Abstract
Study Objectives:
To measure sleep duration in 7-year-old children; identify the determinants of sleep duration; and assess the association between short sleep duration and obesity, cognitive functioning, and behaviour.
Design:
Longitudinal study with disproportionate sampling of the participants.
Setting:
Community.
Participants:
591 seven-year-old children, of whom 519 had complete sleep data.
Interventions:
Not applicable.
Measurements:
Sleep duration was assessed by actigraphy. Other measurements included height, weight, BMI, percentage body fat as assessed by bioimpedance assay, intelligence (WISC-III) and behaviour (Strengths & Difficulties questionnaire, parent and teachers Conners Rating Scales).
Results:
Mean time in bed according to parental report was 10.9 hours (SD 0.8). Mean sleep duration by actigraphy was 10.1 (SD 0.8) hours. In multivariable analysis, sleep duration was longer on weekdays vs. weekend nights (31.5 min, P = 0.002), in winter (40.5 min), autumn (31.1 min), and spring (14.8 min) compared with summer (P <0.0001), and in those with younger siblings (11.7 min, P = 0.03). Sleep duration was shorter when bedtime was after 21:00 (−41.1 min, P <0.0001). In multivariable analysis, sleep duration <9 hours was associated with being overweight/obese (BMI: OR = 3.32; 95% CI = 1.40, 7.87) with an increase of 3.34% body fat (P = 0.03), and this was not explained by physical activity or television watching. Short sleep duration was also associated with higher emotional lability scores (Conners Rating Scale Parent Form; P = 0.03). IQ (WISC-III) and attention deficit / hyperactivity disorder scores (both parent and teachers Conners Rating Scales) did not differ with sleep duration.
Conclusions:
Sleep duration in 7-year-old children varies considerably among individuals. The duration is affected by weekday, season, and having younger siblings. Importantly, short sleep duration was shown to be an independent risk factor for obesity/overweight.
Citation:
Nixon GM; Thompson JMD; Han DY; Becroft DM; Clark PM; Robinson E; Waldie KE; Wild CJ; Black PN; Mitchell EA. Short sleep duration in middle childhood: risk factors and consequences. SLEEP 2008;31(1):71-78.
Keywords: Sleep duration, actigraphy, IQ, behavior, obesity, season
INTRODUCTION
AN ADEQUATE AMOUNT OF GOOD QUALITY SLEEP IS IMPORTANT FOR OPTIMAL HEALTH AND FUNCTIONING THROUGHOUT LIFE. NORMAL RANGES FOR SLEEP duration in childhood have been published from several parts of the world;1–3 however, this has mostly been assessed by parental reports rather than objective measures. In adults, reduced sleep duration and sleep disturbance have been demonstrated to be associated with cognitive deficits and mood disturbance.4 There is mounting evidence of the same kind of effects in children.5–7 However, within any population there is a wide range of sleep duration, and the potential consequences of reduced sleep have received little attention in community-based studies of children.
In addition to the adverse effects of inadequate sleep on mood and cognitive function, several authors have recently demonstrated an association between reduced sleep and obesity in both adults and children.8–12 It is thought that this relationship may be mediated via changes in the levels of some of the neuropeptides that regulate appetite.12 Specifically, sleep restriction leads to increased levels of ghrelin from the stomach and reduced leptin release from adipocytes, changes that stimulate appetite. However, little research has focused on what role environmental or behavioral factors such as daily exercise might play in the relationship between obesity and sleep duration.
Our study has gathered detailed daytime activity and sleep data from a large cohort of children recruited at birth. The aims of this study were: (1) to determine sleep duration by objective means and compare that to parent-reported time in bed; (2) to identify the determinants of sleep duration, and; (3) to assess the association between short sleep duration and obesity, cognitive functioning, behavior, activity, and blood pressure. We hypothesised that sleep duration is affected by environmental factors, and that short sleep duration, measured in a child's home environment, is associated with measurable adverse effects on health (obesity and blood pressure) and well-being (cognition and behavior) compared to children with longer sleep duration. Furthermore we hypothesised that the relationship between sleep duration and obesity is mediated by levels of daytime activity.
METHODS
Overview of the Auckland Birthweight Collaborative (ABC) Study
This study forms part of a larger longitudinal study, the ABC study, originally designed to study differences between healthy term infants who were born at an appropriate weight for gestational age (AGA) and those who were small for gestational age (SGA).13,14 Between 16 October 1995 and 12 August 1996, babies born and resident in the Waitemata Health or Auckland Healthcare regions were eligible for inclusion, and from 12 August 1996 to 30 November 1997 babies born in the Auckland Healthcare region were eligible to participate. All SGA infants and a random sample of AGA infants were selected. SGA was defined as equal to or below the sex-specific 10th percentile for gestational age in the New Zealand population. AGA babies weighed greater than the 10th percentile. Preterm infants (<37 completed weeks of gestation), multiple births, and those with congenital abnormalities were excluded.
Variables/Data Collection
The longitudinal study has involved 4 phases, of which the most recent, including the sleep data, was collected at age 7 years:
Phase 1 (birth):
Mothers were interviewed in the week following delivery. Data collected included pregnancy and obstetric history (gestation, primiparity, smoking during pregnancy), family history, and sociodemographic (socioeconomic status, age that mother left school, marital status) and infant (gender) variables. Socioeconomic status was defined at birth using the higher of the parents' occupations and classified according to the Elley-Irving scale.15 Maternal weight before pregnancy and height were provided by the women by self-recall.
Phase 2 (12 months old):
Parents were posted a questionnaire including data on growth, development, social/family/home environment, and health.
Phase 3 (3.5 years of age):
Children attended the Children's Research Centre at Starship Children's Hospital for assessment of growth, development, home environment, respiratory symptoms, and atopic symptoms.
Phase 4 (7 years of age):
The parents were interviewed and data collected in relation to sociodemographic (maternal smoking, marital status), family circumstances (older and younger siblings), allergic disease (wheeze, rhinitis, eczema), childhood environment (sharing a bedroom, day of week, school holidays, season), activity. The parents were asked 3 questions relating to activity:
“Compared with other children, does your child run around outside?” Possible responses were “A lot,” “the same,” or “less.”
“How many times a week does your child engage in vigorous physical activity long enough to make him/her breathe hard?” Possible responses were “Never” or “occasionally,” “once or twice a week,” “three or more times a week.”
“During a normal week, how many hours a day (24 hours) does your child watch television?” Possible responses were “<1,” “1-3,” or “>3 hours.”
In addition activity was also assessed objectively by accelerometer as described below.
Height and weight were measured and body mass index (BMI) calculated by the standard formula BMI = weight (kg)/(height (m). Internationally referenced gender-specific BMI cut-offs for defining obesity and overweight in childhood were used.16
Bioelectrical impedance analysis was performed to assess body composition using a BIM4 machine (Impedimed Pty Ltd, Australia). Leads were attached to each child's wrists and ankles when he or she was lying down (after voiding the bladder). Resistance, reactance, and impedance were recorded, and from these fat free mass was calculated as Fat free mass (FFM) = 0.65*(height2/impedance) + 0.68*Age(yrs) +0.15.17 From this percentage body fat (PBF) was calculated as (weight-FFM)/weight x100).
Respiratory and atopic symptoms were assessed using the International Study of Asthma and Allergy in Childhood (ISAAC) questionnaires.18 Eczema was defined using the U.K. Working Party's diagnostic criteria for atopic dermatitis.19,20
Season was defined by months, with December to February being defined as summer, June-August as winter, and spring and autumn the appropriate 3-month periods in between. School terms in New Zealand have Ministry of Education defined dates, and these dates were used to define school holidays.
Intelligence was measured by trained psychologists using the Wechsler Intelligence Scale for Children – Third Edition (WISC-III).21 Behavior was assessed by the Strengths & Difficulties Questionnaire22 and Conners teacher and parent rating scales.23 The presence of sleep problems was evaluated using the Children's Sleep Habits Questionnaire (CSHQ),24 including a question on whether the parent felt that the child usually, sometimes, or rarely got enough sleep, and a subscale assessing excessive daytime sleepiness.
Blood pressure was measured using a Dinamap and a cuff appropriate for arm circumference. Blood pressure was taken 3 times with the child in a lying position with the head slightly raised on a pillow, after 5 minutes at rest in that position. Blood pressure measurements that differed from the others by ≥10 mm Hg were excluded, and the average of the valid readings (usually 3) was used for the analysis.
In addition, children had 24-hour body movements recorded using actigraphy (details below).
Actigraphy
Actigraphy is a noninvasive method used to study sleep-wake patterns and circadian rhythms by assessing movement. Each subject wore an actigraph (MTI accelerometer, Manufacturing Technology Inc, Fort Walton Beach, FL, USA) on the right side of the waist for a continuous 24-hour period beginning at 09:00 on the day before attending the study centre for the other assessments. In a small number of cases, the monitor was worn on the day after the visit when there was not time to deliver the monitor to the child's home (when appointments were made or changed at short notice), or the parent forgot to use the monitor on the correct day.
Data were collected by the actigraph in 1-sec epochs, stored in the internal memory of the device and then downloaded to a computer. Actigraphic files were imported into SAS version 9.1 for Windows (SAS institute, Cary, NC, USA) and converted to 1-min epochs. Epochs of sleep were defined using a validated algorithm (see below).25
Parents completed a diary on the day the actigraphy was performed, including what time the child went to bed and rose in the morning. They were also asked to document any periods where the actigraph was taken off the child, such as for a bath. The analysis period for the sleep data presented here (the scoring interval) included the period from the bedtime to the rise time as given in the diary, as recommended by the American Academy of Sleep Medicine.26 Analysis of actigraphy data required a complete collection of data throughout the night (i.e., no time with monitor off or missing data within the scoring interval).
Sleep onset time was defined as the first of 3 consecutive min of sleep in the scoring interval after the reported bedtime.27 Sleep end time was defined as the time of the last of 5 consecutive min of sleep prior to the reported rise time.27 Sleep period time (sleep duration) was the time from sleep onset to sleep end time.
Analysis of actigraphy data required a reasonable correspondence between the parent-completed diary (detailing bedtime and rise time) and the actigraphic data. To allow for small inaccuracies in parent-reported times, a window of 30 min before the parent-reported bedtime and after the rise time was visually inspected.27 In cases where the child was asleep by actigraphic criteria at the reported bedtime and sleep continued unbroken beyond the bedtime (n = 5), sleep latency was defined as zero if sleep onset was within 30 min of the bedtime. Likewise, if the child had unbroken sleep beyond the reported rise time (n = 1), sleep end time was defined up to 30 min after the reported rise time. If the diary times differed from the actigraphic times by >30 min, the child was omitted from further analyses (n=3, included in exclusions detailed in Results section).
The activity variables derived from actigraphy estimated the total physical activity counts, mean activity counts, and minutes of sedentary, moderate, and vigorous activity during waking hours. The cut-offs used for sedentary, moderate, and vigorous activity were those previously defined using the same actigraph (MTI accelerometer).28
Subject Participation
The study at 7 years was restricted from the original birth cohort to children of European mothers, because of the poor response rate of non-European participants at previous phases. There were 871 infants born to mothers who identified as European, of whom 385 (44.2%) were SGA and 486 were AGA. Participants were significantly more likely to have had a tertiary education, to be married, to have higher socioeconomic status, not to have smoked in pregnancy, and to have been older than those who did not continue to participate in the study.
Of the 871 European babies enrolled at birth, 744 (85.4%) participated at 1 year, 550 (63.2%) at 3.5 years and 591 (67.9%) at 7 years of age. There were 241 (40.8%) participants at 7 who were born SGA and 350 who were born AGA.
Statistical Analysis
Sleep duration was analyzed using techniques that included weighting for each subject based on the disproportionate sampling of SGA infants at birth. The analysis was carried out using proc surveyreg in SAS v9.1 (SAS Institute Inc., Cary, NC, USA). The SURVEYREG procedure performs regression analysis for sample survey data. This procedure can handle complex survey sample designs, including designs with stratification, clustering, and unequal weighting. Results are reported as the change in sleep duration in minutes for each group from the baseline group. A multivariable regression was constructed including the variables that were significant at the 10% level in the univariate analysis (listed in Table 1) except for the actigraphy variables (total and mean counts), and then variables were removed in a stepwise manner until all variables remaining in the model remained statistically significant at the 5% level. The activity variables were added individually to this multivariate model.
Table 1.
Associations of Actigraphy-Defined Sleep Duration. Results are Expressed as Effect Size in Minutes (95% Confidence Interval).
| Variable | N | (%) | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|---|---|
| Female gender | 262 | (50.5) | 6.0 (−18.0, 5.9) | P = 0.32 | ||
| Younger siblings | 290 | (56.8) | 12.3 (0.3, 24.4) | P = 0.04 | 11.7 (0.3, 23.2)* | P = 0.03 |
| Older siblings | 246 | (47.5) | −4.8 (−16.8, 7.2) | P = 0.43 | ||
| Season | P <0.0001 | P <0.0001 | ||||
| Winter | 143 | (28.1) | 37.8 (21.4, 54.2) | 40.5 (24.2, 56.8)* | ||
| Spring | 147 | (28.8) | 14.0 (−2.5, 30.4) | 14.8 (1.6, 31.2)* | ||
| Summer | 95 | (18.6) | ref | ref* | ||
| Autumn | 125 | (24.5) | 27.9 (11.1, 44.8) | 31.1 (14.5, 47.7)* | ||
| Weekend night | 71 | (13.9) | −26.9 (−47.7, −6.1) | P = 0.01 | −31.5 (−51.6, −11.4)* | P = 0.002 |
| TV watching | P = 0.20 | |||||
| <1 h/day | 133 | (25.6) | 23.7 (−4.0, 52.2) | |||
| 1-3 h/day | 332 | (64.0) | 15.9 (−10.6, 42.4) | |||
| 3+ h/day | 54 | (10.4) | ref | |||
| Runs around a lot outside (parent report) | 139 | (26.8) | 7.9 (−5.3, 21.0) | P = 0.24 | ||
| Vigorous activity at least once a week (parent report) | 494 | (95.4) | 19.0 (−7.1, 45.0) | P = 0.15 | ||
| Total daytime movement counts (change in sleep duration per 105 counts)** | 519 | (100) | −3.0 (−6.0, 0.0) | P = 0.04 | −3.0 (−5.4, 0.0)† | P = 0.05 |
| Mean daytime movement counts/min (change in sleep duration per 102 counts/min)*** | 519 | (100) | 1.1 (−1.5, 3.7) | P = 0.42 | 0.9 (−1.4, 3.3)† | P = 0.44 |
| Bedtime after 21:00 | 105 | (20.2) | −49.9 (−63.7, −36.0) | P <0.0001 | −41.1 (−55.7, −26.5)† | P <0.0001 |
Multivariable model using season, younger siblings and weekday only.
Multivariable model includes each variable individually together with season, younger siblings, and weekday.
Mean (95% CI) total daytime movement counts 649,441 (625,908, 672,974)
Mean (95% CI) mean daytime movement counts/min 812 (783, 841)
To determine the effect of short sleep duration on comorbidities, sleep duration was dichotomized into <9 h vs ≥9 h. This was chosen because 9 hours is close to the 10th centile of the distribution of sleep duration in our study, and reflects a clinically important degree of sleep restriction in this age group. For categorical variables, odds ratios (OR) were calculated to determine the increase in risk of comorbidity amongst short sleep sleepers. For continuous variables the differences were estimated continuously. Analysis was carried out in proc surveylogistic and proc surveyreg of SAS v9.1. For percentage body fat, a further multivariate model was fitted to control for variables we have shown to be independently associated with PBF in this group of children (maternal BMI, maternal age, female sex, hours of television viewing, and sedentary activity).29
Ethical Approval
Each phase of the study was approved by the local ethics committee. In addition, at each phase a parent gave written informed consent for the participation of themselves and their child.
RESULTS
Of the 591 children whose parents consented to take part in Phase 4, 40 (6.8%) did not have a complete sleep diary, 20 (3.4%) failed to wear the actigraph for part or all of the night, and 12 (2.0%) had technical problems with the actigraph precluding use of the data. Thus, complete sleep data was available for 519 children (88%). The mean age of this group was 7.26 years (SD 0.19), with 262 girls (50%), and 212 SGA (41%). There were no statistical differences in gender, birth weight, gestation, or sociodemographic factors in those for whom activity data was available and those for whom it was not (results not shown).
Sleep Duration
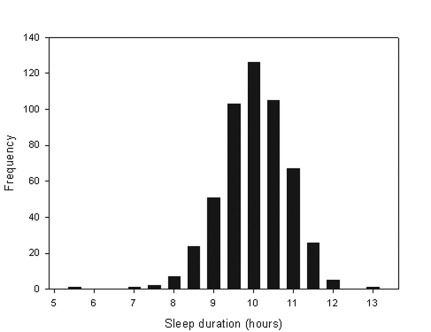
Sleep duration did not differ between children born SGA and AGA (10.1 vs. 10.0 respectively, P = 0.44). The mean time spent in bed on the actigraphy night according to parental report was 10.9 (SD 0.8) hours, with a range of 7.5 to 13 hours. The mean bedtime was 20:15 (SD 48 min) and rise time 07:07 (SD 38 min). As expected, parental report of time in bed was greater than actual sleep time, with mean sleep duration measured by actigraphy being 10.1 (SD 0.8) hours (Figure 1), on average 50 minutes less than time in bed (P <0.0001). However, sleep duration measured by actigraphy was significantly less for children whose parents who reported that their child sometimes or often got insufficient sleep (−16.1 min, 95% CI −2.4, −29.7, P = 0.02).
Figure 1.
Distribution of Sleep Duration
Bedtime after 21:00 was associated with a significantly shorter sleep duration (41.1 min, P <0.0001). The threshold of 21:00 was chosen because there is a 10-h period (average sleep duration) between that time and the average approximate rise time of 07:00.
Factors identified as determinants of sleep duration are shown in Table 1. Those with younger siblings slept for 11.7 minutes longer than the rest of the group (P = 0.03), but there was no significant difference in relation to those with older siblings. Sleep duration was 26.9 minutes less on weekend nights (Friday and Saturday) than on school nights. This was explained by a later bedtime (mean 20:42 on weekends and 20:11 on weekdays, P <0.001) but only a slightly later rise time on weekends (07:17 on weekends and 07:08 on weekdays, P = 0.07). There was also a later sleep onset time by actigraphy (weekends 21:08, weekdays 20:43, P = 0.001), but sleep end time was not later on weekends (weekends 6:50, weekdays 6:47, P = 0.68). There was a significant effect of season on sleep duration, with sleep duration in winter being on average 40.5 minutes longer, in autumn 31.1 minutes longer, and in spring 14.8 minutes longer than sleep in summer (P <0.0001). Usual daytime activity by parental report was not associated with sleep duration. An increase in total daytime activity (total daytime activity counts) measured by actigraphy during the same 24-h period as the night study was associated with a slightly shorter sleep duration (P = 0.05), however mean movement counts/min (which takes into account the increased hours of the day spent awake in those who slept less) did not show this effect (P = 0.44).
The multivariable analysis showed an independent effect on sleep duration of younger siblings, weekend night, and season. The effect of total counts and mean counts when added separately to this multivariable model were similar to that in the univariate analyses (Table 1).
Consequences of Short Sleep Duration
Results for analysis of associations of short sleep duration are presented in Table 2. Children who slept <9 hours were more likely to be overweight or obese (P = 0.0064), and to have higher percent body fat (P = 0.03) than those who slept for more than nine hours. The univariate odds ratio for being overweight or obese was 3.92 (95% CI = 1.91, 8.06) for children who slept <9 hours. Children who slept <9 h spent more time in sedentary activity but not more in moderate or vigorous activity. These relationships held when season, weekday, and younger siblings were controlled for. Using a multivariable model that also adjusted for factors affecting percentage body fat (maternal BMI, maternal age, female sex, hours of television viewing, and sedentary activity), sleep duration <9 h continued to have a strong effect on the risk of overweight/obesity (adjusted OR = 3.32; 95% CI = 1.40, 7.87). Television viewing and sedentary activity continued to be independently associated with overweight/obesity.
Table 2.
The Effect of Short Sleep Duration (Less than 9 Hours) on Outcomes
| Variable | Univariate analysis | Multivariable model 1* | Multivariable model 2† | |||
|---|---|---|---|---|---|---|
| Overweight/ Obese (OR)# | 3.92 (1.91, 8.06) | P = 0.0002 | 3.86 (1.83, 8.13) | P = 0.0004 | 3.32 (1.40, 7.87) | P = 0.0064 |
| Percentage body fat (%) | 4.53 (1.51, 7.55) | P = 0.0034 | 4.58 (1.50, 7.65) | P = 0.0036 | 3.34 (0.31, 6.38) | P = 0.03 |
| Weight (kg) | 3.61 (1.34, 5.89) | P = 0.0019 | 3.78 (1.43, 6.12) | P = 0.0017 | ||
| Height (cm) | 1.82 (−0.50, 4.14) | P = 0.12 | 2.08 (-0.24, 4.41) | P = 0.08 | ||
| IQ points | 0.41 (−4.00, 4.82) | P = 0.86 | ||||
| Total counts (x104) | 5.13 (−3.04, 13.30) | P = 0.22 | ||||
| Mean counts | −21 (−125, 83) | P = 0.69 | ||||
| Sedentary activity (min) | 69 (35, 103) | P <0.0001 | 64 (30, 97) | P = 0.0002 | ||
| Moderate activity (min) | 14 (−7, 35) | P = 0.18 | ||||
| Vigorous activity (min) | 2 (−8, 12) | P = 0.38 | ||||
| SDQ (OR for abnormal)# | 1.39 (0.52, 3.72) | P = 0.45 | ||||
| Emotional lability‡ | 0.17 (0.01, 0.33) | P = 0.03 | 0.18 (0.02, 0.34) | P = 0.03 | ||
| Systolic blood pressure (mmHg) | 3.43 (0.55, 6.31) | P = 0.02 | 3.62 (0.64, 6.61) | P = 0.02 | ||
| (not significant when adjusted for body weight or BMI) | ||||||
Multivariable model 1 adjusting for season, weekday and younger siblings.
Multivariable model 2 adjusting for maternal age, BMI and marital status, gender, TV watching and time spent in sedentary activity.
Parent Conners Rating Scale for emotional lability.
As this is a dichotomous variable the estimate is an odds ratio.
There were no significant associations between sleep duration and IQ or sleep duration and behavioral problems, apart from a negative correlation between sleep duration and the parental Conners Rating Scale subscale for emotional lability (P = 0.03). Short sleep was not significantly associated with higher daytime sleepiness scores on the CSHQ (P = 0.35).
Systolic blood pressure was increased in the short sleep group (P = 0.02), but this effect disappeared after controlling for either current weight or BMI.
DISCUSSION
Sleep Duration
The mean sleep duration defined by actigraphy in this community cohort of 7-year-old children was 10.1 hours, which was 50 min shorter than the time in bed reported by their parents. Children who went to bed before 21:00 had longer sleep duration, owing simply to the hours available for sleep before the rise time (which was consistent regardless of week day). Children had shorter sleep duration on weekend nights and in the summer, whereas the presence of younger siblings was associated with longer sleep duration.
Time in bed by parental report in our cohort of New Zealand Caucasian children (mean 10.9 h) was very similar to that previously reported in English children.1 In contrast, our cohort spent slightly more time in bed than recent studies of US3 and Swiss children2 (both mean 10.6 h, SD 0.7), and nearly two hours longer than Chinese children3 (mean 9.1 h, SD 0.7), owing to both a later bedtime and earlier rise time in Chinese children. Our study demonstrates that time in bed reported by parents used in all these studies underestimates sleep duration measured objectively by actigraphy. Parental report is consistent with nighttime sleep by actigraphy in infants and toddlers,27 but in older children who are usually not directly observed by their parents after bedtime, parental estimate of sleep duration is less likely to be accurate. This should be taken into account when making recommendations for bed time in this age group.
Bedtime is an important determinant of sleep duration in children.30 Longer sleep in winter has previously been demonstrated in a study of Icelandic children.31 That study also found longer sleep duration at weekends after the age of 9 years,31 in contrast to our finding in 7-year-old children who actually slept less on weekend nights due to a later bedtime without a compensatory delay in rise time. Reduced sleep duration on weekend nights in middle childhood was also seen in a large community study of 5- to 11-year-old children in the United Kingdom, which found a reduction of a similar magnitude to our study in their subset of 7-year-old children.1 Although the authors of that study do not point it out, children aged 5–7 years slept less on the weekend, those aged 8-10 years slept the same amount regardless of weekday, and those aged 11 years slept longer on the weekend. These studies are consistent, and together nicely demonstrate the effect of age on sleeping patterns as children move through middle childhood towards adolescence, when weekend sleep is substantially longer than weekday sleep, reflecting a later rise time.32
Studies of the effect of daytime exercise on sleep duration have shown variable results. Duration of exercise had only a weak impact on total sleep time in one meta-analysis, unless the exercise was prolonged (>1 h).33 Another study in adults using actigraphically quantified exercise found no within-subject correlations between physical activity and sleep duration.34 In our study, children who slept less than nine hours (and therefore had more time out of bed) spent more time in sedentary activity without spending any more time in moderate or vigorous activity. However, the converse is also true—the short sleepers did not do less moderate or vigorous exercise than their peers either, despite presumably being more tired.
Our study includes detailed sociodemographic variables recorded from birth, and unlike previous studies,35, 36 we did not find an influence of gender, socioeconomic status, maternal education, maternal stress, or maternal social support on sleep duration. However, our cohort is restricted to children of European descent, and participants at the 7-year follow-up were more likely to have high socioeconomic status than nonparticipants, thus restricting the range of social situations studied. We did not show a statistically significant effect of hours of television watching in our study, although the effect was in the same direction as other studies (Table 1).37
Consequences of Short Sleep Duration
Several authors in Australia,8 Japan,9 Canada,11 and the United Kingdom10 have reported an association between parent-reported short sleep duration and obesity. We have confirmed this association having measured sleep duration objectively by actigraphy. Several mechanisms may explain the association between short sleep duration and obesity: sleep restriction leads to metabolic derangement (decreased leptin, elevated ghrelin, increased appetite)12; both obesity and sleep restriction are markers of related behaviors and/or parenting style38,39; daytime tiredness due to sleep restriction might reduce daytime activity, previously shown to have a weak effect on sleep quality34; and/or increased time awake may lead to increased caloric intake.40 Our data show a strong effect of short sleep duration on risk of obesity even when levels of daytime activity are adjusted for; these data do not support the contention that decreased daytime activity in short sleepers is the principal mechanism behind the association. Furthermore, we showed that short sleep duration, television watching and sedentary activity are all independently associated with obesity. This is to our knowledge the first time that this has been reported.
Reduced sleep duration was associated with higher emotional lability scores in this study, as has been seen in other studies of sleep disturbance.41 Reduced sleep duration was not associated with other daytime behavioral problems or decrements in IQ in this study. Sadeh et al. demonstrated detrimental effects on neurobehavioral functioning (in tests of vigilance and motor reaction) in children in an experimental paradigm that restricted nocturnal sleep by only one hour for three nights.7 Several other groups have shown reduced attention, impaired school performance and hyperactivity in children with sleep disorders.5,42 Our study did not find such an association in a community group of children, suggesting that in the majority of otherwise healthy children in middle childhood, “natural” sleep duration (i.e., that achieved under normal circumstances in the home as distinct from controlled experimentally) is sufficient to avoid a measurable effect on behavior or intellectual performance.
Sleepiness in drivers has a well-recognized association with traffic crashes.43 There is evidence that inadequate sleep duration44 and sleep disturbances45 increases the risk of non-intentional injury in children. It is postulated that short sleep duration and sleep disturbances lead to excessive daytime sleepiness, resulting in inattentive and externalizing behaviors, thus increasing injury risk behavior.45 In this study we did not measure injuries. However, we did not find a relationship between short sleep duration and daytime sleepiness measured by the CSHQ, suggesting that these children are getting sufficient sleep. This is consistent with the findings of an earlier study using the same questionnaire that did not find an association between sleep duration and daytime sleepiness (both as measured by the CSHQ) in US children.3 Frank sleepiness as perceived by parents is unusual in children with sleep disturbance caused by obstructive sleep apnea (OSA),46 and it may thus not be surprising that sleep restriction does not lead to problematic daytime sleepiness.
We found an increase in systolic blood pressure in short sleepers, but this relationship did not hold after adjustment was made for body weight. In adults, the relationship holds but is weaker when adjusted for BMI.47 One previous study in children showed lower blood pressure in children with short sleep duration, even after correction for BMI.48
Strengths and Weaknesses of the Study
The strengths of our study include a large dataset of children recruited from the community. We have both subjective and objective information about sleep at age 7 years, complemented by comprehensive data about potential influences on health outcomes from the time of birth. Because the wider study of which this report forms a part was designed to study daytime activity, a uniaxial (vertical) accelerometer was used.49 Polysomnography (PSG) is the gold standard for sleep studies. However, it is expensive and subject burden is high, and thus it is not suitable for large epidemiological studies. Actigraphy correlates well with sleep duration as measured by PSG.50 Sadeh's algorithm has been shown to slightly overestimate total sleep time, but only by 2%.51 Instrument reliability and between-unit variability has been demonstrated to be excellent.52 A limitation of the study is that the activity monitors were worn only for 24 hours. The duration of monitoring used in studies varies, but generally the longer the duration the more accurate the estimate. Current practice parameters for actigraphy recommend at least 3 consecutive days of recordings.26 This is based on clinical use in individuals, however, aiming to minimize loss of data for technical reasons, and the impact of measuring for a single night only in a healthy community population has not been studied. Recording from a single night does not allow us to assess intra-subject variability, but the focus of this study is on associations in the group as a whole. We have demonstrated that parental report of short sleep duration was confirmed by actigraphy, supporting the validity of our data. Despite this, associations with short sleep duration were found with a number of factors including sedentary activity and obesity.
Summary and Conclusions
Knowledge of the influences on sleep habits of early school age children and the consequences of sleep restriction in this age group is important in relation to education programmes about healthy sleep for parents and children. Our findings suggest that sleep duration may be at least in part a modifiable risk factor for other poor health outcomes in childhood, including obesity.
ACKNOWLEDGMENTS
We sincerely thank the parents and children for participating in these studies. The 3.5 and 7 year follow-up studies were conducted in the Children's Research Centre which is supported in part by the Starship Foundation and Auckland District Health Board. GM Nixon, JMD Thompson, and EA Mitchell are partially supported by the Child Health Research Foundation.
The initial study was funded by the Health Research Council of New Zealand. The 12-month postal questionnaire was funded by Hawkes Bay Medical Research Foundation. The 3.5-year follow-up study was funded by Child Health Research Foundation, Becroft Foundation and Auckland Medical Research Foundation. The 7-year follow-up study was funded by Child Health Research Foundation.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Nixon has financial interests in a private clinical practice in pediatric respiratory and sleep medicine. The other authors have indicated no conflicts of interest.
REFERENCES
- 1.Gulliford MC, Price CE, Rona RJ, Chinn S. Sleep habits and height at ages 5 to 11. Arch Dis Child. 1990;65:119–22. doi: 10.1136/adc.65.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115(1) Suppl:241–9. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- 4.Dinges DF. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 5.Owens J, Opipari L, Nobile C, Spirito A. Sleep and daytime behavior in children with obstructive sleep apnea and behavioral sleep disorders. Pediatrics. 1998;102:1178–84. doi: 10.1542/peds.102.5.1178. [DOI] [PubMed] [Google Scholar]
- 6.Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Percept Mot Skills. 2001;93:213–29. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- 7.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74:444–55. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 8.Eisenmann JC, Ekkekakis P, Holmes M. Sleep duration and overweight among Australian children and adolescents. Acta Paediatrica. 2006;95:956–63. doi: 10.1080/08035250600731965. [DOI] [PubMed] [Google Scholar]
- 9.Sekine M, Yamagami T, Handa K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28:163–70. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 10.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int J Obes. 2006;30:1080–5. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 12.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell EA, Robinson E, Clark PM, et al. Maternal nutritional risk factors for small for gestational age babies in a developed country: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2004;89:F431–5. doi: 10.1136/adc.2003.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson J, Clark P, Robinson E, et al. Risk factors for small for gestational age babies: the Auckland Birthweight Collaborative Study. J Paediatr Child Health. 2001;37:369–75. doi: 10.1046/j.1440-1754.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- 15.Elley WB, Irving JC. Revised socioeconomic index for New Zealand. NZ J Educ Stud. 1976;11:25–30. [Google Scholar]
- 16.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer F, Georgi M, Zieger A, Scharer K. Usefulness of bioelectric impedance and skinfold measurements in predicting fat-free mass derived from total body potassium in children. Pediatr Res. 1994;35:617–24. [PubMed] [Google Scholar]
- 18.Asher MI, Keil U, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Resp J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 19.Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party's diagnostic criteria for atopic dermatitis III. Independent hospital validation. Br J Dermatol. 1994;131:406–16. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams HC. How do I make the diagnosis of atopic eczema? A practical manual for researchers wishing to define atopic eczema. ( http://www.nottingham.ac.uk/dermatology/eczema/contents.html)
- 21.Wechsler D. WISC-III: Wechsler Intelligence Scale for Children- Third Edition: Manual. San Antonio: Harcourt Brace & Company; 1992. [Google Scholar]
- 22.Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791–9. [PubMed] [Google Scholar]
- 23.Conners K. Conners Rating Scales - Revised Technical Manual. North Tonawanda, NY: Multi-Health Systems; 1997. [Google Scholar]
- 24.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 25.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 26.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 27.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28:1568–77. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- 28.Trost SG, Pate RR, Sallis JF, et al. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc. 2002;34:350–5. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Blair NJ, Thompson JMD, Black PN, et al. Risk factors for obesity in 7 year old European children: The Auckland Birth weight Collaborative Study. Arch Dis Child. 2007;29:866–71. doi: 10.1136/adc.2007.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohyama J, Shiiki T, Hasegawa T. Sleep duration of young children is affected by nocturnal sleep onset time. Pediatr Int. 2000;42:589–91. doi: 10.1046/j.1442-200x.2000.01304.x. [DOI] [PubMed] [Google Scholar]
- 31.Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–37. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 32.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 33.Youngstedt SD, O'Connor PJ, Dishman RK. The effects of acute exercise on sleep: a quantitative synthesis. Sleep. 1997;20:203–14. doi: 10.1093/sleep/20.3.203. [DOI] [PubMed] [Google Scholar]
- 34.Youngstedt SD, Perlis ML, O'Brien PM, et al. No association of sleep with total daily physical activity in normal sleepers. Physiol Behav. 2003;78:395–401. doi: 10.1016/s0031-9384(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin Crabtree V, Beal Korhonen J, Montgomery-Downs HE, Faye Jones V, O'Brien LM, Gozal D. Cultural influences on the bedtime behaviors of young children. Sleep Med. 2005;6:319–24. doi: 10.1016/j.sleep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Spilsbury JC, Storfer-Isser A, Drotar D, et al. Sleep behavior in an urban US sample of school-aged children. Arch Pediatr Adolesc Med. 2004;158:988–94. doi: 10.1001/archpedi.158.10.988. [DOI] [PubMed] [Google Scholar]
- 37.Olds T, Ridley K, Dollman J. Screenieboppers and extreme screenies: the place of screen time in the time budgets of 10-13 year-old Australian children. Aust N Z J Public Health. 2006;30:137–42. doi: 10.1111/j.1467-842x.2006.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 38.Rhee KE, Lumeng JC, Appugliese DP, Kaciroti N, Bradley RH. Parenting styles and overweight status in first grade. Pediatrics. 2006;117:2047–54. doi: 10.1542/peds.2005-2259. [DOI] [PubMed] [Google Scholar]
- 39.Spilsbury JC, Storfer-Isser A, Drotar D, Rosen CL, Kirchner HL, Redline S. Effects of the home environment on school-aged children's sleep. Sleep. 2005;28:1419–27. doi: 10.1093/sleep/28.11.1419. [DOI] [PubMed] [Google Scholar]
- 40.Hicks RA, Rozette E. Habitual sleep duration and eating disorders in college students. Percept Mot Skills. 1986;62:209–10. doi: 10.2466/pms.1986.62.1.209. [DOI] [PubMed] [Google Scholar]
- 41.Rosen CL, Storfer-Isser A, Taylor HG, Kirchner HL, Emancipator JL, Redline S. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics. 2004;114:1640–8. doi: 10.1542/peds.2004-0103. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatrics. 2004;145:458–64. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 43.Horne JA, Reyner LA. Sleep related vehicle accidents. BMJ. 1995;310:565–7. doi: 10.1136/bmj.310.6979.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valent F, Brusaferro S, Barbone F. A case-control study of sleep and childhood injury. Pediatrics. 2001;107:E23. doi: 10.1542/peds.107.2.e23. [DOI] [PubMed] [Google Scholar]
- 45.Owens JA, Fernando S, McGuinn M. Sleep disturbance and injury risk in young children. Behav Sleep Med. 2005;3:18–31. doi: 10.1207/s15402010bsm0301_4. [DOI] [PubMed] [Google Scholar]
- 46.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108:610–8. doi: 10.1378/chest.108.3.610. [DOI] [PubMed] [Google Scholar]
- 47.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 48.Sampei M, Dakeishi M, Wood DC, Murata K. Impact of total sleep duration on blood pressure in preschool children. Biomed Res. 2006;27:111–5. doi: 10.2220/biomedres.27.111. [DOI] [PubMed] [Google Scholar]
- 49.Kelly LA, Reilly JJ, Fairweather SC, Barrie S, Grant S, Paton JY. Comparison of two accelerometers for assessment of physical activity in preschool children. Pediatr Exerc Sci. 2004;16:324–33. [Google Scholar]
- 50.Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep-wake patterns. Pediatrics. 1991;87:494–9. [PubMed] [Google Scholar]
- 51.de Souza L, Benedito-Silva AA, Pires MLN, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;1:81–5. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 52.Tryon WW, Williams RW. Fully proportional actigraphy: a new instrument. Instruments and Computer. 1996;28:392–403. [Google Scholar]