Abstract
Objective:
We report a series of seven consecutive cases of catathrenia (sleep related groaning) that differ from limited previous reports in the literature with regard to sleep stage and response to treatment.
Background:
Catathrenia was recently defined as a parasomnia in the International Classification of Sleep Disorders Diagnostic and Coding Manual (ICSD-2), but there is debate about its classification, and its response to CPAP is unknown.
Methods:
We present 7 consecutive patients presenting with catathrenia over a 5-year period. They were all young women, ranging in age from 20 to 34 years with a body mass index (BMI) <25. They underwent standard clinical evaluation, questionnaires, physical exam, craniofacial evaluations, and nocturnal polysomnography. All seven were titrated on continuous passive airway pressure (CPAP) treatment for sleep disordered breathing then offered surgical treatment if unable to tolerate or adhere to CPAP recommendations.
Results:
Groaning was present throughout all stages of sleep. The mean (SD) AHI and RDI were 3.2 (0.56) and 13.1 (2.4) respectively. CPAP resolved groaning in all cases. 5 patients (71%) elected subsequent surgical intervention. Three of the 4 that followed up after surgery required adjuvant oral appliance treatment, but all four ultimately had resolution of groaning.
Conclusions:
Catathrenia may have subtypes related to sleep stage specificity or presence of sleep disordered breathing. In our heterogeneous group of non-obese women with a normal AHI and elevated RDI, CPAP and select soft tissue surgeries of the upper airway (often augmented with an oral appliance) successfully treated nocturnal groaning.
Citation:
Guilleminault C; Hagen CC; Khaja AM. Catathrenia: parasomnia or uncommon feature of sleep disordered breathing?. SLEEP 2008;31(1):132-139.
Keywords: Cathatrenia, parasomnia, NREM sleep, sleep disordered breathing, nasal CPAP, nomenclature
INTRODUCTION
CATATHRENIA, (SLEEP RELATED GROANING) WAS RECENTLY INTRODUCED INTO THE AMERICAN ACADEMY OF SLEEP MEDICINE NOMENCLATURE WITH ITS inclusion into The International Classification of Sleep Disorders Diagnostic and Coding Manual (ICSD-2) as a parasomnia.1 The quality of groaning in catathrenia is monotone, and often presents with a morose or sexual connotation, causing a social problem for some patients. The term catathrenia comes from combining two Greek words kata (below) and threnia (to lament) referring to the morose connotation typically encountered. The differential diagnosis includes sleep talking (somniloquy), stridor, moaning during epileptic seizures, laryngospasm, and snoring.
Clearly a rare disorder, its actual incidence and prevalence are unknown, and a Medline search yielded a limited number of cases presented in the literature thus far.2–6 A positive family history was noted in 14.8% of cases presented by Oldani et al.4 Thus far, most reports found a majority of expiratory groaning occurring during REM sleep, while others reported that it occurred exclusively during REM sleep.3–5 Both catathrenia's underlying mechanism and ISCD-2 classification are the subject of discussion within the literature.3,5,6 Its entry in ICSD-2 is qualified by the statement that information is needed about its response to continuous positive airway pressure (CPAP) treatment.1
The absence of general population studies for such a rare condition results in most reports coming from clinical data. As with many parasomnias, more severe cases that burden family or bed partners prompt clinical attention and are included in clinically based case reports, thereby underrepresenting milder forms in the literature. Despite these limitations, we present a case series of 7 patients. We report novel findings regarding stage of sleep specificity and treatment response that contrast significantly with previously reported cases and series.
SUBJECTS
Seven patients presented to the Stanford Sleep Disorders Clinic over a 5-year span, with a chief complaint of involuntary groaning during sleep that was brought to their attention by family members. All patients were women ranging in age from 20 to 34 years (mean 26.7). Additional demographic data is presented in table 1. Their groaning was a nightly occurrence at the time of consultation and was considered a significant problem for various social reasons.
Table 1.
Patient Demographics and Clinical Evaluation
| Patient | Age | BMI | Reason for Consult | Onset of groaning | Associated Symptoms | History | Craniofacial examination | ESS |
|---|---|---|---|---|---|---|---|---|
| 1 | 23 | 23 | Social problem, Family and bed partner. | Childhood | Snoring, Mouth breathing, Dry mouth | Wisdom teeth extracted, orthodontic treatment, nasal allergies. | Long narrow face, Mallampati 3, tonsils 3+, 3 mm overjet, high narrow hard palate | 5 |
| 2 | 34 | 24 | Family problem; loud with sexual connotation | Childhood | Dry mouth | Somniloquy; somnambulation (2 to 14 years of age); bruxism; childhood adeno-tonsillectomy. | Mandibular deficiency, Mallampati 4, tonsils 2+, deviated nasal septum, collapse of internal valve, 4 mm overjet | 6 |
| 3 | 28 | 24 | Social problem— family and husband. | 12 years of age | Mouth breathing; dry mouth, morning headache. | Orthodontics; enuresis at 6–7 yr; nasal allergies. | Maxillo-mandibular deficiency, Mallampati 3, high narrow hard palate, no overjet, enlarged turbinates, tonsils 2+ | 4 |
| 4 | 20 | 22 | Social problem—, family and bed partner | 9 years of age | Mouth breathing; fatigue | Wisdom teeth extracted; sleep terrors as a child (18 months to 4 years of age.) | Mandibular and maxillary deficiency, Mallampati 2, high narrow hard palate, long face, small chin, no overjet, tonsils 2+ | 3 |
| 5 | 31 | 23 | Social problem | Childhood, (absent during pregnancy) | Mouth breathing; sleep disruption; bouts of insomnia | Impacted wisdom teeth; depression; nasal allergies. | Mandibular deficiency, Mallampati 3, has long face, overjet 4-5 mm, tonsils 1+ | 4 |
| 6 | 26 | 23 | Social problem | 14 years of age | Mouth breathing, Dry mouth | Orthodontics, Incisor extractions. | Mandibular deficiency, Mallampati 4, 3 mm overjet, tonsils 2+. | 5 |
| 7 | 25 | 23 | Social problem, particularly loud volume | 12–13 years of age | Mouth breathing; dry mouth; fatigue | None mentioned | Mandibular and maxillary problems, long face, Mallanpati 3, no overjet, tonsils 2+, presence of redundant soft tissue in palate, long uvula. | 4 |
BMI = body mass index (in kg/m2), ESS = Epworth sleepiness scale
METHODS
All patients completed the same clinical evaluation. They completed standardized forms including the Sleep Disorder Questionnaire7 and the Epworth Sleepiness Scale,8 clinical interview, and physical examination. Past medical history and medication histories were reviewed. A detailed sleep history was obtained. Physical evaluation included body mass index, neck circumference, and standardized scales for the evaluation of head and neck anatomy such as the Friedman tonsil scale9 and the Mallampati scale.10 Patients were also examined by an ENT and craniofacial specialist during a sleep surgery specialty clinic. Septum deviation was evaluated, and inferior nasal turbinate size was scored between 1 and 3 (1 indicating nearly nonexistent and 3 indicating very enlarged). Nasal evaluation also included an assessment for internal nasal valve collapse and normal or abnormal external nasal valves.11 Facial features were also analyzed following scales derived from Kholar and Salter12 scoring the presence of a mandible in normal position, retroplaced, or with prognathism, as well as presence of a maxillary deficiency or normal position, (based on physical exam and reexamination of frontal and lateral photos obtained at the initial visit). Position of the posterior molars in one of the defined 3 dental classes was determined.13
All subjects completed one night of nocturnal polysomnography and bed partners were asked to complete a sleep log for the 10 preceding nights noting the presence or absence of groaning and time of onset during the night.
Polysomnography
All subjects had the same montage that included eight EEG channels (C3/A2, C4/A1, Fp1/T3, Fp2/T4, T3/O1, T4/O2, O1/C3, O2/C4), two channels for right and left eye movements, chin EMG and 2 leg EMGs, one ECG derivation (modified V2 lead), body position, and a sound recording channel. Respiration was monitored with neck microphone, finger pulse oximetry, nasal cannula-pressure transducer, oral thermistor, and chest and abdominal piezoelectric bands. Sounds were continuously monitored through a room microphone and a videotape was obtained during the entire recording. Polysomnograms were scored by two scorers unaware of the patient's history, and all scored records were reviewed by the same sleep specialist.
Sleep was scored following Rechtschaffen and Kales,14 but also included identification of short (>3-sec) EEG arousals.15 Apneas and hypopneas were scored following published definitions to determine an apnea-hypopnea index (AHI).16 Presence of flow limitation was determined as previously described.17 Flow limitation was scored when there was a change in the nasal cannula flow pattern (either “flattening” of the nasal curve or indication of abnormal decrease in flow during inspiration).18,19 Each event of flow limitation had to persist at least 4 successive breaths, and the decrease in flow must not have been more than 30% compared to basal normal nasal breathing. The number of events called “flow limitation” was tabulated over the total sleep time in parallel with the apnea and hypopnea scoring; even if the “flow limitation” lasted several minutes, the “event” was only scored as one event. The combination of AHI and episodes of flow limitation combined are reported as the respiratory disturbance index (RDI).
Treatment Trial
Based on findings from the above evaluation (anatomic findings) and polysomnograms (indicating abnormal RDI and flow limitation) all 7 patients began nasal CPAP treatment after one full night of nasal CPAP titration using the previously described montage, but replacing the nasal cannula pressure transducer with a CPAP flow output. CPAP titration goals included both a reduction of hypopneas and elimination of flow limitation. Effects of CPAP use and treatment compliance were monitored with continued bed partner reporting of the presence or absence of catathrenia, and downloadable data from the patients' CPAP machines (Table 3).
Table 3.
Treatment and Response to Therapy
| Patient | CPAP Pressure (cm of water) | Groaning Response during CPAP | Surgical intervention | Oral appliance | Response to surgery/oral appliance | Follow-up duration/Result |
|---|---|---|---|---|---|---|
| 1 | 7 | Resolution | Adenotonsillectomy | Yes | Resolution with combined treatment | 3 years/Resolved |
| 2 | 9 | Resolution | None | No | NA | 12 months/ Resolved with CPAP nightly |
| 3 | 8 | Resolution | Tonsillectomy, pharyngoplasty | Yes | Persistent postoperatively, but resolved with combined therapy | 3 years/Resolved |
| 4 | 8 | Resolution | Adenotonsillectomy, septoplasty, and turbinate reduction. | No | Significantly reduced groaning | 3 years/Resolved |
| 5 | 10 | Resolution | None | No | NA | One month/ Ambivalent about CPAP |
| 6 | 10 | Resolution | Adenotonsillectomy, pharyngoplasty, septoplasty | Yes | Improved with oral appliance, resolved with combined treatment | 3 years/Resolved |
| 7 | 7 | Resolution | UPPP, after consultation at an outside center | No | Unknown | No follow-up. |
CPAP = continuous positive airway pressure; cm = centimeter; UPPP = uvulopalato-pharyngoplasty
Six out of the seven subjects did not want continued nasal CPAP as treatment for their catathrenia or SDB. As there was anatomic evidence of a small upper airway and presence of inspiratory flow limitation, we suggested proceeding to surgical treatment for these documented abnormalities. One underwent uvulopalatopharyngoplasty at an outside facility and did not return for follow-up. Five others had upper airway surgery. Three of these used an adjunct oral appliance device after surgery.
Analysis
Descriptive statistics include the mean and standard deviation (Microsoft, Redmond Washington). Additional statistical analysis of the response to treatment was deemed inappropriate given the small sample size and categorical nature of the response to treatment.
RESULTS
Demographic results are summarized in Table 1, as is the history related to the problem and past medical history. Interestingly, 3 (43%) of individuals had a personal history of another parasomnia in childhood, and 6 (86%) had prior orthodontic intervention. Five (71%) had tooth extraction (mostly third molar) during their teenage years, usually an indicator of small jaw structure. Two subjects (29%) reported another family member with likely catathrenia, and both thought it to be of lesser severity than their own.
All subjects had an abnormally high Mallampati scale score and some evidence of a small jaw. Table 1 presents results of the craniofacial evaluations and anatomic scales.
Table 2 presents the results of the ESS and polysomnograms. Mean (SD) on the ESS was 4.4 (0.98). The mean (SD) AHI and RDI were 3.2 (0.56) and 13.1 (2.4) respectively. All patients had a normal ESS and an AHI less than 5, and therefore an absence of obstructive sleep apnea syndrome (OSAS). Nasal cannula pressure transducers permit detection of flow limitation, and this demonstrated flow limitation and an abnormal RDI in all subjects despite their normal AHI. No important oxygen saturation (SpO2) drop was noted, as the lowest monitored value during total sleep time was 92% in one subject.
Table 2.
Polysomnographic Findings
| Patient | TST | NREM % | REM % | AHI | RDI | Min O2 Sat | Groaning* Epochs NREM/REM | Features |
|---|---|---|---|---|---|---|---|---|
| 1 | 379 | 86 | 16 | 3 | 11 | 93 | 599/40 | Expiratory groaning, ↓ in REM |
| 2 | 392 | 72 | 18 | 4 | 14 | 92 | 501/47 | Expiratory groaning, pushes tongue out, hypopneas during REM |
| 3 | 371 | 79 | 21 | 3.2 | 9 | 94 | 514/56 | Expiratory groaning, sound ↓ during REM, hypopneas during REM. |
| 4 | 407 | 81 | 19 | 2.5 | 14 | 93 | 587/66 | Expiratory groaning, sound ↓ in REM, hypopneas during REM |
| 5 | 366 | 84 | 16 | 4 | 16 | 92 | 572/36 | Expiratory groaning, hypopneas during REM. |
| 6 | 391 | 82 | 18 | 3 | 13 | 93 | 568/54 | Expiratory groaning, louder supine, hypopneas during REM. |
| 7 | 360 | 84 | 16 | 3 | 15 | 93 | 517/42 | Expiratory groaning, loud in NREM, hypopneas in supine REM. |
TST = total sleep time, NREM = non rapid eye movement sleep, REM = rapid eye movement sleep, Sat. = saturation, AHI = apnea/hypopnea index, RDI = respiratory disturbance index
Number of 30-second epochs with at least 2 breaths containing a monotone non-snoring sound
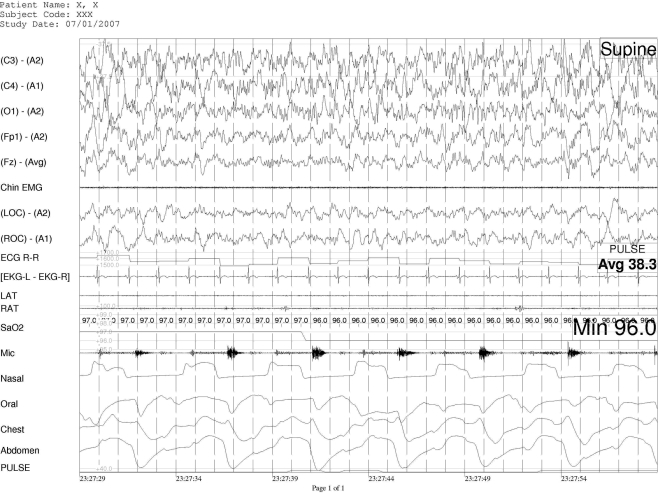
No snoring was noted on the neck microphone during the recording, but a monotone groan was found in all subjects. It was present throughout expiration, with a different intensity and pitch for each patient. In one patient, as reported by their family members, the sound emitted was quite loud and produced a distinct sexual connotation. The video camera was zoomed on face and mouth during segments of sleep in each patient; this demonstrated that the mouth was always partially opened during this expiratory sound. Sound was interrupted by an EEG arousal and re-occurred immediately following continuous stage 1 or presence of stage 2. Pitch and frequency of occurrence did not change during stages 3–4 NREM sleep (see Figures 1 and 2). During REM sleep there was a variability of pitch and volume reduction to the point of being barely audible during bursts of rapid eye movements (phasic REM sleep).
Figure 1.
Thirty-second example of expiratory groaning during NREM sleep in patient #3
Legend: The groaning is recorded on the microphone (mic) channel, it is seen in the expiratory phase (inspiration on nasal cannula, mouth thermistor, chest and abdominal channels is presented as an upward movement.).
Channels from top to bottom are: EEG (5 channels), chin EMG, Electro-oculogram (2 channels) ECG and RR interval, leg EMG (2 channels), microphome, nasal cannula, mouth thermistor, chest and abdominal piezoelectric band, pulse oximetry.
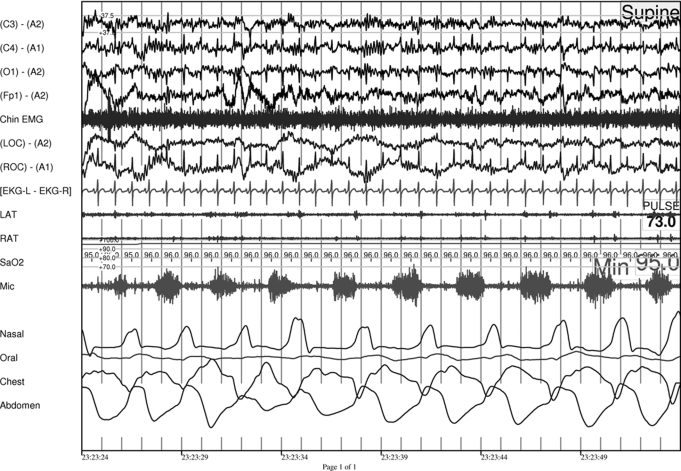
Figure 2.
Thirty-second example of expiratory groaning during NREM sleep in patient #2
Note the presence of some degree of flow limitation shown on the nasal cannula (inspiration is presented as an upward movement). (Groaning is recorded on microphone: mic.)
Legend: Channels from top to bottom are: EEG (4 channels) chin EMG, EOG (2 channels) ECG (1 channel), EOG (2 channels), oxygen saturation, microphone, nasal cannula, mouth thermistor, chest and abdominal piezoelectric bands
Treatment Trial
All patients agreed to have nasal CPAP titration in the laboratory, and following the titration agreed to use nasal CPAP at home for one month. Results of treatment with nasal CPAP are presented in Table 3. When used, nasal CPAP eliminated the expiratory sound. The elimination of the sound persisted and was confirmed at home by bed partner report during the month-long trial. Except for one patient (#2) who was particularly burdened by the sexual connotation produced by her catathrenia, none of the other subjects continued CPAP treatment. Two patients were lost for further follow-up (#5 and #7), but one of them (#7) underwent uvulopalatopharyngoplasty performed by a community surgeon without additional follow-up in our clinic. Four subjects sought surgical intervention or oral appliance therapy from providers affiliated with our sleep clinic and returned for further follow-up after these interventions. In the 5 patients that had long-term follow-up (4 post surgery and one with nasal CPAP), treatment of the flow limitation (and small upper airway) resolved the expiratory groaning sounds for >3 years in each case.
DISCUSSION
Our 7 catathrenia patients had a relatively homogeneous presentation (BMI, female, normal AHI with abnormal RDI, and groaning throughout REM and NREM sleep) and they all responded to CPAP. Groaning responded to both CPAP and soft tissue surgeries such as adenotonsillectomy—either alone or in conjunction with an oral appliance. Strengths of our study include a thorough craniofacial examination which identified findings of a narrow upper airway in all seven patients and aggressive CPAP titrations with the intention of resolving flow limitation. We were also persistent in our efforts and provided treatment options for their catathrenia and sleep disordered breathing if they were unable to tolerate or adhere to CPAP recommendations.
Like other case series, we are limited by a selection bias that influences who presents for treatment and at what point in their lifespan. Obviously, our data should not be mistaken for epidemiologic data or a description of the typical catathrenia presentation, as this is still unclear for this rare disorder. Unlike previous reports, all of our patients were non-obese women with an elevated RDI, but a normal AHI. Other reports have presented a broad range of catathrenia patients, including men and patients with and without OSA. All of these have indicated a poor response with various medication trials including clonazepam, gabapentin, pramipexole, carbamazepine, trazodone, paroxetine and dosulepine, therefore we did not offer pharmacologic treatment options.2–4,6
It is unclear how our patients would respond to our treatment plan if they were a heterogeneous group of men and women with higher BMIs and more variable SDB severity. Iriarte et al3 presented a 62-year-old woman with OSA and catathrenia that responded to CPAP. Combining this report with our response in patients with SDB we suspect that men and women with catathrenia and obstructive sleep apnea may respond to CPAP therapy despite earlier isolated reports to the contrary. No data are currently available on how catathrenia will respond to soft tissue surgeries in this broader population.
ICSD-2 is unclear about the timing of the groaning during sleep, as it indicates in its diagnostic criteria that it is present in “sleep” or “REM sleep.” One may question if vocalizations during REM sleep might be mistakenly identified as catathrenia in some patients. This clearly did not occur in our series as groaning was quite striking and present throughout NREM and REM sleep (Table 2). Our cases are clearly different from those reported by Oldani et al2 and Vetrugno et al5 wherein they emphasized that nocturnal groaning occurred occasionally in stage 2 NREM sleep, but predominantly during REM sleep. We observed a decrease in intensity or volume of the groaning during REM. Although contradictory to earlier reports of worsening in REM or specificity to REM, our findings are more consistent with the groaning noted by Iriarte et al, which was present throughout the entire night and in all stages of sleep.3 These differences in presentation raise new questions regarding heterogeneity within catathrenia. It is unclear whether different types of catathrenia are being described in the literature which may ultimately be discerned by the presence or absence of OSA, or its REM specificity.
One may question why 5 (71%) of our patients elected surgical intervention rather than accepting nasal CPAP as an indefinite nightly treatment for their problem. We and others have previously noted that nasal CPAP is not an easily accepted treatment for any type of SDB, if excessive sleepiness is not present. For example, we have seen some women with complaints of insomnia and mild OSA or upper airway resistance syndrome quickly reject nasal CPAP treatment despite indication of improvement of their daytime fatigue. These women have then opted for nasal and pharynx soft tissue surgeries and/or dental appliances20. The rationale for their switch in treatments was that nasal CPAP was a new cause of sleep disturbance. In the current study, subjects did not suffer from a type of sleep disturbance that they sensed themselves; rather the original pressure to seek treatment was essentially a response to third party or family pressure. Like subjects mentioned above, the amount of sleep disordered breathing and sleep fragmentation was improved with CPAP, but the treatment itself introduced a new and perceptible sleep disturbance experienced as a persistent nightly nuisance and inconvenience that did not abate after a one month trial. Availability of treatment alternatives in our clinic led them to consider other treatment avenues.
The groaning we observed was remarkably loud and gave the appearance of strain or effort. We question whether this is an expiratory behavior similar to vocalizations from tennis players, weight lifters, and other athletes during exertion. We did not monitor expiratory muscles, such as abdominal muscle activity, to see if there was an abnormal expiratory muscle contraction with the groaning. Groaning was lower in volume during REM sleep but present in all stages, which supports a role for reduced expiratory muscle activity during REM sleep related atonia. It must be emphasized that none of the patients had OSAS, and none complained of sleepiness. However, they clearly had flow limitation at the nasal cannula recording; with the addition of episodes of flow limitation incorporated into the scoring, all had an abnormal RDI. All of our patients had signs of a narrow upper airway and small jaws: history of wisdom teeth extraction during teenage years, Mallampatti scale ratings of 3 and 4, and presence of small mandible or maxilla. Recognition of small jaws can be early in life as 60% of the adult face is built by 4 years of age. As reported by other groups, groaning in our subjects was also noted early in life and one may question if it was not an adaptive behavior to enhance normal breathing. We speculate that the groaning may therefore represent a positive end-expiratory pressure maneuver used to maintain airway patency at end expiration, preparing the upper airway for inspiration, and maintaining a valid inspiratory flow.
Distress from the social impact of their groaning, rather than a concern for their health, was the primary motivation for seeking treatment in each of our patients. One patient (#2) elected to continue with CPAP therapy for her catathrenia and UARS for the past 3½ years while none of the other subjects wished to keep nasal CPAP. Her loud groaning had a conspicuous sexual connotation which was observed not only by her husband, but also her two daughters or anyone else that may be nearby. We highlight this aspect, as her case demonstrates the type of social impact that catathrenia can have on an individual and their household. Guilleminault et al already reported the relationship between SDB and distressing sexually related noises in 2002.21 Such noises may have a familial pattern. Two of our 7 cases had a positive history of sleepwalking or sleep terrors and a third was enuretic during childhood, again possibly related to an association between parasomnias and SDB. Recently, Schenck et al reviewed the published literature on the association between sleep disorders and sexual behavior related to sleep.22 Atypical sexual behavior during sleep may be related to NREM sleep parasomnia, epilepsy, and REM behavior disorder. The comorbidity of parasomnias with abnormal breathing during sleep is not uncommon. Multiple studies have demonstrated the association between parasomnias and sleep disordered breathing indicating that it is most common in this population for both children and adults.23–28 Guilleminault et al have shown that NREM sleep instability, indicated by increased amounts of cyclic alternating pattern type A2 and A3, may predispose the occurrence of parasomnias in both adults and children.25,26
Our patients' groaning responded completely as long as they were using CPAP. Iriarte et al reported improvement in groaning in their one patient, but other groups reported poor responses to CPAP therapy.3–6 We speculate this discrepancy may result from different strategies employed during CPAP titration. We have extensive experience treating women with upper airway resistance syndrome and we are aware that in pre-menopausal women nasal CPAP pressures well above 8 cm of water are often needed. The narrowest segment of the upper airway is typically behind the base of the tongue in these cases and is difficult to maintain patent at lower pressures. Our goal during CPAP titration was to achieve resolution of flow limitation which often arises from this stricture point. Pursuing this, we were somewhat surprised at the amount of positive pressure needed to eliminate flow limitation, but pleased to find the associated resolution of catathrenia.
With the exception of one patient, most in our series sought surgical treatment alternatives despite their favorable response to CPAP. Of those pursuing surgery, three required addition of an oral appliance to achieve adequate improvement in upper airway patency and adequate control of their catathrenia. All patients were premenopausal young women, and we do not know if their upper airway interventions will remain effective after menopause.
The women in our series clearly have a different sleep related presentation than the limited cases previously published. This raises several questions. Should the ICSD-2 categorization of catathrenia be subdivided, reserved for patients without SDB, or categorized as a SDB related phenomenon? Our results and the most recent case report in the literature3 both suggest that catathrenia is a SDB related phenomenon that responds appropriately to interventions for SDB. Discussion in the literature is generating more consistent support for this view. Like both the Iriarte and Ortega-Alba's groups, we believe that catathrenia is a SDB related phenomenon and is currently misclassified as a parasomnia in ICSD-2.1,29,30 Future studies are needed to clarify conflicting reports of sleep stage specificity, how catathrenia evolves over time, and its long term response to treatment.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.AASM. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The International Classification of Sleep Disorders, Diagnostic and Coding Manual, pp. 165–67. [Google Scholar]
- 2.De Roek J, Van Hoof E, Cluydts R. Sleep-related expiratory groaning. A case report. J Sleep Res. 1983;12:237. [Google Scholar]
- 3.Iriarte J, Alegre M, Urrestarazu U, Viteri C, et al. Continuous positive airway as treatment for catathrenia (nocturnal groaning) Neurology. 2006;66:609–10. doi: 10.1212/01.WNL.0000198503.93340.10. [DOI] [PubMed] [Google Scholar]
- 4.Oldani A, Manconi M, Zucconi M, Castronovo V, Ferini-Strambi L. Nocturnal groaning: just a sound or parasomnia? J Sleep Res. 2005;14:305–10. doi: 10.1111/j.1365-2869.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 5.Vetrugno R, Provini F, Plazzi G, Vignatelli L, Lugaresi E, Montagna P. Catathrenia (nocturnal groaning): a new type of parasomnia. Neurology. 2001;56:681–83. doi: 10.1212/wnl.56.5.681. [DOI] [PubMed] [Google Scholar]
- 6.Pevernagie D, Boon PA, Mariman ANN, Verhaeghen DB, Pauwels RA. Vocalization during episodes of prolonged expiration: a parasomnia related to REM sleep. Sleep Med. 2001;2:19–30. doi: 10.1016/s1389-9457(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 7.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire I: creation and multivariate structure of SDQ. Sleep. 1994;17:160–67. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 8.Johns M W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–45. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 9.Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109:1901–7. doi: 10.1097/00005537-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–34. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 11.Chabolle F, Fleury B, editors. ORL et troubles du sommeil. Paris: Societe francaise d'ORL et de Chirurgie de la Face et du Cou; 2006. p. 133. (publ.) [Google Scholar]
- 12.Kolar JC, Salter EM. Springfield, IL: Charles C. Thomas; 1997. Craniofacial anthropometry (practical measurement of the head and face for clinical, surgical and research use) [Google Scholar]
- 13.Enlow DH, Poston WR. Facial Growth. 3rd ed. Philadelphia: Saunders; 1990. [Google Scholar]
- 14.Rechtschaffen A, Kales A. Manual of Standardized Terminology: Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 15.American Sleep Disorders Association Atlas Task Force. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine Task Force: Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurements techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.Guilleminault C, Poyares D, Palombini L, et al. Variability of respiratory effort in relation to sleep stages in normal controls and upper airway resistance syndrome patients. Sleep Med. 2001;2:397–406. doi: 10.1016/s1389-9457(01)00111-3. [DOI] [PubMed] [Google Scholar]
- 18.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med. 1998;157:1461–67. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 19.Epstein MD, Chicoine SA, Hanumara RC. Detection of upper airway resistance syndrome using a nasal cannula/pressure transducer. Chest. 2000;117:1073–77. doi: 10.1378/chest.117.4.1073. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C, Palombini L, Poyares D, Chowdhury S. Chronic insomnia, post menopausal women, and SDB. Part 2: Comparison of non drug treatment trials in normal breathing and UARS post menopausal women complaining of insomnia. J. Psychosomat Res. 2002;53:617–23. doi: 10.1016/s0022-3999(02)00463-4. [DOI] [PubMed] [Google Scholar]
- 21.Guilleminault C, Moscovitch A, Yuen K, Poyares D. Atypical sexual behavior during sleep. Psychosomat Med. 2002;64:328–36. doi: 10.1097/00006842-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Schenck C, Arnulf I, Mahowald MW. Sleep and sex: What can go wrong? A review of the literature on sleep related disorders and abnormal sexual behaviors and experiences. Sleep. 2007;30:683–702. doi: 10.1093/sleep/30.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilleminault C, Leger D, Philip P, Ohayon MM. Nocturnal wandering and violence: review of a sleep clinic population. J Forensic Sci. 1998;43:158–63. [PubMed] [Google Scholar]
- 24.Espa F, Ondze B, Deglise P, Billiard M, Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;111:929–39. doi: 10.1016/s1388-2457(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 25.Guilleminault C, Lee JH, Chan A, Lopes MC, Huang Y, Da Rosa A. Non-REM-sleep instability in recurrent sleepwalking in pre-pubertal children. Sleep Medicine. 2005;6:515–21. doi: 10.1016/j.sleep.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Guilleminault C, Kirisoglu C, da Rosa AC, Lopes MC, Chan A. Sleepwalking, a disorder of NREM sleep instability. Sleep Med. 2006;7:163–70. doi: 10.1016/j.sleep.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Ohayon M, Guilleminault C, Priest R. How frequent are night terrors, sleep walking and confusional arousals in the general population. Their relationship to other sleep and mental disorders. J Clin Psychiatry. 1999;60:268–76. doi: 10.4088/jcp.v60n0413. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin JL, Kaeming KL, Fregosi RF, et al. Parasomnias and sleep disordered breathing in Caucasian and Hispanic children- The Tucson children's assessment of sleep apnea study. BMC Medicine. 2004. pp. 2–14. ( www.biomedical.com/1741–7015/2/14) [DOI] [PMC free article] [PubMed]
- 29.Ortega-Albas JJ, Diaz JR, Serrano AL. Entrambasaguas. Correspondence. Neurology. 2006 May 25; [Google Scholar]
- 30.Iriarte J, Alegre M, Urrestarazu E, Artieda J. Correspondence: Reply from the authors. Neurology. 2006 May 25; [Google Scholar]