Abstract
Study Objectives:
Extensive data implicate serotonin (5-hydroxytryptamine [5-HT]) in the regulation of sleep. Jouvet has hypothesized that 5-HT promotes wakefulness, yet is necessary for subsequent non-rapid eye movement (NREM) sleep, actions he proposes to be mediated by sleep factors. Studies in rat support this dual role for 5-HT. The objectives of this study were to (1) determine effects of serotonergic activation on sleep of mice and (2) elucidate a potential role for the cytokine interleukin-6 as a sleep factor mediating serotonergic effects on sleep.
Design:
C57BL/6J and B6.129S6-Il6tm1Kopf (interleukin-6 knockout [IL-6 KO]) mice were purchased from the Jackson Laboratory and instrumented for recording the electroencephalogram and body temperature. After recovery, separate groups of mice were injected intraperitoneally at either light or dark onset with vehicle or with the 5-HT precursor 5-hydroxytryptophan (5-HTP). Sleep-wake behavior was determined and body temperature recorded for 24 hours after injections.
Results:
5-HTP induced hypothermia in both mouse strains. When injected at dark onset, the highest dose of 5-HTP (200 mg/kg) increased NREM sleep. Light onset administration initially increased wakefulness, with increases in NREM sleep apparent only during the subsequent dark period. For most parameters, there were no differences in responses between strains. However IL-6 KO mice at some doses exhibited a greater increase in NREM sleep.
Conclusions:
5-HTP alters sleep-wake behavior and body temperature of mice in a manner similar to that of rats. Increases in NREM sleep after 5-HTP are apparent only during the dark period, which may represent a fundamental property of the serotonergic system. These results suggest that 5-HT should not be considered either wake promoting or NREM sleep promoting. Rather, the role of 5-HT in the regulation of sleep-wake behavior must be considered within the context of the degree to which the system is activated and the time at which the activation occurs.
Citation:
Morrow JD; Vikraman S; Imeri L; Opp MR. Effects of serotonergic activation by 5-hydroxytryptophan on sleep and body temperature of C57BL/6J and interleukin-6-deficient mice are dose and time related. SLEEP 2008;31(1):21-33.
Keywords: 5-HT, serotonin, thermoregulation, behavior, cytokine, IL-6, IL-1
THE ROLE THAT SEROTONIN (5-HT; 5-HYDROXYTRYPTAMINE) PLAYS IN THE REGULATION OF SLEEP-WAKE BEHAVIOR HAS BEEN OF INTEREST SINCE BRODIE and colleagues reported, in 1955, that reserpine depletes 5-HT in brain and induces sedation.1 Since that seminal publication, numerous studies implicate 5-HT in multiple brain functions, including arousal state regulation, thermoregulation, and locomotion [reviewed 2,3]. It is now thought that 5-HT, release of which is maximal during wakefulness, promotes wakefulness per se by actions on 5-HT2 receptors and subsequently triggers sleep via stimulation of sleep-promoting systems.4,5 Observations of the effects of increasing serotonergic activity by intraperitoneal (IP) injection of the 5-HT precursor L-5-hydroxytryptophan (5-HTP) in rats directly support this dual role for 5-HT in the regulation of arousal state. The effects of serotonergic activation by 5-HTP on non-rapid eye movement (NREM) sleep of rats are dose and time dependent. The IP administration of 5-HTP into rats initially increases wakefulness, irrespective of whether 5-HTP is given prior to dark onset6 or prior to light onset.7 During this period of 5-HTP-induced wakefulness, NREM sleep and rapid eye movement (REM) sleep are suppressed. When given prior to dark onset, the initial increase in wakefulness is transient, and NREM sleep increases after a delay of only 2 to 3 hours.6 When given prior to light onset, 5-HTP-induced increases in NREM sleep are not apparent until the subsequent dark period, 12 hours after administration.7 Serotonergic activation by this means may or may not affect REM sleep of rats, depending on dose and time of administration.6,7 Serotonergic activation by 5-HTP administration in rats during either the dark or light period induces hypothermia. When 5-HTP is administered at dark onset, the duration of hypothermia is dose dependent, and hypothermia encompasses periods of both increased wakefulness and enhanced NREM sleep.
The use of 5-HT precursors as a method of activating the serotonergic system of cats is limited by side effects.2 Mice, in part because of the relative ease with which the mouse genome may be manipulated, are now increasingly used in studies of sleep-wake behavior. Reports demonstrate alterations in sleep of mice lacking 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT2C receptors or the serotonin transporter.8–13 Although extracellular 5-HT is likely to increase in mice lacking the serotonin transporter, to our knowledge no studies of the impact of serotonergic activation by 5-HTP on sleep of mice have been reported. As such, the first aim of this study was to investigate, in C57BL/6J mice, the effects on sleep-wake behavior of 5-HTP administration.
Most cytokines were originally described as products of the immune system. However, it is now known that multiple cytokines and their receptors are produced and are biologically active within the central nervous system.14 The constitutive presence of cytokines and cytokine receptors in normal, healthy brain suggests that at least some cytokines may play a role in the regulation or modulation of complex behavior and normal physiologic processes controlled by the central nervous system. Indeed, there is now ample evidence indicating that several cytokines play a direct role in the regulation of sleep-wake behavior [reviewed 15,16] in addition to mediating responses to immune stimulation during pathologic conditions.
Although interleukin (IL)-1 and tumor necrosis factor (TNF) are the most investigated cytokines with regard to the regulation of sleep [reviewed 15], IL-6 may play a role in alterations of sleep during some sleep disorders or other pathologies. IL-6 mediates facets of sickness behavior and responses to IL-1 and TNF. IL-6 in plasma exhibits a diurnal rhythm with peak values during sleep and nadirs during wakefulness. Sleep deprivation of human volunteers increases IL-6 in plasma, and subcutaneous injection of IL-6 increases slow wave sleep and reduces REM sleep of humans.18 Although studies of human subjects in which the IL-6 system has been directly antagonized have not been conducted, the TNFα receptor antagonist etanercept, when administered intravenously into volunteers, reduces IL-6 in plasma and decreases daytime sleepiness in patients with sleep apnea.17 Finally, several pathologies characterized by excessive daytime sleepiness (e.g., narcolepsy, sleep apnea, insomnia) are associated with elevated IL-6 [reviewed 15].
IL-6 administration increases NREM sleep of rats.19 However, antagonizing the IL-6 system of rats with anti-IL-6 antibodies does not alter sleep.19 Furthermore, NREM sleep of IL-6–deficient (knock-out) mice does not differ from that of C57BL/6J control mice,20 although the increase in NREM sleep of IL-6 KO mice in response to immune challenge is reduced.21 Collectively, these preclinical studies suggest that, although IL-6 may not play a role in the regulation of sleep–wake behavior of healthy animals, it can modulate sleep-wake behavior when this cytokine is elevated, as in pathologic conditions.
Previous studies have demonstrated that interactions between IL-1 and the serotonergic system are of functional consequence for the regulation of NREM sleep [e.g.22–25]. However, IL-6 may be another cytokine that interacts with the serotonergic system to alter sleep, particularly during pathologic conditions. 5-HT increases IL-6 mRNA in rat astrocytes.27 Therefore, the second aim of the study was to test the hypothesis that mice lacking IL-6 will differ from C57BL/6J mice with respect to 5-HTP-induced alterations in sleep-wake behavior. Specifically, because 5-HT induces IL-6 mRNA27 and IL-6 is a powerful inducer of 5-HT in brain,28–30 we hypothesized that 5-HTP would increase wakefulness less in IL-6 KO mice due to a less robust stimulation of the serotonergic system in brain.
We now report that 5-HTP administration into mice alters wakefulness and sleep. As previously demonstrated in rats,6 5-HTP administered into mice at light onset initially increases wakefulness, whereas dark-onset administration enhances NREM sleep. Lack of IL-6 alters the magnitude, but not timing, of some responses to 5-HTP, suggesting that this cytokine may modulate some of the behavioral responses to serotonergic activation by this method.
METHODS
Substances
5-HTP was purchased as lyophilized powder from Sigma-Aldrich (St. Louis, MO). Prior to each use, 5-HTP was dissolved in pyrogen-free NaCl (PFS; Abbott, North Chicago, IL) and brought to an appropriate concentration. PFS served as vehicle control.
Animals and Surgical Procedures
All procedures used in this study were approved by the University of Michigan Committee on Care and Use of Animals and in accordance with the US Department of Agriculture Animal Welfare Act, and the Public Health Service policy on Humane Care and Use of Laboratory Animals.
Two strains of adult male mice (approximately 25 g at time of surgery) were used in this experiment: C57BL/6J mice and B6.129S6-Il6tm1Kopf (IL-6 KO) mice. All mice were initially purchased from the Jackson Laboratory (Bar Harbor, ME). The IL-6 KO strain was generated on a 129S6 background and backcrossed for 11 generations with C57BL/6J mice to produce a fully congenic strain. After 10 generations of backcrossing, mice are 99.9% identical to the backcross strain at all unlinked loci.31 As such, the geneticists at the Jackson Laboratory consider C57BL/6J to be the appropriate control for the IL-6 KO strain. Breeding pairs of IL-6 KO mice were used to establish a breeding colony under the direction of the University of Michigan Unit for Laboratory Animal Medicine. The mice were housed in standard shoeboxes in environmentally controlled chambers at a temperature of 29°C ± 1°C and on a 12:12 hour light:dark cycle. Food (Lab Diet 5001, PMI Nutrition International, Brentwood, MO) and water were provided ad libitum.
Transmitters (ETA10-F20, Data Sciences International, St. Paul, MN) were surgically implanted under isoflurane anesthesia into the abdominal cavity, as previously described.20,21 Insulated biopotential leads were routed to the head and connected to miniature screws that served as electroencephalographic (EEG) recording electrodes. Ibuprofen (0.2 mg/mL) was provided in the drinking water as an analgesic, beginning 24 hours before surgery and continuing until 48 hours after surgery. A broad-spectrum antibiotic (imipenem, 25 mg/kg) was given immediately following surgery. The mice were allowed a minimum of 28 days recovery prior to the beginning of experiments. During recovery and for the duration of the experimental protocol, the mice were housed in the recording chamber in the same conditions as used for recordings.
Recording Apparatus
Signals from the transmitter were detected by a receiver (RPC-1, DSI) located directly underneath the mouse's shoebox. This signal was digitized and fed into a DSI analog converter (ART Analog-8 CM), which then converted the EEG and temperature voltages using calibration factors supplied by the manufacturer. The analog signal from the DSI system was fed into an A/D board (model PCI 3033E, National Instruments, Austin, TX) in a separate computer and captured and digitized at 128 Hz with 16-bit precision. The EEG was digitally filtered into delta (0.5–4.0 Hz) and theta (6.0–9.0 Hz) frequency bands. These digitally filtered frequency components were integrated over 1-second periods. Voltage values from the temperature channel were converted to engineering units (°C) by regression using coefficients specific for each transmitter and obtained by calibration using a water bath. Movements of the mice in their shoeboxes were detected using an infrared sensor (BioBserve, GmbH, Bonn, Germany). All signals (EEG, body temperature, movement, integrated values for frequency bands) were stored as binary files until further processing.
A custom program, ICELUS (M. Opp, University of Michigan) written in LabView for Windows (National Instruments), was used to assign arousal state for artifact-free 10-second epochs based on measures of the EEG and movement. Arousal state was assigned by visual/manual scoring as NREM sleep, REM sleep, or wakefulness, based on evaluation of EEG, body movements, and integrated delta and theta values. Briefly, wakefulness was defined on the basis of a low-amplitude, mixed-frequency (delta, theta) EEG accompanied by body movements. Increases in body temperature occur as a function of activity. NREM sleep was identified by an increased absolute EEG amplitude, integrated values for the delta frequency band greater than those for theta, and lack of body movements. Body temperature declines upon entry into NREM sleep until it reaches a regulated asymptote. REM sleep was characterized by a low-amplitude EEG, with integrated values for the delta frequency band less than those for the theta frequency band. During the assignment of arousal state, any epoch containing movement artifact or electrical noise was tagged and excluded from subsequent spectral analyses.
Experimental Protocol
After recovery, 24-hour baseline recordings were obtained from undisturbed animals. Following this baseline recording, the mice were given 0.2 mL PFS (vehicle) IP as a control. These injections of PFS were given either 15 minutes before dark onset or at light onset. Immediately after the injections, the mice were returned to their cages and recordings were obtained for the next 24 hours. After control recordings were completed, each animal was injected IP with 0.2 mL PFS containing 1 of 3 doses of 5-HTP: 50, 100, or 200 mg/kg. Injections of 5-HTP were given at the same time as control injections of PFS, and 24-hour recordings were obtained. As such, each animal received both vehicle and 5-HTP and served as its own control. Injections of 5-HTP were separated by a minimum of 72 hours, and no animal received more than 2 doses of 5-HTP. Sample sizes for each dose of 5-HTP were as follows: Dark Onset—C57BL/6J mice: n = 6–11/dose, IL-6 KO mice: n = 6–10/dose; Light Onset—C57BL/6J mice: n = 5–8/dose; IL-6 KO mice—n = 5–8/dose.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows (SPSS, Inc., Chicago, IL). All values are presented as the mean ± SEM. Two types of statistical comparisons were made. Comparisons within mouse strain between treatments for a single 5-HTP dose were analyzed with one-way analyses of variance (ANOVA). The duration of each vigilance state (NREM sleep, REM sleep, wakefulness), and the core body temperature values were the dependent variables in these analyses, and manipulation (PFS, 5-HTP dose) was the independent variable (factor). The ANOVA analyses were conducted on data from 4-hour time blocks across the 24-hour recording period. These time blocks were defined on the basis of visual inspection of the results. An α level of P ≤ 0.05 was taken as indicating a statistically significant difference between values obtained after administration of vehicle and 5-HTP. To determine whether there were dose-related responses to 5-HTP, difference scores were calculated (control value—experimental value) for each parameter (duration of NREM sleep, REM sleep, wakefulness) for each hour. Difference scores for core body temperature were calculated using the same formula, but for each 10-minute interval. These difference scores were the independent variables in one-way ANOVA, whereas the dependent variable (factor) was 5-HTP dose. When significant differences among the three 5-HTP doses were revealed, posthoc pairwise comparisons were made by the method of Scheffé to determine responses to which dose contributed to the overall effect. Comparisons between mouse strain were made for each independent variable (NREM sleep, REM sleep, wakefulness, core body temperature) by using the strain-specific difference scores. These strain comparisons were made with one-way ANOVAs, in which mouse strain (C57BL/6J, IL-6 KO) was the dependent variable (factor). These analyses were also conducted on 4-hour time blocks using an α level of P ≤ 0.05 as indicating values that differed statistically between strains.
RESULTS
Dark-Onset Administration of 5-HTP
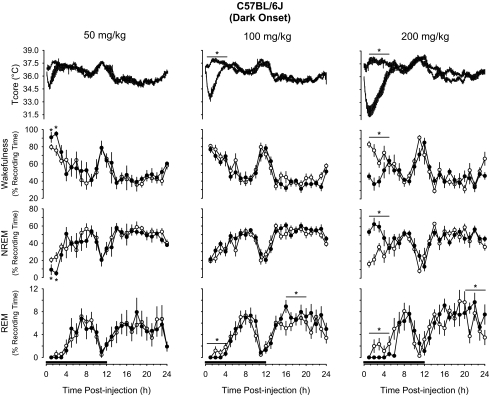
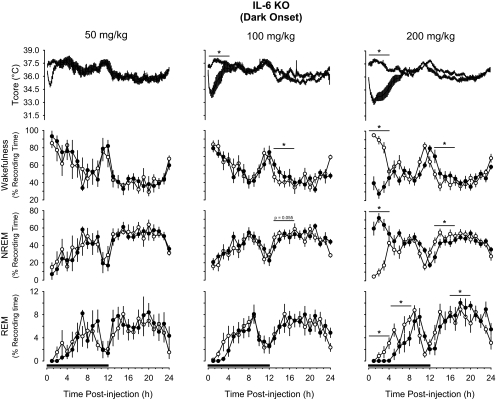
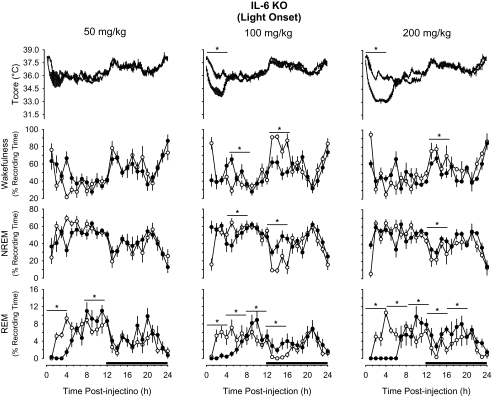
IP administration of 5-HTP prior to dark onset induced hypothermia, increased NREM sleep, and reduced REM sleep and wakefulness in a dose-dependent manner in both mouse strains (Figures 1, 2). Analysis of the 4-hour time blocks indicated the 2 lowest doses of 5-HTP used in this study (50, 100 mg/kg) did not substantially alter sleep-wake behavior of either mouse strain. During the subsequent light period, these doses of 5-HTP did modestly suppress REM sleep of C57BL/6J mice and increase wakefulness of IL-6 KO mice (Figures 1, 2). When statistical analyses were confined to the initial 2 hours after injection, C57BL/6J mice responded to 50 mg/kg 5-HTP with a transient increase in wakefulness and reduction in NREM sleep (Figure 1). After administration of 200 mg/kg of 5-HTP, there were dramatic effects on sleep-wake behavior in both mouse strains. During the initial 4 hours after administration of this dose of 5-HTP, NREM sleep of C57Bl/6J mice increased by nearly 125%, from 24.9% ± 2.9% of recording time after vehicle to 55.8% ± 3.4% of recording time after 5-HTP (Figure 1). This increase in NREM sleep was concomitant with reductions in REM sleep and wakefulness. These 5-HTP–induced alterations in sleep-wake behavior of C57BL/6J mice were confined to the first 4 hours after injection, although there was a modest increase in REM sleep during postinjection hours 21 to 24 (Figure 1). The effects of 5-HTP on NREM sleep and wakefulness of C57BL/6J mice were dose dependent. During the first 4 hours after injection, the increase in NREM sleep and reduction in wakefulness following the 200 mg/kg dose of 5-HTP were statistically larger than those following administration of 50 mg/kg or 100 mg/kg of 5-HTP (Figure 1). There was no statistical difference among the 3 doses of 5-HTP administered into C57BL/6J mice with respect to suppression of REM sleep. Responses of IL-6 KO mice to 5-HTP administered at dark onset were generally confined to the 200-mg/kg dose (Figure 2). NREM sleep of IL-6 KO mice increased during the initial 4 hours after administration by about 230%, from 18.9% ± 3.1% of recording time after vehicle to 62.7% ± 3.6% of recording time after 5-HTP. As with C57BL/6J mice, the increase in NREM sleep was concomitant with reductions in REM sleep and wakefulness. The suppression of REM sleep after this dose of 5-HTP in IL-6 KO mice lasted for 8 hours after injection (Figure 2), and was followed by a modest increase in REM sleep during the subsequent light period that achieved statistical significance during postinjection hours 13 to 18. During this same postinjection period, there were alterations in NREM sleep and wakefulness that mirrored those observed immediately after injection of 5-HTP. That is, the initial increase in NREM sleep was followed by a reduction in this sleep stage and the reduction in wakefulness was followed by an increase (Figure 2). As for C57BL/6J mice, the effects of 200 mg/kg of 5-HTP on NREM sleep and wakefulness of IL-6 KO mice differed in magnitude from responses following administration of 50 mg/kg or 100 mg/kg 5-HTP (Figure 2).
Figure 1.
Impact of 5-hydroxytryptophan (5-HTP) on core body temperature (Tcore), wakefulness, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep of C57BL/6J mice when administered at dark onset. Symbols are means ± SEM for 10-min (Tcore) or hourly (wakefulness, NREM, REM) values after intraperitoneal administration of vehicle (pyrogen-free saline, open symbols, thin lines) or 5-HTP (closed symbols, thick lines). Sample sizes are: 50 mg/kg, n = 6; 100 mg/kg, n = 11; 200 mg/kg, n = 6. The black bars on the x-axes denote the dark period of the light:dark cycle. Asterisks and horizontal lines with asterisks identify 4-hour time blocks during which values differed statistically between conditions (P < 0.05).
Figure 2.
Impact of 5-hydroxytryptophan (5-HTP) on core body temperature (Tcore), wakefulness, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep of B6.129S6-Il6tm1Kopf (IL-6 KO) mice when administered at dark onset. Symbols are means ± SEM for 10-min (Tcore) or hourly (wakefulness, NREM, REM) values after intraperitoneal administration of vehicle (pyrogen-free saline, open symbols, thin lines) or 5-HTP (closed symbols, thick lines). Sample sizes are: 50 mg/kg, n = 6; 100 mg/kg, n = 10; 200 mg/kg, n = 10. The black bars on the x-axes denote the dark period of the light:dark cycle. Horizontal lines with asterisks identify 4-hour time blocks during which values differed statistically between conditions (P < 0.05).
5-HTP induced dramatic hypothermic responses in both mouse strains (Figures 1, 2). The magnitude and duration of the hypothermia were dose related. The nadirs in core body temperatures after 5-HTP occurred in postinjection hour 1 or 2 in both mouse strains. The drop in core body temperature of C57BL/6J mice amounted to 1.6°C ± 0.4°C after the 50-mg/kg dose of 5-HTP, 3.1°C ± 0.4°C after the 100-mg/kg dose of 5-HTP, and 5.7°C ± 0.6°C after the 200-mg/kg dose of 5-HTP. These drops in core body temperature differed statistically from each other. These same 5-HTP doses reduced body temperatures by 1.9°C ± 0.2°C, 3.0°C ± 0.3°C, and 4.4°C ± 0.4°C, respectively, in IL-6 KO mice. As in C57BL/6J mice, hypothermic responses of IL-6 KO mice to these doses of 5-HTP each differed statistically from the other. In both mouse strains, these hypothermic responses lasted about 2 hours after the 50-mg/kg dose, 4 hours after the 100-mg/kg dose, and approximately 6 hours after the 200-mg/kg dose (Figures 1, 2).
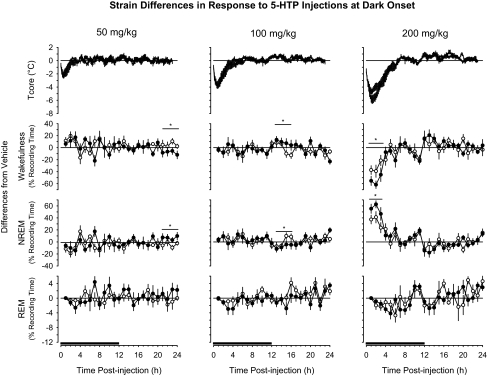
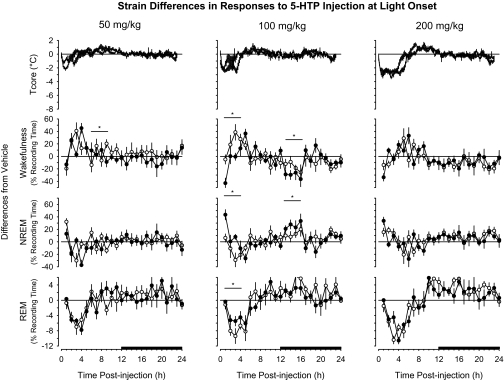
Analyses of difference scores revealed that administration of 5-HTP at dark onset generally induced similar responses in both mouse strains with respect to most parameters determined (Figure 3). The strains differed modestly in responses to 50 mg/kg or 100 mg/kg of 5-HTP. After 50 mg/kg of 5-HTP, the IL-6 KO mice demonstrated significantly more NREM sleep and less wakefulness during postinjection hours 21 to 24 than did the C57BL/6J mice. After 100 mg/kg of 5-HTP, IL-6 KO mice spent less time in NREM sleep and more time awake during the first 4 hours of the subsequent dark period. There were more substantive strain differences in sleep-wake behavior after injection of 200 mg/kg of 5-HTP (Figure 3). During the first 4 hours after injection of 200 mg/kg of 5-HTP, IL-6 KO mice spent significantly more time in NREM sleep than did C57BL/J mice (Figure 3). The proportionally greater amount of 5-HTP–induced NREM sleep of IL-6 KO mice was mirrored by a significantly greater reduction in wakefulness than was exhibited by C57BL/6J mice. There were no strain differences in the hypothermic responses to 5-HTP (Figure 3).
Figure 3.
Comparison of responses of C57BL/6J mice (open symbols, thin lines) and B6.129S6-Il6tm1Kopf (IL-6 KO) mice (closed symbols, thick lines) to intraperitoneal administration of 5-HTP at dark onset. Values for core body temperature (Tcore), wakefulness, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep are expressed as differences from vehicle control (depicted by the zero line) and are means ± SEM for 10-min (Tcore) or hourly (wakefulness, NREM, REM) intervals. Sample sizes are as indicated in Figure 1 and Figure 2 for C57BL/6J and IL-6 KO mice, respectively. The black bars on the x-axes denote the dark period of the light:dark cycle. Horizontal lines with asterisks identify 4-hours time blocks during which values differed statistically between the 2 mouse strains (P < 0.05).
Light-Onset Administration of 5-HTP
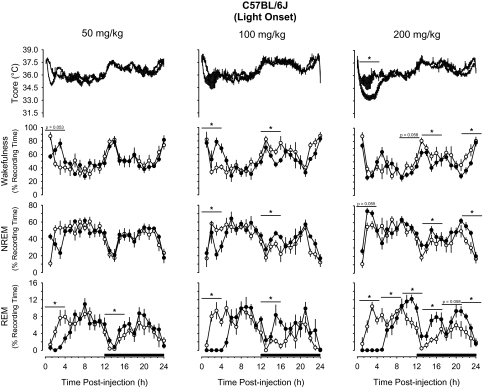
IP administration of 5-HTP at light onset induced hypothermia and initially increased wakefulness and reduced NREM sleep and REM sleep (Figure 4, Figure 5). In C57BL/6J mice, the 50-mg/kg and 100-mg/kg doses tended to increase wakefulness during the initial 4 hours after injection, an effect that achieved statistical significance after the 100-mg/kg dose (Figure 4). These increases in wakefulness after 5-HTP were mirrored by reductions in NREM sleep and REM sleep (Figure 4). The increases in wakefulness and reductions in NREM sleep during the initial postinjection period after 100 mg/kg of 5-HTP were followed by reductions in wakefulness and increases in NREM sleep during the early part of the subsequent dark period (Figure 4). In contrast with the 2 lower doses, the 200-mg/kg dose of 5-HTP did not alter wakefulness during the initial postinjection periods. Although there was a tendency for an increase in NREM sleep during the first 4 hours after injection of this dose of 5-HTP, this effect did not achieve statistical significance (Figure 4). However, during the subsequent dark period, NREM sleep was significantly increased and wakefulness was decreased. These effects were apparent during postinjection hours 13 to 16 and 21 to 24. The effects of 5-HTP on NREM sleep and wakefulness of C57BL/6J mice were dose dependent. The reduction in NREM sleep and increase in wakefulness during the initial 4 hours after administration of the 100-mg/kg dose at the beginning of the light period were statistically greater than after the 50-mg/kg or 200-mg/kg doses.
Figure 4.
Impact of 5-hydroxytryptophan (5-HTP) on core body temperature (Tcore), wakefulness, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep of C57BL/6J mice when administered at light onset. Symbols are means ± SEM for 10-min (Tcore) or hourly (wakefulness, NREM, REM) values after intraperitoneal administration of vehicle (pyrogen-free saline, open symbols, thin lines) or 5-HTP (closed symbols, thick lines). Sample sizes are: 50 mg/kg, n = 8; 100 mg/kg, n = 5; 200 mg/kg, n = 7. The black bars on the x-axes denote the dark period of the light:dark cycle. Horizontal lines with asterisks identify 4-hour time blocks during which values differed statistically between conditions (P < 0.05).
Figure 5.
Impact of 5-hydroxytryptophan (5-HTP) on core body temperature (Tcore), wakefulness, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep of B6.129S6-Il6tm1Kopf (IL-6 KO) mice when administered at light onset. Symbols are means ± SEM for 10-min (Tcore) or hourly (wakefulness, NREM, REM) values after intraperitoneal administration of vehicle (pyrogen-free saline, open symbols, thin lines) or 5-HTP (closed symbols, thick lines). Sample sizes are 50 mg/kg, n = 6; 100 mg/kg, n = 8; and 200 mg/kg, n = 5. The black bars on the x-axes denote the dark period of the light:dark cycle. Horizontal lines with asterisks identify 4-hour time blocks during which values differed statistically between conditions (P < 0.05).
Responses of IL-6 KO mice to 5-HTP administration at light onset were generally similar to those of C57BL/6J mice. Although no statistically significant effects on NREM sleep or wakefulness were apparent after 50 mg/kg of 5-HTP, there were increases in wakefulness and reductions in NREM sleep of these animals during the light period following administration of 100 mg/kg of 5-HTP (Figure 5). The increase in wakefulness and reduction in NREM sleep of these animals was delayed by 4 hours relative to responses of C57BL/6J mice. As with C57BL/6J mice, IL-6 KO mice responded to 200 mg/kg of 5-HTP at light onset with modest increases in NREM sleep and reductions in wakefulness, although these effects were of shorter duration (Figures 4, 5). These effects of 5-HTP on NREM sleep and wakefulness of IL-6 KO mice were dose dependent, with responses to 50 mg/kg of 5-HTP differing from those to 100 mg/kg or 200 mg/kg.
Perhaps the most striking effect of 5-HTP administered at light onset on sleep-wake behavior of mice used in this study is the dose-related impact on REM sleep (Figures 4, 5). 5-HTP initially suppressed REM sleep, irrespective of dose or mouse strain. The extent of the initial REM sleep suppression in C57BL/6J and IL-6 KO mice was of 4 hours' duration following 50 mg/kg of 5-HTP and for 8 hours after 100 mg/kg and 200 mg/kg of 5-HTP (Figures 4, 5). After the initial period of REM sleep suppression, there were increases in REM sleep that lasted 4 to 12 hours, depending on dose and mouse strain (Figures 4, 5). In C57BL/6J mice, the increases in REM sleep after 50 mg/kg or 100 mg/kg were limited to the first 4 hours of the subsequent dark period. After the 200-mg/kg dose of 5-HTP, however, REM sleep of C57BL/6J mice was increased essentially for the last 16 hours of the recording period (Figure 4). The magnitude and duration of REM sleep enhancement in C57BL/6J mice following 5-HTP administration were dose related; responses during postinjection hours 8 to 24 after each dose differed statistically from each other. Although there were increases in REM sleep of IL-6 KO mice after each dose of 5-HTP, the greatest effect was after the 200-mg/kg dose, during which time REM sleep was increased for 12 hours (Figure 5). The effects of 200 mg/kg of 5-HTP on REM sleep of IL-6 KO mice differed statistically from those after either the 50-mg/kg dose or the 100-mg/kg dose.
As observed following dark-onset administration, injection of 5-HTP at light onset induced hypothermic responses in both mouse strains (Figures 4, 5). The nadirs in core body temperature of C57BL/6J mice after 50 mg/kg and 100 mg/kg of 5-HTP occurred during the first postinjection hour and were of 1.3°C ± 0.1°C and 1.4°C ± 0.9°C magnitude, respectively. Following administration of 200 mg/kg of 5-HTP, the nadir of core body temperatures of C57BL/6J mice was 2.6°C ± 0.2°C during the third postinjection hour. The hypothermic response after this dose of 5-HTP was prolonged, lasting 4 hours, during which time average body temperatures were 2.4°C ± 0.1°C lower than during the same period following vehicle administration (Figure 4). These hypothermic responses of C57BL/6J mice to 5-HTP administration at light onset did not differ statistically among doses.
IL-6 KO mice also responded to 5-HTP administration at light onset with hypothermic responses. Nadirs in body temperature of IL-6 KO mice were 0.8°C ± 0.2°C in hour 1 and 2.1°C ± 0.2°C in hour 2 after administration of 50 mg/kg and 100 mg/kg respectively. After injection of 200 mg/kg of 5-HTP, the nadir of body temperature for IL-6 KO mice was 3.0°C ± 0.2°C in the third postinjection hour. Core body temperatures of IL-6 KO mice were reduced for 6 hours after injection after this does of 5-HTP, during which time average body temperatures were 2.3°C ± 0.1°C lower than during comparable periods after vehicle administration (Figure 5). The hypothermic responses of IL-6 KO mice to 5-HTP differed statistically among the 3 doses tested, with values after each dose differing from those of the other doses.
C57BL/6J and IL-6 KO mice responded to 5-HTP administration at light onset in a similar fashion for most parameters determined (Figure 6). The greatest differences in the manner in which these mouse strains differed in responses to 5-HTP were apparent after injection of 100 mg/kg. After this dose of 5-HTP, NREM sleep of IL-6 KO during the first 4 hours after injection was not statistically altered, although there was an increase in NREM sleep during postinjection hour 1 (Figure 5). During this same 4-hour postinjection period, NREM sleep of C57BL/6J mice was suppressed (Figure 4). As such, when comparing strain differences during this time block (Figure 6), there was a proportionally greater impact on NREM sleep of IL-6 KO mice than on NREM sleep of C57BL/6J mice. The same is true of effects on REM sleep and wakefulness; when comparing the manner in which these strains responded to 100 mg/kg of 5-HTP, there were differences between strains, even if the responses to 5-HTP did not differ from vehicle within each strain. There were no statistically significant strain differences in the hypothermic responses of C57BL/6J and IL-6 KO mice to these doses of 5-HTP (Figure 6).
Figure 6.
Comparison of responses of C57BL/6J mice (open symbols, thin lines) and B6.129S6-Il6tm1Kopf(IL-6 KO) mice (closed symbols, thick lines) to intraperitoneal administration of 5-HTP at light onset. Values for core body temperature (Tcore), wakefulness, non-rapid eye movements (NREM) sleep, and rapid eye movements (REM) sleep are expressed as differences from vehicle control (depicted by the zero line) and are means ± SEM for 10-min (Tcore) or hourly (wakefulness, NREM, REM) intervals. Sample sizes are as indicated in Figure 4 and Figure 5 for C57BL/6J and IL-6 KO mice, respectively. The black bars on the x-axes denote the dark period of the light:dark cycle. Horizontal lines with asterisks identify 4-hour time blocks during which values differed statistically between the 2 mouse strains (P < 0.05).
DISCUSSION
The results obtained demonstrate that 5-HTP–induced changes in sleep-wake behavior of C57BL/6J mice depend on dose and time of administration. When administered at the beginning of the dark period, 5-HTP transiently enhances wakefulness at a low dose (50 mg/kg), does not affect either wakefulness or NREM sleep at an intermediate dose (100 mg/kg), and enhances NREM sleep at a high, yet physiologic dose (200 mg/kg). There are modest dose-related reductions in REM sleep that are followed by increases in REM sleep during the subsequent light period. When administered at the beginning of the light period, the low dose (50 mg/kg) of 5-HTP enhances wakefulness and inhibits NREM sleep, whereas, after the 100-mg/kg dose, these increases in wakefulness and reductions in NREM sleep are followed by increases in NREM sleep during the subsequent dark period. There is a tendency for NREM sleep to be enhanced immediately after highest dose used (200 mg/kg), but the major effect of this dose is enhanced NREM sleep during the subsequent dark period. Each dose of 5-HTP used in this study suppresses REM sleep after light-onset administration. These periods of REM sleep suppression are followed by dose-related increases in REM sleep that are most apparent during the subsequent dark period.
The results of this study investigating effects of serotonergic activation in mice are strikingly similar to observations previously obtained in rats using similar doses of 5-HTP and the same injection protocol.6,7 Changes induced in rat sleep-wake activity by 5-HTP administration at dark onset are time and dose related. Changes in sleep of rats during the dark period progress in a specific pattern: low doses of 5-HTP in rats increase wakefulness and reduce NREM sleep and REM sleep, whereas high physiologic doses enhance NREM sleep and reduce wakefulness.6 When given to rats at the beginning of the light period, the same doses of 5-HTP initially increase wakefulness, with increases in NREM sleep apparent only after a long delay, during the subsequent dark period.7
Despite extensive experimental and clinical evidence implicating 5-HT in sleep regulation, the exact role of the serotonergic system in this regard remains poorly understood. Historic data suggest that the serotonergic system is necessary for NREM sleep. For example, the destruction of raphe nuclei or the administration of the 5-HT synthesis inhibitor p-chlorophenylalanine induces insomnia that is selectively antagonized or reversed by administration of the 5-HT precursor 5-HTP [reviewed in2,32]. Other data suggest that the preoptic area and the basal forebrain are brain regions at which 5-HT promotes NREM sleep. For example, the anterior hypothalamus is the only brain area where stimulation of serotonergic activity can restore sleep in cats that have been made insomniac by brain 5-HT depletion.33 Serotonin hyperpolarizes basal forebrain cholinergic neurons responsible for cortical activation.34 Furthermore, 5-HT stimulates IL-1 mRNA expression in the hypothalamus,22 and, in the preoptic area/basal forebrain IL-1 inhibits the discharge of wake-active neurons and increases NREM sleep.35
Although evidence briefly summarized above suggests that 5-HT is necessary for sleep, contradictory data suggest, rather, that 5-HT is a wake-promoting or sleep-suppressing neurotransmitter [reviewed in 2,32]. For instance, experimental manipulations that increase 5-HT release and synaptic availability, such as the electrical stimulation of dorsal raphe nuclei, enhance wakefulness.36 In agreement with the interpretation of 5-HT as a wake-inducing substance, blockade of 5-HT2 receptors increases NREM sleep of rats and humans.37 Moreover, neurophysiologic3,38–40 and neurochemical23,41–43 activity of the serotonergic system increases during wakefulness and decreases during sleep. Furthermore, 5-HT inhibits sleep-active presumptive GABAergic neurons in the ventral lateral preoptic area.44 As a unifying hypothesis, it has been proposed that 5-HT released during wakefulness promotes wakefulness, per se, but triggers subsequent sleep through the induction of the synthesis and/or release of as yet to be identified sleep-inducing factors.5
Data from mouse (this study) and rat6,7 suggest that the question is not whether 5-HT specifically promotes NREM sleep or waking, but, rather, that the role played by 5-HT in the regulation of arousal states depends on the degree of activation of the serotonergic system, the specific time-point at which the system is activated, or the interval after the activation itself. Thus, when 5-HT release is enhanced by the administration of low doses of 5-HTP, NREM sleep is inhibited and wakefulness is enhanced. This may be a direct 5-HT effect because it occurs with little delay. Such low doses of 5-HTP may not, however, sufficiently activate the serotonergic system to stimulate synthesis of sleep-inducing factors. With increasing activation of the serotonergic system induced by higher doses of 5-HTP, enhanced 5-HT release may rapidly induce release of some preformed (stored) sleep-inducing factors that counteract 5-HT itself. These higher doses of 5-HTP may also induce de novo synthesis of sleep-inducing factor or factors responsible for the increased NREM sleep occurring later. We have previously hypothesized that the cytokine IL-1 might represent one of the sleep factors through which 5-HT promotes NREM sleep,6,7,22 perhaps by acting at the level of preoptic area/basal forebrain.35
Since rats and mice are highly circadian in nature, the effects of serotonergic activation are strongly modulated by the light:dark cycle. We did not measure 5-HT levels in this study and, as such, do not know if there is a different effect of the phase of the light:dark cycle at which 5-HTP is administered on the conversion of 5-HTP to 5-HT per se. Although this possibility cannot be ruled out, we are unaware of studies demonstrating circadian effects on the synthesis of 5-HT from 5-HTP. The doses of 5-HTP used in this study may be considered to be within physiologic limits; 80 mg/kg of l-tryptophan is equivalent to about 40% of the daily intake in rat.45 Aromatic l-amino acid decarboxylase, the enzyme converting 5-HTP to 5-HT, is not saturated under physiologic conditions.46 As such, increased 5-HTP availability is expected to result in increased 5-HT regardless of the phase of the light-dark cycle. Moreover, 5-HTP administration results in the formation of 5-HT in serotonergic, as well as in catecholaminergic, neurons because both types of neurons express aromatic l-amino acid decarboxylase.46
Although 5-HT formation following 5-HTP administration may be independent of the light-dark cycle, 5-HT release and metabolism depend on neuronal firing.46 At least in cats, activity of raphe neurons is strictly activity and state dependent with no diurnal variation.47 Serotonin release and metabolism in rat are maximal when the animal is most active, i.e., during the dark phase.48 As in rats, mice are nocturnal animals with most sleep occurring during the light phase of the light-dark cycle, whereas, during the dark phase, wakefulness predominates.
Nevertheless, our data indicate that, when 5-HTP is administered at the beginning of the dark period, when mice are most awake, increases in wakefulness are limited to the first 2 hours after injection; it is probably difficult to enhance wakefulness above what could represent a physiologic upper limit. Under these conditions of higher basal levels of wakefulness, the 5-HTP–induced increase in NREM sleep is robust and, after high doses, occurs with short delay. In contrast, when 5-HTP is administered at the beginning of the light period, during which mice sleep the most, wakefulness is increased after low and intermediate doses and with short delay, whereas NREM sleep is enhanced only with intermediate and high doses and with long delay, during the subsequent dark phase. The timing of responses of mice to 5-HTP are strikingly similar to previous data obtained in rats.6,7
In the present study, 5-HTP–induced enhancement of NREM sleep occurs only during the dark period, irrespective of whether administered at dark onset or at light onset. These observations are in close agreement with previous data obtained in rats; NREM sleep enhancement induced by 5-HTP administration into rats only occurs during the dark period.6,7 Observations that, in both rats and mice, increases in NREM sleep following administration of 5-HTP are limited to the dark period suggest that such responses represent a fundamental property of the serotonergic system. Though additional experiments are necessary to determine the precise mechanisms involved, these time-dependent differences in responses to 5-HTP underscore the importance of interactions between circadian and sleep-regulatory mechanisms.
Much is known about the neuroanatomic and neurochemical systems responsible for REM-sleep generation [reviewed49–51]. Serotonergic neurons in the raphe nuclei (as well as noradrenergic neurons in the locus coeruleus) either directly49,50 or indirectly51 inhibit neuronal systems responsible for REM sleep generation. Data in the present study, showing that REM sleep of mice is inhibited by stimulation of the serotonergic system by 5-HTP administration, are in agreement with extensive evidence that experimental and pharmacologic manipulations that increase 5-HT levels in brain inhibit REM sleep [reviewed 2,32]. As such, data obtained in mice are consistent with the current models of REM sleep regulation mentioned above and with previous data obtained from rats.6,7
5-HTP administration into mice initially suppresses REM sleep. Subsequent increases in REM sleep may be considered a physiologic and compensatory rebound. Specifically, the highest 5-HTP dose tested (200 mg/kg), when given to C57BL/6J or IL-6 KO mice at light onset, results in a REM sleep rebound that is remarkably similar in amount and time course to that observed in C57BL/6J mice following 6 hours of sleep deprivation by gentle handling at light onset.20 The REM sleep rebound observed in the present study is also very similar to the rebound observed in C57BL/6J mice following REM sleep inhibition induced by the IP administration of lipopolysaccharide,21 a cell-wall component of gram-negative bacteria and a potent activator of the immune system. Collectively, these data suggest that regardless of these causes of REM sleep loss (activation of the serotonergic system, sleep deprivation by gentle handling, or immune challenge), mice subsequently compensate with increased amounts of REM sleep.
5-HTP administration into mice induces dose-dependent hypothermia, which is more pronounced when given at the beginning of the dark period when body temperatures of mice are normally at their highest. The present data obtained in mice are in agreement with data previously obtained in rats6,7 and suggest that 5-HT may play a role in lowering body and brain temperature. Although the role of 5-HT in thermoregulation is complex, and there is evidence that implicates 5-HT in elevating body temperature [reviewed in 52,53], 5-HT may lower body temperature because this neurotransmitter excites hypothalamic warm-sensitive neurons and inhibits cold-sensitive neurons,54,55 actions that cause heat loss and subsequent decreases in body temperature. In addition, 5-HT stimulates release of pro-opiomelanocortin–derived peptides, such as α-melanocyte-stimulating hormone [α-MSH56,57], which is well established as an endogenous cryogen [e.g.58–60]. As such, it is possible that the hypothermic response to 5-HTP observed in this present study may result from stimulation of α-MSH or other pituitary-derived peptides.
One important aspect of observations of the hypothermic response to serotonergic activation in this and previous studies in rats6,7 is the relationship between sleep and thermoregulation. Although sleep-wake activity and thermoregulation are normally closely associated [reviewed in61], the data in the present study contribute to a growing list of conditions in which these processes may be dissociated [e.g.,25,62 and reviewed in63]. In the present study, 5-HTP reduces core body temperature irrespective of whether NREM sleep is reduced, unchanged, or enhanced. The observation that 5-HTP reduces body temperature implies that maintenance of body temperature need not depend on vigilance state or vice versa.
Although many of the responses of IL-6 KO mice to 5-HTP reported herein are generally similar to those of C57BL/6J mice, we initially hypothesized that mice lacking a functional IL-6 gene would exhibit less wakefulness and more NREM sleep following 5-HTP administration. This hypothesis is based on extant literature indicating that 5-HT upregulates IL-6 mRNA expression in rat hippocampal astrocytes27 and IL-6 stimulates the release of 5-HT in brain.28,29,64,65 Therefore, lack of IL-6 could result in less robust stimulation by 5-HTP of the serotonergic system in brain and, as a consequence, less wakefulness. We do not have data concerning the extent to which 5-HTP increases 5-HT in mouse brain under the conditions of this study, but responses of mice to 100 mg/kg of 5-HTP given at light onset are consistent with our stated hypothesis. Under these conditions, during postinjection hours 1 to 4, wakefulness of C57BL/6J mice increases, whereas wakefulness of IL-6 KO mice is suppressed. Furthermore, the increase in NREM sleep of IL-6 KO mice is proportionally greater following administration of 200 mg/kg of 5-HTP at dark onset than is observed in C57BL/6J mice, consistent with reduced concentrations in brain of wake-promoting 5-HT. Given that IL-6 induces 5-HT in brain of genetically intact mice, it is possible that the brain serotonergic system of IL-6 KO mice may be fundamentally altered. Whether the lack of IL-6 alters basal serotonergic tone with subsequent effects on sleep and wakefulness remains to be determined. However, the observation that IL-6 KO mice exhibit more REM sleep under basal conditions20 is consistent with the hypothesis that 5-HT is reduced in mice lacking IL-6. Nevertheless, results of the present study indicate that IL-6 does not play a critical role in the sleep and temperature responses to increased serotonergic activation by means of 5-HTP.
In conclusion, serotonergic activation by 5-HTP administration into mice results in alterations in sleep-wake behavior and body temperature that are dose and time dependent. The similarities between responses of mice (this study) and of rats6,7 to 5-HTP reinforce our previously stated conclusions that 5-HT is not specifically a wake-promoting or a sleep-promoting neurotransmitter. Instead, 5-HT, although generally promoting wakefulness, may also induce the synthesis or release of as yet to be identified sleep-promoting factors that trigger subsequent NREM sleep. That 5-HTP promotes NREM sleep of rats and mice only during the dark period, irrespective of when administered, also reinforces the idea that the serotonergic system exerts effects on sleep-wake behavior that differ depending on the degree to which the system is stimulated and the precise period after or during which serotonergic activity is increased. We previously hypothesized that the cytokine IL-1 may be one factor by which 5-HT induces subsequent NREM sleep.23,24 The present study demonstrates that lack of the cytokine IL-6 modulates some aspects of, but does not dramatically alter, the impact of serotonergic stimulation by 5-HTP on sleep-wake behavior or body temperatures of mice. As such, future studies of interactions between the IL-6 system and the serotonergic system with respect to sleep-wake behavior may not be warranted.
ACKNOWLEDGMENTS
The authors wish to acknowledge the excellent technical assistance of Ms. Jill Priestley. This study was supported by the National Institutes of Health grant MH64843 and the Department of Anesthesiology at the University of Michigan.
Financial support for this study was provided by the National Institutes of Health grant MH64843 to MRO and LI.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Brodie BB, Pletscher A, Shore PA. Evidence that serotonin has a role in brain function. Science. 1955;122(3177):968. doi: 10.1126/science.122.3177.968. [DOI] [PubMed] [Google Scholar]
- 2.Adrien J. The serotoninergic system and sleep-wakefulness regulation. In: Kales A, editor. The Pharmacology of Sleep. Berlin: Springer-Verlag; 1995. pp. 91–116. [Google Scholar]
- 3.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Jouvet M, Sallanon M, Petitjean F, Boblier P. Serotonergic and non-serotonergic mechanisms in sleep. In: Gibson CJ, Chase MH, editors. Sleep Disorders: Basic and Clinical Research. New York: Spectrum; 1983. pp. 557–571. [Google Scholar]
- 5.Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24s–27s. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 6.Imeri L, Mancia M, Bianchi M, Opp MR. 5-hydroxytryptophan, but not L-tryptophan, alters sleep and brain temperature in rats. Neuroscience. 2000;95(2):445–452. doi: 10.1016/s0306-4522(99)00435-2. [DOI] [PubMed] [Google Scholar]
- 7.Imeri L, Bianchi S, Opp MR. Antagonism of corticotropin-releasing hormone alters serotonergic-induced changes in brain temperature, but not sleep, of rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R1116–R1123. doi: 10.1152/ajpregu.00074.2005. [DOI] [PubMed] [Google Scholar]
- 8.Boutrel B, Franc B, Hen R, Hamon M, Adrien J. Key Role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. J Neurosci. 1999;19(8):3204–3212. doi: 10.1523/JNEUROSCI.19-08-03204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Gimenez JF, Tecott LH, Palacios JM, Mengod G, Vilaro MT. Serotonin 5- HT (2C) receptor knockout mice: autoradiographic analysis of multiple serotonin receptors. J Neurosci Res. 2002;67(1):69–85. doi: 10.1002/jnr.10072. [DOI] [PubMed] [Google Scholar]
- 10.Boutrel B, Monaca C, Hen R, Hamon M, Adrien J. Involvement of 5-HT1A receptors in homeostatic and stress-induced adaptive regulations of paradoxical sleep: studies in 5-HT1A knock-out mice. J Neurosci. 2002;22(11):4686–4692. doi: 10.1523/JNEUROSCI.22-11-04686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monaca C, Boutrel B, Hen R, Hamon M, Adrien J. 5-HT 1A/1B receptor-mediated effects of the selective serotonin reuptake inhibitor, citalopram, on sleep: studies in 5-HT 1A and 5-HT 1B knockout mice. Neuropsychopharmacology. 2003;28(5):850–856. doi: 10.1038/sj.npp.1300109. [DOI] [PubMed] [Google Scholar]
- 12.Popa D, Lena C, Fabre V, et al. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25(49):11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisor JP, Wurts SW, Hall FS, et al. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. NeuroReport. 2003;14(2):233–238. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- 14.Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- 15.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J Clin Endocrinol Metab. 2004;89(9):4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 18.Späth-Schwalbe E, Hansen K, Schmidt F, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- 19.Hogan D, Morrow JD, Smith EM, Opp MR. Interleukin-6 alters sleep of rats. J Neuroimmunol. 2003;137(1-2):59–66. doi: 10.1016/s0165-5728(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin-6-deficient mice to sleep deprivation. Brain Behav Immun. 2005;19(1):28–39. doi: 10.1016/j.bbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav Immun. 2005;19(1):40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Gemma C, Imeri L, Opp MR. Serotonergic activation stimulates the pituitary-adrenal axis and alters interleukin-1 mRNA expression in rat brain. Psychoneuroendocrinology. 2003;28(7):875–884. doi: 10.1016/s0306-4530(02)00103-8. [DOI] [PubMed] [Google Scholar]
- 23.Imeri L, Mancia M, Opp MR. Blockade of 5-HT2 receptors alters interleukin-1-induced changes in rat sleep. Neuroscience. 1999;92:745–749. doi: 10.1016/s0306-4522(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 24.Imeri L, Bianchi S, Mancia M. Muramyl dipeptide and IL-1 effects on sleep and brain temperature after inhibition of serotonin synthesis. Am J Physiol Regul Integr Comp Physiol. 1997;273(5):R1663–R1668. doi: 10.1152/ajpregu.1997.273.5.R1663. [DOI] [PubMed] [Google Scholar]
- 25.Gemma C, Imeri L, De Simoni MG, Mancia M. Interleukin-1 induces changes in sleep, brain temperature, and serotonergic metabolism. Am J Physiol Regul Integr Comp Physiol. 1997;272(2):R601–R606. doi: 10.1152/ajpregu.1997.272.2.R601. [DOI] [PubMed] [Google Scholar]
- 26.Silverman DHS, Imam K, Karnovsky ML. Muramyl peptide/serotonin receptors in brain-derived preparations. Peptide Res. 1989;2(5):338–344. [PubMed] [Google Scholar]
- 27.Pousset F, Fournier J, Legoux P, Keane P, Shire D, Soubrie P. Effect of serotonin on cytokine mRNA expression in rat hippocampal astrocytes. Mol Brain Res. 1996;38:54–62. doi: 10.1016/0169-328x(95)00324-l. [DOI] [PubMed] [Google Scholar]
- 28.Barkhudaryan N, Dunn AJ. Molecular mechanisms of actions of interleukin-6 on the brain, with special reference to serotonin and the hypothalamo-pituitary-adrenocortical axis. Neurochem Res. 1999;24(9):1169–1180. doi: 10.1023/a:1020720722209. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Dunn AJ. Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem Int. 1998;33:143–154. doi: 10.1016/s0197-0186(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Dunn AJ. The role of interleukin-6 in the activation of the hypothalamo-pituitary-adrenocortical axis and brain indoleamines by endotoxin and interleukin-1β. Brain Res. 1999;815:337–348. doi: 10.1016/s0006-8993(98)01091-9. [DOI] [PubMed] [Google Scholar]
- 31.Flaherty L. Congenic strains. In: Foster HL, Small JD, Fox JG, editors. The Mouse in Biomedical Research: History, Genetics, and Wild Mice. New York: Academic Press; 1981. pp. 215–221. [Google Scholar]
- 32.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6(1):55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 33.Denoyer M, Sallanon M, Kitahama K, Aubert C, Jouvet M. Reversibility of para-chlorophenylalanine-induced insomnia by intrahypothalamic microinjection of L-5-hydroxytryptophan. Neuroscience. 1989;28(1):83–94. doi: 10.1016/0306-4522(89)90234-0. [DOI] [PubMed] [Google Scholar]
- 34.Khateb A, Fort P, Alonso A, Jones BE, Muhlethaler M. Pharmacological and immunohistochemical evidence for serotonergic modulation of cholinergic nucleus basalis neurons. Eur J Neurosci. 1993;5(5):541–547. doi: 10.1111/j.1460-9568.1993.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 35.Alam MN, McGinty D, Bashir T, et al. Interleukin-1β modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20(1):207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 36.Cespuglio R, Gomez ME, Walker E, Jouvet M. Effets du refroidissement et de la stimulation des noyaux du systeme du raphe sur les etats de vigilance chez le chat. Electroencephalogr Clin Neurophysiol. 1979;47:289–308. doi: 10.1016/0013-4694(79)90281-5. [DOI] [PubMed] [Google Scholar]
- 37.Dugovic C, Meert TF, Ashton D, Clincke GHC. Effects of ritanserin and chlordiazepoxide on sleep-wakefulness alterations in rats following chronic cocaine treatment. Psychopharmacology. 1992;108:263–270. doi: 10.1007/BF02245110. [DOI] [PubMed] [Google Scholar]
- 38.Cespuglio R, Faradji H, Gomez ME, Jouvet M. Single unit recordings in the nuclei raphe dorsalis and magnus during sleep-waking cycle of semi-chronic prepared cats. Neurosci Lett. 1981;24:133–138. doi: 10.1016/0304-3940(81)90236-6. [DOI] [PubMed] [Google Scholar]
- 39.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 40.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 41.Cespuglio R, Sarda N, Gharib A, et al. Voltammetric detection of the release of 5-hydroxyindole compounds throughout the sleep-waking cycle of the rat. Exp Brain Res. 1990;80:121–128. doi: 10.1007/BF00228853. [DOI] [PubMed] [Google Scholar]
- 42.Portas CM, McCarley RW. Behavioral state-related changes of extracellular serotonin concentration in the dorsal raphe nucleus: a microdialysis study in the freely moving cat. Brain Res. 1994;648:306–312. doi: 10.1016/0006-8993(94)91132-0. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson LO, Auerbach SB, Jacobs BL. Extracellular serotonin levels change with behavioral state but not with pyrogen- induced hyperthermia. J Neurosci. 1991;11:2732–2741. doi: 10.1523/JNEUROSCI.11-09-02732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallopin T, Fort P, Eggermann E, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404(6781):992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 45.Bakalian MJ, Fernstrom JD. Effects of L-tryptophan and other amino acids on electroencephalographic sleep in the rat. Brain Res. 1990;528:300–307. doi: 10.1016/0006-8993(90)91671-3. [DOI] [PubMed] [Google Scholar]
- 46.Frazer A, Hensler JG. Serotonin. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic Neurochemistry. New York: Raven Press; 1994. pp. 283–308. [Google Scholar]
- 47.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: lack of diurnal variation. Neurosci Lett. 1983;36(3):285–290. doi: 10.1016/0304-3940(83)90014-9. [DOI] [PubMed] [Google Scholar]
- 48.Faradji H, Cespuglio R, Jouvet M. Voltammetric measurements of 5-hydroxyindole compounds in the suprachiasmatic nuclei: circadian fluctuations. Brain Res. 1983;279(1–2):111–119. doi: 10.1016/0006-8993(83)90168-3. [DOI] [PubMed] [Google Scholar]
- 49.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26(11):578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3(8):591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 51.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 52.Myers RD. Serotonin and thermoregulation: Old and new views. J Physiol (Paris) 1981;77:505–513. [PubMed] [Google Scholar]
- 53.Rothwell NJ, Stock MJ. Effect of diet and fenfluramine on thermogenesis in the rat: possible involvement of serotonergic mechanisms. Int J Obes. 1987;11:319–324. [PubMed] [Google Scholar]
- 54.Jell RM. Responses of hypothalamic neurones to local temperature and to acetylcholine, noradrenaline and 5-hydroxytryptamine. Brain Res. 1973;55(1):123–134. doi: 10.1016/0006-8993(73)90492-7. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T, Morimoto A, Murakami N. Effect of amine on temperature-responsive neuron in slice preparation of rat brain stem. Am J Physiol. 1986;250(4 Pt 2):R553–R559. doi: 10.1152/ajpregu.1986.250.4.R553. [DOI] [PubMed] [Google Scholar]
- 56.Fuller RW. Serotonergic stimulation of pituitary-adrenocortical function in rats. Neuroendocrinology. 1981;32:118–127. doi: 10.1159/000123142. [DOI] [PubMed] [Google Scholar]
- 57.Carr JJ, Saland LC, Samora A, Benavidez A, Krobert K. In vivo effects of serotonergic agents on alpha-melanocyte-stimulating hormone secretion. Neuroendocrinology. 1991;54:616–622. doi: 10.1159/000125968. [DOI] [PubMed] [Google Scholar]
- 58.Feng JD, Dao T, Lipton JM. Effects of preoptic microinjections of α-MSH on fever and normal temperature control in rabbits. Brain Res Bull. 1987;18(4):473–477. doi: 10.1016/0361-9230(87)90111-0. [DOI] [PubMed] [Google Scholar]
- 59.Murphy MT, Richards DB, Lipton JM. Antipyretic potency of centrally administered α-melanocyte stimulating hormone. Science. 1983;21:192–193. doi: 10.1126/science.6602381. [DOI] [PubMed] [Google Scholar]
- 60.Opp MR, Krueger JM. Effects of α-MSH on sleep, behavior, and brain temperature: interactions with IL-1. Am J Physiol. 1988;255:R914–R922. doi: 10.1152/ajpregu.1988.255.6.R914. [DOI] [PubMed] [Google Scholar]
- 61.Heller HC, Glotzbach S, Grahn D, Radeke C. Sleep-dependent changes in the thermoregulatory system. In: Lydic R, Biebuyck JF, editors. Clinical Physiology of Sleep. Bethesda: American Physiological Society; 1988. pp. 145–158. [Google Scholar]
- 62.Imeri L, Bianchi S, Angeli P, Mancia M. Stimulation of cholinergic receptors in the medial preoptic area affects sleep and cortical temperature. Am J Physiol. 1995;269(2 Pt 2):R294–R299. doi: 10.1152/ajpregu.1995.269.2.R294. [DOI] [PubMed] [Google Scholar]
- 63.Krueger JM, Takahashi S. Thermoregulation and sleep: closely linked but separable. In: Blatteis CM, editor. Annals of the New York Academy of Sciences; Thermoregulation: Proceedings of the 10th International Symposium on the Pharmacology of Thermoregulation; New York: New York Academy of Sciences; 1997. pp. 281–286. [DOI] [PubMed] [Google Scholar]
- 64.Wang P, Barks JDE. Tat, a human immunodeficiency virus-1-derived protein, augments excitotoxic hippocampal injury in neonatal rats. Neuroscience. 1999;88:585–597. doi: 10.1016/s0306-4522(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y, Shaghaghi EK, Jacquot C, Pallardy M, Gardier AM. Synergism between interleukin-6 and interleukin-1β in hypothalamic serotonin release: a reverse in vivo microdialysis study in F344 rats. Eur Cytokine Net. 1999;10(1):57–63. [PubMed] [Google Scholar]