445 found in this study could be through either the wake-promoting or the sleep-promoting cell types in the hypothalamus. For instance, if 5-HT6 receptors are located on cells containing hypocretin or histamine, then blockade of these receptors would disfacilitate these neurons, thus creating a condition in which sleep is more likely. Such a mechanism might account for the delay to increased sleep. Conversely, 5-HT6 receptors might reside on local GABAergic interneurons that are connected to the sleep-active cells of the ventrolateral preoptic area or the median preoptic nucleus. If this were the case, then 5-HT6 receptor blockade would disinhibit the sleep-active cells that would promote sleep. These hypotheses are highly speculative, and further studies are needed to determine the cellular localization and electrophysiologic effects of 5-HT6 receptors.
The results of this study present a novel effect of sleep promotion via 5-HT6 receptor blockade while confirming the effect of 5-HT2A receptor antagonism on sleep promotion. The established roles and neural mechanisms of 5-HT6 and 5-HT2A receptors do not fully explain the observed effects of these receptors on sleep and suggest that other, possibly region-specific, signaling cascades remain to be elucidated.
Footnotes
Disclosure Statement
This study was supported by Roche Palo Alto. Dr. Martin and Ms. Hedley are employees of Roche Palo Alto. Drs. Moriarity, Kilduff, and Ms. Flores are employees of SRI International and received funding from Roche Palo Alto to perform the studies. Dr. Kilduff has received an honorarium from Roche Palo Alto for a speaking engagement.
REFERENCES
- 1.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 2.Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6:341–51. [PubMed] [Google Scholar]
- 3.Idzikowski C, Mills FJ, Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res. 1986;378:164–8. doi: 10.1016/0006-8993(86)90299-4. [DOI] [PubMed] [Google Scholar]
- 4.Dugovic C, Wauquier A. 5-HT2 receptors could be primarily involved in the regulation of slow-wave sleep in the rat. Eur J Pharmacol. 1987;137:145–6. doi: 10.1016/0014-2999(87)90196-8. [DOI] [PubMed] [Google Scholar]
- 5.Dursun SM, Patel JK, Burke JG, Reveley MA. Effects of typical antipsychotic drugs and risperidone on the quality of sleep in patients with schizophrenia: a pilot study. J Psychiatry Neurosci. 1999;24:333–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Cohrs S, Meier A, Neumann AC, Jordan W, Ruther E, Rodenbeck A. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66:989–96. doi: 10.4088/jcp.v66n0805. [DOI] [PubMed] [Google Scholar]
- 7.Sharpley AL, Elliott JM, Attenburrow MJ, Cowen PJ. Slow wave sleep in humans: role of 5-HT2A and 5-HT2C receptors. Neuropharmacology. 1994;33:467–71. doi: 10.1016/0028-3908(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 8.Dugovic C, Wauquier A, Leysen JE, Marrannes R, Janssen PA. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology (Berl) 1989;97:436–42. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- 9.Fish LR, Gilligan MT, Humphries AC, et al. 4-Fluorosulfonylpiperidines: selective 5-HT2A ligands for the treatment of insomnia. Bioorg Med Chem Lett. 2005;15:3665–9. doi: 10.1016/j.bmcl.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 10.Kantor S, Jakus R, Bodizs R, Halasz P, Bagdy G. Acute and long-term effects of the 5-HT2 receptor antagonist ritanserin on EEG power spectra, motor activity, and sleep: changes at the light-dark phase shift. Brain Res. 2002;943:105–11. doi: 10.1016/s0006-8993(02)02698-7. [DOI] [PubMed] [Google Scholar]
- 11.Adrien J, Alexandre C, Boutrel B, Popa D. Contribution of the ≪knock-out≫ technology to understanding the role of serotonin in sleep regulations. Arch Ital Biol. 2004;142:369–77. [PubMed] [Google Scholar]
- 12.Boutrel B, Monaca C, Hen R, Hamon M, Adrien J. Involvement of 5-HT1A receptors in homeostatic and stress-induced adaptive regulations of paradoxical sleep: studies in 5-HT1A knock-out mice. J Neurosci. 2002;22:4686–92. doi: 10.1523/JNEUROSCI.22-11-04686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutrel B, Franc B, Hen R, Hamon M, Adrien J. Key role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. J Neurosci. 1999;19:3204–12. doi: 10.1523/JNEUROSCI.19-08-03204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–7. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Wisor JP, Wurts SW, Hall FS, et al. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport. 2003;14:233–8. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- 16.Popa D, Lena C, Fabre V, et al. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25:11231–8. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank MG, Stryker MP, Tecott LH. Sleep and sleep homeostasis in mice lacking the 5-HT2c receptor. Neuropsychopharmacology. 2002;27:869–73. doi: 10.1016/S0893-133X(02)00353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolley ML, Marsden CA, Fone KC. 5-HT6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- 19.Gerard C, el Mestikawy S, Lebrand C, et al. Quantitative RT-PCR distribution of serotonin 5-HT6 receptor mRNA in the central nervous system of control or 5,7-dihydroxytryptamine-treated rats. Synapse. 1996;23:164–73. doi: 10.1002/(SICI)1098-2396(199607)23:3<164::AID-SYN5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Lieben CK, Blokland A, Sik A, Sung E, van Nieuwenhuizen P, Schreiber R. The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacology. 2005;30:2169–79. doi: 10.1038/sj.npp.1300777. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber R, Vivian J, Hedley L, et al. Effects of the novel 5-HT(6) receptor antagonist RO4368554 in rat models for cognition and sensorimotor gating. Eur Neuropsychopharmacology. 2007;17:277–88. doi: 10.1016/j.euroneuro.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Kehne JH, Baron BM, Carr AA, et al. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–81. [PubMed] [Google Scholar]
- 23.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- 24.Vickers SP, Easton N, Malcolm CS, et al. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–52. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 25.Scott DO, Heath TG. Investigation of the CNS penetration of a potent 5-HT2a receptor antagonist (MDL 100,907) and an active metabolite (MDL 105,725) using in vivo microdialysis sampling in the rat. J Pharm Biomed Anal. 1998;17:17–25. doi: 10.1016/s0731-7085(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 26.Meneses A, Terron JA, Hong E. Effects of the 5-HT receptor antagonists GR127935 (5-HT1B/1D) and MDL100907 (5-HT2A) in the consolidation of learning. Behav Brain Res. 1997;89:217–23. doi: 10.1016/s0166-4328(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 27.Edgar DM, Seidel WF, Gee KW, et al. CCD-3693: an orally bioavailable analog of the endogenous neuroactive steroid, pregnanolone, demonstrates potent sedative hypnotic actions in the rat. J Pharmacol Exp Ther. 1997;282:420–9. [PubMed] [Google Scholar]
- 28.Brunner DP, Dijk DJ, Munch M, Borbély AA. Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 1991;104:1–5. doi: 10.1007/BF02244546. [DOI] [PubMed] [Google Scholar]
- 29.Kopp C, Rudolph U, Tobler I. Sleep EEG changes after zolpidem in mice. Neuroreport. 2004;15:2299–302. doi: 10.1097/00001756-200410050-00031. [DOI] [PubMed] [Google Scholar]
- 30.van Laar M, Volkerts E, Verbaten M. Subchronic effects of the GABA-agonist lorazepam and the 5-HT2A/2C antagonist ritanserin on driving performance, slow wave sleep and daytime sleepiness in healthy volunteers. Psychopharmacology (Berl) 2001;154:189–97. doi: 10.1007/s002130000633. [DOI] [PubMed] [Google Scholar]
- 31.Danjou P, Warot D, Hergueta T, Lacomblez L, Bouhours P, Puech AJ. Comparative study of the psychomotor and antistress effects of ritanserin, alprazolam and diazepam in healthy subjects: some trait anxiety-independent responses. Int Clin Psychopharmacol. 1992;7:73–9. [PubMed] [Google Scholar]
- 32.Neale AC, Jenkins H, Amend D, Lesem M. A 14 day, dose escalation, double blind, randomized, placebo-controlled study of SGS518 in adult patients with schizophrenia. Neuropsychopharmacology. 2005;30:S54. [Google Scholar]
- 33.Terry AV, Jr, Buccafusco JJ, Bartoszyk GD. Selective serotonin 5-HT2A receptor antagonist EMD 281014 improves delayed matching performance in young and aged rhesus monkeys. Psychopharmacology (Berl) 2005;179:725–32. doi: 10.1007/s00213-004-2114-1. [DOI] [PubMed] [Google Scholar]
- 34.Landolt HP, Meier V, Burgess HJ, et al. Serotonin-2 receptors and human sleep: effect of a selective antagonist on EEG power spectra. Neuropsychopharmacology. 1999;21:455–66. doi: 10.1016/S0893-133X(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 35.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 36.Arvanov VL, Wang RY. M100907, a selective 5-HT2A receptor antagonist and a potential antipsychotic drug, facilitates N-methyl-D-aspartate-receptor mediated neurotransmission in the rat medial prefrontal cortical neurons in vitro. Neuropsychopharmacology. 1998;18:197–209. doi: 10.1016/S0893-133X(97)00126-7. [DOI] [PubMed] [Google Scholar]
- 37.Wang RY, Arvanov VL. M100907, a highly selective 5-HT2A receptor antagonist and a potential atypical antipsychotic drug, facilitates induction of long-term potentiation in area CA1 of the rat hippocampal slice. Brain Res. 1998;779:309–13. doi: 10.1016/s0006-8993(97)01174-8. [DOI] [PubMed] [Google Scholar]
- 38.Arvanov VL, Liang X, Magro P, Roberts R, Wang RY. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci. 1999;11:2917–34. doi: 10.1046/j.1460-9568.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- 39.Lena I, Parrot S, Deschaux O, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep--wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–9. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- 40.Campbell IG, Feinberg I. Comparison of MK-801 and sleep deprivation effects on NREM, REM, and waking spectra in the rat. Sleep. 1999;22:423–32. doi: 10.1093/sleep/22.4.423. [DOI] [PubMed] [Google Scholar]