Abstract
Question of the study:
Prevalence and determinants of daytime hypoxemia in patients with obstructive sleep apnea (OSA) syndrome are not well established. The aims of this study, conducted in a large series of OSA patients, were to estimate the prevalence of daytime hypoxemia, to assess the reciprocal effects between daytime PaO2 and nocturnal SpO2, and to investigate the direct and indirect role of sleep apnea severity in determining feedback gas exchange abnormalities.
Materials and methods:
In 456 patients a daytime hypoxemia-nocturnal hypoxia feedback structural equations model was designed. PaO2 adjusted for age (% of predicted), percent sleep time spent with SpO2 <90% (TST90), oxygen desaturation index and the apnea-hypopnea index, were determined as the measures of daytime hypoxemia, nocturnal hypoxia, and sleep apnea severity, respectively, after adjusting for the severity of obesity and lung volumes.
Results:
The TST90-PaO2 feed-back structural equations modeling showed that daytime PaO2 was inversely related (P<0.001) to nocturnal hypoxia (−4.0% of PaO2 per 1 SD of TST90). The severity of OSA (−1.0%) was an indirect determinant of daytime PaO2 via the TST90 pathway. In contrast, daytime PaO2 did not influence (P>0.05) the extent of nocturnal hypoxia.
Conclusions:
In OSA patients, the extent of nocturnal hypoxia seems to be both a direct determinant and a mediator of the indirect effect of sleep apnea on the development of daytime hypoxemia.
Citation:
Fanfulla F; Grassi M; Taurino AE; Lupo ND; Trentin R. The relationship of daytime hypoxemia and nocturnal hypoxia in obstructive sleep apnea syndrome. SLEEP 2008;31(2):249–255.
Keywords: Sleep apnea, hypoxemia, structural equation model, respiratory function, polysomnography
SLEEP APNEA IS A CHRONIC CONDITION CHARACTERIZED BY UPPER AIRWAY COLLAPSE DURING SLEEP. THE RESULTING DECREASE OR CESSATION OF airflow is generally associated with recurrent drops in oxyhemoglobin saturation. Gas exchange during sleep may be severely affected in certain patients, especially in those who are grossly obese or have chronic respiratory disorders, such as chronic obstructive pulmonary disease (COPD).1–2 Daytime hypoxemia has been reported to develop in patients with obstructive sleep apnea (OSA).3–8 In the past, looking for the physiological determinants of nocturnal arterial oxygenation in OSA patients, it was found that derangements of pulmonary mechanics and awake PaO2 were of major importance in establishing the severity of nocturnal hypoxemia.9 Other studies showed that the main determinant of daytime hypoxemia is not the OSA per se but rather concomitant comorbidity such as COPD.7–8,10 However, all these studies were performed on heterogeneous populations with a very high prevalence of COPD patients, so that airway obstruction and lung hyperinflation had major roles in determining the lower values of PaO2. Moreover, the PaO2 level at rest is highly dependent on age, and none of the studies previously cited corrected the level of PaO2 for age, thus invalidating any comparisons of the level of oxygenation in patients with widely different ages.
The aims of the current study were: (i) to estimate the prevalence of alterations in daytime oxygenation in a very large group of OSA patients with a very broad spectrum of disease severity and obesity; (ii) to assess the reciprocal (feedback) effects between daytime PaO2 and nocturnal SaO2, adjusted for comorbidities such as obesity and lung volumes; and (iii) to investigate the direct and indirect role of sleep apnea severity in determining feedback gas exchange abnormalities. Some of the results of this study have been previously reported in the form of an abstract.11
METHODS
Patients and Study Design
We studied 456 consecutive patients (49.4 ± 10.7 years, 160 females) who complained of snoring and excessive daytime sleepiness and had been consecutively referred to our laboratory for investigations of possible OSA. These investigations consisted of standard polysomnography and, the day after, determination of static and dynamic lung volumes and blood gas measurements. Patients with previously diagnosed or radiologically evident respiratory diseases, radiologically evident lung lesions (e.g., previous tuberculosis treated physically, pulmonary abscesses, pneumothorax), neuromuscular disorders (e.g., post-polio lesions), chest-wall defects, previous use of appetite suppressants or a clinical history of venous thromboembolic disease, a bronchopulmonary infection, or cardiac or respiratory failure in the preceding 6 months were excluded from the study, as were patients with a previous diagnosis of pulmonary arterial hypertension.
The protocol was submitted to the Technical and Ethical Committees. The former approved the protocol and the latter stated that approval was waived since, on admission to hospital, patients are asked to sign whether they consent or not to the use of their medical records and “routine” examination results for research purposes. We analyzed only records of patients who agreed to the use of their data.
Measurements
Sleep Study
Full standard in-laboratory polysomnography (Embla, Medcare, Reykyiavik, Iceland) was performed using current procedures and scored manually according to the criteria of Rechtschaffen and Kales.12 The polysomnography included: EEGs (C3-A2, C4-A1), EOGs, submental EMG, anterior tibialis electromyograms, nasal cannula airflow signal using a nasal cannula/pressure transducer system, oral thermistor, ECG and body position. SpO2 was recorded by means of an in-built pulse oximeter (Nonin Medical Inc., Minneapolis, MN, USA) with an Oximax sensor (Nellcore, Pleasanton, CA, USA). The equipment provides both an averaged (4 seconds) and a beat-to-beat SpO2 value. Respiratory efforts were monitored by the plethysmographic method (X-trace, Medcare, Iceland), so that the sum of thoracic and abdominal activities was obtained.
Arousals were scored according to standard criteria.13 Apnea was defined as a cessation of airflow for ≥10 seconds, while hypopnea as a clear amplitude reduction of a validated measure of breathing during sleep (but less than a 50% reduction from baseline) associated with an oxygen desaturation of >3% or an arousal.14
We determined the apnea-hypopnea index (AHI), and the oxygen desaturation index (ODI), and the percent sleep time spent with SpO2 <90% (TST90).
Evaluation of Daytime Respiratory Function and Blood Gas Assays
Pulmonary function tests (Masterlab, Jaeger, Hochberg, Germany) were performed as described in the European Respiratory Society statement.15 Respiratory function data were compared with predicted normal values obtained using the European Community for Steel and Coal (ECSC) 1983 regression equations. Diffuse airway obstruction was defined as a FEV1 < 70% of predicted and FEV1/VC <60%, according to a previous study.6
Arterial blood-gases were analyzed by an automated, computerized gas analyzer (ABL 550, Radiometer, Copenhagen, Denmark). PaO2 was adjusted for age and expressed as percent of predicted according to a reference equation for the Italian population.16 All the measurements were performed between 10:00 and 12:00 a.m. Daytime hypoxemia was defined as PaO2% <90%, while nocturnal hypoxia was defined as TST90 > 1%.
Statistical Analysis
Results are expressed as mean ± SD, and t-tests considering equal or unequal variances were used to compare data among the two groups of patients classified by the dichotomized definitions of TST90 and PaO2. Pearson's r correlation coefficients and forward stepwise regression analyses were performed to evaluate bivariate associations between pairwise variables, and the predictors that explain the variance of continuous measurements of TST90 and PaO2, respectively.
The relationship between daytime hypoxemia and nocturnal hypoxia was investigated by structural equations modeling (SEM).17,18 This is a statistical multivariate approach based on the use of a system of simultaneous equations to describe a priori relationships of observed (manifest) and unobserved (latent) variables of the physical process that generates the data.
A special type of structural equations modeling is the Path Analysis model in which only observed variables are considered in simultaneous equations. Path Analysis distinguishes three types of effects: direct, indirect, and total effects. The direct effect of an explanatory variable on a response variable is the net effect of a predictor setting all the other predictors in the built-in equations being equal. The indirect effect of an explanatory variable is the effect mediated by the pathway relationships of the other variables built-in the equations. The total effect is the sum of both the direct and indirect effects: total effect = direct effect + indirect effect. The decomposition of effects is always with respect to a specific model.
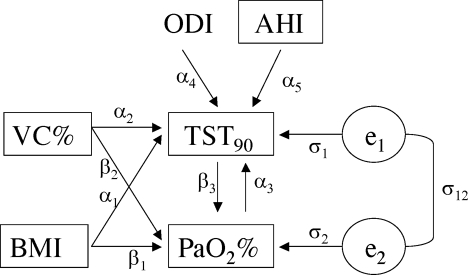
Based on a preliminary statistical analysis (a stepwise regression analysis), and clinical evidence, a Path Analysis of a TST90−PaO2% feedback model was designed (Figure 1) to assess the reciprocal effects between TST90 and PaO2%, and the direct and indirect effects of sleep apnea severity (expressed by AHI and ODI) in determining TST90−PaO2% feedback, adjusted for obesity (expressed by BMI), and lung volumes (expressed by VC%). A gender stratification analysis was also planned.
Figure 1.
Path diagram of the dependencies of the TST90−PaO2 feed back structural equations designed (*). The observed variables are enclosed in boxes, and the unobserved variables (error terms) are circled. An arrow from one variable to another indicates that the first variable has a direct influence on the second, two straight single-headed arrows connecting two variables indicate a reciprocal direct influence, and a curved two-headed arrow signifies a correlation between two variables. The path coefficients displayed on the arrows are the regression coefficients for the observed variables, and the residual variances/covariances for the unobserved errors.
(*) VC%: vital capacity in % of predicted; BMI: body mass index (Kg/m2); TST90: percent sleep time spent with SaO2 <90%; PaO2%: partial oxygen arterial pressure in % of predicted; ODI: oxygen desaturation index; AHI: apnea-hypopnea index. The SEM is written as:
TST90 = α1 BMI + α2 VC% + α3 PaO2% + α4 ODI + α5 AHI + e1
PaO2% = β1 BMI + β2 VC% + β3 TST90 + e2
var (e1)= σ1; var (e2)= σ2; cov (e1; e2) = σ12
The structural equations were fitted using maximum likelihood estimates (MLE). The χ2 difference test of “fitted” vs. “saturated” models was used for hypothesis testing to evaluate the appropriateness of the feed-back model; large P-value (P > 0.05) was the criteria for data support. The regression parameter estimates (direct, indirect, and total effects) of simultaneous equations were re-expressed in a standardized form multiplying the unstandardized one by 1 standard deviation (SD) of the explanatory variables. The P-values of the direct, indirect, and total estimates were evaluated by t-tests (=estimate/standard error) using robust standard errors; the level of statistical significance was set at P < 0.05, two-sided.
Descriptive and exploratory statistics were analyzed with SPSS 13.0 software (www.spss.com), while the feedback structural equations modeling was performed with Mplus 3.11 software (www.statmodel.com).
RESULTS
All patients enrolled completed the study. The mean PaO2 value was lower than expected on the basis of age (80.2% ± 10.7% of predicted). Fourteen patients (3% of 456 patients) had diffuse airway obstruction but they did not differ (P >0.05) from the remaining for PaO2% of predicted (76.3% ± 12.3% vs. 80.3% ± 10.7%), AHI (46.8 ± 22.3 vs. 48.4 ± 33.4) or TST90 (31.2 ± 39.6 vs. 24.2 ± 30.1). As expected, the patients with diffuse airway obstruction were older (age 60.7 ± 6.6 vs. 49.1 ± 12.2 yrs, P<0.001) and less obese (BMI 34.8 ± 6.9 vs. 42.7 ± 10.9 kg/m2, P = 0.01) than the entire sample.
The great majority of patients (81.6% of the 456 patients, 95% CI: 78.0% to 85.1%) had a PaO2 <90% of predicted. As shown in Table 1, the subjects with preserved PaO2 were older, and less obese than the patients with PaO2 <90% of predicted. The absolute FEV1/VC ratio was similar in these two groups of patients; while patients with daytime PaO2 >90% of predicted showed lower mean values of AHI, ODI, and TST90 in comparison with patients with daytime hypoxemia.
Table 1.
Anthropometric, Lung Volumes, Blood Gas, and Sleep Data of Patients Classified by the Presence of Daytime Hypoxemia (PaO2 %<90 of Predicted)
| PaO2%<90 (n = 372) | PaO2%>90 (n = 84) | P-value (#) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Sex (% (n) male) | 63.7 (237) | 70.2 (52) | 0.311 |
| Age (yr) | 48.1 ± 11.9 | 55.2 ± 12.1 | <0.001 |
| BMI (Kg/m2) | 44.3 ± 10.5 | 35.8 ± 12.1 | <0.001 |
| BSA (m2) | 2.36 ± 0.32 | 2.09 ± 0.26 | <0.001 |
| VC (% pred.) | 92.9 ± 14.5 | 99.2 ± 16.1 | 0.0013 |
| FEV1 (% of pred.) | 91.3 ± 17.1 | 110.3 ± 18.1 | <0.001 |
| FEV1/VC (abs value) | 78.3 ± 8.4 | 79.4 ± 7.7 | 0.2531 |
| FRC (% of pred.) | 75.5 ± 20.5 | 83.3 ± 20.6 | 0.0017 |
| RV (% of pred.) | 90.4 ± 23.9 | 90.1 ± 17.7 | 0.9162 |
| TLC (% of pred.) | 90.7 ± 12.3 | 94.0 ± 13.4 | 0.0302 |
| MEF50 (% of pred.) | 90.9 ± 34.5 | 96.2 ± 36.3 | 0.2083 |
| PaO2 (mmHg) | 68.9 ± 7.7 | 83.9 ± 6.4 | <0.001 |
| PaCO2 (mmHg) | 40.4 ± 4.7 | 37.0 ± 3.1 | <0.001 |
| AHI (event-hour-1) | 50.7 ± 34.2 | 38.2 ± 25.7 | 0.0021 |
| ODI (event-hour-1) | 52.1 ± 34.2 | 37.6 ± 25.3 | <0.001 |
| TST90 (%) | 13.1 ± 42.7 § | 1.45 ± 15.6 § | <0.001 |
BMI= body mass index; BSA = body surface area; RV = residual volume; MEF50: expiratory flow at 50% of FVC. AHI = apnea and hypopnea index; ODI = oxygen desaturation index; TST90 = total sleep time spent with SaO2 <90% (% of total sleep time).
P-values were computed by t-tests of two independent populations with equal or unequal variances, Fisher's exact χ2 test for sex, and Mann-Whitney U test for TST90.
Median ± Interquartile Range
The great majority of patients (79.4% of the 456 patients, 95% CI: 75.7% to 83.1%) had altered gas exchange during sleep, defined as TST90 >1% of total sleep time. As shown in Table 2, patients with nocturnal hypoxia had smaller lung volumes, but similar FEV1/VC ratio, functional residual capacity and residual volumes. In contrast, they had more severe sleep apnea and more impaired daytime PaO2.
Table 2.
Anthropometric, Lung Volumes, Blood Gas, and Sleep Data of Patients Classified by Presence of Nocturnal Hypoxia (TST90≥1.)
| TST90<1 % (n=94) | TST90 ≥1 % (n=362) | P-value (#) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Sex (% (n) male) | 56.4 (53) | 67.1 (243) | 0.068 |
| Age (yr) | 50.5 ± 13.4 | 49.2 ± 11.9 | 0.3466 |
| BMI (Kg/m2) | 40.0 ± 12.6 | 43.4 ± 10.9 | 0.0086 |
| BSA (m2) | 2.19 ± 0.31 | 2.34 ± 0.32 | <0.001 |
| VC (% pred.) | 98.6 ± 15.1 | 92.9 ± 14.8 | <0.001 |
| FEV1 (% of pred.) | 96.7 ± 18.1 | 92.0 ± 17.4 | 0.0195 |
| FEV1/VC (abs value) | 78.5 ± 8.1 | 78.5 ± 8.4 | 0.9547 |
| FRC (% of pred.) | 80.5 ± 19.6 | 76.0 ± 21.0 | 0.0605 |
| VR (% of pred.) | 94.0 ± 20.7 | 89.4 ± 23.4 | 0.0878 |
| TLC (% of pred.) | 94.2 ± 12.4 | 90.5 ± 12.5 | 0.0117 |
| MEF50 (% of pred.) | 92.4 ± 36.4 | 91.7 ± 34.4 | 0.8713 |
| PaO2 (mm Hg) | 77.3 ± 8.0 | 70.2 ± 9.3 | <0.001 |
| PaCO2 (mm Hg) | 37.7 ± 3.7 | 40.3 ± 4.7 | <0.001 |
| AHI (event-hour−1) | 26.8 ± 23.2 | 54.0 ± 33.0 | <0.001 |
| ODI (event-hour−1) | 28.4 ± 26.0 | 54.9 ± 32.8 | <0.001 |
| PaO2 (% of pred) | 87.0 ± 9.2 | 78.5 ± 10.4 | <0.001 |
BMI = body mass index; BSA = body surface area; RV = residual volume; AHI = apnea and hypopnea index; ODI = oxygen desaturation index; TST90 = total sleep time spent with SaO2 <90%.
P-values were computed by t-tests of two independent populations with equal or unequal variances, and Fisher's exact χ2 test for sex.
A stepwise regression procedure included the predictors VC, BMI, AHI, ODI, and PaO2% in the regression equation of TST90 from all the potential predictor variables listed in Table 2; while VC, BMI, and TST90 were the selected predictors in the regression equation of PaO2% from all the potential predictor variables listed in Table 1 (data not shown). The results of separate linear regression models for PaO2% and TST90 were used to define the Path Analysis model with the addition of the feedback loop.
The maximum likelihood estimates and robust 95% CI of the standardized regression coefficient parameters (=average change of the response variable by 1 standard deviation (SD) increment of the explanatory variable) for the TST90-PaO2 feedback structural equations of Figure 1 are reported in Table 3. The chi-square testing (χ2 = 3.242, df = 1, P = 0.072 NS) provided evidence of the appropriateness of the feedback model given the observed data. The two feedback structural equations explained 34.2% and 31.3% of the total variance of continuous measurements of TST90 and PaO2%, respectively, while the residual correlation between TST90 and PaO2% was fixed to 0 to ensure that the regression parameters of the estimated structural model were identified.
Table 3.
Maximum Likelihood Estimates, and Robust 95% CI of Direct and Indirect Effect Parameters of the Fitted (*) TST90-PaO2 Feedback Structural Equations of Figure 1
| TST90 (%) |
PaO2 (%) |
|||
|---|---|---|---|---|
| β (95%CI) § | P-value | β (95%CI) § | P-value | |
| Feed-back loop: | ||||
| TST90 (%) | − | − | −3.96 (−2.11 to −5.82) | <0.001 |
| PaO2 (%) | +0.15 (−6.35 to +6.65) | 0.965 | − | − |
| Cor (TST90; PaO2) | 0 # | 0 # | ||
| Direct effects: | ||||
| BMI (kg/m2) | +3.91 (+0.61 to +7.21) | 0.020 | −3.33 (−2.14 to −4.53) | <0.001 |
| Vital capacity (%) | −4.91 (−2.24 to −7.58) | <0.001 | +1.50 (+0.59 to +2.41) | 0.002 |
| AHI (event-hour−1) | +7.99 (+3.63 to +12.3) | <0.001 | − | − |
| ODI (event-hour−1) | +7.01 (+2.45 to +11.6) | 0.003 | − | − |
| Indirect effects: | ||||
| AHI (event-hour−1) via TST90 | − | − | −1.03 (−0.25 to −1.81) | 0.008 |
| ODI (event-hour−1) via TST90 | − | − | −0.93 (−0.28 to −1.58) | 0.009 |
| R-square | 0.342 | 0.313 | ||
χ2 = 3.242, df = 1, P = 0.072 NS
Fixed parameter in the structural equations
β = average change of the response variable for an increment of 1 SD in the explanatory variable
The daytime PaO2 % of predicted was statistically significantly associated (P<0.001) with the feedback effect of nocturnal hypoxia (TST90) and with the effects of obesity (BMI) and lung volumes (VC%). Specifically, an increment of 1 SD (30.5%) of TST90 produced a reduction of 3.96% (95% CI: −2.11% to −5.82%) in the PaO2 % of predicted; an increment of 1 SD (11.3 kg/m2) of BMI produced a decline of 3.33% (95% CI: −2.14% to −4.53%); and an increment of 1 SD (15%) of VC% produced a rise of 1.5% (95% CI: +0.59% to +2.41%).
In contrast, the daytime PaO2 feedback effect did not influence (P = 0.965) the extent of nocturnal hypoxia, which was statistically significantly related with the severity of OSA (P <0.001), and background measurements (BMI, P = 0.020 and VC%, P<0.001). Specifically, an increment of 1 SD (33 events/1 hour) in the AHI or ODI increased TST90 by 7.99% (95% CI: +3.63% to +12.3%) or 7.01% (95% CI: +2.45% to +11.6%), respectively; TST90 also increased by 3.91% (95% CI: +0.61% to +7.21%) for 1 SD of BMI and declined 4.91% (95% CI: −2.24% to −7.58%) for 1 SD of VC%.
The above described changes were net effects (direct effects); however, the severity of OSA, expressed by AHI and ODI, also had statistically significant indirect effects (P = 0.009) on daytime hypoxemia via the nocturnal hypoxia pathway, i.e., TST90 is a variable that mediates the effect of OSA on PaO2 outcome. Specifically, via the TST90 path, an increment of 1 SD in the AHI or ODI decreased PaO2 % of predicted by about 1% (95% CI: −0.25% to −1.81% for AHI, and −0.28% to −1.58% for ODI).
None of the results changed if PaO2 was considered in absolute values rather than in % of predicted.
Stratification according to gender did not show any major differences in feedback, direct or indirect effects between subgroups (data not shown).
DISCUSSION
The main findings of this study are: (i) patients with OSA, in the absence of lung comorbidity such as diffuse airway obstruction, had lower values of PaO2 than those expected on the basis of age; and (ii) a feedback model of daytime hypoxemia-nocturnal hypoxia (Figure 1) was not supported, even after adjustment for obesity and lung volume, and gender stratification. An increase of percent sleep time with reduced SpO2 is associated with a decrease in daytime PaO2, while the level of nocturnal SpO2 is not related to the level of daytime PaO2. Moreover, because nocturnal hypoxia is related to the severity of OSA (expressed by AHI and ODI), the level of nocturnal SaO2 is also a mediator of the severity of the OSA effect on daytime hypoxemia.
We performed the present study in an attempt to determine the direction of the relationship between daytime hypoxemia and nocturnal hypoxia. This issue is related to an appropriate definition of daytime hypoxemia and an appropriate identification of the population of patients. Firstly, most of the previous papers reported that a normal value of PaO2 at rest is ≥80 mm Hg, hypoxemia is present when the PaO2 is below 80 mm Hg, and severe hypoxemia when the PaO2 is ≤65 mm Hg.1,3,7,8,10 None of these studies adjusted the PaO2 values for age, obesity or lung volumes. We corrected the PaO2 data according to the reference equation for the Italian population, thus defining the PaO2 as a percent of predicted. In this way, we found that the great majority of subjects had reduced PaO2 values. Indeed, only 84 (18%) patients had a PaO2 higher than 90% of predicted. This result did not change when different reference values of PaO2 published in the literature were applied (data not shown),19,20 or when the PaO2 was considered as an absolute value.
The second point regards the choice of the population of the patients. In the present study we included consecutive patients with sleep apnea, so that all biases related to disordered respiratory mechanics could be excluded. This was not the case in the previous studies in which a significant proportion of patients, ranging from 11% to 25%, were affected by concomitant COPD, with the prevalence of COPD, sometimes with FEV1 < 1 litre.1,3,7–8,10 In COPD, peripheral airway obstruction, parenchymal destruction, and pulmonary vascular abnormalities reduce the lung's capacity for gas exchange, producing hypoxemia and, later on, hypercapnia.21 Since daytime PaO2 data were not corrected for age or lung function and, since subjects with concomitant COPD were older than those with simple OSA, the effect of age and lung function impairment in determining lower values of PaO2 were mutually enhanced.22
We found that TST90, an index of nocturnal hypoxia, was a valuable determinant of the development of daytime hypoxemia, even after adjustment for the degree of obesity, lung volumes and sex stratification. AHI and ODI, expressions of the severity of sleep apnea, were also significant determinants although their effect was mediated through TST90. Of interest, the level of TST90 appears independent of daytime PaO2, because the feedback (reciprocal) effect was not supported. This latter is an intriguing observation since in the past it was hypothesized that a low level of daytime PaO2 may account for the severity of the nocturnal hypoxia.10 Indeed, Bradley et al. observed, in a group of OSA patients, that the main predictors of nocturnal desaturation, expressed by the mean value of SaO2 across the night, were daytime PaO2, alterations in respiratory mechanics (again mainly related to airway obstruction and the degree of obesity), and the sleep time spent in apnea. On the contrary, our results are in agreement with data, reported by Sanders et al, obtained from the Sleep Heart Health Study.23 They found that patients with sleep apnea without obstructive airway disease had a 20-fold higher odds ratio for nocturnal desaturation than that of healthy subjects, even after adjustment for age, sex, height, weight, race, smoking status and awake SaO2.
The development of daytime hypoxemia in OSA patients has generally been related to the presence of subclinical arterial pulmonary hypertension (PH).3–6 However, this association with pulmonary hypertension is now a matter of discussion since most of the studies produced conflicting results. It should be considered that all the studies that gave negative results were the oldest ones and the ones with a relevant percentage of COPD patients, as stated above.1–3,7–8,10 There is, however, a growing body of evidence that highlights the role of sleep apnea in the development of daytime gas exchange abnormalities through the pathophysiological effect of chronic intermittent hypoxia. The occurrence of PH in OSA patients without concomitant lung disease ranged from 20% to 43%.4–6,24 In all these studies PH patients had a more altered nocturnal gas exchange and in two of them a lower level of daytime PaO2.6,24
The increasing of pulmonary arterial pressure has been associated with hypoxia-induced pulmonary vasoconstriction.5,25,26 Animal models showed increases in right ventricular mass, hematocrit level and pulmonary arterial pressure during exposure to chronic intermittent hypoxia or asphyxia.27,28 Chronic intermittent hypoxia activates homeostatic mechanisms in the respiratory system that induce changes in gene expression by mediation of several transcription factors, such as hypoxia-inducible factor (HIF).29 Experimental data showed that hypoxia inducible factors (both HIF-1α and HIF-2α) are involved in physiological responses of pulmonary arterioles to chronic intermittent hypoxia, collectively producing hypertrophy and depolarization of pulmonary arteriolar smooth muscle.29
Recent studies suggested that the hypoxia inducible factor-1 pathway response to chronic intermittent hypoxia seems to be activated only in patients with moderate to severe nocturnal gas exchange alterations.30 These patients showed a significantly increased level of erythropoietin during sleep, differently from patients with less severe OSA.31 On the contrary, in these latter chronic intermittent hypoxia activated a different pathway mediated through the transcription factor nuclear factor-κB that, in turn, increased the expression of tumor necrosis factor-α, which may contribute to endothelial dysfunction.32 Finally, treatment with CPAP has been demonstrated to reduce pulmonary arterial pressure levels also in patients with a pressure <20 mm Hg.26,33
However, daytime hypoxemia in OSA patients may develop through mismatching of ventilation and perfusion, as a result of different mechanisms, such as changes in mechanical properties of the lung or development of subclinical interstitial pulmonary edema. An increase in elastance of the whole lung during sleep was found in patients with OSA.34 Sleep may induce a loss in the mechanical coupling between airways and lung parenchyma which, in turn, allows the airways to narrow more easily.35 Alternatively, the reduction of lung volumes during sleep could not only decrease parenchymal attachments but also increase the forces arising from surface tension, which would lead to an increase in elastance. Indeed, it was shown that the severity of apnea-induced desaturation was correlated with lung volume, particularly with the difference between supine expiratory reserve volume and seated closing volume.36 These mechanical modifications may induce closure of some respiratory units during upper airway obstruction, which is reversed at the end of the obstruction. These transient abnormalities in the recruitment of lung units resulting in air space closure reduce the gas exchange capacity of the lungs independently from the level of daytime gas exchange.34
Chronic intermittent hypoxia induces proliferation of the vasculature due to angiogenesis but can also change the integrity of vessels, leading to changes in vascular permeability.37 Fletcher et al., in a canine model of OSA, demonstrated that pulmonary edema can develop after recurrent obstructive apneas.38 They found a significant deterioration of gas exchange in those animals with histologic and/or electron microscopy evidence of lung edema compared to those without edema and controls and a substantial fall in the amplitude of apnea desaturation related to deterioration of gas exchange. It has been demonstrated that hypoxia induces angiogenesis upregulating the vascular endothelial growth factor (VEGF).39 Schulz et al. found that the serum levels of VEGF are elevated in severely hypoxic patients with OSA and are related to the degree of nocturnal oxygen desaturation.40 Of interest, patients with the highest degree of nocturnal desaturation were those with the lowest level of daytime PaO2.
Recently, Guardiola et al. suggested that the mechanical changes and increased interstitial lung water described above were not immediately reversed since they found that OSA patients had lower PaO2 values in the morning than in the evening.41
A possible limitation of our study, like that of the other studies, is the lack of information about the intensity and duration of exposure to nocturnal hypoxia. Different durations of disease, or, more probably, an early onset of OSA may contribute to explain the variability between the results we obtained and those of the previous studies. One particularly complex methodological issue in characterizing the progression of OSA is the worsening epidemic of obesity in western countries such that it can be very difficult to separate the effects on gas exchange of prolonged OSA from those related to increasing body weight.42 Indeed, a longitudinal study on sleep disordered breathing performed in a nonclinic population found that changes in the respiratory disturbance index over time do not vary uniformly with age, sex, and weight.43
Finally, most of the patients enrolled in our study were obese and therefore caution needs to be exercised regarding the generalizability of our findings. However, all the results were statistically adjusted for the level of obesity so that the effect of this anthropometric parameter in our specific cohort could be minimized.
In conclusion, our data support the evidence that nocturnal hypoxia seems to be both a direct determinant and a mediator of the indirect effect of sleep apnea on the development of daytime hypoxemia. Follow-up studies are needed to evaluate the time course of daytime gas exchange abnormalities and to assess the role of therapy in stopping or slowing down this evolution.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest. 1996;109:380–6. doi: 10.1378/chest.109.2.380. [DOI] [PubMed] [Google Scholar]
- 2.Laks L, Lehrhaft B, Grunstein RR, Sullivan CE. Pulmonary hypertension in obstructive sleep apnoea. Eur Respir J. 1995;8:537–41. [PubMed] [Google Scholar]
- 3.Bradley TD, Rutherford R, Grossman RF, et al. Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis. 1985;161:835–9. doi: 10.1164/arrd.1985.131.6.835. [DOI] [PubMed] [Google Scholar]
- 4.Sanner BM, Doberauer C, Konermann M, Sturm A, Zidek W. Pulmonary hypertension in patients with obstructive sleep apnea syndrome. Arch Intern Med. 1997;157:2483–7. [PubMed] [Google Scholar]
- 5.Sajkov D, Cowie RJ, Thornton AT, Espinoza HA, McEvoy RD. Pulmonary hypertension and hypoxemia in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1994;149:416–22. doi: 10.1164/ajrccm.149.2.8306039. [DOI] [PubMed] [Google Scholar]
- 6.Bady E, Achkar A, Pascal S, Orvoen-Frija E, Laaban JP. Pulmonary arterial hypertension in patients with sleep apnoea syndrome. Thorax. 2000;55:934–9. doi: 10.1136/thorax.55.11.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieger J, Sforza E, Apprill M, Lampert E, Weitzenblum E, Ratomaharo J. Pulmonary hypertension, hypoxemia, and hypercapnia in obstructive sleep apnea patients. Chest. 1989;96:729–37. doi: 10.1378/chest.96.4.729. [DOI] [PubMed] [Google Scholar]
- 8.Chaouat A, Weitzemblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:82–6. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 9.Bradley TD, Martinez D, Rutherford R, et al. Physiological determinants of nocturnal arterial oxygenation in patients with obstructive sleep apnea. J Appl Physiol. 1985;59:1634–8. doi: 10.1152/jappl.1985.59.5.1364. [DOI] [PubMed] [Google Scholar]
- 10.Weitzenblum E, Krieger J, Appril M, et al. Daytime pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am Rev Respir Dis. 1988;138:345–9. doi: 10.1164/ajrccm/138.2.345. [DOI] [PubMed] [Google Scholar]
- 11.Taurino AE, Bruschi C, Trentin R, D'Artavilla Lupo N, Montemartini S, Fanfulla F. The reversibility of hypoxemia in OSA patients is related to CPAP therapy. Eur Respir J. 2005;26:112S. [Google Scholar]
- 12.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages in human adults. Washington. DC: U.S. Government Printing Office; 1968. [Google Scholar]
- 13.Atlas Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 14.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault J-C. Report Working Party. Standardization of lung function tests. Eur Respir J. 1993;6(Suppl.16):5–40. [PubMed] [Google Scholar]
- 16.Cerveri I, Zoia MC, Fanfulla F, et al. Reference values of arterial oxygen tension in the middle-aged and elderly. Am J Respir Crit Care Med. 1995;152:934–41. doi: 10.1164/ajrccm.152.3.7663806. [DOI] [PubMed] [Google Scholar]
- 17.Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- 18.Pearl J. Models, reasoning, and inference. New York: Cambridge University Press; 2000. Causality. [Google Scholar]
- 19.Sorbini CA, Grassi V, Solinas E, Muiesan G. Arterial oxygen tension in relation to age in healthy subjects. Respiration. 1968;25:3–13. doi: 10.1159/000192549. [DOI] [PubMed] [Google Scholar]
- 20.Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1400 meters. Am J Respir Crit Care Med. 1999;160:1525–31. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- 21.Global Initiative for Chronic Obstructive Lung Disease. [Accessed September 21, 2006];Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. doi: 10.1111/j.1440-1843.2005.00692.x. Update 2005. Available at: http//www.goldcopd.com. [DOI] [PMC free article] [PubMed]
- 22.Fletcher EC, Schaaf JW, Miller J, Fletcher JG. Long-term cardiopulmonary sequelae in patients with sleep apnea and chronic lung disease. Am Rev Respir Dis. 1987;135:525–33. doi: 10.1164/arrd.1987.135.3.525. [DOI] [PubMed] [Google Scholar]
- 23.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 24.Alchantis A, Tourkohoriti G, Kakouros S, Kosmas E, Podaras S, Jordanoglou JB. Daytime pulmonary hypertension in patients with obstructive sleep apnea. Respiration. 2001;68:566–72. doi: 10.1159/000050574. [DOI] [PubMed] [Google Scholar]
- 25.Sajkov D, Wang T, Saunders NA, Bune AJ, Neill AM, McEvoy RD. Daytime pulmonary hemodynamics in patients with obstructive sleep apnea without lung disease. Am J Respir Crit Care Med. 1999;159:1518–26. doi: 10.1164/ajrccm.159.5.9805086. [DOI] [PubMed] [Google Scholar]
- 26.Sajkov D, Wang T, Saunders NA, Bune AJ, McEvoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 165:152–8. doi: 10.1164/ajrccm.165.2.2010092. [DOI] [PubMed] [Google Scholar]
- 27.Fagan KA. Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol. 2001;90:2502–7. doi: 10.1152/jappl.2001.90.6.2502. [DOI] [PubMed] [Google Scholar]
- 28.Bradford A. Effects of chronic intermittent asphyxia on haematocrit, pulmonary arterial pressure and skeletal muscle structure in rats. Exp Physiol. 2004;89:44–52. doi: 10.1113/expphysiol.2003.002656. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–7. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- 30.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/Calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–8. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 31.Winnicki M, Shamsuzzaman A, Lanfranchi P, et al. Erythropoietin and obstructive sleep apnea. Am J Hypertens. 2004;17:783–6. doi: 10.1016/j.amjhyper.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 33.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martinez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure. Eur Heart J. 2006;27:1106–13. doi: 10.1093/eurheartj/ehi807. [DOI] [PubMed] [Google Scholar]
- 34.Bijaoui EL, Champagne V, Baconnier PF, Kimoff J, Bates JHT. Mechanical properties of the lung and upper airways in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:1055–61. doi: 10.1164/ajrccm.165.8.2107144. [DOI] [PubMed] [Google Scholar]
- 35.Irvin CG, Pak J, Martin RJ. Airway-parenchyma uncoupling in nocturnal asthma. Am J Respir Crit Care Med. 2000;161:50–6. doi: 10.1164/ajrccm.161.1.9804053. [DOI] [PubMed] [Google Scholar]
- 36.Series F, Cormier Y, La Forge. Role of lung volumes in sleep apnoea-related oxygen desaturation. Eur Respir J. 1989;2:26–30. [PubMed] [Google Scholar]
- 37.Neubauer JA. Physiological and pathophysiological response to intermittent hypoxia. J Appl Physiol. 2001;90:1593–9. doi: 10.1152/jappl.2001.90.4.1593. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher EC, Proctor M, Yu J, et al. Pulmonary edema develops after recurrent obstructive apneas. Am J Respir Crit Care Med. 1999;160:1688–96. doi: 10.1164/ajrccm.160.5.9810003. [DOI] [PubMed] [Google Scholar]
- 39.Sinor AD, Irvin SM, Cobbs CS, Chen J, Graham SH, Greenberg DA. Hypoxic induction of vascular endothelial growth factor (VEGF) protein in astroglial cultures. Brain Res. 1998;812:289–91. doi: 10.1016/s0006-8993(98)00976-7. [DOI] [PubMed] [Google Scholar]
- 40.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum level of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 41.Guardiola J, Yu J, Hasan N, Fletcher EC. Evening and morning blood gases in patients with obstructive sleep apnea. Sleep Med. 2004;5:489–93. doi: 10.1016/j.sleep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 43.Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26:703–9. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]