Abstract
Study Objective:
To evaluate whether cardiovascular responses to maximal exercise testing and recovery are altered with obstructive sleep apnea (OSA) in overweight young adult men.
Design:
Three sedentary subject groups were recruited: Overweight with OSA (OSA), overweight without OSA (No-OSA), and normal weight without OSA (Control). Presence of OSA was screened via portable diagnostic device. Body composition was measured with dual-energy X-ray absorptiometry. Subjects performed maximal ramping exercise testing (RXT) on a cycle ergometer with 5 minutes of active recovery. Exercise measurements included heart rate (HR), blood pressure (BP), respiratory exchange ratio (RER), and oxygen consumption (VO2). Recovery HR was converted to a HR difference (HRdiff) calculation (HRpeak − HR each minute recovery), and BP was converted to a recovery ratio for each minute.
Setting:
The study was carried out on the campus of Virginia Tech, Department of Human Nutrition, Foods, and Exercise, Blacksburg, Virginia.
Participants:
14 OSA, 16 No-OSA, and 14 Control volunteers.
Intervention:
N/A
Measurements and Results:
In OSA subjects, HR recovery was significantly attenuated compared to the No-OSA and Control groups throughout recovery (P = 0.009). No differences were noted in the HR or BP response to exercise in any group. The VO2, adjusted for fat-free soft tissue mass, did not differ between groups.
Conclusions:
We found that OSA elicits alterations in the cardiovascular response post exercise, reflected by an attenuated HR recovery. This may indicate an imbalance in the autonomic regulation of HR. Exercise tests may provide utility in risk stratification for those at risk for OSA.
Citation:
Hargens TA; Guill SG; Zedalis D; Gregg JM; Nickols-Richardson SM; Herbert WG. Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. SLEEP 2008;31(1):104-110.
Keywords: Obstructive sleep apnea, exercise testing, heart rate recovery
INTRODUCTION
OBSTRUCTIVE SLEEP APNEA (OSA) AFFECTS APPROXIMATELY 2% TO 4% OF MIDDLE-AGED ADULTS,1 WITH ESTIMATES REACHING 20% AND 7% FOR MILD AND moderate-to-severe OSA, respectively, in overweight individuals with a body mass index (BMI) of 25–28 kg/m2.2 Furthermore, it is estimated that 93% of females, and 82% of males with moderate-to-severe OSA, those that would most benefit from treatment, remain undiagnosed clinically.3 This disorder represents a significant public health concern, as OSA markedly increases the risk for motor vehicle accidents through daytime sleepiness,4 as well as increasing the risk for several adverse health conditions including stroke, insulin resistance and glucose intolerance, congestive heart failure, cardiac arrhythmias, and hypertension (HTN).2,5–7 In addition, OSA may result in an increased risk for the development of cardiovascular disease (CVD),8 although no longitudinal, randomized interventional studies have been conducted to conclusively demonstrate independent causation.9
The underlying mechanisms linking OSA to HTN and CVD remain unclear. At night, repeated apneas and hypopneas result in decreased arterial oxygen saturation and carbon dioxide retention which cause sympathetic nervous system (SNS) activation and stressful arousals to reestablish breathing. In OSA patients, exaggerated SNS activation persisting into waking hours has been demonstrated,10,11 which can lead to surges in heart rate (HR) and blood pressure (BP). In response to short-term increases in HR and BP, normal baroreceptor activation results in a net decrease in central nervous system outflow which decreases HR and BP. With OSA patients, an alteration in the baroreflex activation appears to occur. Over time, depressed baroreflex sensitivity in OSA patients has been demonstrated,12 suggesting an impairment of cardiovascular autonomic function, which may result in increased risk for HTN and CVD.
Maximal ramping exercise testing (RXT) has long been used as an effective tool for the identification of those at high risk for CVD.13,14 Ramping exercise test protocols offer several advantages over protocols with discrete increments including near-continuous and uniform increases in workload, flexible timing for sampling physiological responses, and a more accurate estimate of exercise capacity.13,14 Furthermore, alterations in the HR and BP response to exercise and immediately post-exercise have shown prognostic value and may reflect impairment in autonomic regulation. Exaggerated BP response during exercise is predictive for the development of future HTN.15 Following exercise, attenuated BP16,17 and HR18,19 recovery have both shown to predict future CVD and mortality. Given the predisposition for HTN as a result of OSA, the hemodynamic responses to exercise in the OSA individual may provide useful information to further risk stratify those in need of additional diagnostic testing. Research into the exercise response of OSA subjects is limited and equivocal. Several studies report a decreased functional capacity in OSA subjects compared to controls,20,21 while others report no difference.22–24 One recent investigation reported a significantly blunted HR response to exercise and a delayed systolic BP response post-exercise in middle-aged OSA subjects vs. non-OSA controls.22
To date, no published literature has examined the cardiovascular responses associated with RXT in young overweight men with untreated OSA. In recent years, HR and BP have been identified as important surrogates of autonomic dysfunction in RXT and appear to have promise in forecasting future cardiac risk.15,25 Whether these changes occur early in the development and progression of the disorder has not been examined. The purpose of this study was to evaluate whether autonomic control of the cardiovascular responses during and after exercise was distorted by OSA in young overweight men. We hypothesized that the HR response to exercise and recovery would be blunted, the BP response with exercise would be exaggerated, and the recovery BP response delayed with RXT in overweight, sedentary young men with untreated OSA, in comparison to overweight and normal weight control groups of sedentary young men without OSA.
METHODS
Subjects
Sedentary overweight men with untreated OSA (OSA; n = 14), and men matched for age, BMI, and central adiposity, but without OSA (No-OSA; n = 16) were recruited from the local university community through campus notices as well as newspaper advertisements. Subjects were between 18 and 26 years of age and were classified as overweight or obese according to BMI criteria.13 Sedentary, normal weight control men without OSA (Control; n = 14) were also recruited. All subjects underwent a prescreening, which included an initial qualification questionnaire as well as a detailed health history questionnaire to identify any potential exclusion criteria. All subjects were nonsmokers, were free from acute respiratory infections during the previous 6 weeks, and did not report current tonsillitis and adenoiditis. Subjects were free from significant cardiovascular, pulmonary, metabolic, or musculoskeletal disorders that would preclude maximal aerobic exercise testing. Subjects were not taking any prescribed vasoactive medications, hypnotics, sedatives, analgesics, psychotropics, steroids, or sympathomimetics. Individuals who had participated in regular physical activity (>3 days/week, >30 min/day) for the previous 6 months were considered active and were thus excluded.22 All methods and procedures, approved by the Institutional Review Board of Virginia Tech, Blacksburg, VA, were explained to the subjects, who then read and signed a written informed consent form.
Home Sleep Evaluation
Subjects underwent an unattended, limited home sleep evaluation to screen for OSA, utilizing a valid device (Embletta, Embla, Broomfield, CO).26 The evaluation consisted of: 1) nasal flow detection via nasal cannula; 2) finger pulse oximetry; 3) respiratory effort detection via belts positioned on the upper and lower torso; and 4) body position detection. Sleep data were interpreted by a sleep technician and transposed into an apnea-hypopnea index (AHI; events/h) and oxygen desaturation index (ODI; events/h) score, with results verified by a physician specialized in sleep medicine. Apnea was defined as a cessation of airflow for ≥10 sec. Hypopnea was defined as ≥50% reduction in airflow for ≥10 sec coupled with a decrease in oxygen saturation (≥4%).27 Average and lowest nocturnal oxygen saturation values were also measured in each subject. Normal weight subjects (BMI <25 kg/m2) with an AHI score ≥5 events/h were excluded. The OSA group included men with an AHI score of >5 events/h, the No-OSA group included men with an AHI score <5 events/h, and the Control men had AHI scores <5 events/h.
Body Composition Measurement
Total body dual-energy X-ray absorptiometry (DXA) (version 8.26a:3*, QDR4500A, Hologic Inc., Bedford, MA) was used to measure percent body fat, fat-free soft tissue mass (FFM), and fat mass (FM). Central abdominal fat (CAF) was measured from total body DXA scans by the method of Kamel et al.28 One investigator conducted and analyzed all DXA measures. Quality control for soft tissue mass measurements was completed with weekly scans of an external soft tissue bar (Hologic Inc.). Test-retest reliability for the DXA unit has been previously reported.29
Ramp Exercise Testing
Subjects completed a maximal cycle RXT. Prior to the test, standing height, body weight, and neck, waist, and hip circumferences were measured. HR and BP were measured in a sitting posture. Exercise tests were performed on an electronically braked cycle ergometer (SensorMedics, Yorba Linda, CA) utilizing a standardized protocol previously described.22 Respiratory gas exchange measurements including oxygen consumption (VO2), minute ventilation (VE), and respiratory exchange ratio (RER) were obtained during the exercise test using a computer controlled, breath-by-breath system (SensorMedics Vmax 229, Yorba Linda, CA) and values were calculated to 10-sec averages. Peak VO2 (VO2pk) was defined as the highest VO2 achieved during the last min of exercise. To summarize results across study groups that included subjects with different VO2pk, HR and VO2 values were input into spreadsheet software (Microsoft Excel, Microsoft Corp, Bellevue, WA) as time-down columns from the start of exercise to peak. HR and VO2 were designated the y- and x-axis, respectively. Polynomial regression was employed with the line of best fit option of the spreadsheet software to establish response values that corresponded to 20%, 40%, 60%, and 80% of VO2pk. A mean R2 > 0.80 was achieved by assessing the lowest order polynomial regression that produced the highest R2 for each subject. For exercise recovery, BP data was converted to a recovery BP ratio (BPR) [i.e., systolic BP (SBP) at 1-min recovery/SBP at peak] for the 5-min recovery period. Recovery HR was converted to a HR difference (HRdiff) calculation. The difference between HR peak (HRpk) and HR at each post-exercise minute (i.e., HRpk − HR at 1 min post exercise) was calculated for the 5-min recovery period.
Statistical Analysis
All statistical analyses were performed using SPSS (version 14.0, SPSS Inc., Chicago, IL). Comparisons for the effect of group and exercise intensity for the cardiovascular measures were made with repeated measures ANOVA, with exercise intensity as the within-subject factor, and group as the between-subject factor. Comparisons for the effect of group and time for the post-exercise cardiovascular measures were also made with repeated measures ANOVA with time as the within-subject factor, and group as the between-subject factor. When ANOVA results showed significant differences between groups, post hoc multiple comparisons were made with Bonferroni tests. Pearson correlations were calculated to identify potential relationships between select cardiovascular measures and AHI. Cardiovascular measures included HR, SBP, diastolic BP (DBP), mean arterial pressure (MAP), post-exercise systolic blood pressure ratio (SBPR), post-exercise diastolic blood pressure ratio (DBPR), and heart rate recovery (HRR) measurements. A value of P < 0.05 was considered statistically significant.
RESULTS
Subject Characteristics
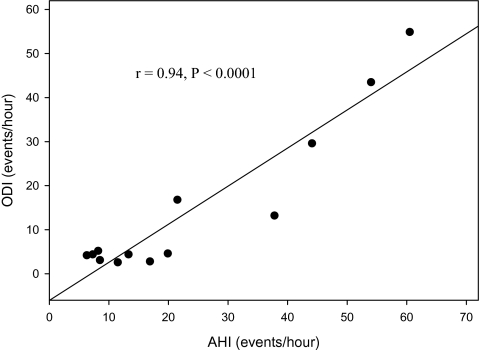
Baseline subject characteristics for each group are presented in Table 1. By study design, AHI scores were significantly higher in the OSA group vs. No-OSA (P < 0.005) and Control (P < 0.005) groups. Similarly, ODI scores were also higher in the OSA group vs. No-OSA (P < 0.01) and Control (P < 0.01) groups. The AHI and ODI were highly correlated (r = 0.94, P < 0.001) in subjects with an AHI > 5 (Figure 1). Lowest oxygen saturation was lower in the OSA group vs. Control (P < 0.05). No significant differences were noted between the three groups with respect to age, height, average nocturnal oxygen saturation, HRrest, or SBPrest, and MAPrest. DBPrest in the No-OSA group was significantly higher than Control group (P = 0.03). The OSA and No-OSA groups also did not differ from each other in BMI, neck circumference, waist circumference, and CAF; however, as intended by study design, the Control group had significantly lower weight (P < 0.05), BMI (P < 0.05), and CAF (P < 0.05). For the total subject sample, AHI was positively correlated with BMI (r = 0.45, P = 0.002), neck circumference (r = 0.31, P = 0.05), fat mass (r = 0.43, P = 0.004) and CAF (r = 0.51, P < 0.0001). However, when adjusted for body weight, only BMI (r = 0.34, P = 0.03) and CAF (r = 0.42, P = 0.006) remained significant.
Table 1.
Baseline Subject Characteristics of Young Men Completing RXT
| OSA (n = 14) | No-OSA (n = 16) | Control (n = 14) | |
|---|---|---|---|
| Age (yr) | 22.4 (2.8) | 21.4 (2.6) | 21.4 (2.1) |
| AHI (events/hr) | 22.7 (18.5) | 2.5 (1.3)† | 2.0 (1.1)† |
| ODI (events/hr) | 14.6 (17.3) | 1.8 (1.3)† | 2.1 (2.8)† |
| Avg O2 (%) | 95.5 (1.7) | 95.6 (1.9) | 96.4 (0.5) |
| Low O2 (%) | 86.2 (4.3)‡ | 88.3 (4.3) | 90.0 (2.8) |
| Height (cm) | 171.6 (18.6) | 178.2 (6.1) | 177.1 (6.7) |
| Weight (kg) | 99.6 (13.4) | 99.4 (12.4) | 69.1 (6.6)* |
| BMI (kg/m2) | 32.0 (3.7) | 31.4 (3.7) | 22.0 (1.3)* |
| NC (cm) | 40.8 (2.1) | 40.6 (2.6) | 36.2 (1.5)* |
| WC (cm) | 100.5 (8.1) | 95.4 (9.7) | 77.5 (6.3)* |
| CAF (kg) | 8.7 (2.4) | 7.0 (1.9) | 3.4 (0.98)* |
| HR (bts.min−1) | 92.4 (14.2) | 86.0 (14.0) | 85.9 (11.6) |
| SBP (mmHg) | 125.9 (9.9) | 124.9 (10.9) | 121.4 (9.1) |
| DBP (mmHg) | 85.7 (5.4) | 87.6 (8.1) | 80.9 (6.2)** |
| MAP (mmHg) | 99.1 (5.5) | 100.0 (8.2) | 94.4 (5.3) |
Values are means with SD in parentheses. Values were taken at rest.
AHI = apnea/hypopnea index; ODI = oxygen desaturation index; Avg O2 = average nocturnal oxygen saturation; Low O2 = lowest nocturnal oxygen saturation; BMI = body mass index; NC = neck circumference; WC = waist circumference; CAF = central abdominal fat; HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure
Significantly different from OSA, No-OSA (P < 0.05)
Significantly different from No-OSA (P < 0.03)
Significantly different from OSA (P < 0.01)
Significantly different from Control (P < 0.05)
Figure 1.
Association between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI) in young, sedentary men with obstructive sleep apnea (AHI > 5).
Exercise Test Responses
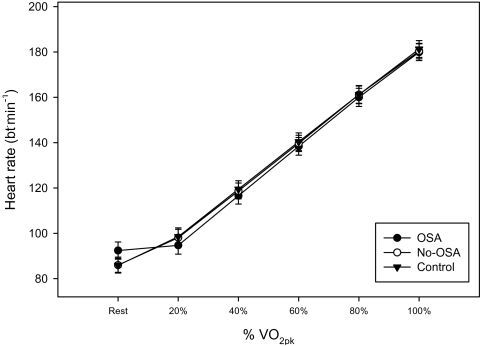
When the VO2 response was compared between the 2 overweight groups (OSA vs. No-OSA), no differences were noted across any exercise intensities, including peak (see Table 2). However, the normal weight control group had higher VO2 responses at submaximal intensities and at peak vs. both OSA (P < 0.02) and No-OSA (P < 0.05) groups. When VO2 was adjusted for FFM, however, no significant differences were noted between the OSA, No-OSA, or Control groups at any submaximal intensity or at peak exercise (P = 0.25). In addition, HR (Figure 2), SBP, DBP, RER, and MAP did not differ between subject groups at any submaximal workload or at peak exercise. All three groups achieved maximal exercise test endpoints, confirmed by Borg Scale (6–20) ratings of perceived exertion at peak >16 and peak RER >1.10.
Table 2.
Peak RXT Data
| OSA (n = 14) | No-OSA (n = 16) | Control (n = 14) | |
|---|---|---|---|
| HR (bt.min−1) | 179.9 (3.7) | 180.4 (3.4) | 181.3 (3.7) |
| SBP (mmHg) | 196.9 (7.0) | 202.6 (6.5) | 193.4 (7.2) |
| DBP (mmHg) | 90.7 (3.1) | 91.4 (2.9) | 89.5 (3.2) |
| MAP (mmHg) | 126.1 (14.9) | 128.4 (14.5) | 124.5 (9.8) |
| RER | 1.14 (0.06) | 1.13 (0.04) | 1.17 (0.07) |
| VO2pk (ml.kg−1.min−1) | 27.1 (4.5) | 28.0 (5.8) | 33.2 (6.2)* |
| VO2pk (ml.kg FFM−1.min−1) | 37.8 (4.9) | 39.6 (6.9) | 41.9 (6.3) |
Values are means with SD in parentheses.
FFM = Fat free mass
Significantly different from OSA, No-OSA (P < 0.05)
Figure 2.
HR responses to cycle exercise testing as a function of percent VO2pk in young, sedentary men.
Exercise Recovery Responses
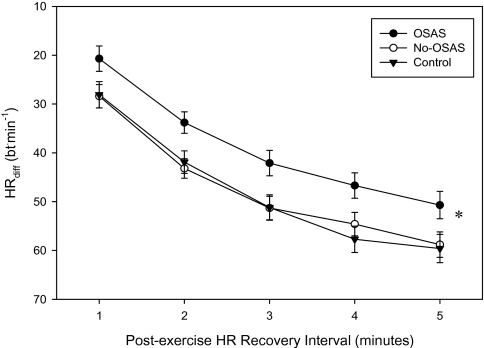
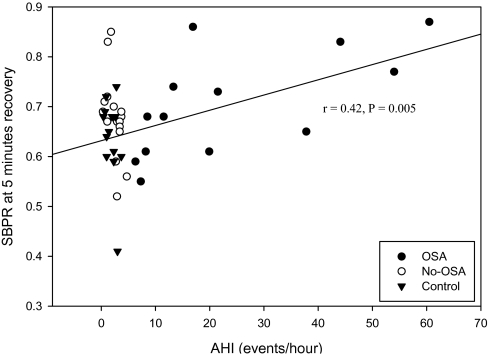
Data from one Control subject were excluded due to symptomatic hypotension during the exercise recovery period. The HR recovery was significantly attenuated in the OSA group throughout the recovery period when compared to the No-OSA (P < 0.03) and Control (P < 0.03) groups (Figure 3). No significant group differences were noted in SBPR or DBPR during the recovery exercise period. AHI was negatively correlated with HRdiff for minutes 1–4 of the recovery period (r = −0.34, P = 0.023; r = −0.48, P = 0.001; r = −0.33, P = 0.033; r = −0.39, P = 0.011 for minutes 1–4, respectively), and positively correlated with SBPR at 4 min (r = 0.36, P = 0.02) and 5 min (r = 0.42, P = 0.005) post-exercise, DBPR at 5 min post-exercise (r = 0.33, P = 0.03), for all subjects. When corrected for body weight, these relationships remained significant with the exception of HRdiff at 3 min (P = 0.069). Correlation analysis of recovery BP responses, only for subjects with an AHI > 5 events/h, revealed that severity of OSA explained a significant portion of the variance in the decline of SBPR at 4 min (r = 0.58, P = 0.03) and 5 min (r = 0.64, P = 0.01) (Figure 4) and in DBPR at 3 (r = 0.58, P = 0.03) and 4 min (r = 0.70, P = 0.005).
Figure 3.
HRdiff responses during post-exercise recovery following cycle ergometer exercise in young, sedentary men, obtained during physically active, low intensity pedaling. (* P = 0.009)
Figure 4.
Recovery systolic blood pressure ratio (SBPR) at 5 minutes recovery in all study subjects. Regression line is for OSA subjects only
DISCUSSION
To our knowledge, this study represents the first evaluation of cardiovascular responses to exercise in young overweight men with untreated OSA. Our hypothesis was supported by the finding in the OSA group of attenuated HR recovery in the OSA group that persisted through at least 5 min following maximal exercise (Figure 3). When compared to subjects with similar body mass and adiposity but without OSA, young overweight men with OSA showed a blunted heart rate recovery response (Avg. ↓ HRdiff = 8.4 bt.min−1 for the post-exercise period). We did not, however, find any group differences in the SBP or DBP recovery response due to the presence of OSA. This may be related to the fact that our OSA group was comprised primarily of individuals with mild-to-moderate disease, with only 4 having clinically severe status (AHI > 30 events/h). However, the associations between AHI score and degree of blunting in both SBPR and DBPR for subjects in the OSA group indicated that 33% to 49% of this response pattern was explained by severity of OSA. Thus, although this subset with severe OSA was too small to evaluate statistically for a differential response, all of them showed this pattern in their cardiovascular recovery responses for HR, SBP, and DBP. Thus, these results suggest that RXT may be a useful tool in identifying significant clinical signs in the early stages of OSA progression, which may aid clinicians in improving risk stratification and patient selection for overnight polysomnography.
Published data on the cardiovascular responses to exercise testing in OSA patients are limited. Available literature regarding effects of OSA on functional capacity has shown conflicting results. Decreased VO2pk in overweight, moderate to severe OSA patients, compared to age- and BMI-matched control subjects has been reported20,21 as has no difference in VO2pk.20,22,23 Ozturk et al.24 further reported that measured VO2pk in the OSA patients was significantly lower than predicted VO2pk. These previous investigations were all conducted on middle-aged to older individuals, who likely have been afflicted with significant OSA longer than the young subjects included in our study. We support results of Ozturk et al.24 and Alonso-Fernandez et al.23 and extend those findings to a younger population, who likely are not as far along in their disease progression. Utilizing the same prediction formula as Ozturk,24 measured VO2pk (27.1 ± 1.2 ml.kg−1.min−1) was significantly lower than predicted (42.4 ± 0.28 ml.kg−1.min−1) in our OSA group. Our No-OSA and Control groups also showed significantly lower measured VO2pk (ml.kg−1.min−1) than predicted (28.0 ± 1.5 vs. 43.8 ± 0.24 ml.kg−1.min−1 and 33.2 ± 6.2 vs. 42.8 ± 0.97 ml.kg−1.min−1 for No-OSA and control, respectively). All 3 of our groups were previously sedentary (regular exercise <3 days/week; 30 min/day) for more than 6 months. Differences in measured and predicted VO2pk likely reflect a lack of conditioning. Several other studies report data for sedentary subject groups,21,23,24 but do not specify criteria for qualification as a sedentary individual.
Only 2 previous studies comparing OSA patients to matched controls have reported the HR and BP responses at rest, submaximal exercise intensities, and at peak exercise.21,22 Kaleth et al.22 reported a significantly lower HR at all submaximal workloads and at peak exercise despite a lack of differences in VO2pk between groups. They suggest that the impaired chronotropic response to exercise may be due to downregulation of beta-adrenergic receptors in response to exaggerated sympathetic activation. In addition, a significantly greater DBP in their OSA group vs. control group at rest, across all submaximal intensities, and at peak was observed.22 In contrast, Vanuxem et al.21 reported no significant differences in the HR response with exercise in OSA subjects vs. controls at any submaximal exercise level or at peak, but reported greater SBP (P < 0.05) and DBP (P < 0.005) at rest in OSA vs. controls, as well as a greater DBP at peak exercise (P < 0.05).21 Results from our study agree with those of Vanuxem et al.21 in that no differences were observed in the HR response to exercise. We show that DBP is higher in both overweight subject groups at rest (OSA and No-OSA) vs. Controls (Table 1), but that DBP is not different between these groups, indicating that this higher DBP may be a result of obesity and not OSA. At peak exercise, those differences disappeared (Table 2). Other studies report resting and/or peak HR and BP values only,20,23 and show no significant differences in HR or BP at rest or at peak exercise.
Kaleth et al.22 is the only other study that reports recovery HR and BP data. In contrast to our study, no difference in HR recovery response between the OSA group and control group (when differences in peak HR response were taken into account) were found; however, SBP recovery was significantly attenuated with OSA. A delayed recovery response in SBP may indicate an autonomic imbalance, in response to chronic sympathetic activation with OSA, which slows the normal rapid response in cardiac output and peripheral vascular function to cessation of exercise.22 The different recovery cardiovascular responses seen in the current study vs. that of Kaleth et al.22 may indicate an age effect of OSA, as subjects in the previous investigation were middle-aged, compared to our younger age group.
Attenuated HR recovery has been identified as an independent predictor of cardiovascular and all-cause mortality in individuals undergoing diagnostic symptom-limited exercise testing 18,19 as well as in a generally healthy adult cohort,30 utilizing relatively short recovery periods of 1 to 2 min. This may be attributable to a reduction in parasympathetic activity, which predominates during the recovery phase of exercise.18, 31 However, Cheng and colleagues demonstrated that a decreased HR recovery, measured for as long as 5 min, was also independently predictive of cardiovascular and all-cause mortality in men with diabetes mellitus.32 Our results are similar to Cheng et al.,32 in that a significantly blunted HR recovery was seen in the OSA group through 5 min of recovery.
The mechanism for attenuated HR recovery in OSA is unclear. During exercise, HR is under the control of both the sympathetic and parasympathetic branches of the autonomic nervous system.33 During the initial phases of exercise, HR increase is mediated primarily by withdrawal of parasympathetic activity. After a HR of approximately 100 beats.min−1, the HR increase is due primarily to increased sympathetic activity, which acts much slower on HR than the parasympathetic system.33 Following exercise, the decrease in HR is due to sympathetic withdrawal and parasympathetic activation.34 Belozeroff et al.,35 utilizing a model-based approach, concluded that OSA results in abnormal sympathetic and parasympathetic control of HR. Their model was able to control for the fluctuations in HR due to respiration, known as the respiratory sinus arrhythmia.35 Exaggerated sympathetic activation has previously been reported in OSA patients,10,11 which persists throughout normal waking hours. Attenuation of the HR recovery response in OSA may reflect predominance and/or slower withdrawal of sympathetic influence; how this pattern may be affected by parasympathetic reactivation that normally slows HR in early post-exercise recovery is uncertain.
Obesity is itself an independent risk factor for the development of HTN,36 and is associated with elevated SNS activation.37,38 Distinguishing between the independent effects of OSA on sympathetic activity vs. that previously noted in obesity presents a challenge in OSA research. In the current study, both overweight subject groups were matched for body composition variables, including CAF, which may be a significant link between obesity and exaggerated sympathetic activation.39 We have shown that in subjects matched for age, body size, and body adiposity, those with OSA have a significant attenuation of their recovery HR response, suggestive of autonomic imbalance. In addition, those in the No-OSA group did not differ from the normal weight Control group in recovery HR response, suggesting a limited effect of obesity on autonomic control of HR during recovery.
One limitation of our study is the lack of direct evidence of SNS activity, either through muscle sympathetic nerve activation or plasma catecholamine measurements. Future studies need to examine these direct measures of sympathetic activation in younger OSA patients to determine if exaggerated sympathetic activity is an early sign in the development and progression of OSA, and to the development of HTN and CVD. Additionally, studies examining cardiovascular remodeling and endothelial dysfunction in young OSA patients are needed. Early signs of cardiovascular remodeling and vascular impairment have been reported in middle-aged OSA patients, suggesting that these OSA-related modifications may take time to manifest.40 Studies specifically targeted to establish the timeline for the onset of cardiovascular and endothelial modifications are needed. Another limitation is that cycle ergometry was utilized as the exercise mode rather than treadmill walking. Cycle ergometry can result in lower VO2pk values compared to treadmill walking. However, in the current study, peak RER values for each subject group (Table 2) were above maximal exercise criteria (>1.10), suggesting that maximal or near maximal efforts were achieved in all subjects.
In conclusion, this is the first study to examine the cardiovascular responses to ramp exercise testing and post-exercise recovery in young, overweight men with untreated OSA. Results indicate that OSA elicits unique cardiovascular responses during recovery from maximal exercise. These results suggest an imbalance in the autonomic control of HR during recovery, and may be an early clinical sign in the progression of OSA. These findings also suggest the potential for RXT in improving risk stratification and clinical decision making leading to patient selection for diagnostic investigation for OSA. Further clinical studies, across a wider variety of age groups, are needed to examine whether there is an age-related influence in the exercise and post-exercise responses in OSA.
ACKNOWLEDGMENTS
Parts of the research were supported by a grant from the ResMed Foundation, LaJolla, CA, and ResMed Corporation, San Diego, CA. Respiratory gas exchange equipment for this research was provided by SensorMedics, Yorba Linda, CA, a Division of VIASYS Healthcare, Inc.
Research Conducted in the Laboratory for Health and Exercise Science, Department of Human Nutrition, Foods and Exercise, on the campus of Virginia Polytechnic Institute and State University, Blacksburg, VA, and the Sleep Disorders Network of Southwest Virginia, Christiansburg, VA
Footnotes
Disclosure Statement
Parts of the research were supported by a grant from the ResMed Foundation, LaJolla, CA, and ResMed Corporation, San Diego, CA. Respiratory gas exchange equipment for this research was provided by SensorMedics, Yorba Linda, CA, a Division of VIASYS Healthcare, Inc. Dr. Nickols-Richardson has consulted and participated in speaking engagements for Bayer HealthCare. Dr. Herbert is a member of the advisory board for Life Fitness. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–97. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 4.Ellen RLB, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Song-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 5.Wolk R, Somers VK. Sleep Apnoea – Hypertension: Physiological bases for a causal relation: Sleep and the metabolic syndrome. Exp Physiol. 2007;92:67–78. [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Kuniyoshi FH, Somers VK. Sleep apnea in hypertension: when, how, and why should we treat? Hypertension. 2006;47:818–9. doi: 10.1161/01.HYP.0000217130.01867.4b. [DOI] [PubMed] [Google Scholar]
- 10.Grassi G, Facchini A, Trevano FQ, et al. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46:321–5. doi: 10.1161/01.HYP.0000174243.39897.6c. [DOI] [PubMed] [Google Scholar]
- 11.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–43. doi: 10.1161/01.hyp.32.6.1039. [DOI] [PubMed] [Google Scholar]
- 13.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 7th ed. Philadelphia, PA: Lippincott Williams – Wilkins; 2005. [Google Scholar]
- 14.Myers J, Buchanan N, Walsh D, et al. Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol. 1991;17:1334–42. doi: 10.1016/s0735-1097(10)80144-5. [DOI] [PubMed] [Google Scholar]
- 15.Singh JP, Larson MG, Manolio TA, et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham Heart Study. Circulation. 1999;99:1831–6. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 16.McHam SA, Marwick TH, Pashkow FJ, Lauer MS. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34:754–9. doi: 10.1016/s0735-1097(99)00269-7. [DOI] [PubMed] [Google Scholar]
- 17.Acanfora D, De Caprio L, Cuomo S, et al. Diagnostic value of the ratio of recovery systolic blood pressure to peak exercise systolic blood pressure for the detection of coronary artery disease. Circulation. 1988;77:1306–10. doi: 10.1161/01.cir.77.6.1306. [DOI] [PubMed] [Google Scholar]
- 18.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–8. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 19.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 20.Lin CC, Hsieh WY, Chou CS, Liaw SF. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol. 2006;150:27–34. doi: 10.1016/j.resp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Vanuxem D, Badier M, Guillot C, Delpierre S, Jahjah F, Vanuxem P. Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med. 1997;91:551–7. doi: 10.1016/s0954-6111(97)90089-5. [DOI] [PubMed] [Google Scholar]
- 22.Kaleth AS, Chittenden TW, Hawkins BJ, et al. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007;8:160–8. doi: 10.1016/j.sleep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Obstructive sleep apnoea-hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–15. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- 24.Ozturk LM, Metin G, Cuhadaroglu C, Utkusavas A, Tutluoglu B. Cardiopulmonary responses to exercise in moderate-to-severe obstructive sleep apnea. Tuberk Toraks. 2005;53:10–9. [PubMed] [Google Scholar]
- 25.Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. 2006;48:342–62. doi: 10.1016/j.pcad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 27.Gould GA, Whyte KF, Rhind GB, et al. The sleep hypopnea syndrome. Am Rev Respir Dis. 1988;137:895–8. doi: 10.1164/ajrccm/137.4.895. [DOI] [PubMed] [Google Scholar]
- 28.Kamel EG, McNeill G, Van Wijk MC. Usefulness of anthropometry and DXA in predicting intra-abdominal fat in obese men and women. Obes Res. 2000;8:36–42. doi: 10.1038/oby.2000.6. [DOI] [PubMed] [Google Scholar]
- 29.Miller LE, Nickols-Richardson SM, Wootten DF, Ramp WK, Herbert WG. Relationships among bone mineral density, body composition, and isokinetic strength in young women. Calcif Tissue Int. 2004;74:229–35. doi: 10.1007/s00223-003-0060-2. [DOI] [PubMed] [Google Scholar]
- 30.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–5. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 31.Arai Y, Saul JP, Albrecht P, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256:H132–41. doi: 10.1152/ajpheart.1989.256.1.H132. [DOI] [PubMed] [Google Scholar]
- 32.Cheng YJ, Lauer MS, Earnest CP, et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003;26:2052–7. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]
- 33.Rowell LB. Human cardiovascular control. New York: Oxford University Press; 1993. [Google Scholar]
- 34.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–35. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 35.Belozeroff V, Berry RB, Khoo MC. Model-based assessment of autonomic control in obstructive sleep apnea syndrome. Sleep. 2003;26:65–73. doi: 10.1093/sleep/26.1.65. [DOI] [PubMed] [Google Scholar]
- 36.Dyer AR, Elliott P. The INTERSALT study: relations of body mass index to blood pressure. INTERSALT Co-operative Research Group. J Hum Hypertens. 1989;3:299–308. [PubMed] [Google Scholar]
- 37.Grassi G, Seravalle G, Colombo M, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–42. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 38.Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–3. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–6. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 40.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–86. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]