Abstract
Study Objectives:
To assess whether noninvasive application of nCPAP is a mechanical stimulus inducing early nasal inflammation.
Design:
Prospective controlled animal study.
Setting:
University laboratory.
Patients or Participants:
32 male Sprague-Dawley rats (250–300 g).
Interventions:
The rats were anesthetized and subjected to nCPAP=10 cm H2O and sham-CPAP through a mask for 3 h and 5 h (n=8 each).
Measurements and Results:
After nCPAP or sham, nasal scraping was carried out to detect neutrophils, and septum and dorsal nasal concha were excised to assess gene expression of inflammatory markers by real time PCR. Percentage of neutrophils in nucleated cells in the nasal scrapings was significantly (P = 0.006) higher after 5 h of nCPAP (3.51% ± 0.73%; m ± SEM) than in the sham group (1.12% ± 0.39%). When compared with sham, the mRNA of macrophage inflammatory protein-2 (MIP-2) in nasal tissue was significantly overexpressed after both 3 h (2.28-fold ± 0.43–fold; P = 0.034) and 5 h (5.56-fold ± 1.88–fold; P = 0.002) of nCPAP=10 cm H2O. No significant changes were found in the gene expressions of tumor necrosis factor-α, nerve growth factor and tachykinin-1 receptor.
Conclusions:
The compression applied by nCPAP (10 cm H2O, 5 h) on the nasal wall of healthy rats is a mechanical stimulus that triggers an early inflammatory process mediated by MIP-2, resulting in neutrophil extravasation.
Citation:
Almendros I; Acerbi I; Vilaseca I; Montserrat JM; Navajas D; Farré R. Continuous positive airway pressure (CPAP) induces early nasal inflammation. SLEEP 2008;31(1):127-131.
Keywords: Sleep apnea, CPAP, rhinitis, mechanical stimulus, neutrophil extravasation
INTRODUCTION
THE OBSTRUCTIVE SLEEP APNEA (OSA) SYNDROME IS A PREVALENT DISORDER CHARACTERIZED BY RECURRENT PARTIAL OR TOTAL AIRWAY CLOSURE CAUSED by increased upper airway collapsibility. The most widespread treatment for avoiding upper airway obstructions and for preventing their consequences is the nocturnal application of nasal continuous positive airway pressure (nCPAP).1 The rationale for using nCPAP is to mechanically splint the upper airway by applying positive pressure in the airway lumen to counterbalance the abnormal upper airway collapsibility. The treatment with nCPAP is extremely effective in normalizing breathing during sleep, but in a large number of patients this therapy is associated with nasal side effects such as congestion and rhinorrhea.2,3 As a consequence, a substantial number of OSA patients are unable to benefit from this therapy.4
The nasal wall, and more specifically its mucosa, is subjected to a continuous compression stimulus during the application of nCPAP. Mechanical compression triggers a biological response in a variety of cells and tissues,5–8 and particularly in the airways.9,10 Recent data indicating that nCPAP increases airway hyperresponsiveness in patients with OSA11 lend further support to the notion that mechanical compression induces an inflammatory response in the airways. Accordingly, the available data from non-nasal cells and tissues suggest that nCPAP could be an inflammatory mechanical stimulus causing de novo nasal symptoms or exacerbating a previously existing rhinitis.
The aim of this work was to study the early inflammatory effects of nCPAP on the nasal wall tissue. The study was conducted in a novel sham-controlled setting for noninvasive application of nCPAP in rats. The inflammatory response induced by nCPAP was detected by directly assessing neutrophil extravasation in the nasal mucosa. The potential mechanisms determining CPAP-induced nasal inflammation were investigated by analyzing gene expression in the nasal wall tissue. In addition to studying common inflammatory markers such as macrophage inflammatory protein-2 (MIP-2) and tumor necrosis factor-α (TNF-α), the potential involvement of the neurotrophin nerve growth factor (NGF) and of the receptor of the neuropeptide substance P (TACR1) in the tissue response to CPAP was also investigated. The results obtained clearly show that nCPAP induces early nasal inflammation in healthy rats.
METHODS
Animals
The study, which was approved by the Ethics Committee for Animal Experimentation of the University of Barcelona, was conducted on 32 Sprague-Dawley male rats (250–300 g) intraperitoneally anesthetized with urethane 10% (1g/kg).
Experimental Setting
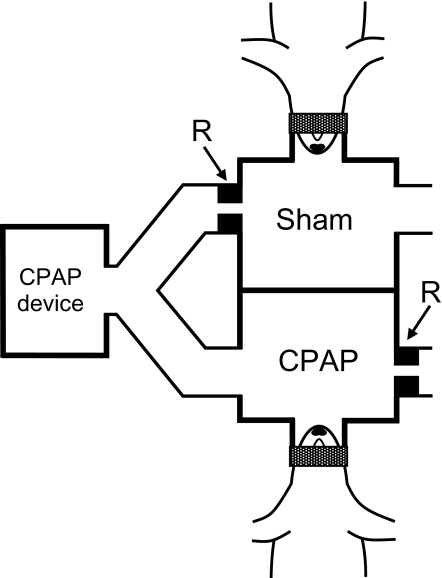
A novel setup was devised to noninvasively apply nCPAP to one rat and sham-CPAP to another rat simultaneously. A conventional CPAP device set to 10 cm H2O was connected to a bifurcated tube (Figure 1). One branch was connected to a chamber with 2 short cylindrical tubes (1.5-cm length, 2-cm diameter). One of these tubes was used as a facemask and the other one was open to the atmosphere through a resistor (R) consisting of a 1 mm diameter hole. With the rat in place, this chamber was pressurized to 10 cm H2O (CPAP) and the air was renewed by a flow of 25 mL/s. The other branch from the bifurcation was connected to another similar chamber (Figure 1). However, in this case the resistor (R) was connected at the inlet of the chamber instead of at the outlet, which was directly open to the atmosphere (Figure 1). Therefore, in this chamber (sham) the pressure was zero and the airflow to avoid rebreathing was the same as in the CPAP chamber.
Figure 1.
Diagram of the experimental setup to noninvasively apply nasal CPAP and sham-CPAP in anesthetized rats. R are resistors inducing positive pressure in the CPAP chamber and nil pressure in the sham chamber. The airflow to avoid rebreathing was the same in both chambers. See text for detailed explanation.
Protocol
The effects of 3 h and 5 h of nCPAP (and simultaneous sham control, Fig. 1) were studied in 4 groups of 8 rats each. The anesthetized animal was placed in supine and connected to the mask, ensuring a leakless sealing by using a lubricant paste and adhesive tape (Figure 1). After nCPAP (or sham), the animal was sacrificed by exsanguination. A nasal scraping sample was obtained from the left nostril, suspended in 0.9% NaCl solution and maintained in ice. The mandibular symphysis was cut to separate the 2 halves of the lower jaw and a sagittal cut through the head was made along the left nostril to expose the nasal wall structures. Septum and dorsal nasal concha from the intact right nostril were excised and frozen (−80°C).
Assessment of Neutrophils in the Nasal Scrapings
Each scraping sample was pooled on a glass slide (Cytospin IV, Shandon Scientific, UK), fixed and Wright-stained. A minimum of 200 total cells were counted by randomly scanning the sample (bright-field, 40×, Eclipse TE2000, Nikon, Japan). Counted cells were classified to compute the percentage of neutrophils.
Analysis of Gene Expression in Nasal Wall Tissue
The septum and dorsal nasal concha samples were disrupted (Polytron 2100, Kinematica, Switzerland). The content of mRNA was isolated and gene expression of MIP-2, TNF-α, NGF, and TACR1 were quantified in triplicate (7300 Real Time PCR-RT, Applied Biosystems, CA), using GAPD as endogenous control. The gene expression assays were obtained from Applied Biosystems (Rn00586403_m1, Rn00562055_m1, Rn00824646_m1, Rn00562004_m1 and Rn99999916_s1, respectively). Quantification of each gene relative to its respective control was estimated using the conventional comparative Ct method12 implemented in the PCR device.
Statistical Analysis
Data are presented as mean ± SEM. Comparisons between different groups were carried out by the Student's t-test or the Mann-Whitney Rank Sum Test. Statistical significance was assumed for P<0.05.
RESULTS
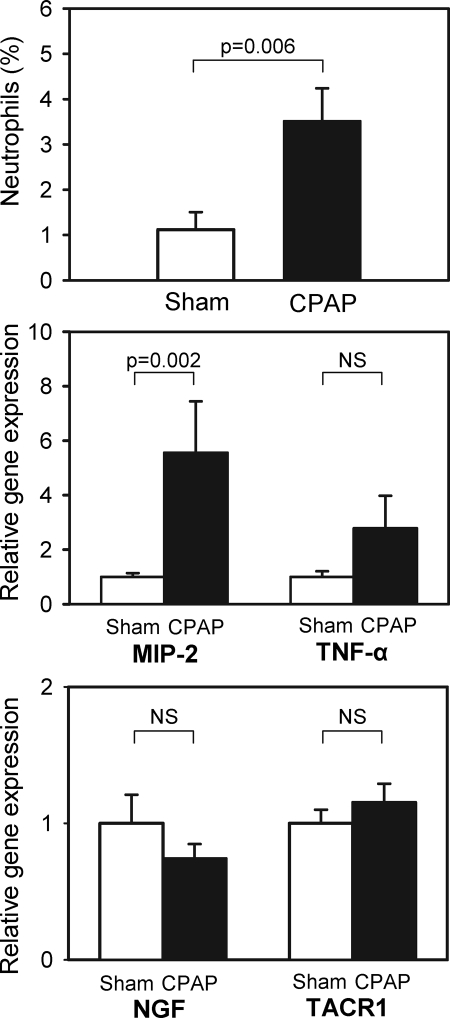
Application of nCPAP for 5 h caused a 3-fold increase in the number of neutrophils found in the nasal mucosa. The percentage of neutrophils in the total nucleated cells in the nasal scrapings was 3.51% ± 0.73% (mean ± SE) in the nCPAP group and 1.12% ± 0.39% in controls (Figure 2, top).
Figure 2.
Top: Percentage of neutrophils in total cells in the nasal scraping samples obtained from rats subjected to 5-h of CPAP = 10 cmH2O and to sham-CPAP. Center: Relative gene expression of macrophage inflammatory protein (MIP-2) and tumor necrosis factor-α (TNF-α) in the nasal wall tissue of rats subjected to 5 h of CPAP = 10 cm H2O and to sham-CPAP. Bottom: Relative gene expression of nerve growth factor (NGF) and of tachykinin-1 receptor (TACR1) in the nasal wall tissue of rats subjected to 5 h of CPAP = 10 cm H2O and to sham-CPAP.
Five hours of nCPAP induced a significant increase in the gene expression of inflammatory markers in the nasal wall tissue. The mRNA of the pro-inflammatory chemokine MIP-2 was markedly upregulated: 5.56 ± 1.89 vs. 1.00 ± 0.14 in controls (Figure 2, center). This figure also shows that, although nonsignificant, an overexpression trend was observed in the mRNA of cytokine TNF-α in the nCPAP group: 2.78 ± 1.19 vs. 1.00 ± 0.21 in controls. By contrast, minor nonsignificant changes were found when comparing the gene expression of NGF and TACR1 between both groups (Figure 2, bottom).
When analyzing the effects of applying nCPAP for 3 h, the only significant difference found between nCPAP and control groups was a gene over expression of MIP-2: 2.28 ± 0.43 vs. 1.00 ± 0.33 in controls (P = 0.034).
DISCUSSION
The present work provides evidence that the compression exerted by nCPAP on the nasal wall is a mechanical stimulus that per se triggers an early local inflammation. Five hours with 10 cm H2O of nCPAP (values typical of a one-night treatment in OSA patients), resulted in neutrophil extravasation in the nasal mucosa.
To our knowledge, this is the first time that rats have been subjected to nCPAP to mimic its application through a mask in patients. The setting designed allowed us to precisely control the experimental conditions. On the one hand, confounding factors such as systemic13 and upper airway inflammation14 and earlier rhinitis3 common in patients with OSA were avoided. On the other hand, methodological factors concerning the application of nCPAP that could interfere with nasal inflammation were also avoided.15,16 To this end, we made sure that there were no air leaks caused by the mouth opening or by incorrect mask fitting. Moreover, the use of control rats simultaneously subjected to sham-CPAP ensured that the only factor causing the observed results was the application of positive pressure. The sham-CPAP system was based on an approach previously described for application in patients.17 As shown in Figure 1, the CPAP and the sham-CPAP chambers were connected in parallel to a pressure source. Given that each identical chamber was connected to an equal serial resistor, both circuits open to the atmosphere presented the same impedance to the pressure source and, consequently, identical flows renewed the air in the chambers. Connecting the resistor at the outlet or at the inlet of the chamber (Figure 1) ensured that the pressure was positive in one chamber (CPAP) and zero in the other (sham). The resistor magnitude was selected to ensure sufficient, though not excessive, flow for air renewal through the chambers taking into account rat minute ventilation.18 Despite the advantages of the rodent model in isolating the inflammatory effects of nCPAP, it is debatable whether the results obtained can be translated to humans. This should not constitute an obstacle to the present work given that our aim was to determine whether compression induces nasal tissue inflammation. Indeed, as assumed in other studies,19–22 the basic response mechanisms observed at cell and tissue levels are similar in all mammals.
The finding that compression is a local pro-inflammatory stimulus in the nasal mucosa is consistent with data obtained when nonphysiologic compression was applied in other cells and tissues. Indeed, data available indicate that this mechanical stimulus is able to activate mechanotransduction pathways leading to neurogenic and nonneurogenic inflammation. For instance, the compression forces applied on the periodontium during orthodontic tooth movements induce the secretion of inflammatory cytokines and the neuropeptide substance P in the gingival crevicular fluid.6 Moreover, increased inflammatory cytokines5 and overexpression of the neurotrophic factor NGF7 were found in response to nerve and spinal cord compression, respectively. It has also been shown that compression is an inflammatory stimulus in the airways, particularly in the bronchi and the trachea. Indeed, mitogen activated protein kinase pathways are activated in bronchial epithelial cells subjected to a continuous compression stimulus.23 Furthermore, it has been found that a constant positive pressure induces leukocyte recruitment in the tracheal circulation in rats.9
As expected from published data on the effects of different mechanical stimuli in cells,24,25 the nasal inflammation process triggered by nCPAP was time dependent. Indeed, after 3 h of nCPAP the only significant change detected was a 2.28-fold increase in MIP-2 mRNA. This rodent chemokine, homologous to human interleukin 8, is a potent neutrophil chemo-attractant playing a key role in the initiation of inflammation induced by mechanical stress in bronchial and alveolar epithelial cells25,26 and in the upper airway.27 However, 3 h of nCPAP were not enough to produce neutrophil extravasation. By contrast, after 5 h of stimulus the inflammatory process was consolidated. In addition to a further increase in the expression of MIP-2, a tendency to increase in TNF-α gene expression was also observed. This cytokine plays a crucial role in inflammation by regulating the expression of adhesion molecules and the production of other inflammatory mediators and reactive oxygen species. After 5 h of nCPAP it was possible to detect neutrophils in the nasal mucosa scrapings showing that this time period was sufficient to complete the basic inflammatory events.
The absence of early gene over expression of NGF or TACR1 does not exclude the possibility that the compression stimulus could give rise to further neurogenic nasal inflammation. Indeed, the early inflammatory markers induced by CPAP could stimulate the nasal sensory nerves,28,29 thereby inducing the synthesis and secretion of the neuropeptides that regulate symptoms of rhinitis.29 It is possible that the inflammatory cytokines induce the synthesis of NGF in the nasal mucosa in a way similar to that observed in bronchial epithelial cells30 and in smooth muscle cells.31 Such an increase in NGF would in turn activate the nasal sensory nerves and, hence, the symptoms of rhinitis.29 Finally, as reported in colon epithelial cells32 and in peritoneal macrophages,33 the early inflammatory cytokines induced by CPAP could over express the substance P receptor TACR1 in the nasal mucosa, facilitating the manifestation of symptoms of rhinitis. However, further data from chronic exposure to compression are warranted to confirm the long term neurogenic inflammatory pathways activated by nasal CPAP.
Under normal physiological conditions the early inflammatory process triggered by nCPAP could be compensated by adaptive antiinflammatory mechanisms. This would explain why in a majority of OSA patients treated with nCPAP or in patients subjected to long-term noninvasive ventilation by nasal positive pressure34 the symptoms of rhinitis are not documented or are so small that they allow treatment tolerance. However, it is possible that in some patients the potential adaptive antiinflammatory mechanisms are not effective because of preconditioning. In such a case the compression stimulus would act as a second-hit source of injury. For instance, it has been reported that CPAP directly induces nasal tissue inflammation in premature neonates with respiratory failure subjected to nasal positive pressure,35 and that CPAP causes acute lung injury in rats with circulating endotoxin.36 In the specific case of OSA patients with previous rhinitis,37 the upper airway inflammation could enhance the inflammatory stimulus of mechanical compression, resulting in intolerance to the therapy. It is also possible that the continuous flow of relatively cold and dry air38 through the open mouth would enhance symptoms of rhinitis even after several months of CPAP use.
In conclusion, this study shows that nasal tissue compression caused by conventional nCPAP triggers an early local inflammation. This pressure-sensitive mechanotransduction response resulting in nasal inflammation could account for the rhinitic side effects of CPAP in patients with OSA.
ACKNOWLEDGMENTS
The authors wish to thank Mr. Miguel A. Rodriguez for his technical assistance.
Support: This work was supported in part by Ministerio de Ciencia y Tecnología (SAF2005-00110 and SAF2004-00684) and Ministerio de Sanidad y Consumo (FIS-PI040929 and CibeRes-ISCiii-CB06/06).
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Sullivan CE, Berthonjones M, Issa FG, Eves L. Reversal of obstructive sleep-apnea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–65. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 2.Pepin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side-effects of nasal continuous positive airway pressure in sleep-apnea syndrome - study of 193 patients in 2 French sleep centers. Chest. 1995;107:375–81. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 3.Brander P, Soirinsuo M, Lohela P. Nasopharyngeal symptoms in patients with obstructive sleep apnea syndrome. Effect of nasal CPAP treatment. Respiration. 1999;66:128–35. doi: 10.1159/000029354. [DOI] [PubMed] [Google Scholar]
- 4.Janson C, Noges E, Svedberg-Brandt S, Lindberg E. What characterizes patients who are unable to tolerate continuous positive airway pressure (CPAP) treatment? Respir Med. 2000;94:145–9. doi: 10.1053/rmed.1999.0703. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi S, Baba H, Uchida K, et al. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine. 2005;30:1699–1705. doi: 10.1097/01.brs.0000171910.97937.0e. [DOI] [PubMed] [Google Scholar]
- 6.Dudic A, Kiliaridis S, Mombelli A, Giannopoulou C. Composition changes in gingival crevicular fluid during orthodontic tooth movement: comparisons between tension and compression sides. Eur J Oral Sci. 2006;114:416–22. doi: 10.1111/j.1600-0722.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 7.Kasahara K, Nakagawa T, Kubota T. Neuronal loss and expression of neurotrophic factors in a model of rat chronic compressive spinal cord injury. Spine. 2006;31:2059–66. doi: 10.1097/01.brs.0000231893.21964.f2. [DOI] [PubMed] [Google Scholar]
- 8.Tan JC, Kalapesi FB, Coroneo MT. Mechanosensitivity and the eye: cells coping with the pressure. Br J Ophthalmol. 2006;90:383–8. doi: 10.1136/bjo.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim LH, Wagner EM. Airway distension promotes leukocyte recruitment in rat tracheal circulation. Am J Respir Crit Care Med. 2003;168:1068–74. doi: 10.1164/rccm.200207-690OC. [DOI] [PubMed] [Google Scholar]
- 10.Tschumperlin DJ, Drazen JM. Chronic effects of mechanical force on airways. Annu Rev Physiol. 2006;68:563–83. doi: 10.1146/annurev.physiol.68.072304.113102. [DOI] [PubMed] [Google Scholar]
- 11.Devouassoux G, Levy P, Rossini E, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol. 2007;119:597–603. doi: 10.1016/j.jaci.2006.11.638. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–6. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 15.Togias AG, Naclerio RM, Proud D, et al. Nasal challenge with cold, dry air results in release of inflammatory mediators—possible mast-cell involvement. J Clin Invest. 1985;76:1375–81. doi: 10.1172/JCI112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest. 2004;126:1248–54. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 17.Farre R, Hernandez L, Montserrat JM, Rotger M, Ballester E, Navajas D. Sham continuous positive airway pressure for placebo-controlled studies in sleep apnoea. Lancet. 1999;353:1154. doi: 10.1016/S0140-6736(99)01056-9. [DOI] [PubMed] [Google Scholar]
- 18.Strohl KP, Thomas AJ, StJean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82:317–23. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher EC. Physiological and genomic consequences of intermittent hypoxia: invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–5. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 20.Farre R, Rotger M, Montserrat JM, Calero G, Navajas D. Collapsible upper airway segment to study the obstructive sleep apnea/hypopnea syndrome in rats. Respir Physiol Neurobiol. 2003;136:199–209. doi: 10.1016/s1569-9048(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 21.Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167:1540–7. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- 22.Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155:93–6. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Tschumperlin DJ, Shively JD, Swartz MA, Silverman ES, Haley KJ, Raab G, Drazen JM. Mechanotransduction in the lung - Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am J Physiol Lung Cell Mol Physiol. 2002;282:L904–11. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- 24.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 1999;277:L167–73. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 25.Puig F, Rico F, Almendros I, Montserrat JM, Navajas D, Farre R. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammation. Sleep. 2005;28:1312–16. doi: 10.1093/sleep/28.10.1312. [DOI] [PubMed] [Google Scholar]
- 26.Li LF, Ouyang B, Choukroun G, et al. Stretch-induced IL-8 depends on c-Jun NH2-terminal and nuclear factor-kappa B-inducing kinases. Am J Physiol Lung Cell Mol Physiol. 2003;285:L464–75. doi: 10.1152/ajplung.00031.2003. [DOI] [PubMed] [Google Scholar]
- 27.Almendros I, Acerbi I, Puig F, Montserrat JM, Navajas D, Farre R. Upper-airway inflammation triggered by vibration in a rat model of snoring. Sleep. 2007;30:225–7. doi: 10.1093/sleep/30.2.225. [DOI] [PubMed] [Google Scholar]
- 28.Belvisi MG. Sensory nerves and airway inflammation: role of A delta and C-fibres. Pulm Pharmacol Ther. 2003;16:1–7. doi: 10.1016/S1094-5539(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 29.Sarin S, Undem B, Sanico A, Togias A. The role of the nervous system in rhinitis. J Allergy Clin Immunol. 2006;118:999–1014. doi: 10.1016/j.jaci.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006;117:787–94. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- 31.Freund V, Pons F, Joly V, Mathieu E, Martinet N, Frossard N. Upregulation of nerve growth factor expression by human airway smooth muscle cells in inflammatory conditions. Eur Respir J. 2002;20:458–63. doi: 10.1183/09031936.02.00269202. [DOI] [PubMed] [Google Scholar]
- 32.Goode T, O'Connor T, Hopkins A, et al. Neurokinin-1 receptor (NK-1R) expression is induced in human colonic epithelial cells by proinflammatory cytokines and mediates proliferation in response to substance P. J Cell Physiol. 2003;197:30–41. doi: 10.1002/jcp.10234. [DOI] [PubMed] [Google Scholar]
- 33.Marriott I, Mason MJ, Elhofy A, Bost KL. Substance P activates NF-kappa B independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J Neuroimmunol. 2000;102:163–71. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- 34.Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest. 1999;116:667–75. doi: 10.1378/chest.116.3.667. [DOI] [PubMed] [Google Scholar]
- 35.Yong SC, Chen SJ, Boo NY. Incidence of nasal trauma associated with nasal prong versus nasal mask during continuous positive airway pressure treatment in very low birthweight infants: a randomised control study. Arch Dis Child Fetal Neonatal Ed. 2005;90:F480–3. doi: 10.1136/adc.2004.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuchida S, Engelberts D, Roth M, McKerlie C, Post M, Kavanagh BP. Continuous positive airway pressure causes lung injury in a model of sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L554–64. doi: 10.1152/ajplung.00143.2005. [DOI] [PubMed] [Google Scholar]
- 37.Canova CR, Downs SH, Knoblauch A, Andersson M, Tamm M, Leuppi JD. Increased prevalence of perennial allergic rhinitis in patients with obstructive sleep apnea. Respiration. 2004;71:138–43. doi: 10.1159/000076674. [DOI] [PubMed] [Google Scholar]
- 38.Cruz AA, Naclerio RM, Proud D, Togias A. Epithelial shedding is associated with nasal reactions to cold, dry air. J Allergy Clin Immunol. 2006;117:1351–8. doi: 10.1016/j.jaci.2006.01.054. [DOI] [PubMed] [Google Scholar]