Abstract
Study Objectives:
To evaluate long-term efficacy and safety of zolpidem extended-release 3 to 7 nights/week for chronic primary insomnia.
Design:
Multicenter, 25-week, phase IIIb, randomized, double-blind, placebo-controlled, parallel-group.
Setting:
Outpatient; visits every 4 weeks.
Patients:
Aged 18 to 64 years; DSM-IV criteria for chronic primary insomnia; ≥3 months of difficulty initiating or maintaining sleep or experiencing nonrestorative sleep.
Interventions:
Single-dose zolpidem extended-release 12.5 mg (n = 669) or placebo (n = 349), self-administered from a minimum of 3 nights/week to a maximum of 7 nights/week.
Measurements and Results:
Patient's Global Impression (PGI) and Clinical Global Impression-Improvement (CGI-I) were assessed every 4 weeks up to week 24. Patient Morning Questionnaire (PMQ), recorded daily, assessed subjective sleep measures—sleep onset latency (SOL), total sleep time (TST), number of awakenings (NAW), wake time after sleep onset (WASO), and quality of sleep (QOS)—and next-day functioning. At week 12, PGI, Item 1 (aid to sleep), the primary endpoint, was scored as favorable (i.e., “helped me sleep”) by 89.8% of zolpidem patients vs. 51.4% of placebo patients (P < 0.0001, based on rank score) and at week 24 by 92.3% of zolpidem extended-release patients vs. 59.7% of placebo patients. Zolpidem extended-release also was statistically significantly superior to placebo at every time point for PGI (Items 1–4) and CGI-I (P < 0.0001, rank score), TST, WASO, QOS (P < 0.0001), and SOL (P ≤ 0.0014); NAW (Months 2–6; P < 0.0001). Sustained improvement (P < 0.0001, all time points) was observed in morning sleepiness and ability to concentrate (P = 0.0014, month 6) with zolpidem extended-release compared with placebo. Most frequent adverse events for zolpidem extended-release were headache, anxiety and somnolence. No rebound effect was observed during the first 3 nights of discontinuation.
Conclusions:
These findings establish the efficacy of 3 to 7 nights per week dosing of zolpidem extended-release 12.5 mg for up to 6 months. Treatment provided sustained and significant improvements in sleep onset and maintenance and also improved next-day concentration and morning sleepiness.
Citation:
Krystal AD; Erman M; Zammit GK; Soubrane C; Roth T. Long-Term Efficacy and Safety of Zolpidem Extended-Release 12.5 mg, Administered 3 to 7 Nights Per Week for 24 Weeks, in Patients With Chronic Primary Insomnia: A 6-Month, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter Study. SLEEP 2008;31(1):79-90.
Keywords: Zolpidem extended-release, randomized controlled trial, chronic insomnia, long-term, rebound, sleep maintenance
INTRODUCTION
CHRONIC INSOMNIA, DEFINED AS A COMPLAINT OF PERSISTENT DIFFICULTY FALLING ASLEEP, MAINTAINING SLEEP, AND/OR EXPERIENCING nonrestorative sleep accompanied by significant dysfunction in next-day cognitive, physical, or social functioning, affects approximately 10% of the population.1–3 This condition continues to represent an under-recognized public health burden and clinical challenge.3 One particularly challenging aspect of clinical management is the implementation of effective treatment that takes into account the chronic nature of this disorder. Patients often continue to report symptoms for many years after the disorder's onset, with approximately 45% of patients remaining symptomatic after 10 years.2,4 Correspondingly, many chronic insomnia patients take sedative-hypnotics for longer durations (∼5 years) than clinically evaluated and traditionally recommended.5–7 So far, only one placebo-controlled trial of 6 months' duration has been carried out with a medication of this type,8 although two open-label studies of 12 months' treatment have been completed.9,10 The fact that long-term use of insomnia medications is widespread and that little of the treatment research has been conducted over extended periods underscores the need for more studies of long-term pharmacotherapy of insomnia.
An important methodological point is that, in the few long-term pharmacotherapy studies that have been done, patients with insomnia received nightly medication dosing throughout the treatment period. As the duration of treatment increases, the costs and risks of adverse effects associated with taking a medication nightly become increasingly important considerations. There is good reason to believe that, for many insomnia patients, it may be possible to achieve effective treatment while decreasing risks and costs by employing non-nightly medication dosing. Studies of patient dosing behaviors have, in fact, indicated that approximately 41% of chronic insomnia patients take hypnotic medications intermittently on an “as needed” basis, as opposed to consistent, nightly administration.11 A potential explanation for this dosing behavior is the waxing and waning severity of insomnia12–15 and the unpredictable variability in the presentation of specific symptoms over time (e.g., difficulties with sleep onset, sleep maintenance, or both).16 Thus, utilizing a dosing strategy that allows for some degree of patient choice may better match the pattern of sleep difficulties in many insomnia patients and reduce the incidence of their taking medications when not needed.17
The efficacy and safety of optional dosing of a hypnotic within a prespecified range has thus far only been demonstrated for zolpidem tartrate, a short-acting nonbenzodiazepine agent indicated for the short-term treatment of insomnia,18 in clinical trials of 8 and 12 weeks' duration.19,20 Similar to other short-acting nonbenzodiazepine hypnotics (e.g., zaleplon, eszopiclone), zolpidem selectively binds to the alpha-1 subunit subtype of the gamma-aminobutyric acid receptor.21 Characterized by a relatively short half-life (2.5 h), zolpidem is effective at reducing the latency time to persistent sleep (LPS) and at increasing total sleep time (TST), but it is not consistently efficacious for the treatment of sleep maintenance symptoms,22–24 which occur in 60% to 73% of insomnia patients.22,25,26
Zolpidem tartrate extended-release 12.5 mg, developed to extend the duration of action of the original zolpidem formulation, is a dual-layered tablet that provides a biphasic release of zolpidem: an initial release of drug to facilitate sleep onset, and a delayed release to benefit the maintenance of sleep throughout the middle of the night.27,28 While zolpidem extended-release 12.5 mg and the original zolpidem 10 mg formulation share similar rapid onsets of peak plasma concentrations (Tmax: 1.5 h vs. 0.88 h, respectively) and elimination half-lives (T1/2: 2.8 ho vs. 2.6 h, respectively), zolpidem extended-release exhibits higher, prolonged plasma concentrations than original zolpidem, beyond 3 h postdose.28
The efficacy and safety of zolpidem extended release 12.5 mg has been evaluated only in a short-term trial.29 This was a placebo-controlled study involving adult patients with primary insomnia who received nightly administration of zolpidem. Polysomnographic evaluation showed that, in comparison with placebo, treatment with zolpidem extended-release 12.5 mg significantly improved LPS, a measure of sleep onset, and it significantly reduced the duration of wake time after sleep onset (WASO) and the number of awakenings (NAW), both measures of sleep maintenance, on the first 2 nights of treatment and after 2 weeks of treatment.
The present study expands upon this finding by examining the long-term efficacy and safety of zolpidem extended-release 12.5 mg, self-administered from a minimum of 3 to a maximum of 7 nights per week for 24 weeks, in adults with chronic primary insomnia who exhibit difficulties with both sleep onset and sleep maintenance.
METHODS
Study Objectives
The primary objective of the study was to evaluate the efficacy of 6 months of treatment with zolpidem extended-release 12.5 mg, as compared with placebo, when taken 3 to 7 nights per week by patients with chronic primary insomnia. Additional objectives included evaluation of the safety and tolerability of zolpidem extended-release 12.5 mg, compared with placebo, when taken for a long-term period of time, and evaluation of the potential for rebound effects after abrupt discontinuation. Next-day functioning, including the ability to concentrate and level of sleepiness in the morning, was also subjectively assessed throughout the treatment period.
Study Design
This was a national (United States), multicenter, phase IIIb, randomized, double-blind, placebo-controlled, two-parallel-group study in adult patients with chronic primary insomnia. The 26-week study was divided into the following three phases: (1) a run-in period of 7 days (±3 days), comprising screening and baseline determination; (2) a post-randomization treatment period of 24 weeks (±3 days), in which patients received study medication; and (3) a run-out period, with no medication for 7 days (±3 days), to assess rebound effects after abrupt discontinuation of study medication. During the treatment period, patients received either zolpidem extended-release 12.5 mg tablets or identical placebo tablets. Patients were instructed to take the medication on those nights when they judged it to be necessary, with the caveat that they were required to self-administer study medication at least 3 nights per week. Patients were given sleep hygiene instructions, which included a directive to refrain from alcohol. Patients were scheduled to report to their study center every 4 weeks for clinical evaluation; those who completed the trial were evaluated at a total of 9 study center visits (Figure 1). Each patient signed an informed consent form before the conduct of any study-related procedure. The appropriate Institutional Review Board at each investigative site approved the protocol.
Figure 1.
Study design.
Patient Selection and Screening
Eligible study participants included male and female patients, 18 to 64 years of age, who met criteria for chronic primary insomnia from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).1 These criteria included a history of at least 3 months (before screening) of difficulty falling asleep, difficulty maintaining sleep, or experiencing nonrestorative sleep, with reports of clinically significant impairment in social, occupational, or other important areas of functioning. Women of childbearing potential were required to have a negative serum pregnancy test at the screening visit and to agree to use an acceptable form of contraception throughout the study. In addition, patients were required to have experienced ≥1 h of wakefulness for at least 4 nights per week over the past month and to have spent >6.5 h but <8.5 h per night in bed trying to sleep over the past 2 weeks. During the run-in period, when patients completed a daily questionnaire, the data were required to confirm a mean TST of >3 h but <6.5 h per night and a mean WASO of ≥40 min.
Patients were excluded if they were shift workers or if they napped more than 3 times per week. Also excluded were patients who consumed more than 5 xanthine-containing beverages per day, as well as patients who had been using over-the-counter sleep remedies or prescription sleep medications within 2 weeks or 5 half-lives (whichever was longer) before screening. Use of any substance associated with effects on sleep/wake function within 1 week or 5 half-lives (whichever was longer) before screening was not permitted. In addition, patients were ineligible if they had primary hypersomnia, narcolepsy, breathing-related sleep disorders, circadian-rhythm sleep disorders, parasomnia, or dyssomnia not otherwise specified (i.e., periodic leg movement disorders). Patients who had a current severe neuropsychiatric disorder (i.e., psychosis, obsessive compulsive disorder, major depression, anxiety disorders, panic disorders, dementia of Alzheimer or vascular type) according to DSM-IV criteria, a history of substance abuse or dependence (including alcohol) within the past year, myasthenia gravis, severe respiratory insufficiency, hepatic insufficiency, any unstable medical condition, or sensitivity to zolpidem or its excipients were not entered into the study. Women who were lactating or pregnant were also excluded, as were any patients who had participated in another clinical trial within the 2 months prior to screening.
Treatment and Compliance
After successful completion of the screening visit and run-in period, eligible patients were randomly assigned to receive either zolpidem extended-release 12.5 mg or placebo in a 2:1 ratio, according to a randomization schedule supplied by Sanofi-Aventis. Placebo tablets were the same size, shape, color, and taste as zolpidem extended-release tablets and were therefore indistinguishable from active treatment. Patients were instructed to take no more than 1 tablet per night of study medication immediately before bedtime with a glass of water, for a minimum of 3 tablets and a maximum of 7 tablets per week. The 3-tablet minimum was judged to be necessary to produce a minimum drug-treatment effect in this chronically ill population. During week 25, the run-out period, patients took no medication.
Patient kits contained blister packages of the study medication sufficient for 4 weeks of treatment. At each visit, patients returned the packet from the previous 4 weeks, and the study staff recorded the number of remaining tablets along with the dates on which the patient omitted dosing, as noted on the questionnaire that patients completed each morning. The investigator reviewed the patient questionnaire at each study visit to determine drug compliance and the recording of daily sleep parameters. Patients were considered compliant with treatment if they took between 3 and 7 tablets per week. Patients could withdraw from the study at any time and for any reason. Study staff recorded the reasons for withdrawal or early discontinuation and made every effort to have the patients complete an early termination visit.
Assessments
Patient's Global Impression (PGI)
The PGI is a 4-item, subjective, patient self-report that assesses treatment aid to sleep (Item 1), treatment benefit to sleep induction (Item 2), treatment benefit to sleep duration (Item 3), and appropriateness of study medication strength (Item 4). Each item, presented to patients as a survey document for them to complete, consists of a 3-point categorical scale, with a recorded score of 1 representing a treatment benefit/advantage on items 1 to 3 (“too strong” on item 4), a rank score of 2 representing no effect/change on items 1 to 3 (“just right” on item 4), and a rank score of 3 representing a worsening/disadvantage on items 1 to 3 (“too weak” on item 4). Each patient completed the PGI at every 4-week study center visit during the 24-week treatment period and at the final visit at week 25 during the run-out period. PGI assessments at each visit were based on the patient's global perception of the effects of treatment on sleep during that treatment period (including nights with and without dosing), as compared to their sleep prior to entering the study.
Clinical Global Impression (CGI)
The CGI is a clinician-rated scale composed of two subscales that measure disease severity and degree of improvement, respectively. CGI-Severity (CGI-S) is a single-item, global scale of disease severity that requires the investigator to compare the patient's symptoms with those of all other patients who have the disorder. It is scored from 1 (normal) to 7 (among the most extremely ill). CGI-S was assessed at the baseline visit. CGI-Improvement (CGI-I) is a single-item scale of symptomatic improvement or worsening that requires the investigator to compare the patient's status at the time of assessment with baseline severity (baseline CGI-S). CGI-I is scored from 1 (very much improved) to 7 (very much worsened). CGI-I was assessed at each 4-week study center visit during the 24-week treatment period and at the final visit at week 25 during the run-out period.
Patient's Morning Questionnaire (PMQ)
Patients completed the PMQ each morning upon awakening and recorded their responses via the interactive voice response system (or Internet). The PMQ asks the respondent whether he or she took the study medication the previous evening and assesses the following subjective sleep measures: duration of sleep onset latency (SOL), TST, WASO, NAW, and quality of sleep (QOS). QOS was measured using a 4-point categorical scale scored as excellent (1), good (2), fair (3), and poor (4). In addition, each respondent assessed the level of next-day functioning by rating the level of morning sleepiness using numerical values from 0 (very sleepy) to 10 (not at all sleepy) and by rating ability to concentrate using a 4-point categorical scale scored as excellent (1), good (2), fair (3), and poor (4). The PMQ was completed daily beginning after the screening visit and continuing through to study termination. Data from this questionnaire were used in various assessments: patient eligibility during the run-in period, the effects of treatment on secondary efficacy variables during the 24-week treatment period, the frequency of self-administration of study medication during the 24-week treatment period, and the occurrence of rebound insomnia during the run-out period (for TST and WASO only).
Epworth Sleepiness Scale (ESS)
The ESS was used to determine daytime sleepiness and was completed by each patient at each 4-week study center visit during the 24-week treatment period and at the final visit at week 25 of the run-out period. The scale consisted of 8 items assessing the degree of sleepiness during the performance of everyday activities, each item being scored from 0 (would never fall asleep) to 3 (high chance of falling asleep).
Efficacy and Safety Analyses
Primary Efficacy Variable
The primary efficacy variable was the score on the PGI Item 1, treatment aid to sleep, assessed at week 12 of the treatment period in the intent-to-treat (ITT) population. The ITT population consisted of all randomized patients who had received at least one dose of study medication and had undergone at least one post-baseline efficacy assessment. The PGI Item 1 included three ordered categories related to the patient's impression that treatment (1) helped me sleep, (2) did not affect my sleep, or (3) worsened my sleep.
Secondary Efficacy Variables
The main secondary efficacy variables were the scores on the following additional assessments performed every fourth week during the treatment period: CGI-I; PGI Items 1, 2, 3, and 4; and the following assessment as summarized for each month of the study treatment period: PMQ parameters TST, WASO, SOL, QOS, and NAW in the ITT population. Tablet-taking behavior was analyzed over each 4-week period of treatment. Measures of daytime ability to function and sleepiness in the morning, provided daily as part of the PMQ, were analyzed on a monthly basis.
Safety Assessments
Safety was assessed by physical examination during screening and at the last visit, by measurement of vital signs (heart rate, supine and standing systolic and diastolic blood pressure) during each visit, and by documentation of spontaneously reported or observed adverse events (AEs) throughout the study.
Rebound Effect
A rebound insomnia effect was defined as a worsening of sleep from baseline values. Rebound effect was assessed by patients' TST and WASO scores as recorded in the patient's daily questionnaire during the run-out period.
Statistical Analyses
A sample size of 1000 patients was required in a ratio of 2:1 (zolpidem extended-release 12.5 mg to placebo) to provide ≥99% power to detect a difference of 0.6 (standard deviation [SD], 0.5) between zolpidem extended-release 12.5 mg and placebo in scores on the primary endpoint, PGI Item 1 (P ≤ 0.05, 2-sided test). This sample size was also powered sufficiently to detect a difference between groups in scores on the secondary endpoints (WASO and TST) at month 3, taking into account a dropout rate of 20% at month 3 (P ≤0.05, 2-sided test).
Efficacy assessments were analyzed in the ITT population. Safety assessments were analyzed in the population of randomized patients who had received at least one dose of study medication.
In the event of missing assessments of the primary endpoint at week 12, data from the week 16 assessments were used, and for missing assessments of the main secondary PMQ endpoints at month 3, data from the month 4 assessments were used. If assessments were not available for week 12 and week 16, then week 8 values were used, and for missing assessments for month 3 and month 4, month 2 values were used. No other replacements were performed.
Unless otherwise noted, all statistical analyses were performed at the 0.05 level of significance using 2-sided tests. For PGI Items 1 through 3 and the CGI, the Cochran-Mantel-Haenszel test with rank scores was used. For PGI Item 4, a chi-square test (on proportion favorable ratings) was used. These variables were also described using mean values ± SD, as well as the percentage of patients within each of the categorical responses. On the PMQ, for all measurements, the change from baseline was averaged over each month (each 4-week period) during the treatment period up to week 24. The change from baseline for each PMQ measurement and ESS total score was assessed using analysis of covariance (ANCOVA), with treatment group as a fixed factor and the baseline value centered on the grand mean for each group as covariate. From this model, the least-square (LS) mean and the LS mean difference between zolpidem extended-release and placebo was determined; pairwise comparisons between groups were made at the 5% significance level and 95% confidence intervals (of the least means adjusted difference).
Analysis for a rebound effect was conducted on data from patients who took at least one tablet on the last night of the treatment period. During each of the first 3 days of discontinuation, data for the TST and WASO mean change from baseline were analyzed by the same ANCOVA as previously described. Rebound was also assessed for patients who permanently discontinued the study during the treatment period (i.e., before the run-out period) and who provided data for at least 1 actual month and completed a study visit at least 1 night after permanent treatment discontinuation.
RESULTS
Patients
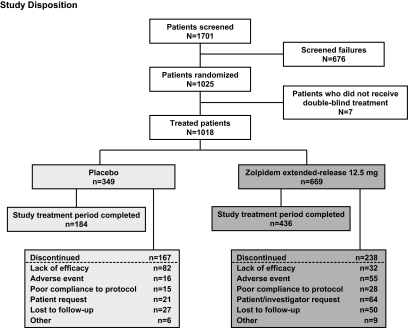
Of the 1701 patients screened, 1025 were randomized into the study and 1018 received at least one dose of study medication (n = 669 for zolpidem extended-release 12.5 mg and n = 349 for placebo) (Figure 2). There were no post-baseline data for 2 randomized patients in the zolpidem extended-release group, and thus the ITT population totaled 1016 patients.
Figure 2.
Summary of inclusion disposition: screened population.
In the zolpidem extended-release group, 436 patients (64.7%) completed the study treatment period, with “patient's request” being the most frequently cited reason for discontinuation (63 [9.3%] patients). In the placebo group, 184 patients (52.4%) completed the study treatment period, with “lack of efficacy/disease progression” cited as the most frequent reason for discontinuation (82 [23.4%] patients) within this group (Figure 2).
Demographic characteristics of the 2 treatment groups were similar (Table 1). The patients ranged in age from 18 to 64 years, and the majority were white and female. Mean weight of all patients was 80.3 (± 19.6) kg. Baseline disease characteristics were comparable between treatment groups. Overall, 34.2% of the patients (348) had had insomnia for ≥10 years, 25.6% (261) for 5 to 10 years, 36.0% (366) for 2 to 5 years, and 4.1% (42) for ≤1 year. Mean baseline CGI-S score was 4.21 (range, 1 to 7) for both groups. Baseline insomnia characteristics recorded from the PMQ during the run-in period were similar between groups (Table 2).
Table 1.
Demographic Characteristics at Baseline: Treated Population
| Zolpidem Extended-Release 12.5 mg | Placebo | Overall | ||
|---|---|---|---|---|
| (n = 669) | (n = 349) | (n = 1018) | ||
| Gender, n (%) | Male | 256 (38.3) | 139 (39.8) | 395 (38.8) |
| Female | 413 (61.7) | 210 (60.2) | 623 (61.2) | |
| Age, y, mean (SD) | Total | 46.0 (10.8) | 45.0 (11.5) | 45.7 (11.0) |
| Male | 45.9 (10.6) | 45.4 (11.4) | 45.7 (10.9) | |
| Female | 46.1 (10.9) | 44.7 (11.6) | 45.6 (11.2) | |
| Body mass index, kg/m2, mean (SD) | Total | 28.2 (6.1) | 28.0 (5.6) | 28.1 (6.0) |
| Male | 28.5 (5.2) | 28.0 (4.8) | 28.4 (5.1) | |
| Female | 28.0 (6.6) | 28.0 (6.1) | 28.0 (6.5) | |
| Race, n (%), mean (SD) | Asian/Oriental | 10 (1.5) | 4 (1.1) | 14 (1.4) |
| Black | 120 (17.9) | 63 (18.1) | 183 (18.0) | |
| White | 432 (64.6) | 230 (65.9) | 662 (65.0) | |
| Other | 107 (16.0) | 52 (14.9) | 159 (15.6) |
SD = standard deviation
Table 2.
Summary of PMQ Findings at Baseline: Treated Population
| Mean (SD) |
||
|---|---|---|
| Zolpidem Extended-Release 12.5 mg (n = 669) | Placebo (n = 349) | |
| TST, min | 295.9 (48.7) | 292.8 (47.3) |
| WASO, min | 99.6 (48.3) | 102.5 (51.2) |
| SOL, min | 74.6 (47.1) | 78.0 (49.2) |
| QOS | 3.21 (0.43) | 3.24 (0.43) |
| NAW, n | 3.2 (7.1) | 3.0 (4.9) |
NAW = nocturnal awakenings; PMQ = Patient's Morning Questionnaire; QOS = quality of sleep; SD = standard deviation; SOL = sleep onset latency; TST = total sleep time; WASO = wake after sleep onset.
Treatment Efficacy: Primary Efficacy Variable (Week 12)
Scores on PGI Item 1 at Week 12 in the zolpidem extended-release group were statistically significantly superior to those in the placebo group, based on rank scores (P < 0.0001). In the zolpidem extended-release group, 89.8% of patients considered that the medication helped them sleep, versus 51.4% in the placebo group.
Treatment Efficacy: Secondary Efficacy Variables (Over 6 Months)
PGI
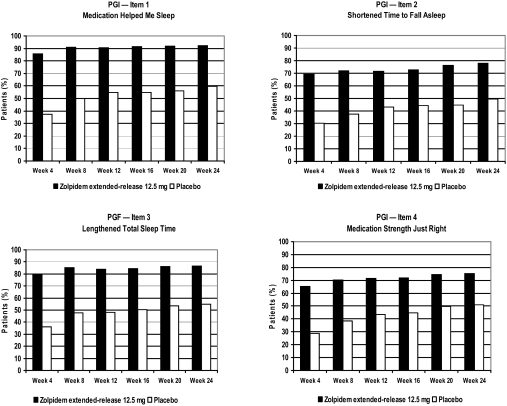
The percentage of patients who reported a treatment benefit on the PGI was higher in the zolpidem extended-release group than in the placebo group for all four PGI items at the conclusion of the first 4-week treatment period, and this difference was sustained throughout the entire study. Based on analysis of rank scores for Items 1, 2, and 3 and the percentage of favorable responses for Item 4, significantly superior improvement was observed in the zolpidem extended-release group versus placebo at each 4-week interval of the 24-week treatment period (P < 0.0001, for each measurement) (Figure 3).
Figure 3.
Improvements in PGI scores for Items 1, 2, 3, and 4 over 6 months of treatment. Scores in the zolpidem extended-release group were higher than those in the placebo group at all 4-week intervals. Improvements in the zolpidem extended-release group were significantly superior at all time points (P < 0.001) based on rank scores for PGI Items 1, 2, and 3 (data not shown) and the percentages displayed for PGI Item 4 (chi square test). PGI = Patient's Global Impression.
CGI-I
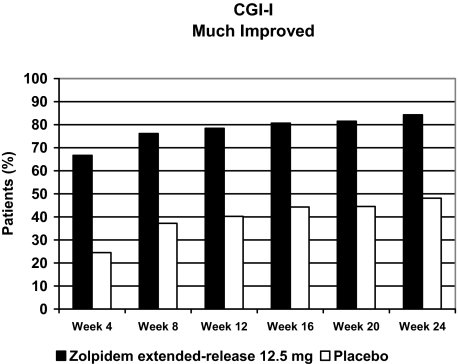
The percentage of patients who obtained a positive evaluation on the CGI-I scale (i.e., “much improved” or “very much improved”) was greater in the zolpidem extended-release group than in the placebo group at the conclusion of the first 4-week treatment period, and this difference was sustained throughout the entire study. Based on rank scores, significantly superior improvement was observed in the zolpidem extended-release group compared with the placebo group at all 4-week intervals of the 24-week treatment period (P < 0.0001 at each 4-week time point) (Figure 4).
Figure 4.
Improvements in CGI scores (“much improved” or “very much improved”) over 6 months of treatment. Based on rank scores (data not shown), the improvement in insomnia symptoms was significantly greater in the zolpidem extended-release group than in the placebo group at all 4-week intervals of the 24-week treatment period (P < 0.001). CGI = Clinical Global Impression.
PMQ
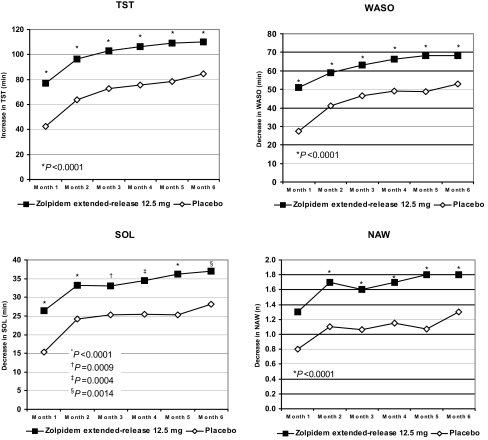
At every time point, results on the PMQ for patients in the zolpidem extended-release group were significantly superior to those for patients in the placebo group for the TST (P < 0.0001), WASO (P < 0.0001), and SOL (P ≤ 0.0014) (Figure 5). The same was true for QOS (P < 0.0001) (Table 3) and for NAW at all but the first month (month 1, P = 0.0515; months 2 to 6, P < 0.0001) (Figure 5). The magnitude of these improvements generally increased over the 24- week treatment period.
Figure 5.
Change from baseline in PMQ over 6 months of treatment. For each of the 6 treatment months, patients who received zolpidem extended-release reported significantly greater improvement in TST (P < 0.0001), WASO (P < 0.0001), and SOL (P ≤ 0.0014) than did patients who received placebo. Improvement in NAW was also significantly greater at months 2 to 6 in the zolpidem extended-release patients (P < 0.0001). NAW = number of nocturnal awakenings; SOL = sleep onset latency; TST = total sleep time; WASO = wake after sleep onset.
Table 3.
Changes in PMQ Scores for QOS Over 6 Months of Treatment
| LS Mean Change From Baseline |
|||
|---|---|---|---|
| Placebo (n = 349) | Zolpidem Extended-Release12.5 mg (n = 667) | P | |
| Month 1 | −0.45 | −0.81 | <0.0001 |
| Month 2 | −0.60 | −0.94 | <0.0001 |
| Month 3 | −0.69 | −0.96 | <0.0001 |
| Month 4 | −0.70 | −1.00 | <0.0001 |
| Month 5 | −0.72 | −1.03 | <0.0001 |
| Month 6 | −0.80 | −1.04 | <0.0001 |
LS = least squares; PMQ = Patient's Morning Questionnaire; QOS = quality of sleep.
QOS was rated as excellent (1), good (2), fair (3), or poor (4). The increasing negative values indicate increasing improvement from baseline.
Next-Day Functioning
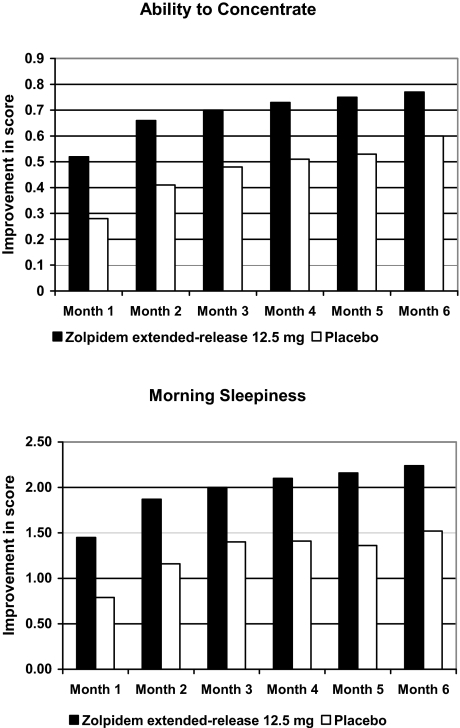
Patients in the zolpidem extended-release group, as compared with those in the placebo group, demonstrated significant and sustained improvements in their ability to concentrate in the morning at each month throughout the treatment period (months 1 to 5, P < 0.0001; month 6, P = 0.0014) (Figure 6). Patients in the zolpidem extended-release group also had sustained reductions in their level of sleepiness in the morning that were significantly greater than those in the placebo group at each month throughout the treatment period (months 1 to 6, P < 0.0001) (Figure 6).
Figure 6.
Improvements from baseline in PMQ scores for ability to concentrate and morning sleepiness. For each of the 6 months of treatment, patients who received zolpidem extended-release reported significantly greater improvement than did patients who received placebo in the ability to concentrate (months 1 to 5, P < 0.0001; month 6, P = 0.0014) and in morning sleepiness (months 1 to 6, P < 0.0001).
At baseline, both groups had a mean baseline ESS of 7.5, which is within the normal range (<10). ESS was overall statistically significantly lower in the zolpidem extended-release group than the placebo group during the double-blind treatment period. Univariate analyses indicated that the groups were statistically significantly different at all time points except month 6 and month 1 (trend for significance) (Table 4).
Table 4.
Change in ESS Total Scores over 6 Months
| LS Mean Change From Baseline |
|||
|---|---|---|---|
| Placebo (n = 349) | Zolpidem Extended-Release 12.5 mg n = 667) | P | |
| Month 1 | −1.1 | −1.5 | 0.0943 |
| Month 2 | −1.2 | −2.0 | 0.0004 |
| Month 3 | −1.3 | −2.3 | 0.0002 |
| Month 4 | −1.5 | −2.4 | 0.0007 |
| Month 5 | −1.8 | −2.5 | 0.0167 |
| Month 6 | −2.0 | −2.3 | 0.3137 |
ESS = Epworth Sleepiness Scale; LS = least squares.
ESS maximum score = 24; minimum = 0.
The increasing negative values indicate increasing improvement from baseline.
Drug-Taking Behavior
Over the 6-month period, the mean number of nights per month that tablets were reported as taken was stable in each treatment group. The mean value was slightly higher for patients in the zolpidem extended-release group than for those in the placebo group (Table 5). Of note, each patient's average tablet intake per month was based on a minimum required dosing of 3 tablets per week, with the option to take up to a maximum of 7 tablets per week.
Table 5.
Summary of Days with Study Medication: Treated Population*
| Placebo (n = 349) | Zolpidem Extended-Release 12.5 mg (n = 669) | |
|---|---|---|
| Overall | ||
| N | 349 | 667 |
| Mean (SD) | 86.3 (57.0) | 111.2 (55.2) |
| Median | 95.0 | 126.0 |
| Month 1 | ||
| N | 349 | 667 |
| Mean (SD) | 16.5 (7.4) | 18.9 (6.3) |
| Median | 17.0 | 20.0 |
| Month 2 | ||
| N | 277 | 589 |
| Mean (SD) | 17.0 (7.5) | 19.4 (6.6) |
| Median | 17.0 | 21.0 |
| Month 3 | ||
| N | 234 | 538 |
| Mean (SD) | 17.7 (6.9) | 19.5 (6.3) |
| Median | 18.0 | 20.0 |
| Month 4 | ||
| N | 212 | 498 |
| Mean (SD) | 17.8 (6.1) | 19.6 (6.2) |
| Median | 18.0 | 21.0 |
| Month 5 | ||
| N | 204 | 467 |
| Mean (SD) | 17.5 (6.6) | 20.1 (5.8) |
| Median | 17.0 | 21.0 |
| Month 6 | ||
| N | 188 | 448 |
| Mean (SD) | 17.9 (6.3) | 19.6 (6.2) |
| Median | 18.0 | 20.0 |
SD = standard deviation.
Study protocol required patients to take a minimum of 3 doses per week
Safety
Of the 1018 patients who received at least one dose of study drug, treatment-emergent AEs were reported by 423 patients (63.2%) in the zolpidem extended-release group and 179 patients (51.3%) in the placebo group. The majority of AEs were mild or moderate in severity. The most common AEs occurring at a slightly higher frequency in the zolpidem extended-release group than in the placebo group were headache, anxiety, somnolence, dizziness, fatigue, disturbance in attention, irritability, nausea, and sinusitis (Table 6). These AEs were similar in nature to those reported in previous studies and to the known safety profile of zolpidem extended-release. Overall, 57 patients (8.5%) in the zolpidem extended-release group and 16 patients (4.6%) in the placebo group discontinued treatment because of AEs. The AEs most commonly leading to discontinuation in the zolpidem extended-release and placebo groups were psychiatric disorders (3.4% versus 1.1%, respectively), nervous system disorders (3.0% versus 2.0%), and general disorders (1.6% versus 0.3%).
Table 6.
Summary of Treatment-Emergent AEs Occurring in >3% of Patients in the Zolpidem Extended-Release Group
| n (%) |
||
|---|---|---|
| Placebo (n = 349) | Zolpidem Extended-Release 12.5 mg (n = 669) | |
| Headache | 33 (9.5) | 70 (10.5) |
| Anxiety | 9 (2.6) | 42 (6.3) |
| Somnolence | 7 (2.0) | 38 (5.7) |
| Dizziness | 7 (2.0) | 32 (4.8) |
| Fatigue | 11 (3.2) | 30 (4.5) |
| Disturbance in attention | 6 (1.7) | 29 (4.3) |
| Irritability | 10 (2.9) | 25 (3.7) |
| Nausea | 8 (2.3) | 23 (3.4) |
| Sinusitis | 3 (0.9) | 22 (3.3) |
AE = adverse event.
A total of 30 serious AEs occurred in 25 patients (in 2.8% of zolpidem extended-release patients and in 1.7% of placebo patients), but none of the serious AEs were considered by the investigator to be related to the study medication.
Rebound Insomnia
Potential rebound insomnia was assessed by examining the mean change in TST and WASO scores from baseline on each of the first 3 nights after abrupt discontinuation of study medication during the run-out period. In neither the zolpidem extended-release group nor the placebo group was any worsening from baseline (i.e., no rebound insomnia) observed in TST and WASO measurements on any of the first 3 discontinuation nights. On night 1 of discontinuation, WASO and TST values were statistically significantly better for the placebo group than for the zolpidem extended-release group (WASO, P <0.001; TST, P <0.0001). However, no differences between these groups were observed on nights 2 and 3. In both groups, on nights 2 and 3, TST improved by 42 to 55 min, and WASO improved by 31 to 38 min, as compared with baseline (Table 7).
Table 7.
Evaluation of Rebound Effect: The First 3 Nights Following Drug Discontinuation, Compared with Baseline
| Increase in TST (min) |
Decrease in WASO (min) |
|||||
|---|---|---|---|---|---|---|
| Zolpidem extended-release 12.5 mg | Placebo | P | Zolpidem extended-release 12.5 mg | Placebo | P | |
| Night 1 n = 416, 193 | 17.7 (4.7) |
55.8 (6.9) |
<0.0001 | −21.1 (3.6) |
−42.2 (5.3) |
0.0010 |
| Night 2 n = 404, 178 | 44.5 (4.3) |
54.4 (6.6) |
0.2106 | −31.4 (3.4) |
−36.9 (5.0) |
0.3648 |
| Night 3 n = 170, 380 | 42.9 (4.5) |
49.8 (6.7) |
0.3969 | −35.9 (3.0) |
−38.3 (4.5) |
0.6543 |
All values are LS mean change from baseline (SE).
n's are for zolpidem extended-release and placebo, respectively.
LS = least square; SE = standard error; TST = total sleep time; WASO = wake time after sleep onset.
DISCUSSION
This study assessed the efficacy and safety of zolpidem extended-release 12.5 mg, as compared with placebo, taken 3 to 7 nights per week over a 24-week period in patients with chronic primary insomnia. A review of the current literature suggests that this is the longest placebo-controlled study completed thus far of intermittent dosing of an insomnia agent. Results of this study demonstrate that patients with chronic insomnia, manifesting sleep onset and/or sleep maintenance difficulties, can be treated safely and effectively with zolpidem extended-release 12.5 mg self-administered on a 3 to 7 nights per week basis for 6 months.
Non-nightly dosing may offer many advantages over nightly dosing, including decreased drug exposure, increased patient control over therapy, and reduced medication expense. It is consistent with the prevailing prescribing recommendations for medications in this class and is also the preferred dosing pattern among many patients.11,17 In two prior randomized, placebo-controlled short-term studies of zolpidem in the treatment of primary insomnia, in which patient eligibility was defined similarly to the criteria used in the present study, a 3 to 5 nights per week dosing protocol (dosing on at least 3 nights but no more than 5 nights per week) was utilized.19,20 Consistent with the present study, both trials showed a statistically significantly greater benefit in the zolpidem group than in the placebo group on patient global ratings of improvement, with no reduction in clinical efficacy over the course of the study and no evidence of rebound insomnia upon drug discontinuation.
Results of the present study confirm the sleep maintenance efficacy of zolpidem extended-release 12.5 mg observed in the previous 3-week study and extend the duration of observed efficacy to 6 months.20,29 As in the prior study, significant efficacy in measures of sleep maintenance, WASO and NAW, were observed in addition to the significant SOL and TST effects noted. With respect to QOS, the largest benefit obtained in the placebo group (0.80, month 6) was less than the smallest benefit obtained with zolpidem extended-release 12.5 mg (0.81, month 1). Furthermore, the absolute magnitude of the benefits obtained with zolpidem extended-release 12.5 mg increased slightly but steadily from the first measurement period to the last, strongly suggesting the absence of tolerance to drug effect.
The primary efficacy parameter, aid to sleep (PGI Item 1), was chosen to capture patients' overall impressions of the medication's ability to improve sleep. Zolpidem extended-release 12.5 mg was highly superior to placebo on this measure at the week 12 end point, with 89.7% of zolpidem extended-release patients reporting that the medication helped them sleep, compared with 51.4% of placebo patients. One of the main concerns with developing a longer-acting formulation of a sedative or hypnotic drug is the potential for continuing drug effects after the patient awakens in the morning. Therefore, it is encouraging to note that patient-reported daily measures of morning sleepiness and ability to concentrate were significantly better in the zolpidem extended-release group than in the placebo group for each 4-week interval throughout the entire study. This is consistent with the pharmacokinetic profile of zolpidem extended-release, which provides higher plasma concentrations beyond 3 h, compared to original zolpidem 10 mg, yet retains a similar elimination half-life, thereby presenting low plasma concentrations at 8 h post-dose.28 These data are consistent with results from the 3-week zolpidem extended-release study, which included polysomnographic data showing no statistically significant drug effects during hours 7 to 8 following drug ingestion29 and no difference in subjective measures of attention or sleepiness upon awakening.29 They are also consistent with the findings of two short-term, double-blind, active (flurazepam 30 mg)- and placebo-controlled, similarly designed trials of zolpidem extended-release in healthy adults30 and elderly volunteers31 (N=18 and N=24, respectively), utilizing five neuropsychological tests to assess the potential impact on cognitive functioning at 8 h post-dose, in which no differences from placebo were found on any of these measures. The present study, however, not only confirms the absence of next-day residual drug effects but also shows an improvement in subjective level of attention and alertness compared to placebo, as demonstrated by statistically significant differences from placebo on the PMQ items “ability to concentrate” and “morning sleepiness” that began at month 1 and were sustained throughout the study.
Scores on the ESS were significantly improved with zolpidem extended-release versus placebo in months 2 to 5, and there was a trend for this effect in month 1. As far as we are aware, this is the first double-blind, placebo-controlled trial demonstrating significant efficacy of an insomnia medication on this measure. This finding lends further support to the conclusion that zolpidem extended-release treatment improved subjective daytime sleepiness in this study. This result is intriguing, however, given that ESS measures were not elevated in this population at baseline and are generally not elevated in adult insomnia patients.32 It is also interesting to note that this effect was not significant at 4 weeks but became so by 8 weeks, suggesting that improvement in ESS may require a longer period of hypnotic pharmacotherapy to be realized.
Rebound insomnia—defined as an acute worsening of insomnia symptoms upon discontinuation of medication to a level of severity greater than baseline—was not observed in this study. In the 3-day washout period, TST and WASO scores remained improved over baseline levels in both subject groups. This result occurred despite the fact that rebound was assessed only in the subset of subjects who had taken study medication on the last night of the double-blind phase, in order to decrease the likelihood that a rebound effect might have been missed by a drug-free period before the run-out phase. However, it is notable that on the first night of the discontinuation phase, there appeared to be a worsening of TST and WASO compared with the second and third nights in those receiving zolpidem extended-release tablets.
The mean number of days that pills were taken was stable over the 6-month period in both treatment groups. This lack of increase over time in the number of pill-taking nights strongly supports the intermittent dosing model examined in this trial. Neither treatment group attained a mean or median frequency greater than 21 pills per month, or roughly 5 pills per week. Furthermore, given that the study protocol required a minimum of 3 tablets per week, or 12 tablets per month, patients in the zolpidem extended-release group used medication on average an additional 8 to 9 nights of the remaining 16 nights per month that they could have elected to use medication (Table 5). This pill-taking behavior reinforces the notion that patients used active medication, which they judged to be effective, in a selective fashion and did not become increasingly dependent on it over this 6-month period of open access. This lack of escalation in frequency of dosing is also highly suggestive that there would be no escalation over time in the dose itself, although the study design limiting intake to one pill per night precludes drawing any definitive conclusions on this issue. Nonetheless, the fact of sustained therapeutic effect throughout the trial also indicates that dose escalation would be unlikely.
It is important to note that this study did not employ a purely “as needed” dosing regimen in which patients would be given complete autonomy to determine whether or not to take a double-blinded dose of either zolpidem extended-release or placebo on any given night. Rather, patients were required to take a dose of study medication at least 3 nights per week. The choice of dosing regimen employed reflects an attempt to balance the desire to study “as needed” dosing with the statistical imperative to have adequate power to compare zolpidem extended-release to placebo. This minimum dosing requirement is an important caveat when considering the frequency of self-administration by patients in this study. It is likely that some patients, during one or more weeks of the study, may have taken one or more doses of study medication solely to adhere to the study protocol and not because of perceived need. To date, however, no studies have been carried out comparing purely “as needed” dosing with the regimen employed in this study. As a result, further studies will be needed to determine how full patient autonomy regarding dosing would affect efficacy, safety, and frequency of dosing outcomes in a long-term study of zolpidem extended-release, or another hypnotic medication.
Zolpidem extended-release 12.5 mg was generally well tolerated throughout the study. The most frequent AEs experienced in the zolpidem extended-release group, all of which occurred at rates higher than in the placebo group, were central nervous system–related AEs. However, these events were not unexpected, as they are consistent with the pharmacologic effects and known safety and tolerability profile of zolpidem.27 Because of this, the rate of withdrawal due to AEs was higher in the zolpidem extended-release group than in the placebo group (8.5% versus 4.6%), but the rates of serious AEs were similar and low in both groups. Overall, a higher percentage of patients withdrew from the placebo group (47.6%) than from the zolpidem extended-release group (35.3%), but this was due primarily to the much higher rate of study withdrawal resulting from a lack of efficacy in the placebo group.
The only other published 6-month, randomized, placebo-controlled trial of a sedative or hypnotic agent in the treatment of patients with primary chronic insomnia was a study evaluating the efficacy and safety of daily eszopiclone 3 mg.8 This trial, like the present one, demonstrated efficacy for the treatment of primary insomnia. This study differed from the present one, however, in at least one important way: fixed nightly dosing was used in the eszopiclone trial. Since sleep parameters are better on pill nights than on non-pill nights, a fixed dosing strategy will maximize efficacy outcomes for the drug-treated group. Despite this difference, zolpidem extended-release demonstrated comparable therapeutic benefit using a dosage strategy that may better approximate the needs of some patients with chronic insomnia.
Potential limitations of this study are the absence of PSG-derived sleep parameters, the exclusive reliance on subjective assessments of next-day functioning and cognitive ability, and the lack of a direct comparison to a nightly control group. Future studies are needed to address these issues. Another limitation of this study is the lack of an active control group. Studies involving active controls are needed to determine the relative utility of zolpidem and other agents when used on a non-nightly basis. In addition, future studies are needed to determine which patients are best managed with intermittent dosing and when nightly therapy is needed.
CONCLUSION
Zolpidem extended-release 12.5 mg, when taken for 3 to 7 nights per week, was well tolerated and effective in improving subjective sleep onset and sleep maintenance symptoms of insomnia throughout 6 months. These benefits were associated with improved subjective next-day functioning, no increase in pill-taking behavior over time, and no rebound insomnia upon drug cessation. These findings extend those from short-term studies, supporting the safety and efficacy of long-term zolpidem extended-release pharmacotherapy for insomnia.
ACKNOWLEDGMENTS
The authors would like to provide their sincere thanks to the investigators of the ZOLONG study:
Michael P. Biber, Newton, MA; Stephen N. Brooks, San Francisco, CA; Richard K. Bogan, Columbia, SC; Camellia P. Clark, San Diego, CA; Martin A. Cohn, Naples, FL; David Mayleben, Cincinnati, OH; Stephen Duntley, St. Louis, MO; Helene A. Emsellem, Chevy Chase, MD; Neil T. Feldman, St. Petersburg, FL; James Ferguson, Salt Lake City, UT; Gregory Ferriss, New Orleans, LA; Jonathan Flescher, Raleigh, NC; June Fry, Lafayette Hill, PA; Yury Furman, Los Angeles, CA; Paul B. Haberman, Santa Monica, CA; Joseph Henkle, Springfield, IL; Max Hirshkowitz, Houston, TX; Andrew O. Jamieson, Plano, TX; Alan D. Lankford, Atlanta, GA; Marc Leibowitz, Glendale, CA; John Marshall, Madison, WI; W. Vaughn McCall, Winston-Salem, NC; Leslie Moldauer, Denver, CO; Dennis J. Munjack, Beverly Hills, CA; Vernon G. Pegram, Birmingham, AL; Michael Perlis, Rochester, NY; John F. Pinto, Las Vegas, NV; Geri E. Poss, San Antonio, TX; Norman Rosenthal, Rockville, MD; Beth Safirstein, Ft. Lauderdale, FL; Carlos Santana, Tampa, FL; Eric A. Sheldon, Miami, FL; David J. Seiden, Pembroke Pines, FL; Thomas M. Shiovitz, Sherman Oaks, CA; Ram Shrivastava, New York, NY; Ed Stepanski, Chicago, IL; John Stoukides, East Providence, RI; Todd Swick, Katy, TX; Steve Thein, San Diego, CA; David P. Walling, Garden Grove, CA; Jan H. Westerman, Jasper, AL; Stasia J. Wieber, New York, NY; David H. Winslow, Louisville, KY; Phyllis Zee, Chicago, IL; Cynthia B. Strout, Mt. Pleasant, SC; Kerri Wilks, Ft. Lauderdale, FL; J. Douglas Hudson, Austin, TX; David Linden, Oklahoma City, OK; William Orr, Oklahoma City, OK; Ernie Riffer, Phoenix, AZ; Thomas C. Marbury, Orlando, FL; Amy Stevenson, Greensboro, NC; John E. Pappas, Lexington, KY; Jeffrey S. Simon, Brown Deer, WI; Daniel Rifkin, Williamsville, NY; Thomas Littlejohn, Winston-Salem, NC; Yvette R. Cook, Cary, NC; Frederico Cerrone, Summit, NJ; Gregory Tarasoff, Las Vegas, NV; Brian Bortnick, Atlanta, GA; John Hohengarten, Colorado Springs, CO; Lawrence Sher, Rolling Hills Estates, CA; Bruce Cleeremans, Irvine, CA.
The authors would also like to thank Cherith Marlowe for her editorial assistance. Funding for this editorial support was provided by sanofi-aventis. The authors of this article were fully responsible for the content and editorial decisions and received no financial support or other form of compensation related to the development of this article.
Footnotes
Disclosure Statement
See page 89 for Disclosure Statement information.
DISCLOSURE STATEMENT
This study was funded by Sanofi-Aventis. Dr. Krystal is a consultant and advisory board member to Astellas Pharma, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, King Pharmaceuticals, Merck, Neurocrine Biosciences, Neurogen, Novartis, Organon, Pfizer, Research Triangle Institute, Respironics, Sanofi-Aventis, Sepracor, Somaxon, Takeda, and TransOral; has received research support from Astellas Pharma, Cephalon, Cyberonics, GlaxoSmithKline, Merck, National Institutes of Health, Neurocrine Biosciences, Neuronetics, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somaxon, Takeda, and TransOral; and has served on the speaker's bureau for Sanofi-Aventis; Sepracor, and Sleep Medicine Education Institute. Dr. Erman is a consultant for Cephalon, Mallinckrodt, Neurocrine Biosciences, Sanofi-Aventis, and Takeda; has received research support from Eli Lilly, GlaxoSmithKline, Merck, Neurocrine Biosciences, Organon, Pfizer, Pharmacia, Sanofi-Aventis, Schwarz Pharma, Takeda, and Wyeth Pharmaceuticals; has been an advisory board member for Cephalon, Neurocrine Biosciences, Sanofi-Aventis, and Takeda; has been a speaker's bureau member for Sanofi-Aventis and Takeda; and has financial interests in Cephalon, Forest Laboratories, Neurocrine Biosciences, Pfizer, Sanofi-Aventis, Sepracor, and Somaxon. Dr. Zammit has received research support from Ancile Pharmaceuticals, Arena Pharmaceuticals, Aventis, Cephalon, Elan Pharmaceuticals, Epix Pharmaceuticals, Evotec AG, Forest Laboratories, GlaxoSmithKline, H. Lundbeck A/S, King, Merck, National Institutes of Health, Neurim Pharmaceuticals, Neurocrine Biosciences, Neurogen, Organon, Orphan Medical, Pfizer, Predix Pharmaceuticals, Respironics, Sanofi-Aventis, Sanofi-Synthelabo, Schering-Plough Corporation, Sepracor, Somaxon, Takeda, UCB Pharma, Vanda Pharmaceuticals, and Wyeth-Ayerst Research; has been a consultant for Aventis; Cephalon, Elan Pharmaceuticals, GlaxoSmithKline, Jazz, King, Merck, Neurocrine Biosciences, Organon, Pfizer, Renovis, Sanofi-Aventis, Select Comfort, Sepracor, Shire, and Takeda; has received honoraria from King, Neurocrine Biosciences, McNeil, Sanofi-Aventis, Sanofi-Synthelabo, Sepracor, Takeda, Vela Pharmaceuticals, and Wyeth-Ayerst Research; and has ownership/directorship of Clinilabs, Inc., Clinilabs IPA, Inc., and Clinilabs Physician Services, PC. Dr. Soubrane is an employee of Sanofi-Aventis. Dr. Roth has received research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine Biosciences, Pfizer, Sanofi, Schering Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth Pharmaceuticals, and Xenoport; has been a consultant for Abbott, Accadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Bristol-Myers Squibb, Cephalon, Cypress, Dove, Elan Pharmaceuticals, Eli Lilly, Evotec AG, Forest Laboratories, GlaxoSmithKline, Hypnion, Jazz, Johnson & Johnson, King, H. Lundbeck A/S, McNeil, MediciNova, Merck, Neurim, Neurocrine Biosciences, Neurogen, Novartis, Orexo AB, Organon, Orginer, Prestwick Pharmaceuticals, Proctor & Gamble, Pfizer, Purdue Pharma L.P., Restiva, Roche, Sanofi-Aventis, ScheringPlough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, VivoMetrics, Wyeth, Yamanuchi Pharma, and Xenoport; and has served as a speaker for Sanofi, and Takeda.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Arlington, VA: American Psychiatric Publishing, Inc.; 1994. (DSM-IV) [Google Scholar]
- 2.Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18:163–76. doi: 10.1002/da.10151. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health. NIH Statement Regarding the Treatment of Insomnia. State of the Science Conference Statement: Manifestations and management of chronic insomnia in adults; June 13-15, 2005. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 4.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-a 10-year prospective population based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 5.Balter MB, Uhlenhuth EH. New epidemiologic findings about insomnia and its treatment. J Clin Psychiatry. 1992;53(Suppl):34–9. [PubMed] [Google Scholar]
- 6.Kramer M. Hypnotic medication in the treatment of chronic insomnia: non nocere' Doesn't anyone care? Sleep Med Rev. 2000;4:529–41. doi: 10.1053/smrv.2000.0122. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon M. Epidemiological study of insomnia in the general population. Sleep. 1996;19(suppl):S7–15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 8.Krystal AD, Walsh JK, Laska E, Caron J, Amato DA, Wessel TC, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–99. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 9.Ancoli-Israel S, Richardson GS, Mangano RM, Jenkins L, Hall P, Jones WS. Long-term use of sedative hypnotics in older patients with insomnia. Sleep Med. 2005;6:107–13. doi: 10.1016/j.sleep.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487–95. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Estivill E. Behavior of insomniacs and implication for their management. Sleep Med Rev. 2002;6(suppl 1):S3–6. doi: 10.1016/s1087-0792(02)80001-6. [DOI] [PubMed] [Google Scholar]
- 12.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. The diagnosis and management of insomnia in clinical practice: a practical evidence-based approach. CMAJ. 2000;162:216–20. [PMC free article] [PubMed] [Google Scholar]
- 13.Neubauer D. Chronic insomnia. Clin Cornerstone. 2004;6(suppl 1C):S17–22. doi: 10.1016/s1098-3597(04)80044-9. [DOI] [PubMed] [Google Scholar]
- 14.Summers MO, Crisostomo MI, Stepanski EJ. Recent developments in the classification, evaluation, and treatment of insomnia. Chest. 2006;130:276–86. doi: 10.1378/chest.130.1.276. [DOI] [PubMed] [Google Scholar]
- 15.Vallieres A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14:447–53. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 16.Hohagen F, Kappler C, Schramm E, Riemann D, Weyerer S, Berger M. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening--temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994;17:551–54. [PubMed] [Google Scholar]
- 17.Cluydts R. Zolpidem ‘as needed’: methodological issues and clinical findings. CNS Drugs. 2004;18(suppl 1):25–33. doi: 10.2165/00023210-200418001-00006. [DOI] [PubMed] [Google Scholar]
- 18.Zolpidem CR. Physician's Desk Reference, 60th ed. Montvale, NJ: Thompson PDR; 2006. [Google Scholar]
- 19.Perlis ML, McCall WV, Krystal AD, Walsh JK. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128–1137. doi: 10.4088/jcp.v65n0816. [DOI] [PubMed] [Google Scholar]
- 20.Walsh JK, Roth T, Randazzo A, Erman M, Jamieson A, Scharf M, et al. Eight weeks of non-nightly use of zolpidem for primary insomnia. Sleep. 2000;23:1087–96. [PubMed] [Google Scholar]
- 21.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs. 2000;59:865–89. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18:49–56. doi: 10.1080/10401230500464711. [DOI] [PubMed] [Google Scholar]
- 23.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22(suppl 2):S354–58. [PubMed] [Google Scholar]
- 24.Scharf MB, Roth T, Vogel GW, Walsh JK. A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192–99. [PubMed] [Google Scholar]
- 25.Leger D, Poursain B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. 2005;21:1785–92. doi: 10.1185/030079905X65637. [DOI] [PubMed] [Google Scholar]
- 26.National Sleep Foundation. [Accessed January 22, 2007];Sleep in America poll. 2002 Last update: 2002. Available at: www/sleepfoundation.org/_content/hottopics/2002SleepInAmericaPoll.pdf.
- 27.Moen MD, Plosker GL. Zolpidem extended-release. CNS Drugs. 2006;20:419–26. doi: 10.2165/00023210-200620050-00006. [DOI] [PubMed] [Google Scholar]
- 28.Weinling E, McDougall S, Andre F, Bianchetti G, Dubruc C. Pharmacokinetic profile of a new modified release formulation of zolpidem designed to improve sleep maintenance. Fundam Clin Pharmacol. 2006;20:397–403. doi: 10.1111/j.1472-8206.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 29.Roth T, Soubrane C, Titeux L, Walsh JK. Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia. Sleep Med. 2006;7:397–406. doi: 10.1016/j.sleep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Blin O, Micallef J, Audebert C, Legangneux E. A double-blind, placebo- and flurazepam-controlled investigation of the residual psychomotor and cognitive effects of modified release zolpidem in young healthy volunteers. J Clin Psychopharmacol. 2006;26:284–89. doi: 10.1097/01.jcp.0000218985.07425.d9. [DOI] [PubMed] [Google Scholar]
- 31.Hindmarch I, Legangneux E, Stanley N, Emegbo S, Dawson J. A double-blind, placebo-controlled investigation of the residual psychomotor and cognitive effects of zolpidem-MR in healthy elderly volunteers. Br J Clin Pharmacol. 2006;62:538–45. doi: 10.1111/j.1365-2125.2006.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krystal AD. Treating the health, quality of life, and functional impairments in insomnia. J Clin Sleep Med. 2007;3:63–72. [PubMed] [Google Scholar]