Abstract
Study Objective:
To examine the prevalence of and risk factors for fatigue and sleep disturbance among adult survivors of childhood cancer.
Design:
Retrospective cohort of childhood cancer survivors.
Setting:
Twenty-six academic institutions treating childhood cancer.
Participants:
Two thousand six hundred forty-five survivors of childhood acute lymphocytic leukemia, central nervous system tumors, Hodgkin lymphoma, soft-tissue sarcomas, or bone tumors diagnosed before age 21, surviving at least 5 years from diagnosis, and a 500-sibling comparison group.
Measurements:
Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale.
Results:
Significant differences were found between survivors and siblings on the Functional Assessment of Chronic Illness Therapy-Fatigue (40.8 vs 42.0, P < 0.02), Pittsburgh Sleep Quality Index (6.1 vs 5.5, P < 0.004), and Epworth Sleepiness Scale (6.2 vs 5.4, P < 0.001). Nineteen percent of survivors were in the most fatigued range, 16.7% reported disrupted sleep, and 14% increased daytime sleepiness. Survivors with a history of radiation therapy were more likely to be fatigued (odds ratio 1.7, 95% confidence interval 1.3–2.3), yet without significantly different mean scores. Female sex, congestive heart failure, pulmonary fibrosis, depression, and being unmarried significantly predicted more fatigue, whereas obesity and an infant in the house predicted more daytime sleepiness. Similar sociodemographic predictors were also identified among the siblings.
Conclusion:
Because of the large sample size, we detected more objectively reported fatigue, sleep disturbance, and daytime sleepiness among adult survivors of childhood cancer. However, the clinical significance of these differences is questionable. Predictors of fatigue and poor sleep were similar in both survivors and the siblings.
Citation:
Mulrooney DA; Ness KK; Neglia JP; Whitton JA; Green DM; Zeltzer LK; Robison LL; Mertens AC. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS). SLEEP 2008;31(2):271–281.
Keywords: Fatigue, sleep, cancer, childhood cancer survivor study
THE LAST 50 YEARS HAVE WITNESSED STEADILY INCREASING SURVIVAL RATES AMONG PEDIATRIC MALIGNANCIES THAT ARE NOW APPROACHING NEARLY 80%.1 With improved survival has come recognition of the late effects of prior cancer treatment, such as second malignancies, infertility, growth delays, cardiopulmonary disease, endocrinopathies, and neuropsychological deficits.2–9 As many as two thirds of childhood cancer survivors report a chronic medical condition, with over 25% being severe or life-threatening.10 Understanding these late effects will enable the medical community to better care for the growing population of childhood cancer survivors.
Fatigue is a distressing symptom frequently reported by adult cancer patients.11 Its etiology is likely multifactorial, involving anemia, inactivity, infection, sleep disturbance, anxiety, and depression. The prevalence of fatigue in the general population has been estimated between 7% and 45%,12 but as high as 70% to 96% in recently treated adult cancer patients.13 Cancer-related fatigue (CRF) includes diminished energy and mental capacity and an increased need to rest disproportionate to any recent change in activity, present nearly every day during any 2-week period in the past month.14,15 CRF is not relieved by rest and can severely diminish a patient's ability to interact socially and maintain a reasonable quality of life.16
Fatigue and disrupted sleep have been described by survivors across diagnoses and may occur months to years following therapy.17–19 The prevalence and impact of excessive fatigue and sleep disturbance among long-term survivors of childhood cancer has been the subject of limited investigation.20–26
The aim of this investigation was to describe the prevalence of and risk factors for fatigue and sleep disturbance among a large population of survivors of childhood and adolescent cancers. We hypothesized that this population would have more complaints of fatigue, disrupted sleep, and daytime sleepiness compared with a sibling comparison group and that there would be significant associations with central nervous system (CNS)-directed radiation therapy and late medical complications, including depression.
METHODS
Study Population and Data Collection
Data for this analysis were from the Childhood Cancer Survivor Study (CCSS), an ongoing multicenter epidemiologic study of survivors of childhood and adolescent cancers.27 The CCSS cohort includes individuals diagnosed before the age of 21 years with leukemia, a CNS malignancy, Hodgkin disease (HD), non-Hodgkin lymphoma, kidney cancer, neuroblastoma, a soft tissue sarcoma (STS), or bone malignancy; at 1 of 26 participating institutions (Appendix); between 1970 and 1986; and who survived at least 5 years following diagnosis. The Human Subjects Research Review Committees at the University of Minnesota (the study coordinating center) and each collaborating institution approved the study's protocols and documents. Each eligible participant or his or her proxy, if younger than age 18 years at interview, provided informed consent for the study and separate consent for release and abstraction of medical records.
The CCSS collaborating institutions originally identified 20,720 five-year survivors, and 14,362 (69.3%) completed the baseline survey. Of 12,126 participants still alive in 2002, 9035 (74.5%) completed the second follow-up survey. As part of this survey, we randomly selected 2645 participants from 5 diagnostic groups, including 398 with acute lymphocytic leukemia (ALL; half with a history of craniospinal radiation), 398 brain tumors (half with a history of cranial radiation), 1452 HD, 198 STS, and 199 bone tumors. Among these, 718 (27%) actively declined participation, 41 (1.6%) had died, and 9 (0.3%) were lost to follow-up. Thus, 1897 responses (72%) were available for evaluation. HD survivors were over sampled due to reports of excessive fatigue in this population.18, 28
At the time of the baseline CCSS evaluation, a random sample of survivors (4790) was asked to contact a nearest-aged sibling for participation in a comparison group. Of these, 3901 (81.4%) completed the baseline questionnaire. In 2002, concurrent with our sleep questionnaire, 2905 siblings (74% of those who completed the baseline questionnaire) completed the second follow-up. We randomly selected 500 siblings to complete the sleep questionnaire with the second follow-up. Of these, 369 (73.8%) returned questionnaires, 129 (25.8%) declined participation, and 1 had died.
Outcome Measures
Fatigue
Fatigue was measured with the fatigue subscale of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue). This 13-item scale, validated in cancer patients, is a measure of physical and functional consequences of fatigue. It is scored on a reverse 4-point Likert scale, ranging from 0 to 52, with lower scores indicating more fatigue. The FACIT-Fatigue has good test-retest reliability (r = 0.90) and internal consistency (α = 0.93-0.95).15, 29
Sleep
The Pittsburgh Sleep Quality Index (PSQI) assesses sleep quality over the preceding 30 days. This 19-question scale measures 7 equally weighted components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medications, and daytime dysfunction. Component scores can be evaluated individually or added to give an overall score from 0 to 21. Higher scores indicate poorer sleep quality. The overall reliability coefficient (Cronbach α) is 0.83, and test-retest reliability has a Pearson correlation of 0.85.30
Daytime Sleepiness
The Epworth Sleepiness Scale (ESS) asks subjects to rank the likelihood of falling asleep during common daily situations. Scores range between 0 to 24, with higher scores suggesting increased daytime sleepiness.31 Cronbach α is 0.88, and test-retest reliability correlation is r = 0.82.32
Treatment variables were obtained from the original medical record abstraction. Medical late effects included self-report of congestive heart failure (CHF), lung fibrosis, and hypothyroidism. Depression was evaluated by the Brief Symptom Inventory (BSI-18). Scores on the depression subscale are in a T-distribution, and a score of 63 or greater is considered depressed.
Health-related quality of life was evaluated by the Medical Outcomes Survey Short Form-36 (SF-36).33 Raw scores were converted to t-scores for analytic comparisons.
Statistical Analysis
Descriptive statistics were calculated for demographic and treatment variables and compared between survivors and siblings with 2-sample t-tests or χ2 tests. Means and standard errors were calculated and compared between survivors and siblings in generalized linear mixed models, adjusted for age at interview and sex. A variance component for intrafamily correlation was included.34 Using the scoring standards provided with each of the questionnaires,15,30,31 only responses from participants who completed 50% or more of each subscale of the PSQI and the total scales of the FACIT-Fatigue and ESS were scored. Completion rates for the FACIT-Fatigue, PSQI, and ESS, were 94%, 96%, and 99%, respectively. Missing items for the FACIT-Fatigue and ESS were assigned an average value based on that participant's answers to the scale. Missing items for the PSQI were assigned a zero value indicating no problem. Participants who did not complete enough of a particular scale to receive a score were dichotomized into the “no-outcome” groups for the multivariate analyses.
The scales used in this study have normative data from populations with age ranges higher than in our cohort. Therefore, to dichotomize the scales, we classified the lowest 10th percentile of the sibling scores on the FACIT-Fatigue as fatigued and the highest 10th percentile of the sibling scores on the PSQI and ESS as having poor sleep quality and daytime sleepiness, representing 1.3 standard deviations from the mean. The prevalence of fatigue, poor sleep quality, or daytime sleepiness was compared by treatment and demographic factors with multivariate logistic regression. Generalized estimating equations were used to account for intrafamily correlation when survivors and siblings were in the same logistic-regression models. Race and educational attainment were not independent predictors of the outcomes, nor did they appreciably alter the estimates, so they were not included in the final models.
Mean t-scores for each of the 8 subscales of the SF-36 were calculated. Survivors and siblings were divided into fatigued/nonfatigued, sleep disturbed/non-sleep–disturbed, and those with/without daytime sleepiness. Comparisons were made with 2-sample t-tests. SAS version 9.1 (SAS, Inc., Cary, NC) was used for all analyses.
RESULTS
Characteristics of the Study Population
Participating survivors were more likely to be female (51% vs 42%) and more educated than nonparticipants (72% postsecondary education vs 62%), and proportionally more bone tumor survivors participated than not (8.5% vs 5.5%). Characteristics of participating survivors and siblings are shown in Table 1. Siblings were younger than survivors and less likely to report their ethnicity as Hispanic. Because of oversampling, HD survivors represented 52.5% of the study population. All participants had survived at least 15 years from diagnosis; over half had received chemotherapy and 70.2% radiation therapy.
Table 1.
Characteristics of the Study Population
| Survivors (n = 1897) | Siblings (n = 369) | P Value | |
|---|---|---|---|
| Sex | |||
| Female | 964 (50.8) | 193 (52.3) | 0.60 |
| Male | 933 (49.2) | 176 (47.7) | |
| Race | |||
| White | 1682 (88.7) | 330 (89.4) | < 0.001 |
| Black | 72 (3.8) | 14 (3.8) | |
| Hispanic | 86 (4.5) | 7 (1.9) | |
| Asian | 25 (1.3) | 4 (1.1) | |
| American Indian/Alaska Native | 19 (1.0) | 1 (0.3) | |
| Other | 13 (0.7) | 13 (3.5) | |
| Age at questionnaire, y | < 0.001 | ||
| 18-29 | 452 (23.8) | 139 (37.7) | |
| 30-39 | 879 (46.3) | 135 (36.6) | |
| 40-49 | 532 (28.0) | 89 (24.1) | |
| 50+ | 34 (1.8) | 6 (1.6) | |
| Highest level of education | |||
| Grade school | 8 (0.4) | 0 (0.0) | 0.75 |
| High school | 290 (15.3) | 58 (15.7) | |
| Technical school | 128 (6.7) | 21 (5.7) | |
| College | 1175 (61.9) | 233 (63.1) | |
| Postgraduate | 281 (14.8) | 55 (14.9) | |
| Not indicated | 5 (0.3) | 12 (3.3) | |
| Marital status | |||
| Married or living as married | 1064 (56.1) | 213 (57.7) | 0.61 |
| Not married | 816 (43.0) | 154 (41.7) | |
| Not indicated | 7 (0.4) | 17 (4.6) | |
| Employment status | |||
| Working full time | 1240 (65.4) | 257 (69.6) | 0.11 |
| Not working full time | 657 (34.6) | 112 (30.4) | |
| Diagnosis | |||
| Leukemia | 298 (15.7) | ||
| CNS malignancy | 299 (15.8) | ||
| Hodgkin disease | 995 (52.5) | ||
| Soft tissue sarcoma | 150 (7.9) | ||
| Bone cancer | 155 (8.2) | ||
| Age at diagnosis, y | |||
| 0-4 | 353 (18.6) | ||
| 5-9 | 390 (20.6) | ||
| 10-14 | 523 (27.6) | ||
| 15+ | 631 (33.3) | ||
| Survival time, y | |||
| 15-19 | 495 (26.1) | ||
| 20-24 | 646 (34.1) | ||
| 25-29 | 493 (26.0) | ||
| 30+ | 263 (13.9) | ||
| Chemotherapy | |||
| Yes | 1122 (59.1) | ||
| No | 775 (40.9) | ||
| Radiation | |||
| Yes | 1332 (70.2) | ||
| No | 565 (29.8) |
Data are presented as number (percentage). CNS refers to central nervous system.
Fatigue and Sleep Scores
Mean FACIT-Fatigue, PSQI, and ESS scores are shown in Table 2. Differences were detected between survivors and siblings on all 3 measures. Survivors of HD scored significantly lower than the comparison group on the FACIT-Fatigue and higher on the PSQI and ESS, suggestive of more fatigue, sleep disruption, and daytime sleepiness. CNS survivors also had a lower FACIT-Fatigue scale than the siblings. Differences on the PSQI were detected in STS and bone tumor survivors and ESS scores among ALL and CNS survivors were higher than among the siblings.
Table 2.
Mean Scores on Fatigue, Sleep Quality, and Daytime Sleepiness Questionnaires for Survivors (by Diagnosis and Radiation Groups for Select Diagnoses) and Siblings
| FACIT-Fatiguea |
PSQIa |
ESSa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Meanb | SEc | P Value | Meanb | SEc | P Value | Meanb | SEc | P Value | |
| Siblings (comparison) | 42.0 | 0.5 | 5.5 | 0.2 | 5.4 | 0.2 | |||
| Total population | 40.8 | 0.2 | 0.02 | 6.1 | 0.1 | 0.004 | 6.2 | 0.1 | 0.001 |
| Diagnosis | |||||||||
| ALL | 42.0 | 0.6 | 0.96 | 5.9 | 0.2 | 0.10 | 6.0 | 0.2 | 0.05 |
| CNS malignancy | 40.3 | 0.6 | 0.03 | 5.8 | 0.2 | 0.28 | 6.7 | 0.2 | < 0.001 |
| Hodgkin disease | 40.3 | 0.3 | 0.005 | 6.2 | 0.1 | 0.003 | 6.1 | 0.1 | 0.004 |
| Soft tissue sarcoma | 41.2 | 0.8 | 0.73 | 6.4 | 0.3 | 0.008 | 5.6 | 0.3 | 0.53 |
| Bone cancer | 41.7 | 0.8 | 0.72 | 6.2 | 0.3 | 0.03 | 6.1 | 0.3 | 0.06 |
| Radiation therapy | |||||||||
| Leukemiad | |||||||||
| No cranial radiation | 42.6 | 0.7 | 5.9 | 0.3 | 5.9 | 0.3 | |||
| Cranial radiation | 41.7 | 0.7 | 0.42 | 6.0 | 0.3 | 0.65 | 6.1 | 0.3 | 0.71 |
| CNS Malignancyd | |||||||||
| No cranial radiation | 40.5 | 0.8 | 6.2 | 0.3 | 6.9 | 0.4 | |||
| Cranial radiation | 40.3 | 0.8 | 0.86 | 5.4 | 0.3 | 0.04 | 6.6 | 0.3 | 0.56 |
| Dose, Gye | |||||||||
| 20-39 | 38.8 | 2.3 | 5.7 | 0.8 | 6.1 | 1.0 | |||
| 40-54 | 41.0 | 1.0 | 0.38 | 5.3 | 0.4 | 0.61 | 6.6 | 0.4 | 0.69 |
| 55+ | 37.3 | 2.8 | 0.67 | 5.3 | 1.0 | 0.71 | 6.7 | 1.2 | 0.72 |
| Hodgkin diseased | |||||||||
| No chest radiation | 41.0 | 0.6 | 6.3 | 0.1 | 6.2 | 0.2 | |||
| Chest radiation | 39.9 | 0.4 | 0.19 | 6.1 | 0.2 | 0.39 | 6.1 | 0.1 | 0.88 |
Lower Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) score reflects increased fatigue, higher Pittsburgh Sleep Quality Index (PSQI) score reflects poorer sleep quality, higher Epworth Sleepiness Scale (ESS) score reflects increased sleepiness.
Adjusted for age at interview and sex.
Standard error with variance component for intrafamily correlation.
p-value for comparisons within diagnostic groups.
p-Value for comparison with 20- to 39-Gy group.
No statistically significant difference between mean scores on the FACIT-Fatigue, ESS, or PSQI was observed between ALL survivors with a history of radiation therapy compared to those without. Among survivors of CNS malignancies, those with a history of midbrain radiation therapy had a mean PSQI score significantly lower than those who had not had mid-rain radiation therapy. There was no evidence of an overall radiation therapy dose response for fatigue, sleep quality, or daytime sleepiness. A history of mediastinal radiation therapy did not influence mean fatigue, sleep quality, or daytime sleepiness among survivors of HD.
Fatigue, Sleep Disturbance, and Daytime Sleepiness
After adjustment for sex, diagnosis, age at diagnosis, and treatment variables, females were more likely than males to be in the most fatigued group (Table 3). Those treated with radiation therapy were more fatigued compared to the group without radiation therapy, and survivors of a STS were more likely than those with leukemia to report disordered sleep.
Table 3.
Multivariate Analysis to Predict Prevalence of Fatigue or Sleep Disturbance by Cancer- or Treatment-Related Variables
| Total in group | Fatigueda |
Disordered Sleepa |
Daytime Sleepinessa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | OR | 95% CI | No. | % | OR | 95% CI | No. | % | OR | 95% CI | ||
| Total | 1897 | 364 | (19.2) | 317 | (16.7) | 257 | (13.5) | ||||||
| Sex | |||||||||||||
| Male | 933 | 131 | (14.0) | 1.0b | 138 | (14.8) | 1.0b | 117 | (12.5) | 1.0b | |||
| Female | 964 | 233 | (24.2) | 1.9 | 1.5–2.4 | 179 | (18.6) | 1.5 | 1.0–1.7 | 140 | (14.5) | 1.2 | 0.9–1.5 |
| Diagnosis | |||||||||||||
| ALL | 298 | 45 | (15.1) | 1.0b | 40 | (13.4) | 1.0b | 33 | (11.1) | 1.0b | |||
| CNS malignancy | 299 | 58 | (19.4) | 1.3 | 0.8–2.1 | 47 | (15.7) | 1.5 | 0.9–2.4 | 49 | (16.4) | 1.6 | 1.0–2.8 |
| Hodgkin disease | 995 | 209 | (21.0) | 1.2 | 0.7–1.8 | 170 | (17.1) | 1.5 | 0.9–2.3 | 134 | (13.5) | 1.2 | 0.7–2.1 |
| Soft tissue sarcoma | 150 | 23 | (15.3) | 1.0 | 0.6–1.7 | 33 | (22.0) | 1.9 | 1.1–3.2 | 15 | (10.0) | 0.9 | 0.5–1.7 |
| Bone cancer | 155 | 29 | (18.7) | 1.3 | 0.7–2.3 | 27 | (17.4) | 1.4 | 0.8–2.6 | 26 | (16.8) | 1.4 | 0.8–2.6 |
| Age at diagnosis, y | |||||||||||||
| 0–4 | 353 | 141 | (39.9) | 0.7 | 0.4–1.2 | 58 | (16.4) | 1.4 | 0.8–2.4 | 46 | (13.0) | 0.7 | 0.4–1.3 |
| 5–9 | 390 | 96 | (24.6) | 0.9 | 0.6–1.4 | 65 | (16.7) | 1.3 | 0.8–1.9 | 46 | (11.8) | 0.7 | 0.4–1.1 |
| 10–14 | 523 | 73 | (14.0) | 0.8 | 0.6–1.1 | 84 | (16.1) | 1.0 | 0.7–1.4 | 70 | (13.4) | 0.9 | 0.8–1.1 |
| 15+ | 631 | 54 | (8.6) | 1.0b | 110 | (17.4) | 1.0b | 95 | (15.1) | 1.0b | |||
| Radiation | |||||||||||||
| No | 565 | 76 | (13.5) | 1.0b | 89 | (15.8) | 1.0b | 85 | (15.0) | 1.0b | |||
| Yes | 1332 | 288 | (21.6) | 1.7 | 1.3–2.3 | 228 | (17.1) | 1.0 | 0.7–1.3 | 172 | (12.9) | 0.8 | 0.6–1.1 |
| Chemotherapy | |||||||||||||
| No | 775 | 147 | (19.0) | 1.0b | 118 | (15.2) | 1.0b | 109 | (14.1) | 1.0b | |||
| Yes | 1122 | 217 | (19.3) | 1.0 | 0.8–1.4 | 199 | (17.7) | 1.3 | 0.9–1.8 | 148 | (13.2) | 1.1 | 0.8–1.5 |
Data are presented as number (%), with odds ratios (OR) and 95% confidence intervals (CI). ALL refers to acute lymphocytic leukemia; CNS, central nervous system.
Defined as those scoring below (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-Fatigue] or above (Pittsburgh Sleep Quality Index [PSQI], Epworth Sleepiness Scale [ESS]) the 10th percentile for the sibling cohort.
Reference group.
The multivariate analyses comparing fatigue, disordered sleep, daytime sleepiness, and medical late effects and sociodemographic factors among cancer survivors and the sibling comparison group are shown in Tables 4 and 5. Female survivors and siblings were more likely to report fatigue, compared with males. Among survivors, those who reported CHF or pulmonary fibrosis were more likely to be in the fatigued group, and those with CHF were also more likely to be in the sleep-disturbed and daytime-sleepy groups.
Table 4.
Multivariate Analysis to Predict Prevalence of Fatigue or Sleep Disturbance by Medical Conditions and Sociodemographic Factors Among Childhood Cancer Survivors
| Number | Fatigueda | Disordered Sleepa | Daytime Sleepinessa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | OR | 95% CI | N | % | OR | 95% CI | N | % | OR | 95% CI | ||
| Total | 1897 | 364 | (19.2) | 317 | (16.7) | 257 | (13.5) | ||||||
| Sex | |||||||||||||
| Male | 933 | 131 | (14.0) | 1.0b | 138 | (14.8) | 1.0b | 117 | (12.5) | 1.0b | |||
| Female | 964 | 233 | (24.2) | 2.1 | 1.6–2.7 | 179 | (18.6) | 1.2 | 0.9–1.6 | 140 | (14.5) | 1.2 | 0.9–1.6 |
| Congestive heart failure | |||||||||||||
| No | 1855 | 343 | (18.5) | 1.0b | 297 | (16.0) | 1.0b | 242 | (13.0) | 1.0b | |||
| Yes | 42 | 21 | (50.0) | 2.9 | 1.4–6.1 | 20 | (47.6) | 4.0 | 2.0–8.0 | 15 | (35.7) | 4.2 | 2.0–8.7 |
| Lung fibrosis | |||||||||||||
| No | 1837 | 333 | (18.1) | 1.0b | 298 | (16.2) | 1.0b | 247 | (13.4) | 1.0b | |||
| Yes | 60 | 31 | (51.7) | 2.9 | 1.5–5.4 | 19 | (31.7) | 1.1 | 0.5–2.1 | 10 | (16.7) | 0.5 | 0.2–1.3 |
| Hypothyroidism | |||||||||||||
| No | 1726 | 322 | (18.7) | 1.0b | 282 | (16.3) | 1.0b | 231 | (13.4) | 1.0b | |||
| Yes | 171 | 42 | (24.6) | 0.9 | 0.7–1.3 | 35 | (20.5) | 1.0 | 0.6–1.5 | 26 | (15.2) | 0.9 | 0.6–1.9 |
| Depressed | |||||||||||||
| No | 1743 | 271 | (15.5) | 1.0b | 247 | (14.2) | 1.0b | 208 | (11.9) | 1.0b | |||
| Yes | 154 | 93 | (60.4) | 7.5 | 5.1–10.9 | 70 | (45.5) | 4.4 | 3.1–6.3 | 49 | (31.8) | 3.4 | 2.3–5.0 |
| BMI 30+ kg/m2 | |||||||||||||
| No | 1505 | 276 | (18.3) | 1.0b | 235 | (15.6) | 1.0b | 182 | (12.1) | 1.0b | |||
| Yes | 392 | 88 | (22.4) | 1.3 | 0.9–1.7 | 82 | (20.9) | 1.5 | 1.1–2.0 | 75 | (19.1) | 1.8 | 1.3–2.4 |
| Marital status | |||||||||||||
| Married | 1064 | 145 | (13.6) | 1.0b | 153 | (14.4) | 1.0b | 132 | (12.4) | 1.0b | |||
| Not married | 816 | 215 | (26.3) | 2.7 | 2.0–3.6 | 162 | (19.9) | 1.5 | 1.1–2.0 | 120 | (14.7) | 1.2 | 0.9–1.6 |
| Not indicated | 17 | 4 | (23.5) | NE | 2 | (11.8) | NE | 5 | (29.4) | NE | |||
| Employment status | |||||||||||||
| Working full time | 1240 | 202 | (16.3) | 1.0b | 146 | (11.8) | 1.0b | 153 | (12.3) | 1.0b | |||
| Not working full time | 657 | 162 | (24.7) | 1.2 | 0.3–1.6 | 171 | (26.0) | 1.3 | 1.0–1.7 | 104 | (15.8) | 1.1 | 0.8–1.5 |
| Infant at home < 6 mo old | |||||||||||||
| No | 1862 | 358 | (19.2) | 1.0b | 309 | (16.6) | 1.0b | 26 | (1.4) | 1.0b | |||
| Yes | 35 | 6 | (17.1) | 1.9 | 0.7–5.0 | 8 | (22.9) | 2.2 | 0.9–5.1 | 9 | (25.7) | 2.9 | 1.3–6.6 |
Data are presented as number (%), with odds ratios (OR) and 95% confidence intervals (CI). ALL refers to acute lymphocytic leukemia; CNS, central nervous system; BMI, body mass index; NE, not estimated.
Defined as those scoring below (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-Fatigue] or above (Pittsburgh Sleep Quality Index [PSQI], Epworth Sleepiness Scale [ESS]) the 10th percentile for the sibling cohort.
Reference group.
Table 5.
Multivariate Analysis to Predict Prevalence of Fatigue or Sleep Disturbance by Medical Conditions and Socioeconomic Factors within the Sibling Comparison Group
| Number | Fatigueda | Disordered Sleepa | Daytime Sleepinessa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | OR | 95% CI | N | % | OR | 95% CI | N | % | OR | 95% CI | ||
| Total | 369 | 40 | (10.8) | 45 | (12.2) | 50 | (13.6) | ||||||
| Sex | |||||||||||||
| Male | 176 | 10 | (5.7) | 1.0b | 18 | (10.2) | 1.0b | 29 | (16.5) | 1.0b | |||
| Female | 193 | 30 | (15.5) | 3.4 | 1.4–7.9 | 27 | (14.0) | 1.3 | 0.6–2.7 | 21 | (10.9) | 1.6 | 0.8–3.2 |
| Congestive heart failure | |||||||||||||
| No | 368 | 40 | (10.9) | 44 | (12.0) | 50 | (13.6) | ||||||
| Yes | 1 | 0 | (0.0) | NE | 1 | (100.0) | NE | 0 | (0.0) | NE | |||
| Lung fibrosis | |||||||||||||
| No | 367 | 40 | (10.9) | 45 | (12.3) | 50 | (13.6) | ||||||
| Yes | 2 | 0 | (0.0) | NE | 0 | (0.0) | NE | 0 | (0.0) | NE | |||
| Hypothyroidism | |||||||||||||
| No | 361 | 39 | (10.8) | 1.0b | 44 | (12.2) | 1.0b | 49 | (13.6) | ||||
| Yes | 8 | 1 | (12.5) | 0.7 | 0.1–6.5 | 1 | (12.5) | 1.1 | 0.1–10.0 | 1 | (12.5) | NE | |
| Depressed | |||||||||||||
| No | 312 | 28 | (9.0) | 1.0b | 31 | (9.9) | 1.0b | 14 | (4.5) | 1.0b | |||
| Yes | 57 | 12 | (21.1) | 9.5 | 3.6–25.0 | 14 | (24.6) | 8.9 | 3.6–21.8 | 36 | (63.2) | 2.2 | 1.3–3.1 |
| BMI 30+ kg/m2 | |||||||||||||
| No | 297 | 28 | (9.4) | 1.0b | 34 | (11.4) | 1.0b | 17 | (5.7) | 1.0b | |||
| Yes | 72 | 12 | (16.7) | 2.3 | 1.0–5.3 | 11 | (15.3) | 1.4 | 0.6–3.2 | 33 | (45.8) | 3.2 | 1.5–6.6 |
| Marital status | |||||||||||||
| Married | 213 | 23 | (10.8) | 1.0b | 24 | (11.3) | 1.0b | 29 | (13.6) | 1.0b | |||
| Not married | 154 | 16 | (10.4) | 1.0 | 0.4–2.2 | 20 | (13.0) | 0.9 | 0.4–1.9 | 20 | (13.0) | 0.9 | 0.4–1.8 |
| Not indicated | 2 | 1 | (50.0) | NE | 1 | (50.0) | NE | 1 | (50.0) | NE | |||
| Employment status | |||||||||||||
| Working full time | 257 | 24 | (9.3) | 1.0b | 24 | (9.3) | 1.0b | 38 | (14.8) | 1.0b | |||
| Not working full time | 112 | 16 | (14.3) | 1.3 | 0.6–2.9 | 21 | (18.8) | 2.2 | 1.1–4.6 | 12 | (10.7) | 0.6 | 0.2–1.3 |
| Infant at home < 6 mo. old | |||||||||||||
| No | 355 | 38 | (10.7) | 1.0b | 44 | (12.4) | 1.0b | 269 | (75.8) | 1.0b | |||
| Yes | 14 | 2 | (14.3) | 2.0 | 0.4–10.6 | 1 | (7.1) | 0.6 | 0.1–5.2 | 3 | (21.4) | 2.3 | 0.5–10.0 |
Data are presented as number (%), with odds ratios (OR) and 95% confidence intervals (CI). ALL refers to acute lymphocytic leukemia; CNS, central nervous system; BMI, body mass index; NE, not estimated.
Defined as those scoring below (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-Fatigue] or above (Pittsburgh Sleep Quality Index [PSQI], Epworth Sleepiness Scale [ESS]) the 10th percentile for the sibling cohort.
Reference group.
The prevalence of depression was higher among the siblings (15.4%) than survivors (8.1%) and strongly associated with each outcome. Depressed survivors were 7.5 times more likely to be in the fatigued group, whereas depressed siblings were 9.5 times more likely to be fatigued. For sleep and daytime sleepiness, depressed survivors were 3 to 4 times more likely to report symptoms. However, depressed siblings were 8.9 times more likely to report disordered sleep and twice as likely to report daytime sleepiness.
Obese survivors were more likely to experience disrupted sleep and daytime sleepiness compared to those with a BMI less than 30, while siblings were also more likely to experience daytime sleepiness. Unmarried survivors were more likely than those who are married to be in the fatigued and sleep-disordered groups. The presence of an infant in the home was associated with more daytime sleepiness but not with increased fatigue or sleep disturbance. Among siblings, no association was found with marital status or having a baby in the home. Unemployed survivors reported more sleep disturbance than did employed survivors. Unemployed siblings were 2.2 times more likely to report sleep disturbance than those fully employed.
The multivariate analysis comparing cancer survivors to the sibling comparison group is shown in Table 6. After adjustment for medical and socioeconomic factors, including depression, survivors were 1.9 times more likely than the siblings to be in the fatigued group. Those with CHF, depression, and obesity were more likely to report fatigue, disordered sleep, and daytime sleepiness.
Table 6.
Multivariate Analysis to Predict Prevalence of Fatigue and Sleep Disturbance by Medical Conditions or Socioeconomic Factors Comparing Survivors to the Sibling Comparison Group
| Number | Fatigueda | Disordered Sleepa | Daytime Sleepinessa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | OR | 95% CI | No. | % | OR | 95% CI | No. | % | OR | 95% CI | ||
| Total | 2266 | 404 | (17.8) | 362 | (16.0) | 307 | (13.5) | ||||||
| Case status | |||||||||||||
| Sibling | 369 | 40 | (10.8) | 1.0b | 45 | (12.2) | 1.0b | 50 | (13.6) | 1.0b | |||
| Survivors | 1897 | 364 | (19.2) | 1.9 | 1.3–2.9 | 317 | (16.7) | 1.4 | 0.9–1.9 | 257 | (13.5) | 1.0 | 0.7–1.4 |
| Sex | |||||||||||||
| Male | 1109 | 141 | (12.7) | 1.0b | 156 | (14.1) | 1.0b | 138 | (12.4) | 1.0b | |||
| Female | 1157 | 263 | (22.7) | 2.2 | 1.7–2.8 | 206 | (17.8) | 1.2 | 1.0–1.6 | 169 | (14.6) | 1.2 | 0.9–1.6 |
| Congestive heart failure | |||||||||||||
| No | 2223 | 383 | (17.2) | 1.0b | 341 | (15.3) | 1.0b | 292 | (13.1) | 1.0b | |||
| Yes | 43 | 21 | (48.8) | 2.6 | 1.2–5.8 | 21 | (48.8) | 4.1 | 2.0–8.5 | 15 | (34.9) | 3.8 | 1.8–8.1 |
| Lung fibrosis | |||||||||||||
| No | 2204 | 373 | (16.9) | 1.0b | 343 | (15.6) | 1.0b | 297 | (13.5) | 1.0b | |||
| Yes | 62 | 31 | (50.0) | 2.8 | 1.4–5.4 | 19 | (30.6) | 1.0 | 0.5–2.1 | 10 | (16.1) | 0.5 | 0.2–1.3 |
| Hypothyroidism | |||||||||||||
| No | 2087 | 361 | (17.3) | 1.0b | 326 | (15.6) | 1.0b | 280 | (13.4) | 1.0b | |||
| Yes | 179 | 43 | (24.0) | 0.9 | 0.6–1.4 | 36 | (20.1) | 1.0 | 0.7–1.4 | 27 | (15.1) | 0.9 | 0.6–1.5 |
| Depressed | |||||||||||||
| No | 2082 | 299 | (14.4) | 1.0b | 278 | (13.4) | 1.0b | 244 | (11.7) | 1.0b | |||
| Yes | 184 | 105 | (57.1) | 7.2 | 5.1–10.4 | 84 | (45.7) | 4.8 | 3.4–6.8 | 63 | (34.2) | 3.9 | 2.7–5.6 |
| BMI 30+ kg/m2 | |||||||||||||
| No | 1802 | 304 | (16.9) | 1.0b | 269 | (14.9) | 1.0b | 215 | (11.9) | 1.0b | |||
| Yes | 464 | 100 | (21.6) | 1.4 | 1.0–1.8 | 93 | (20.0) | 1.5 | 1.1–2.0 | 92 | (19.8) | 1.9 | 1.4–2.5 |
| Marital status | |||||||||||||
| Married | 1277 | 168 | (13.2) | 1.0b | 177 | (13.9) | 1.0b | 161 | (12.6) | 1.0b | |||
| Not married | 970 | 231 | (23.8) | 2.4 | 1.8–3.1 | 182 | (18.8) | 1.4 | 1.1–1.8 | 140 | (14.4) | 1.1 | 0.9–1.5 |
| Not indicated | 19 | 5 | (26.3) | NE | 3 | (15.8) | NE | 6 | (31.6) | ||||
| Employment status | |||||||||||||
| Working full time | 1497 | 226 | (15.1) | 1.0b | 195 | (13.0) | 1.0b | 191 | (12.8) | 1.0b | |||
| Not working full time | 769 | 178 | (23.1) | 1.2 | 1.0–1.6 | 167 | (21.7) | 1.6 | 1.2–2.0 | 116 | (15.1) | 1.0 | 0.8–1.4 |
| Infant at home < 6 mo. old | |||||||||||||
| No | 2217 | 396 | (17.9) | 1.0b | 353 | (15.9) | 1.0b | 295 | (13.3) | 1.0b | |||
| Yes | 49 | 8 | (16.3) | 1.9 | 0.8–4.7 | 9 | (18.4) | 1.7 | 0.8–3.7 | 12 | (24.5) | 2.7 | 1.4–5.4 |
Data are presented as number (%), with odds ratios (OR) and 95% confidence intervals (CI). ALL refers to acute lymphocytic leukemia; CNS, central nervous system; BMI, body mass index; NE, not estimated.
Defined as those scoring below (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-Fatigue] or above (Pittsburgh Sleep Quality Index [PSQI], Epworth Sleepiness Scale [ESS]) the 10th percentile for the sibling cohort.
Reference group.
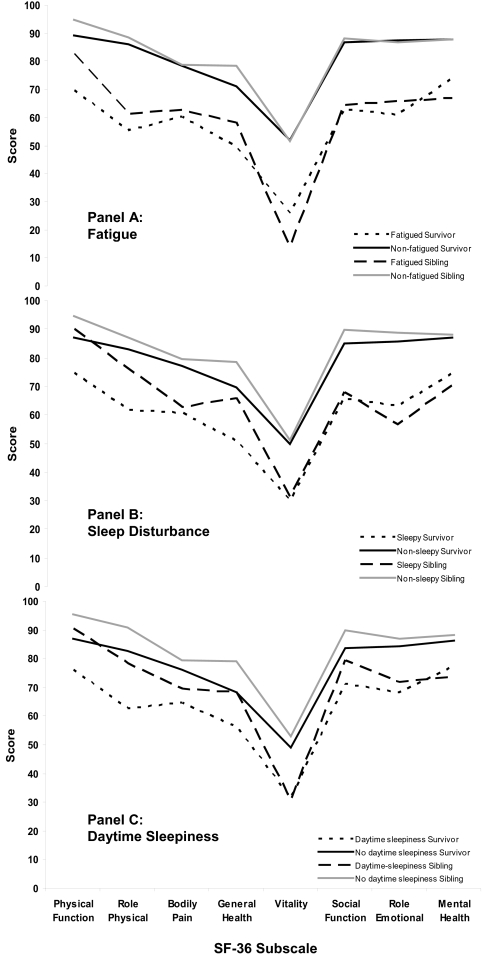
Health-Related Quality of Life
Fatigue, poor sleep quality, and increased daytime sleepiness were associated with lower scores across all domains of the SF-36 (Figure 1). Fatigued survivors scored significantly lower on the physical function (P < 0.01) and general health (P = 0.05) domains than did the fatigued siblings (panel A). However, fatigued siblings had lower mean scores than survivors on vitality (P < 0.01) and mental health (P = 0.05). Those survivors reporting sleep disturbance and daytime sleepiness (panels B and C) had lower physical functioning (P < 0.01), role physical (P = 0.03), and general health (P < 0.01) scores compared with siblings with disrupted sleep and daytime sleepiness. Among those not reporting fatigue, sleep disturbance, or daytime sleepiness mean scores for survivors were lower than those of the siblings on the physical function (P < 0.01), role physical (P < 0.01), general health (P < 0.01), and social function (P < 0.01) domains.
Figure 1.
Mean scores on SF-36 comparing survivors and siblings with and without fatigue, sleep disturbance, and daytime sleepiness.
DISCUSSION
Using the CCSS cohort, we performed the largest, most comprehensive study to date of cancer-related fatigue and sleep disturbance among adult survivors of childhood cancers. We identified statistically significant different mean scores on standardized measures of fatigue, sleep quality, and daytime sleepiness among survivors of ALL, CNS tumors, HD, STS, and bone tumors compared to a sibling control group. We also found that a significant percentage of survivors reported fatigue, but not necessarily disturbed sleep or increased daytime sleepiness, compared with siblings. However, predictors of fatigue, disturbed sleep, and daytime sleepiness were similar among both survivors and siblings. A significant association between fatigue, disordered sleep, and daytime sleepiness with overall health-related quality of life was identified in both groups.
Although these statistical differences on the various fatigue and sleep scales are compelling, their clinical relevance requires clarification. The large size of the CCSS cohort allowed us to identify small differences between means on each of the scales, differences that are unlikely to be recognizable in a clinical setting. A clinically meaningful difference in fatigue, sleep disturbance, or daytime sleepiness between adult survivors of childhood ALL, CNS tumors, HD, STS, and bone tumors and the sibling control group was not apparent.
Our results differ from studies of fatigue and sleep among survivors of adult cancers. In medical oncology, studies of fatigue have largely been performed among survivors of breast cancer or HD and report prevalence rates ranging between 17% to 30% 2 to 23 years following therapy.17,28,35 However, mixed results have been noted among childhood cancer survivors. Langeveld et al21 reported less fatigue in childhood cancer survivors than in the general population 15 years after diagnosis, and Zeltzer et al9 found similar levels of fatigue between survivors of childhood ALL and sibling controls. Yet, Meeske et al9,21,36 reported the prevalence of fatigue to be as high as 30% in 161 childhood ALL survivors an average of 13.9 years off therapy. With the longest follow-up to date (24.6 years; range 17-34 years), we found an overall prevalence of 19% among adult survivors of childhood cancer, and 15% among the ALL survivors.
We speculate that cancer-related fatigue may change or even decrease with increasing time from diagnosis. Visser et al37 has suggested that patients may self-inflate, or reduce, their assessment of fatigue over time, resulting in a “response-shift” to self-reported fatigue levels. It is possible that this shift explains the lower prevalence of fatigue among childhood cancer survivors compared with adult cancer survivors. Additionally, survivors of pediatric malignancies may not be prone to the chronic fatigue reported in the adult population. A recent report among HD survivors, a mean of 18.5 years from diagnosis, evaluated fatigue at 2 time points 8 years apart. Although the prevalence of fatigue remained elevated compared with the general population, investigators found a significant decrease over 8 years.38 With the longer follow-up in survivors of childhood cancers, fatigue levels might be expected to decline over time.
Those who had had a history of radiation therapy were more likely to be in the most fatigued group, yet no significant association was found with sleep quality or daytime sleepiness. Mean scores were not significantly different except among brain tumor survivors, whose PSQI scores actually suggested better sleep than those without radiation therapy. Furthermore, we examined the possibility of an association with mediastinal radiation therapy among survivors of HD and again found no significant difference between those with or without a history of radiation therapy. Additionally, no association between exposure to radiation therapy and depression could be identified. Notably no association between hypothyroidism and fatigue, sleep quality, or daytime sleepiness was identified. However, 93% of the 171 survivors who reported being told by a health care provider that they had hypothyroidism also reported replacement therapy. A history of CHF or pulmonary fibrosis increased the prevalence of fatigue, and those with CHF also experienced more sleep disturbance and daytime sleepiness. It is possible that subclinical medical conditions caused by radiation therapy, such as undiagnosed cardiopulmonary or endocrine disorders, may be responsible for increase fatigued.
Sleep-related issues have received minimal attention among cancer survivors. Insomnia has been reported in adult cancer patients, with a prevalence as high as 30% to 50% during active therapy and 23% to 44% 2 to 5 years following treatment.39 The prevalence in our study was much lower at 16.7%. Again, mixed results can be found among childhood cancer survivors. In our study, 13% of the ALL survivors reported sleep difficulties; however, Meeske et al36 found that as many as 50% of ALL survivors reported sleep problems. This discrepancy may be partially explained by variations in selecting a cut point to dichotomize the scale. The sensitivity (89.6%) and specificity (86.5%) of the PSQI is based upon a score of 5 to distinguish between “good” and “poor” sleepers.30 Although many reports have used this cut point, 1 has suggested that a score of 8 might be more appropriate in cancer survivors. Participants with no reported sleep disturbance were correctly identified by scores of less than 5, but mean scores were greater than 8 in those with disrupted sleep.40 Sixty percent of our study population had a score greater than 5, and 30% greater than 8. Therefore, we compared survivors with a sibling control group and classified individuals as sleep disturbed if their PSQI score was in the highest 10th percentile (≥ 9.5) of the sibling group.
Overall, we found a mean PSQI score of 6.1 and slightly higher scores among survivors of HD, STS, and bone sarcomas, suggesting some level of decreased sleep quality compared with that of the siblings. Yet, the actual difference between survivors and siblings was small and unlikely to be clinically detectable. Interestingly, an increased PSQI score persisted among STS survivors after adjustment for sex, age at diagnosis, a history of radiation therapy, and chemotherapy. To our knowledge, issues of disturbed sleep in sarcoma survivors have not previously been reported, and reasons for this association are not immediately evident. Sleep disturbance among STS survivors warrants further investigation and more detailed clinical correlation.
Being in the most fatigued, sleep disturbed, or daytime sleepiness group was associated with a poorer quality of life for survivors and siblings. Both had lower scores on the SF-36 measures than did survivors or siblings not reporting fatigue or sleep symptoms. Cancer survivors had lower scores, particularly in domains measuring function and physical activity, which persisted even when compared with siblings who also reported fatigue and sleep difficulty. Childhood cancer survivors have previously been reported to have greater performance limitations and participation restrictions, compared with a sibling comparison group.41 Limitations in function and well-being may portend increased fatigue and sleep disturbance.
It is challenging to separate the effects of depression upon fatigue and, likewise, the reverse. Depression was a strong predictor of self-reported fatigue in our study, even after adjustment for case status. Among survivors, approximately 8% reported depression, compared with 19% reporting fatigue, 16.7% poor sleep quality, and 14% daytime sleepiness. These estimates of depression are similar to those reported in other cancer survivor populations.19 Anderson et al42 reported higher levels of fatigue in depressed patients, compared with cancer patients and community subjects, but did not detect a significant difference in sleep scores among the 3. In a prospective study among cancer patients undergoing radiation therapy, Visser et al43 did not observe a strong association between fatigue and depression. In this cross-sectional analysis, we were not able to make causal conclusions; however, it would appear unlikely that cancer-related fatigue can be fully explained by a depressed mood.
These results should not be interpreted without recognizing a number of limitations. The FACIT-Fatigue and PSQI were validated in older populations than ours (median = 45.7 years, range 29-63; mean = 59.9 years, range 24-83, respectively).29,30 We were thus unable to directly compare our results with population norms. However, our use of siblings reflects an appropriate comparison group for this analysis because of their similarity in family-related factors. These data are self-reported and may be influenced by response bias. It is plausible that those who chose not to complete a survey, although demographically similar to our study population, had more medical complications, depression, or poorer physical function that may have resulted in increased fatigue or reduced sleep quality, thus underestimating the prevalence of fatigue and sleep disturbance in this population.
In conclusion, although mean scores for the FACIT-Fatigue, PSQI, and ESS scales were significantly different from those of the siblings, it is unlikely that these small absolute differences would be detected in a clinical setting. There were, however, more cancer survivors than siblings who reported fatigue. The only cancer-related predictor of fatigue was exposure to radiation therapy; for disordered sleep, the only predictor was the diagnosis of a STS. Except for these, our results suggest that survivors who suffer from fatigue or disrupted sleep have similar medical and sociodemographic factors causing these issues as might be expected in the general population in this age range. These factors are increased in cancer survivors due to their prior therapy. The possibility remains that there may be unmeasured or subclinical late effects or functional limitations in survivors of childhood cancer that may contribute to fatigue and sleep quality, yet at a lower rate than that observed in adult cancer survivors. Clearly, further research in adult and pediatric cancer survivors is needed to determine how fatigue and sleep disorders change over time and which medical and sociodemographic factors influence these changes.
ACKNOWLEDGMENTS
This research utilized the Childhood Cancer Survivor Study (CCSS), a resource supported by the National Cancer Institute to promote and facilitate research on long-term survivors of cancer diagnosed in childhood and adolescence. Investigators may apply to use the CCSS by proposing an analysis of existing data or proposing new initiatives that would utilize the cohort. Interested investigators are encouraged to visit the CCSS website at www.stjude.org/ccss to learn more about this unique resource.
APPENDIX
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived 5 or more years after diagnosis of childhood cancer.
CCSS is a retrospectively ascertained cohort of 20,720 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant e U24 CA55727) awarded to St. Jude Children's Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss.
CCSS Institutions and Investigators
| Institution | Investigators |
|---|---|
| St. Jude Children's Research Hospital, Memphis, TN | Leslie L. Robison, PhDbe; Melissa Hudson, MDab; Greg Armstrong, MDb |
| Children's Health Care, Minneapolis, MN | Joanna Perkins, MDa; Maura O'Leary, MDc |
| Children's Hospital and Medical Center, Seattle, WA | Debra Friedman, MD, MPHa; Thomas Pendergrass, MDc |
| Children's Hospital, Denver, CO | Brian Greffe, MDa; Lorrie Odom, MDc |
| Children's Hospital Los Angeles, CA | Kathy Ruccione, RN, MPHa |
| Children's Hospital, Oklahoma City, OK | John Mulvihill, MDb |
| Children's Hospital of Philadelphia, PA | Jill Ginsberg, MDa; Anna Meadows, MDb |
| Children's Hospital of Pittsburgh, PA | Jean Tersak, MDa; A. Kim Ritchey, MDc; Julie Blatt, MDc |
| Children's National Medical Center, Washington, DC | Gregory Reaman, MDa; Roger Packer, MDb |
| Cincinnati Children's Hospital Medical Center | Stella Davies, MD, PhDb |
| City of Hope- Los Angeles, CA | Smita Bhatia, MDa |
| Columbus Children's Hospital, OH | Amanda Termuhlen, MDa; Frederick Ruymann, MDc; Stephen Qualman, MDb; Sue Hammond, MDb |
| Dana-Farber Cancer Institute, Boston, MA | Lisa Diller, MDa; Holcombe Grier, MDc; Frederick Li, MDd |
| Emory University, Atlanta, GA | Lillian Meacham, MDa |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScDab; John Potter, MD, PhDbc |
| Hospital for Sick Children, Toronto, ON | Mark Greenberg, MBChBa |
| International Epidemiology Institute, Rockville, MD | John Boice, ScDb |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MDa; W. Anthony Smithson, MDc; Gerald Gilchrist, MDc |
| Memorial Sloan-Kettering Cancer Center New York | Charles Sklar, MDab; Kevin Oeffinger, MDb |
| National Cancer Institute, Bethesda, MD | Barry Anderson, MDb; Peter Inskip, Sc.D.b |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MDa; Robert Weetman, MDc |
| Roswell Park Cancer Institute, Buffalo, NY | Daniel M. Green, MDab |
| St. Louis Children's Hospital, MO | Robert Hayashi, MDa; Teresa Vietti, MDc |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MDa; Sarah S. Donaldson, MDb; Michael P. Link, MDc |
| Texas Children's Center, Houston, TX | Zoann Dreyer, MDa |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPHa; Jane Sande, MDc; Roger Berkow, MDc |
| University of Alberta, Edmonton, AB | Yutaka Yasui, PhDb |
| University of California-Los Angeles, CA | Lonnie Zeltzer, MDab |
| University of California-San Francisco, CA | Robert Goldsby, MDa; Arthur Ablin, MDc |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MDa |
| University of Minnesota, Minneapolis, MN | Ann Mertens, PhDab; Joseph Neglia, MD, MPHb |
| University of Southern California | Dennis Deapen, DrPHb |
| University of Washington, Seattle, WA | Norman Breslow, PhDb |
| UT-Southwestern Medical Center at Dallas, TX | Dan Bowers, MDa; Gail Tomlinson, MDc; George R. Buchanan, MDc |
| UT MD Anderson Cancer Center, Houston, TX | Louise Strong, MDab; Marilyn Stovall, MPH, PhDb |
Institutional Principal Investigator
Member CCSS Steering Committee
Former Institutional Principal Investigator
Former Member CCSS Steering Committee
Project Principal Investigator (U24 CA55727)
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Zeltzer has participated in a speaking engagement for Ortho-McNeil. Dr. Robison is on the scientific advisory board for Eli Lilly. The other authors have indicated no financial conflicts of interest.
Other investigators and institutions participating in the Childhood Cancer Survivor Study are listed in the appendix.
Supported by grant U24 CA 55727 (L.L. Robison, Principle Investigator), National Cancer Institute, Bethesda, MD, the Children's Cancer Research Fund, Minneapolis, MN, and the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Ries LAG EM, Kosary CL, Hankey BF, et al., editors. SEER Cancer Statistics Review, 1975-2000. Bethesda: National Cancer Institute; 2003. [Google Scholar]
- 2.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–29. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 3.Green DM, Whitton JA, Stovall M, et al. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187:1070–80. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 4.Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. J Clin Endocrinol Metab. 2003;88:4731–9. doi: 10.1210/jc.2003-030784. [DOI] [PubMed] [Google Scholar]
- 5.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97:663–73. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 6.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–41. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 7.Sklar C, Whitton J, Mertens A, et al. Abnormalities of the thyroid in survivors of Hodgkin's disease: data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2000;85:3227–32. doi: 10.1210/jcem.85.9.6808. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255–61. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 9.Zeltzer LK, Chen E, Weiss R, et al. Comparison of psychologic outcome in adult survivors of childhood acute lymphoblastic leukemia versus sibling controls: a cooperative Children's Cancer Group and National Institutes of Health study. J Clin Oncol. 1997;15:547–56. doi: 10.1200/JCO.1997.15.2.547. [DOI] [PubMed] [Google Scholar]
- 10.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 11.Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11:971–5. doi: 10.1023/a:1008318932641. [DOI] [PubMed] [Google Scholar]
- 12.Lewis G, Wessely S. The epidemiology of fatigue: more questions than answers. J Epidemiol Community Health. 1992;46:92–7. doi: 10.1136/jech.46.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Servaes P, van der Werf S, Prins J, Verhagen S, Bleijenberg G. Fatigue in disease-free cancer patients compared with fatigue in patients with chronic fatigue syndrome. Support Care Cancer. 2001;9:11–17. doi: 10.1007/s005200000165. [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–91. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–38. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 16.Flechtner H, Bottomley A. Fatigue and quality of life: lessons from the real world. Oncologist. 2003;8(Suppl 1):5–9. doi: 10.1634/theoncologist.8-suppl_1-5. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 18.Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S. Hodgkin's disease survivors more fatigued than the general population. J Clin Oncol. 1999;17:253–61. doi: 10.1200/JCO.1999.17.1.253. [DOI] [PubMed] [Google Scholar]
- 19.Fossa SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21:1249–54. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 20.Langeveld N, Ubbink M, Smets E. ‘I don’t have any energy': The experience of fatigue in young adult survivors of childhood cancer. Eur J Oncol Nurs. 2000;4:20–8. doi: 10.1054/ejon.1999.0063. [DOI] [PubMed] [Google Scholar]
- 21.Langeveld NE, Grootenhuis MA, Voute PA, de Haan RJ, van den Bos C. No excess fatigue in young adult survivors of childhood cancer. Eur J Cancer. 2003;39:204–14. doi: 10.1016/s0959-8049(02)00629-9. [DOI] [PubMed] [Google Scholar]
- 22.Ioos C, Estournet-Mathiaud B, Pinard JM, Cheliout-Heraut F. Sleep disorders caused by brainstem tumor: case report. J Child Neurol. 2001;16:767–70. doi: 10.1177/088307380101601012. [DOI] [PubMed] [Google Scholar]
- 23.Muller HL, Handwerker G, Wollny B, Faldum A, Sorensen N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J Clin Endocrinol Metab. 2002;87:3993–6. doi: 10.1210/jcem.87.8.8751. [DOI] [PubMed] [Google Scholar]
- 24.Rosen GM, Bendel AE, Neglia JP, Moertel CL, Mahowald M. Sleep in children with neoplasms of the central nervous system: case review of 14 children. Pediatrics. 2003;112:e46–54. doi: 10.1542/peds.112.1.e46. [DOI] [PubMed] [Google Scholar]
- 25.Van Someren EJ, Swart-Heikens J, Endert E, et al. Long-term effects of cranial irradiation for childhood malignancy on sleep in adulthood. Eur J Endocrinol. 2004;150:503–10. doi: 10.1530/eje.0.1500503. [DOI] [PubMed] [Google Scholar]
- 26.Palm L, Nordin V, Elmqvist D, Blennow G, Persson E, Westgren U. Sleep and wakefulness after treatment for craniopharyngioma in childhood;influence on the quality and maturation of sleep. Neuropediatrics. 1992;23:39–45. doi: 10.1055/s-2008-1071310. [DOI] [PubMed] [Google Scholar]
- 27.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 28.Ruffer JU, Flechtner H, Tralls P, et al. Fatigue in long-term survivors of Hodgkin's lymphoma;a report from the German Hodgkin Lymphoma Study Group (GHSG) Eur J Cancer. 2003;39:2179–86. doi: 10.1016/s0959-8049(03)00545-8. [DOI] [PubMed] [Google Scholar]
- 29.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 34.Verbeke G, Molenbergs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag, Inc; 2000. [Google Scholar]
- 35.Loge JH, Abrahamsen AF, Ekeberg, Kaasa S. Fatigue and psychiatric morbidity among Hodgkin's disease survivors. J Pain Symptom Manage. 2000;19:91–9. doi: 10.1016/s0885-3924(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 36.Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J Clin Oncol. 2005;23:5501–10. doi: 10.1200/JCO.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 37.Visser MR, Smets EM, Sprangers MA, de Haes HJ. How response shift may affect the measurement of change in fatigue. J Pain Symptom Manage. 2000;20:12–8. doi: 10.1016/s0885-3924(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 38.Hjermstad MJ, Fossa SD, Oldervoll L, Holte H, Jacobsen AB, Loge JH. Fatigue in long-term Hodgkin's Disease survivors: a follow-up study. J Clin Oncol. 2005;23:6587–95. doi: 10.1200/JCO.2005.09.936. [DOI] [PubMed] [Google Scholar]
- 39.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 41.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143:639–47. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 42.Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25:307–18. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 43.Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer. 1998;6:101–8. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]