Abstract
The discovery and development of medications to treat addiction and notably, cocaine addiction, have been frustrated by both the complexity of the disorder and the lack of target validation in human subjects. The dopamine transporter has historically been a primary target for cocaine abuse medication development, but addictive liability and other confounds of such inhibitors of dopamine uptake have limited clinical evaluation and validation. Herein we describe efforts to develop analogues of the dopamine uptake inhibitors GBR 12909 and benztropine that show promising profiles in animal models of cocaine abuse that contrast to that of cocaine. Their unique pharmacological profiles have provided important insights into the reinforcing actions of cocaine and we propose that clinical investigation of novel dopamine uptake inhibitors will facilitate the discovery of cocaine-abuse medications.
1. Introduction
Drug and alcohol addictions affect more than 30 million people in the United States (US) and Europe [1]. In the US in 2004, the overall cost of drug abuse and addiction was estimated to be greater than $185 billion. This estimate includes costs associated with reduced workplace productivity, health care bills, and expenses related to the criminal justice system. When legal drugs like nicotine and alcohol are included, the projected overall cost of addiction in 2007 may exceed $500 billion [2]. Drug addiction develops over a period of time, evolving from sporadic or intermittent drug use to regular use, and finally to addiction in vulnerable individuals [3]. Numerous factors influence vulnerability to addiction, including genetic factors (40–60%) and environmental factors, such as drug availability, socio-economic status, social support networks, and various life stressors [4].
Many studies indicate that all drugs of abuse, despite their differences in molecular mechanisms, share the common ability to activate mesolimbic dopamine (DA) neurons in the brain. With cocaine and amphetamine, this activation results directly from increases in synaptic DA released from nerve terminals in the nucleus accumbens (NAc). Notably, cocaine binds to the DA transporter to inhibit reuptake of dopamine into the cell, whereas amphetamine is a substrate for the transporter and thereby causes an increase in dopamine release. The ability of drugs of abuse to increase synaptic DA in the NAc underlies their ability to support self-administration behavior, which is one way to measure the reinforcing properties of these agents. Natural reinforcers such as food and sex also stimulate DA transmission in the NAc, but these do not generally lead to addiction, perhaps because natural reinforcers produce elevations in synaptic DA that are substantially lower in magnitude and more discrete in terms of anatomical distribution, when compared to drugs of abuse [4]. Chronic treatment of animals with drugs of abuse produces long-term changes in brain function via alterations in gene expression [5]. Such persistent changes in gene expression are thought to contribute to the progression from occasional drug use to uncontrolled abuse and addiction.
The central role of DA and the NAc in mediating the reinforcing effects of drugs should not obscure the fact that non-dopaminergic neurons and circuits also contribute to the development and maintenance of addictive behavior. For example, chronic stimulant exposure produces deficits in brain serotonin function that resemble those observed in major depression, suggesting drug abuse induces a depressive-like mood state [6]. Other evidence implicates the involvement of central noradrenergic mechanisms in mediating the addictive properties of abused drugs [7, 8]. Clearly, non-dopaminergic mechanisms play some role in addiction [9]. Clinical evidence reveals that brain regions in addition to the NAc are involved in drug-seeking behavior. Childress and others have reported that cocaine craving triggered by cocaine-related cues produces differential activation of specific limbic structures of the brain [10]. Moreover, cocaine-induced changes in the orbital frontal cortex and cingulate cortex are important contributors to the cocaine addiction process [11]. In particular, the loss of frontal lobe function (i.e., hypofrontality) observed in addicts may contribute to their poor judgment and reduced impulse control.
As reviewed elsewhere [12], developing medications to treat stimulant addiction is especially challenging. For example, the high level of co-morbidity between psychiatric illness (e.g., depression and bipolar disorder) and drug dependence complicates the task of recruiting homogeneous patient populations for clinical trials designed to test medication efficacy. Even if a medication is effective in treating an “uncomplicated” cohort of patients, the same medication might not be effective in a more typical community sample of patients with a higher incidence of psychiatric disorders. Since chronic cocaine use alters multiple aspects of brain neurochemistry and circuitry, testing a medication that works via a single well-defined mechanism might be destined to fail. Medications targeting a single neuronal substrate would “normalize” just one of many brain mechanisms dysregulated by chronic cocaine. Viewed from this perspective, perhaps it is not surprising that many controlled trials of medications for cocaine addiction have failed to demonstrate efficacy [13]. One way of addressing this problem is to conduct clinical trials with two or more medications that act via different mechanisms. Alternatively, as suggested for developing treatments for schizophrenia and mood disorders, the best medications for substance use disorders may well be “selectively non-selective drug” [14], i.e. a single molecular entity that targets several receptors, thereby producing greater efficacy then would be possible with a single-target medication.” Another feasible approach is to develop a number of different types of medications that target particular mechanisms involved in the actions of addictive drugs. Such agents might ultimately be part of a pharmacological “tool box” available for use by clinicians treating patients at various stages of detoxification and recovery.
Towards this end, many research groups have sought to develop medications that target monoamine neurotransmitter transporters, since these membrane proteins are the principle sites of action for cocaine and other stimulants. Cocaine binds to monoamine transporters, thereby inhibiting the reuptake of their respective neurotransmitters. The reinforcing actions of cocaine result primarily from inhibition of DA transporters (DAT) and the subsequent increases in extracellular levels of DA, which in turn stimulate post-synaptic DA receptors. Hence, a large investment in discovering and developing novel DA uptake inhibitors has transpired over the past 15 years, resulting in an arsenal of compounds based on the chemical structures of cocaine, GBR12909, benztropine, mazindol, methylphenidate and rimcazole [15–17]. Most of these compounds display a cocaine-like behavioral profile in animal models, and could be potential “agonist” therapy candidates. A few compounds have been identified that are potent and selective DA uptake inhibitors but do not produce cocaine-like behavioral effects. Importantly, some of these compounds attenuate cocaine-induced behaviors in animals, such as locomotor activity and discriminative stimulus effects [18]. The major purpose of this article is to review the work of our respective research groups in developing DAT inhibitors as potential treatment agents for cocaine addiction.
2. Studies with GBR12909
In an attempt to develop a stimulant abuse medication, we wished to identify a high affinity DAT inhibitor that would produce an insurmountable inhibition of the ability of cocaine to elevate extracellular DA [19, 20], i.e. a noncompetitive inhibitor of cocaine’s action. A potential drawback of a medication that competitively blocked the ability of cocaine to elevate extracellular DA is the possibility that the effects of the medication could be overcome by simply self-administering more cocaine. Under these circumstances, high concentrations of cocaine could overwhelm the therapeutic actions of the medication, possibly leading to dangerous side-effects of the abused stimulant. Thus, we believed that it was therapeutically advantageous to develop a DAT ligand that noncompetitively inhibited cocaine’s action. We focused our search on candidate drugs which bound to DAT proteins with high affinity but dissociated very slowly and showed limited intrinsic activity [20]. It was envisioned that if the dissociation rate was slow enough, the agent would cause modest elevations in extracellular DA while functionally neutralizing effects of the abused stimulant in an insurmountable manner. In addition, the candidate medication should produce minimal or no euphoria, resulting in a reduced potential for abuse and dependence. A long-acting noncompetitive DAT inhibitor was thought to have such a profile.
Among the first agents to be characterized as high-affinity and selective inhibitors of DA reuptake were the aryl-1,4-dialk(en)ylpiperazines [21] (See Fig. 1). This series of compounds was originally developed and tested in Europe for the treatment of depression [22]. In particular, GBR12909 (1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine) and GBR12935 (1-{2-benzhydryloxyethyl}-4-(3-phenylpropyl)piperazine) were identified as potent and selective ligands for DAT [21, 23]. Subsequent work showed these compounds are moderately selective inhibitors of DA uptake over serotonin and norepinephrine uptake [22, 24–26]. It was noted that GBR12909 produces modest increases in extracellular DA when administered to rats in vivo, consistent with its ability to block DA uptake in vitro [20, 27]. Further investigations demonstrated that GBR12909 pretreatment is able to reduce the ability of cocaine to increase extracellular DA [28]. GBR12909 was shown to bind tightly to DAT proteins, while being less efficient than cocaine in stimulating DA-mediated locomotor behaviors [29]. Interestingly, a systemically administered dose of GBR12909 that does not affect extracellular DA in rat NAc is able to significantly diminish the effect of a modest dose of cocaine [30] (see Fig 2). Studies in Rhesus monkeys showed that acute and chronic treatment with GBR12909 (Fig. 3) or GBR12935 (Fig. 4) decreases on-going cocaine self-administration behavior [31].
Figure 1.

Structure of GBR12909 and related compounds.
Figure 2.
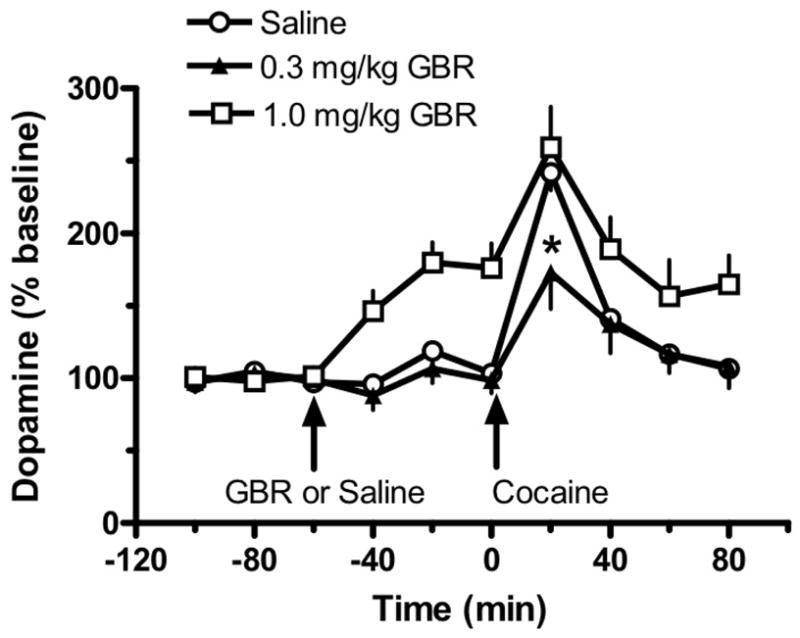
Effect of GBR12909 pretreatment on the elevation of extracellular DA by 1.0 mg/kg i.v. cocaine. DA levels are mean±SEM expressed as a percentage of three baseline samples obtained before treatment (n=6 rats/group). GBR12909 (0.3 or 1.0 mg/kg i.v.) or saline was administered 60 min before cocaine challenge given at time 0. *p<0.05 with respect to the corresponding saline pretreatment group. From: [30].
Figure 3.
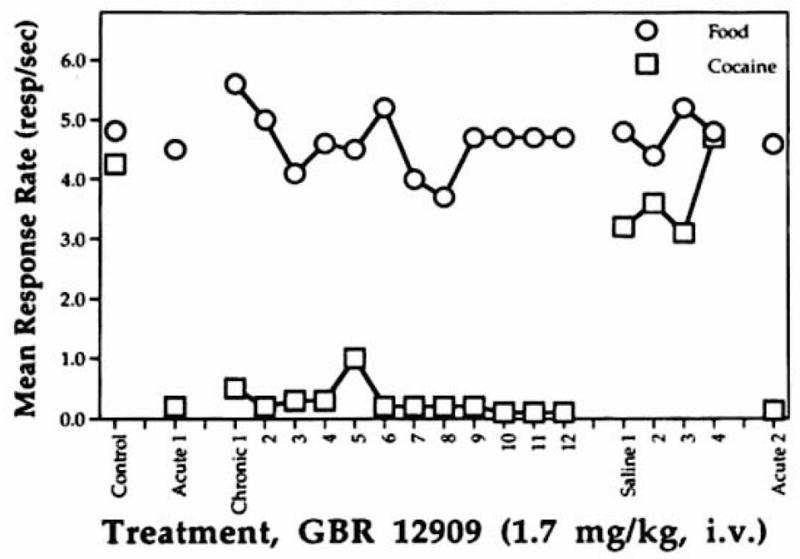
The effects of repeated administration of GBR 12909 (1.7 mg/kg, IV slow injection) on rates of responding maintained under the multiple fixed ratio (FR) food, time out (TO) 3 s (open circle), FR cocaine (10 μg/kg/inj), TO 3 (open square), TO 10-min schedule. Effects are expressed as the mean (n = 4) absolute rate of responding (resp/s) over days (indicated by numbers) of each treatment mode (control = noninjection baseline; acute = replicate single administrations; chronic = repeated daily administrations of GBR 12909; saline = repeated daily administrations of saline). □From Fig 6. in: [31].
Figure 4.

The effects of repeated administration of GBR 12935 (3 mg/kg, IM) on rates of responding maintained under the multiple fixed ratio (FR) food, time out (TO) 3 s (open circle), FR cocaine (10 μg/kg/inj), TO 3 s (open square), TO 10-min schedule. Effects are expressed as the mean (n = 3) absolute rate of responding (resp/s) over days (indicated by numbers) of each treatment mode (control = noninjection baseline; acute = replicate single administration; chronic = repeated daily administrations of GBR 12935). □From: [31].
These early studies demonstrated that GBR12909 differs from cocaine in several key ways [9]. It has a slower onset and longer duration of action than that of cocaine [28, 29, 32, 33]. GBR12909 possesses much higher affinity for and slower dissociation from DAT, when compared to cocaine [25, 28, 34]. It functionally antagonizes the ability of cocaine to elevate extracellular levels of DA [28] and it has a non-stimulant-like profile in normal human volunteers following oral administration [35].
In light of these considerations, many laboratories studied the effects of GBR12909 in various behavioral assays. For example, GBR12909 has been compared with cocaine, methamphetamine, and other DAT inhibitors in drug self-administration studies. These animal studies have generally been accepted as valid and reliable models for evaluating the abuse liability of drugs in humans [36]. In particular, nonhuman primate models of drug self-administration have provided an approach to assessing the reinforcing effects of psychoactive drugs [36, 37]. However, as described in previous reviews, notable exceptions do exist [9].
Not all DA reuptake inhibitors exhibit a profile that would be favorable to the development of an agonist medication [38]. In behavioral studies using Rhesus monkeys, GBR12935 and WIN35,428 ((2S,3S,5R)-methyl-3-(4-fluorophenyl)-8-methyl-8-aza-bicyclo[3.2.1]octane-2-carboxylate) are not able to completely decrease cocaine-maintained responding without affecting normal behavior as assessed by food-maintained responding [31]. In contrast, GBR12909 decreases cocaine-maintained responding without affecting food-maintained responding. Further studies with GBR12909 and several benztropine analogues have shown that compounds with high affinity for DAT can exhibit strong, moderate, weak, or no effectiveness as reinforcers in Rhesus monkeys [39]. Such data demonstrate that factors in addition to DAT binding affinity must be involved in mediating the in vivo reinforcing actions of these drugs.
GBR12909 has been found to serve as a reinforcer in several species [39–42]. Studies showed that GBR12909 can maintain rates of drug-appropriate responding in animals trained to self-administer cocaine, as well as substitute for cocaine [43–49]. However, self-administration studies in rats revealed that pretreatment with GBR12909 produces a dose-dependent decrease in the self-administration of cocaine [49]. Further studies in Rhesus monkeys showed that under multiple fixed-ratio schedules of food and cocaine delivery, GBR12909 selectively decreases cocaine-maintained behavior without affecting food-maintained responding [31]. It is noteworthy that these results are sensitive to the unit dose of cocaine [50]. At a low dose of cocaine (0.01 mg/kg/injection), GBR12909 affords a selective decrease in cocaine-maintained responding, while at a higher dose of cocaine (0.056 mg/kg/injection), this selectivity is lost. In addition, it appears that environmental variables can influence the selectivity of effects mediated by GBR12909 [51].
Positron emission tomography (PET) is a valuable technique for real time measurement of drug binding in vivo. A PET study in baboons using [11C]WIN35,428 has shown that a dose of GBR12909 that completely suppresses cocaine self-administration (10 mg/kg) occupies about 70% of the DAT sites in the brain (Fig. 5) [52]. Similar results have been reported using the phenyltropane derivative, RTI-113 [53, 54]. This level of occupancy at DAT proteins is comparable to the level associated with the euphoric effects of cocaine seen in humans [55].
Figure 5.
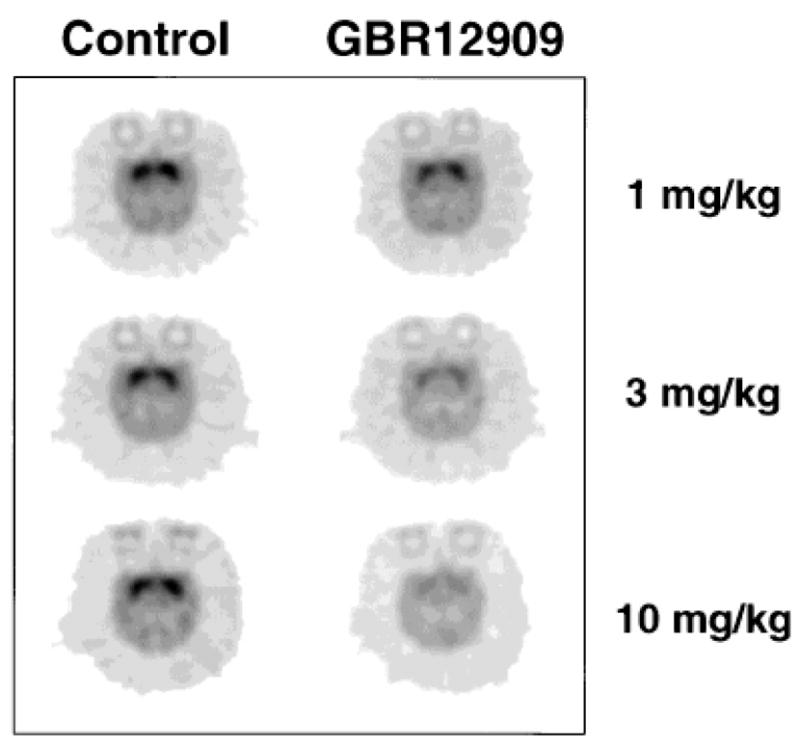
Reductions in DA transporter occupancy are shown in transaxial [11C]WIN-35,428 images in baboons before (left) and after (right) treatment with three different doses of GBR. Each dose was given 90 minutes before [11C]WIN-35,428 injection. Images shown are at mid-striatal level, and represent average PET images (between 70–90 minutes post-injection of the radiotracer) normalized to injected. From [52].
Studies have shown that cocaine self-administration leads to an up-regulation of DAT binding sites in rat brain regions [56]. Interestingly, the substitution of GBR12909 for cocaine completely reverses these cocaine-induced increases in DAT binding. This reversal is not observed when water is substituted for cocaine [56]. Other studies show that continuous infusion of GBR12909 for 7 days in rats causes significant down regulation of DAT binding sites in the caudate putamen and NAc [57]. These results indicate that the reversal of cocaine-induced increases in DAT binding by GBR12909 is most likely due to the specific molecular effects of GBR12909 on DAT proteins [56]. It has been suggested that this and other observed behavioral differences between cocaine and GBR12909 might be the result of their different interactions with DAT proteins [58].
Concurrent with the studies described above, we decided to further explore GBR12909 as a potential stimulant abuse therapeutic. In particular, we sought to enhance its selectivity for the DAT, as well as study its behavioral effects. This structure-activity effort is described in detail in a recent review [17]. One approach that led to a significant advance was to vary the N-alkyl group and develop a potential long-acting formulation [59–62]. The idea here was to find a polar hydroxyl-containing derivative that could easily be converted into a medium or long chain ester [59]. It was thought that this type of preparation might extend the duration of pharmacological effects of GBR12909. An initial study showed that a benzylic hydroxyl group containing analog, i.e. GBR-hydroxy (Fig. 1), has similar affinity and activity at DAT as GBR12909. Furthermore, this alcohol can be converted to a decanoate ester (GBR-decanoate) (Fig. 1) for use as a long-acting formulation. Based on the clinical utility of other decanoate-based prodrugs [63], it is likely that GBR-decanoate will also generate GBR-hydroxy, because esterase activity occurs in both rats and humans. Studies suggested GBR-decanoate is capable of potently inhibiting DAT binding, however it has not been established whether this effect is attributable to intact GBR-decanoate or its hydrolysis product, GBR-hydroxy [59]. In behavioral assays, a single administration of GBR-decanoate decreases cocaine self-administration in Rhesus monkeys for almost a month (Fig. 6) [59]. In rats, a single administration of GBR-decanoate has three important effects that last for at least two weeks: 1) DAT binding is markedly reduced, 2) baseline levels of extracellular DA are elevated about 2.5-fold, and 3) the ability of methamphetamine to release extracellular DA is nearly eliminated [64] (Fig. 7). In addition, GBR-decanoate administration increased the baseline locomotor response to novelty by 37% one week, but not two weeks, after administration, indicating adaptation of this response to the increase in baseline extracellular DA [65]. Taken together, results suggest GBR-decanoate affords long term inhibition of DA uptake, and displays a number of desirable properties consistent with an agonist-like medication for cocaine dependence.
Figure 6.
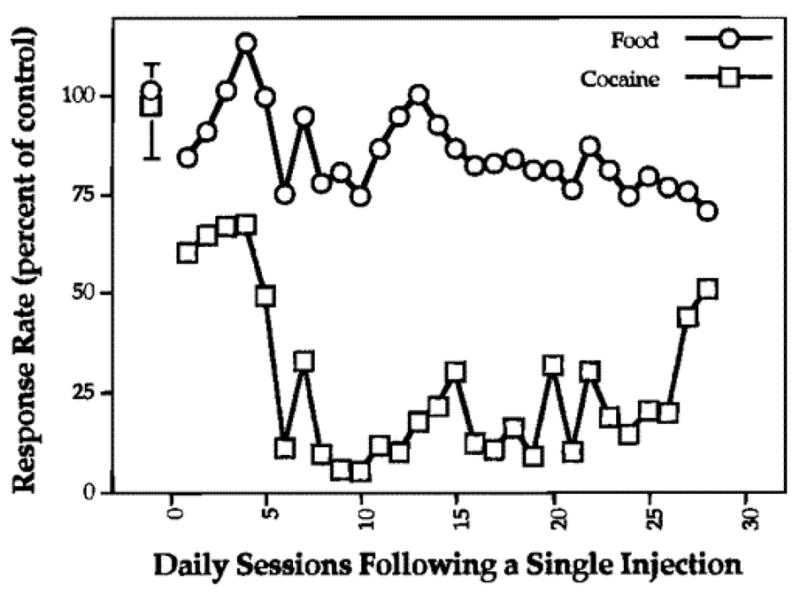
Effects of the 6-mL dose of GBR-decanoate on rates of responding maintained by food or cocaine, for 28 sessions following a single treatment in rhesus monkeys. Effects are expressed as the mean percent of control rates for food- or cocaine-maintained responding (n = 2). From [59].
Figure 7.

Effects of GBR-decanoate (GBR-dec) on METH-induced DA release (left) and 5-HT release (right) in the nucleus accumbens of conscious rats: 2-week test. Rats were treated with single i.m. injections of GBR-dec (1 ml/kg of a 48% solution, or 480 mg/kg) or sesame oil vehicle. Two weeks later, acute i.v. doses of 0.3 and 1.0 mg/kg METH (bottom) were injected at 0 and 60 min, respectively. Acute i.v. saline was administered on the same schedule (top). Data are mean ± S.E.M. for n = 6 to 7 rats/group, expressed as percentage of baseline. Baseline dialysate DA levels for GBR-dec and vehicle groups were 3.95 ± 0.64 and 1.69 ± 0.17 nM. Baseline dialysate 5-HT levels for GBR-dec and vehicle groups were 0.41 ± 0.09 and 0.33 ± 0.05 nM., P < 0.05 with respect to vehicle-pretreated group that received METH. From: [59].
In humans, GBR12909 lacks psychostimulant effects [35]. PET studies conducted in humans showed that after 2 weeks of dosing at either 50, 75 or 100 mg oral GBR12909, DAT occupancy increases with dose, reaching 25 to 35% occupancy at 100 mg [22]. More recently, NIDA terminated its Phase I studies with GBR12909 in cocaine-experienced subjects because of the appearance of QTc interval prolongation in five of five subjects given 75 mg/day of GBR12909 for 11 days [66]. The QT interval is a measure of the time between the start of the Q wave, which marks ventricular depolarization, and the end of the T wave, which marks ventricular repolarization in the heart. Since the QT interval will shorten with a faster heart rate, a corrected value, called the QTc, is calculated that takes into account the heart rate. Certain drugs can increase the QTc interval, thereby increasing the risk of developing ventricular arrhythmias. Given the fact that a relatively low dose of GBR12909 increased the QTc interval, further clinical trials with GBR12909 was deemed too risky. Future work with GBR12909-related analogs will focus on developing compounds that do not increase the QTc interval, a property, which is probably not related to its DA reuptake inhibition actions, but rather to inhibition of a particular potassium voltage gated channel (them “hERG” channel) [67].
3. SAR Studies with Benztropine Analogs
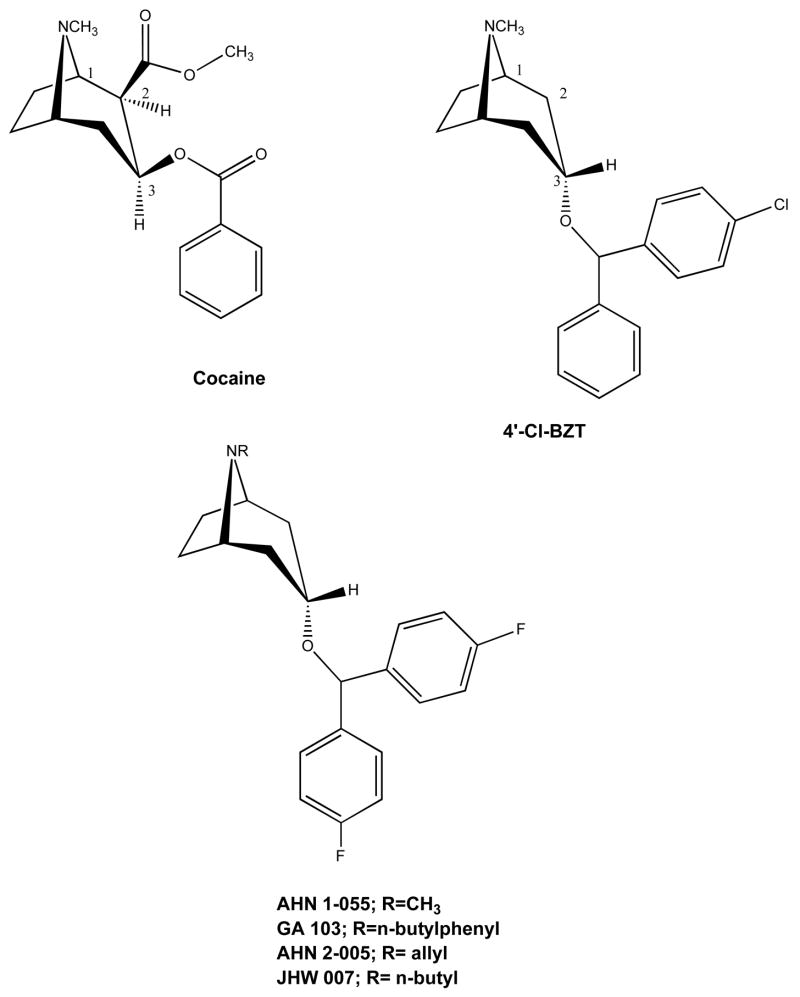
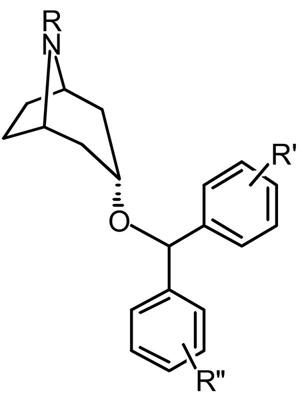
Benztropine (3α-diphenylmethoxytropane, cogentin®) is a tropane-based DA uptake inhibitor that is also a potent anticholinergic agent and is used clinically for the treatment of symptoms associated with Parkinson’s disease. As early as 1970, it was determined that benztropine inhibited DA uptake [68]. Benztropine is a molecule with the shared features of a tropane ring from cocaine and a diphenyl ether of the dialkylpiperazines (e.g. GBR12909) combined to create a unique member of the class of DA uptake inhibitors. Early structure-activity relationships for inhibition of DA uptake in striatum versus hypothalamus in a series of 3-substituted-benztropine analogues demonstrated that optimal potency was achieved with the diphenylmethoxy substituent of the parent compound [68]. Subsequently, it was demonstrated that para-substitution of a chloro-group on one of the pendant phenyl rings of benztropine (4′-Cl BZT, Fig. 8.) resulted in a 10-fold increase in potency for inhibition of dopamine uptake [21]. In addition, this modification caused a decrease in potency for inhibition of serotonin and norepinepherine uptake, resulting in a significantly more DA-selective compound than the parent benztropine [21]. This finding provided the first clue for chemical modification of the benztropine molecule toward the development of highly potent and selective DA uptake inhibitors [69, 70].
Figure 8.
Chemical structures of cocaine and highlighted benztropine analogs.
Initial efforts to synthesize novel, potent and selective DA uptake inhibitors, based on benztropine, focused on the 3-position, with a primary goal of determining the optimal stereochemistry and aryl ether substituents [69–72]. The 3α-stereochemistry provided optimally active compounds as did small substituents such as F or Cl in the para- and or meta- positions, with 4′,4″-diF giving the highest affinity analogue in this series (AHN 1-055, Fig. 8). However, it must be noted that small halogens in these positions uniformly gave high affinity analogues (Ki=11–30 nM), whereas, increasing steric bulk or substitution in the 2′-position caused decreases in DAT affinity [72]. Note that compared to the parent benztropine and cocaine, many of these halogenated analogues displayed superior DAT binding affinities [15]. Moreover, all of the analogues that showed high affinity binding at DAT in rat brain also inhibited DA uptake, and these data were highly and positively correlated (r=0.907; p<0.0001) [71].
In addition, muscarinic receptor affinities were generally decreased, as compared to the parent molecule (Ki =2 nM) so that instead of compounds with 60-fold selectivity for muscarinic receptors, these analogues were more similarly potent at DAT and M1 receptors. Although none of these analogues demonstrated high affinity for SERT or NET and hence they were considered selective DAT inhibitors, the confound of high to moderate muscarinic receptor binding remained. Subsequent studies have failed to correlate muscarinic receptor binding with the benztropines’ lack of cocaine-like effects [73, 74]. It should also be noted that several of these analogues demonstrated high to moderate affinity for histamine H1 receptors [75, 76]. However, a relationship between H1 binding profiles and their behavioral actions was not demonstrated [76].
In an effort to optimize DAT selectivity, replacement of the N-methyl substituent with alkyl and arylalkyl substituents was considered. Indeed, GBR12909 has a phenylpropyl substituent appended to its piperazine N-terminus, and does not bind appreciably to muscarinic M1 receptors. As benztropine can be considered a rigid analogue of GBR 12909, whereby the tropane ring can be considered a structurally rigid piperazine ring sans second nitrogen, a series of N-substituted analogues of AHN 1-055 and several other small halogenated diphenyl ether analogues was designed [15, 77].
In general, N-substituted analogues with the 4′4″-diF substitution on the diphenyl ether resulted in compounds with high affinity at DAT, with several extended alkyl and arylalkyl substituents being well tolerated (DAT Ki = 8–30 nM). However, there was an optimal length of the N-substituents (n-butylphenyl), which if exceeded, resulted in low affinity compounds at DAT [15, 77]. Furthermore, the tropane N must be a secondary or tertiary amine, as amides and other neutral amines were inactive at DAT. When the diphenyl ether substituents increased in steric bulk (e.g. 4′,4″-di Cl) N-substitution resulted in further decreases in DAT affinity [78]. However, a notable separation of DAT from muscarinic M1 receptor binding was achieved, with several analogues in this series having > 100-fold selectivity for DAT [77, 79, 80]. This is in remarkable contrast to the parent benztropine, which is 60-fold selective for muscarinic M1 receptors over DAT. Table 1 shows in vitro binding results for selected benztropine analogues that will be highlighted throughout the rest of this review and in comparison to cocaine and GBR 12909, tested under the same assay conditions.
Table 1.
In vitro binding and inhibition of dopamine uptake data for highlighted benztropine analogues in comparison to cocaine and GBR 12909 tested under the same assay conditions.1
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| compd | N- substitution | 3-subst | DAT | SERT | NET | M1 | H1 | DAUI2 |
| GA 103 | 4″-phenyl-n- butyl | 4′,4″-diF | 8.51 ± 1.2c | 376±51.8m | 2210±240m | 576 ± 10.7g | 141 ± 6.72n | |
| AHN 1-055 | 4′,4″-diF | 4′,4″-diF | 11.8 ± 1.3a | 3260±110k | 610±80.5k | 11.6±0.93g | 19.7±1.32n | 13.8±1.71m |
| JHW 007 | n-butyl | 4′,4″-diF | 24.6 ± 2.0c | 1350±151o | 1670±232o | 399±28.3o | 24.6±1.97d | |
| AHN 2-005 | allyl | 4′,4″-diF | 29.9 ± 3.0c | 2850±62.5o | 1740±242o | 177±21g | 24.9±1.16n | 19.7±0.57q |
| 4-Cl BZT | CH3 | 4′-Cl | 30.0 ± 3.6a | 5120±395i | 1470±180i | 7.90±0.85j | 39.9±.57n | 23.1±1.8i |
| BZT | CH3 | H, H | 118 ± 10.6a | >10,000a | 1390±134o | 2.1±0.29i | 15.7±2.13n | 403±115a |
| cocaine | ---- | ---- | 187 ± 18.7i | 172±15n | 3210±149o | 61400±10900o | 1050±43.0n | 236±20.5i |
| GBR 12909 | ---- | ---- | 12.0±1.9p | 105±11.4p | 497±17.0p | 2.30±0.14p | ||
All binding data are recorded in Ki ± SEM; these data and the methods used to obtain them are published in the cited references
Newman, et al., 1995;
Agoston, et al., 1997a;
Desai, et al., 2005a
Robarge, et al., 2000;
Katz, et al., 2001;
Newman, et al., 2001;
Zou, et al., 2002;
Kulkarni, et al., 2004;
Campbell, et al., 2005;
Katz, et al., 2004;
Newman and Kulkarni 2002;
Newman and Katz, 2007.
The inhibition of dopamine uptake (DAUI) data is recorded as IC50 ± SEM and was obtained in rat brain chopped tissue or brain synaptosomes according to methods detailed in the primary references.
More recently, substitution at the tropane 2-position with carboalkoxy and other isosteric substituents have been explored and these substitutions are also well tolerated at the DAT, as long as the S-(+)- stereochemistry of the compound is maintained [81–83]. It should be noted that this is the opposite enantioselectivity for cocaine and its analogues, at DAT, which further contrasts the structure-activity relationships between these two classes of DA uptake inhibitors. Many of these compounds are the most DAT-selective over SERT, NET, muscarinic and histaminic receptors reported to date and those selected analogues show remarkable diversity in their behavioral pharmacology. However, due to their complexity, only limited preclinical data are available at present [81], so these compounds will not be discussed further in this review.
4. Behavioral Evaluation of the Benztropine Analogues in Animal Models of Cocaine Abuse
Early evaluation of the benztropine analogues, in animal models of cocaine abuse, resulted in the finding that despite having high affinity for the DAT and exhibiting potent inhibition of DA uptake, in vitro, these compounds did not demonstrate a cocaine-like behavioral profile [69, 84]. For example, 4′-Cl BZT did not demonstrate efficacious locomotor stimulation in mice, as compared to cocaine [69]. Furthermore, in rats trained to discriminate 10 mg/kg cocaine from saline, 4′-Cl BZT did not elicit cocaine-like discriminative stimulus effects in a dose range of 0.3–30 mg/kg [69, 84]. Numerous other benztropine analogues demonstrated similar behavioral profiles that contrasted to cocaine [84]. Nevertheless, as these compounds all showed moderate to high affinities for muscarinic receptors, there was a concern that the muscarinic antagonist effects might confound their development as cocaine-abuse therapeutics [39, 73, 85, 86].
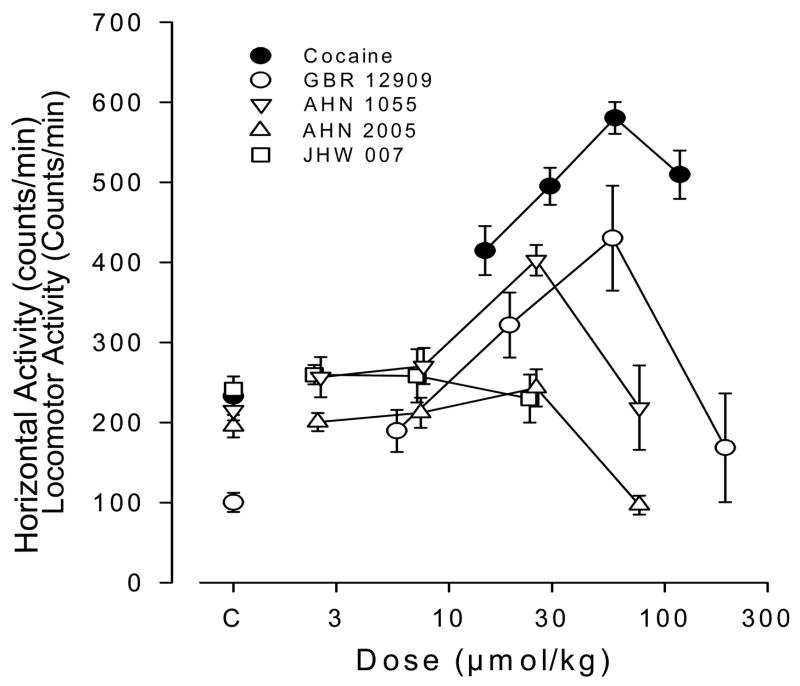
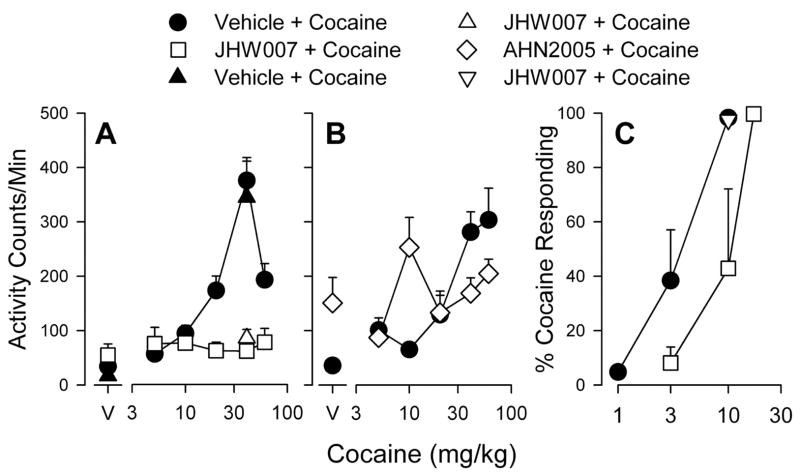
As noted above, several N-substituted 3α-[bis(4′-fluorophenyl)methoxy]tropanes that had much reduced affinity for the M1 receptor and retained high affinity and selectivity for DAT ([79] Fig. 8 and Table 1) have been evaluated extensively in locomotor activity and drug discrimination models of cocaine abuse [18, 85, 87, 88]. One of the early analogues of this series, GA 103 (N-(4″-phenyl-n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane), which demonstrated high affinity and selectivity for DAT [79], did not significantly increase locomotor activity to values greater than obtained after vehicle injection. Further, in rats trained to discriminate 10.0 mg/kg cocaine from saline, there was a dose-related increase in the percentage of responses emitted on the cocaine-appropriate response key as cocaine dose was increased from 1.0 to 10.0 mg/kg however, no dose of GA 103 produced a level of drug-appropriate responding that exceeded 10%. In Figure 9, these results for GA 103 are compared to cocaine and several other N-substituted benztropine analogues with similar affinities for DAT but a range of affinities for muscarinic M1 receptors (see Table 1.) These results suggest that N-substitution in general, while potentially decreasing affinity at muscarinic receptors, does not render these compounds more similar to cocaine with regard to their behavioral effects. As alluded to above, one hypothesis to explain the reduced cocaine-like behavioral effects of benztropine analogs are their antagonist actions at muscarinic M1 receptors. For example, Ranaldi and Woolverton [89] found that combinations of the anticholinergic, scopolamine, and cocaine were generally less effective in maintaining high rates of responding than was cocaine alone. A decrease in rates of responding maintained by cocaine due to co-administration of a muscarinic antagonist is consistent with previous results indicating a lesser effectiveness of benztropine analogs compared with cocaine in self-administration procedures [39, 90]. Nevertheless, Katz et al. showed contrasting results with these agents in rodent models of cocaine abuse [73, 85]. Additional investigation of GA 103 demonstrated that the high lipophilicity of this compound would likely limit further preclinical development, hence additional studies have focused on other analogues with similar pharmacological profiles, but lower clog P (calculated partition coefficient) values [77]. Locomotor activities, in mice, of some of these analogues as compared to cocaine and GBR 12909 are shown in Fig. 10. Although some of the analogues demonstrate locomotor stimulation as compared to vehicle (e.g. AHN 1-055) none of the analogues is as efficacious as cocaine in this model.
Figure 9.
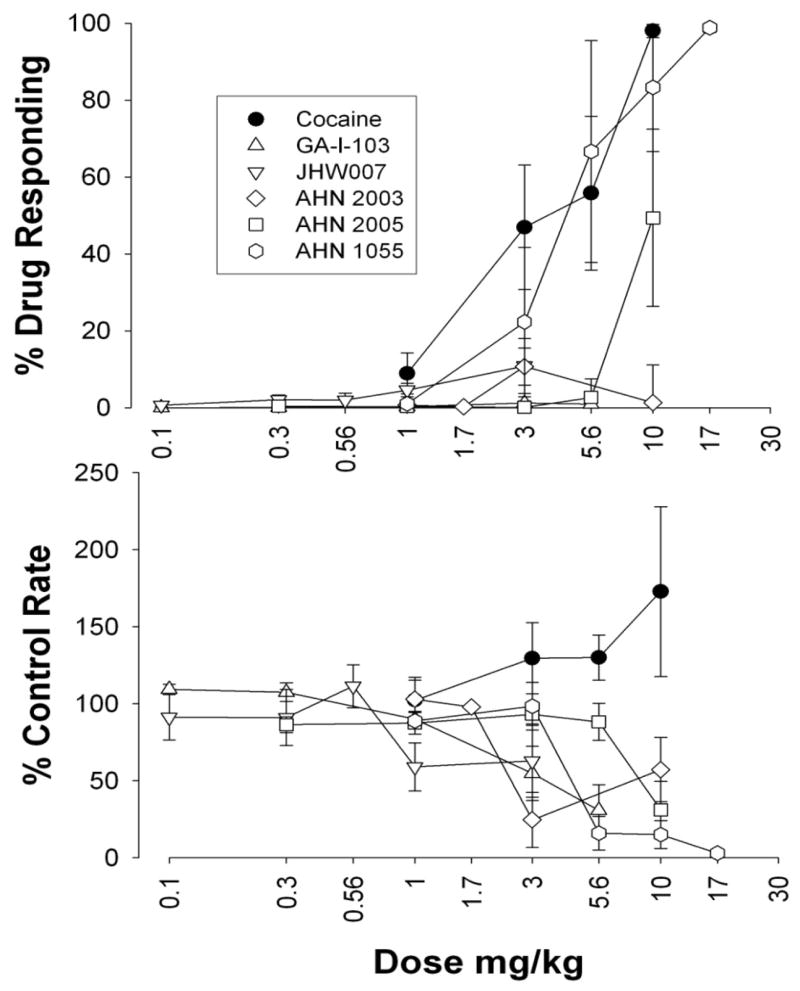
Effects of cocaine, GA 103, JHW 007, AHN 2-003, AHN 2-005 and AHN 1-055 in rats trained to discriminate 10 mg/kg cocaine from saline. Top, ordinate: percentage of responses on the cocaine-appropriate lever. Bottom ordinate: rates at which responses were emitted (as a percentage of response rate after saline administration). Abscissa: drug dose in mg/kg (log scale). Each point represents the effect in 4–16 rats.
Figure 10.
Dose-dependent effects of cocaine, GBR 12909, AHN 1-055, AHN 2-005 and JHW 007 on locomotor activity in mice. Ordinate: horizontal locomotor activity counts after drug administration in counts/minute. Abscissa: dose of drug in μmole/kg, log scale (i.p administration). Each point represents the average effect determined in 8 mice. The data are from the 30 min period immediately after drug administration.
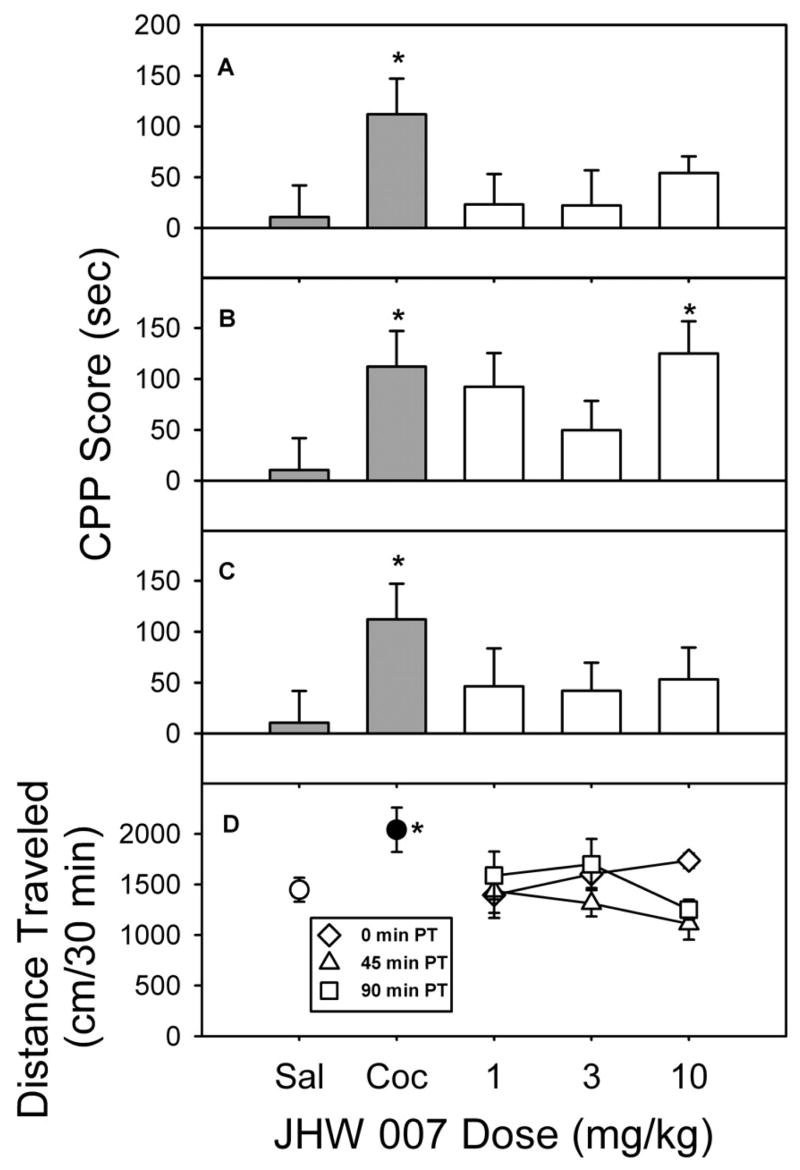
More recently, Li et al. [87] examined three benztropine analogs in which the affinity for the muscarinic M1 receptor varied with changes in chemical structure (see Table 1) but DAT affinities remained similar (Ki=11–20 nM). These benztropine analogues were compared with the effects of the classic muscarinic antagonist atropine and cocaine, using a place-conditioning procedure. Atropine was studied at doses that had previously been shown to potentiate the effects of cocaine [73]. At these doses, atropine did not significantly affect time spent in the drug-paired compartment when compared with the saline group (Fig. 11A). In contrast, the cocaine-conditioning control group, as in the previous study, showed a significant increase in time spent on the drug-paired compartment. Atropine, at doses from 1.0 to 5.6 mg/kg (Fig. 11B) did not significantly alter locomotor activity during the first conditioning session. In contrast, the group given 10.0 mg/kg cocaine showed a significant stimulation of locomotor activity compared with the saline group.
Figure 11.
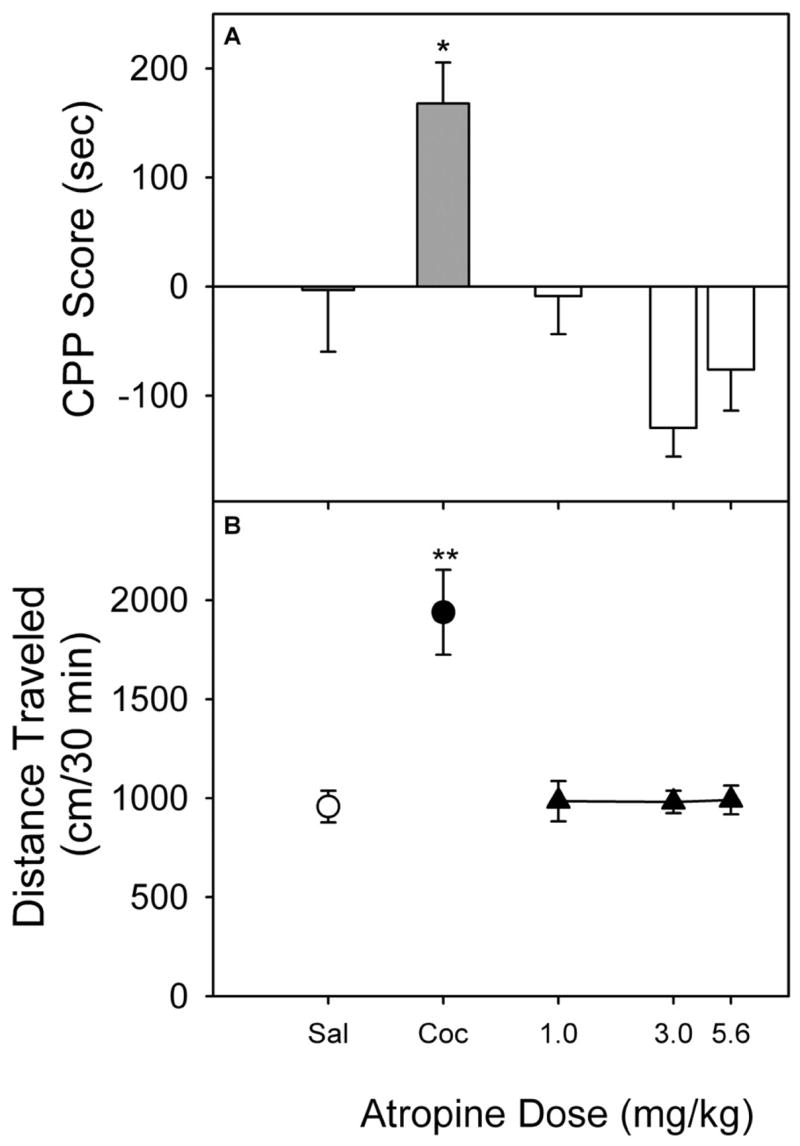
Place conditioning (A) and locomotor effects (B) of atropine in rats. The CPP scores represent the time spent in the drug-paired side during the postconditioning expressed as a difference from that during the last preconditioning session. The locomotor activity was expressed as the total horizontal distance traveled (centimeters) during the first exposure to drug in the first conditioning session. Data are presented as means ± S.E.M. *, p < 0.05; **, p < 0.01, as determined by the Dunnett’s test versus saline group. From [87].
Treatment with the various doses of AHN 1-055, which has a Ki of 12 nM for the M1 receptor, immediately before the conditioning sessions did not produce a significant change in the time spent in either compartment, in contrast to cocaine (Fig. 12A). Because increases in time between injection and testing increased the efficacy of AHN 1-055 in substituting for cocaine [73], the place-conditioning tests were also conducted with injections administered at 45 and 90 min before conditioning sessions. Neither the 45-min (Fig. 12B) nor the 90-min (Fig. 12C) pretreatment time increased the time spent in the drug-paired compartment across the range of doses examined. Moreover, AHN 1-055 did not stimulate activity when it was injected immediately before conditioning (Fig. 12D, diamonds), whereas 10 mg/kg cocaine, as before, significantly increased locomotor activity. Further, when AHN 1-055 was administered 90 min before the first conditioning session, there was a significant dose-related decrease in locomotor activity (Fig. 12D, squares).
Figure 12.

Place conditioning (A–C) and locomotor effects (D) of AHN 1-055 administered to rats at different times before placement in the conditioning chamber. A to C, place conditioning effects of AHN 1-055 given 0, 45, and 90 min before conditioning sessions, respectively. For cocaine and saline treatments, animals were placed in the conditioning chamber immediately after injection. Data are presented as means ± S.E.M. Significant differences from control (p < 0.05) are indicated by *, as determined by Student’s t test versus saline group for the cocaine groups and by ANOVA followed by the Dunnett’s test versus saline group for AHN 1-055 groups. From [87].
Somewhat different results were obtained with AHN 2-005, which has a Ki of 177 nM at the M1 receptor. Whereas with conditioning sessions conducted immediately after injection (Fig. 13A) there was no significant change in the amount of time spent on either 17 side of the chamber, when 1 mg/kg AHN 2-005 was administered 45 min before conditioning sessions (Fig. 13B) there was a small but significant increase in the amount of time spent in the drug-paired compartment. However, this was not sustained at the longer pretreatment time of 90 min, nor was there significant locomotor effects of AHN 2-005 at any dose or pretreatment time tested except at the highest dose (10 mg/kg), there was a decrease in locomotor activity, (Fig. 13D) in contrast to cocaine.
Figure 13.

Same as above except for AHN 2-005
As with the other benztropine analogs, when JHW 007 (M1 Ki=399 nM) was administered immediately before conditioning sessions or at longer pretreatment times (Fig. 14), there were no consistent changes in the amount of time spent on either side of the chamber. Likewise, JHW 007 did not produce a significant change in locomotor activity when it was injected immediately before the first conditioning session or 45 or 90 min before conditioning (Fig. 14D).
Figure 14.
Same as above except for JHW 007
As discussed in detail elsewhere [87], the weight of the evidence in this model and previously tested paradigms suggests that the reduced cocaine-like behavioral effects of these benztropine analogs is not due to a punishment-like effect conferred on the compounds through antimuscarinic effects. Rather the cumulative data support previous suggestions that benztropine analogs act at DAT sites in a manner that is fundamentally different from that for cocaine and that this difference at the molecular level may relate more generally to all of the behavioral effects of these novel benztropine analogs. A recent report using microdialysis showed that preferential muscarinic antagonists did not uniformly affect cocaine-induced extracellular dopamine increases in the nucleus accumbens, nor did this mechanism explain the differences in behavioral actions of cocaine and the benztropine analogues [74]. These data further support our view that the benztropines interact at the DAT differently than cocaine and this is directly related to their distinctive pharmacological profile in vivo.
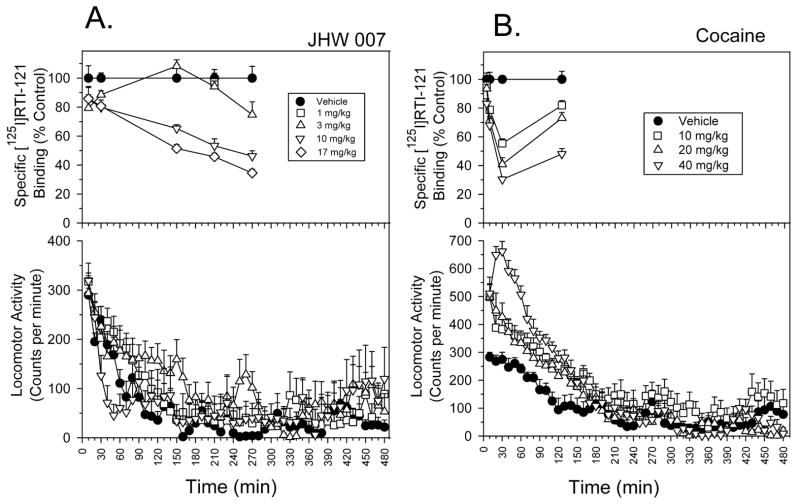
The above findings that JWH 007 lacked cocaine-like behavioral effects despite high affinity inhibition of DA uptake in vitro (IC50 = 24.6 ± 1.97 nM) prompted further study of this compound [18]. As reported in Fig. 15A, ex vivo binding studies with [125I]RTI-121 demonstrated that JHW 007 displaced [125I]RTI-121 in a dose-dependent manner, with no plateau apparent at 270 min after injection. In contrast to cocaine (Fig. 15B), JHW 007 had a slow in vivo apparent association with the DAT; displacement of [125I]RTI-121 by 17 mg/kg JHW 007 occurred at a rate of 0.20 ±0.02%/min, which was significantly (F(1,76) = 39.67; p < 0.0001) less than the 2.04 ± 0.20%/min (5–30 min) obtained with 40 mg/kg cocaine.
Figure 15.
A. Time course of effects of JHW007 on specific [125I]RTI-121 binding (top) and on locomotor activity in mice. x-axis, time after JHW007 injection. y-axis (top), specific [125I]RTI-121 binding expressed as a percentage of vehicle control; y-axis (bottom), horizontal activity counts per minute. Binding data are from 5–10 mice (top). B. Time course of effects of cocaine on specific [125I]RTI-121 binding (top) and locomotor activity in mice. x-axis, time after cocaine injection. Each point represents data from 5–13 (top) or 8 (bottom) mice, with error bars representing +1 SEM. From [18].
The cocaine-induced stimulation of activity was generally in agreement with in vivo DAT occupancy and the correlation was significant (r = 0.61; p = 0.012). However, given the established relationship between DAT actions and behavioral effects [91], the correlation was poorer than predicted, indicating that occupancy is only one determinant of these behavioral effects of cocaine (Figure 16). At doses from 1–10 mg/kg, JHW 007 failed to produce a significant stimulation of locomotor activity throughout the 8 h observation period (Fig. 15A, bottom) (F(3,28) = 1.25; p > 0.05). The correlation of the stimulant effects and DAT occupancy of various doses and times after injection of JHW 007 was not significant (r = 0.002; p = 0.995). Thus, JHW 007 did not show a relationship between DAT occupancy and behavioral stimulation like that observed with cocaine. These data add to previous experiments that established that JHW 007 readily penetrates the brain [92], slowly achieving significant levels of DAT occupancy, without appreciable cocaine-like behavioral effects.
Figure 16.
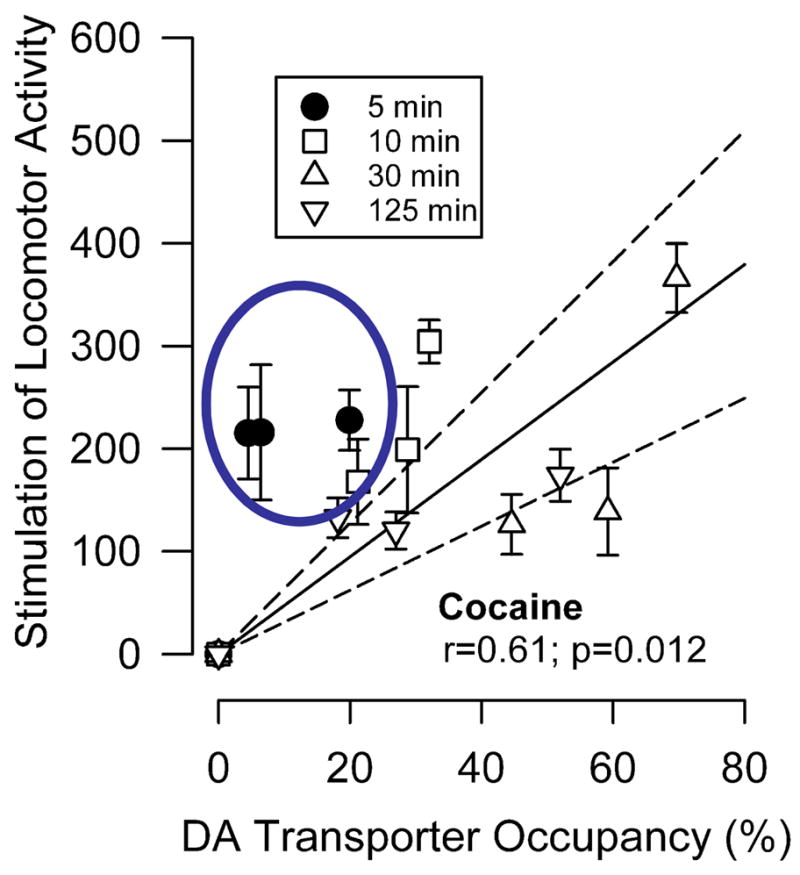
Relationship between percentage occupancy of the DA transporter in striatum and locomotor stimulant effects of cocaine, AHN 1-055, AHN 2-005 or JHW 007. Ordinates: difference between mean horizontal activity counts after drug and after saline. Abscissae: percent occupancy of DA transporter sites. For each drug, the solid line represents the linear regression of percent occupancy of the DA transporter and horizontal locomotor activity when the line is forced to intersect the origin, the point representing no occupancy and no effect. Note that the locomotor stimulant effects of cocaine are less strongly related to DA transporter occupancy than are the effects of AHN 1-055 and AHN 2-005. In contrast to each of the other drugs, the locomotor stimulant effects of JHW 007 were not related to its occupancy at the DA transporter. Modified from [18].
The next set of experiments examined the interactions of cocaine and JHW 007 or AHN 2-005 [18]. As reported in Fig. 17, when injected 270 min after vehicle injection, cocaine produced a dose-related increase in activity, with a maximum at 40 mg/kg (Fig. 17A, filled circles). JHW 007 alone had no significant effects on activity at this time point (Fig. 17A, squares). Moreover, pretreatment with JHW 007 (10 mg/kg) completely antagonized the effects of cocaine (Fig. 17A, squares). In contrast, the N-allyl derivative AHN 2-005 (10 mg/kg), did not antagonize the effects of cocaine (Fig. 17B, compare diamonds, filled circles), although there was a decrease in the effects of the highest doses of cocaine. In cocaine discrimination, there was a dose-related increase in the percentage of responses emitted on the cocaine-appropriate lever, approximating 100% at the training dose (ED50 = 3.31 mg/kg; 95% CL = 2.39–4.59 (Fig. 17C). JHW 007 (10 mg/kg, i.p.) shifted the cocaine dose-effect curve 3.07-fold (95% CL = 2.00–4.86) to the right, suggestive of a competitive antagonism. The following day (28.5 h after injection) (Fig. 17C, inverted triangle), the antagonist effects of JHW 007 were absent.
Figure 17.
Interactions of cocaine and JHW 007 or AHN-2-005. A, B, y-axis, Locomotor activity counts; x-axis, treatment condition, vehicle (V), or dose of cocaine. Each point represents the average effect determined in eight mice, except n = 6 for combination of 10 mg/kg JHW 007 plus 60 mg/kg cocaine. The unconnected points above 40 mg/kg cocaine (A) show a replicate determined several months later in a separate shipment of mice. C, y-axis, Percentage of responses on the cocaine-appropriate lever after drug; x-axis, dose of cocaine. Circles, Effects of cocaine alone; squares, effects of cocaine administered 4.5 h after treatment with 10 mg/kg JHW 007; inverted triangle, effects of cocaine administered 28.5 h after treatment with JHW 007. Each cocaine point represents the average effect determined in five to six mice; for drug combinations, each point represents the effects of three to four mice. The error bars represent +1 SEM. From [18].
These results, in conjunction with others, suggest that the rate of DAT occupancy is an important component of cocaine-like actions and potential for abuse. Furthermore, this study suggests in vivo association as a critical feature contributing to the effects of DAT ligands and suggests that JHW 007 has the attributes necessary to serve as a lead candidate for the treatment of cocaine abuse.
5. Summary
In summary, we have reviewed aspects of the progress made to date in the development of two classes of DA uptake inhibitors as potential therapeutic agents for stimulant addiction. In both these classes of compounds (GBR12909 and benztropine analogs), a behavioral profile in animal models of drug abuse contrast to those of cocaine and hence therapeutic potential has been suggested. However, although extensive preclinical evaluation of these and other DA uptake inhibitors support their development, only the results of clinical investigation will provide needed direction for the discovery of cocaine-abuse medications of the future. Although this article focuses on DAT as a primary target, it is important to note parallel medication development efforts that are underway that exploit other targets [12, 15, 93, 94]. These compounds have provided unique pharmacological profiles that can be used to compare with other DAT ligands and further elucidate mechanistic correlates to cocaine’s reinforcing actions. It is anticipated that these compounds will ultimately lead to a clearer understanding of the neural mechanisms underlying cocaine’s abuse and ultimately lead investigators to the development of agents that will be useful in treating cocaine addiction.
Acknowledgments
This work was supported by the National Institute on Drug Abuse – Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pouletty P. Drug addictions: towards socially accepted and medically treatable diseases. Nat Rev Drug Discov. 2002;1(9):731–6. doi: 10.1038/nrd896. [DOI] [PubMed] [Google Scholar]
- 2.Volkow N, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;8(11):1429–30. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- 3.Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1(9):710–26. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5(12):963–70. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 5.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 6.Baumann MH, Rothman RB. Alterations in serotonergic responsiveness during cocaine withdrawal in rats: similarities to major depression in humans. Biol Psychiatry. 1998;44:578–591. doi: 10.1016/s0006-3223(98)00123-1. [DOI] [PubMed] [Google Scholar]
- 7.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Jasmin L, Narasaiah M, Tien D. Noradrenaline is necessary for the hedonic properties of addictive drugs. Vascul Pharmacol. 2006;45(4):243–50. doi: 10.1016/j.vph.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals and drug seeking behavior, and its implications for medication development: focus on GBR12909. Mol Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- 10.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman AH, Rothman RB. Addiction. In: Williams M, editor. Comprehensive medicinal chemistry II. Vol. 6. Elsevier, Amsterdam; London: 2007. pp. 169–192. [Google Scholar]
- 13.de Lima MS, de Oliveira Soares BG, Reisser AA, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction. 2002;97(8):931–49. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 14.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3(4):353–9. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 15.Newman AH, Kulkarni S. Probes for the dopamine transporter: new leads toward a cocaine-abuse therapeutic--A focus on analogues of benztropine and rimcazole. Med Res Rev. 2002;22(5):429–64. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- 16.Carroll FI. 2002 Medicinal Chemistry Division Award address: monoamine transporters and opioid receptors. Targets for addiction therapy. J Med Chem. 2003;46(10):1775–94. doi: 10.1021/jm030092d. [DOI] [PubMed] [Google Scholar]
- 17.Prisinzano T, Rice KC, Baumann MH, Rothman RB. Development of neurochemical normalization (“agonist substitution”) therapeutics for stimulant abuse: focus on the dopamine uptake inhibitor, GBR12909. Current Medicinal Chemistry (CNSA) 2004;4(1):47–59. [Google Scholar]
- 18.Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci. 2005;25(8):1889–93. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman RB. High affinity dopamine reuptake blockers as potential cocaine antagonists: a strategy for drug development. Life Sci. 1990;46:PL17–PL21. doi: 10.1016/0024-3205(90)90466-5. [DOI] [PubMed] [Google Scholar]
- 20.Rothman RB, Mele A, Reid AA, Akunne H, Greig N, Thurkauf A, Rice KC, Pert A. Tight binding dopamine reuptake inhibitors as cocaine antagonists. A strategy for drug development. FEBS Lett. 1989;257:341–344. doi: 10.1016/0014-5793(89)81566-2. [DOI] [PubMed] [Google Scholar]
- 21.Van der Zee P, Koger HS, Gootjes J, Hespe W. Aryl 1,4 -dialk(en)ylpiperazines as selective and very potent inhibitors of dopamine uptake. Eur J Med Chem. 1980;15:363–370. [Google Scholar]
- 22.Preti A. Vanoxerine National Institute on Drug Abuse. Curr Opin Investig Drugs. 2000;1(2):241–51. [PubMed] [Google Scholar]
- 23.Andersen PH. The dopamine uptake inhibitor GBR12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 24.Sharif NA, Nunes JL, Michel AD, Whiting RL. Comparative properties of the dopamine transport complex in dog and rodent brain: striatal [3H] GBR12935 binding and [3H]dopamine uptake. Neurochem Intl. 1989;15:325–332. doi: 10.1016/0197-0186(89)90140-x. [DOI] [PubMed] [Google Scholar]
- 25.Andersen PH. Biochemical and pharmacological characterization of [3H]GBR 12935 binding in vitro to rat striatal membranes: labeling of the dopamine uptake complex. J Neurochem. 1987;48:1887–1896. doi: 10.1111/j.1471-4159.1987.tb05752.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirate K, Kuribara H. Characteristics of the ambulation-increasing effect of GBR-12909, a selective dopamine uptake inhibitor, in mice. Jpn J Pharmacol. 1991;55:501–511. doi: 10.1254/jjp.55.501. [DOI] [PubMed] [Google Scholar]
- 27.Westerink BH, Damsma G, De Vries JB, Koning H. Dopamine re-uptake inhibitors show inconsistent effects on the in vivo release of dopamine as measured by intracerebral dialysis in the rat. Eur J Pharmacol. 1987;135:123–128. doi: 10.1016/0014-2999(87)90603-0. [DOI] [PubMed] [Google Scholar]
- 28.Rothman RB, Mele A, Reid AA, Akunne HC, Greig N, Thurkauf A, de Costa BR, Rice KC, Pert A. GBR12909 antagonizes the ability of cocaine to elevate extracellular levels of dopamine. Pharmacol Biochem Behav. 1991;40:387–397. doi: 10.1016/0091-3057(91)90570-r. [DOI] [PubMed] [Google Scholar]
- 29.Rothman RB, Grieg N, Kim A, de Costa BR, Rice KC, Carroll FI, Pert A. Cocaine and GBR12909 produce equivalent motoric responses at different occupancy of the dopamine transporter. Pharmacol Biochem Behav. 1992;43:1135–1142. doi: 10.1016/0091-3057(92)90493-y. [DOI] [PubMed] [Google Scholar]
- 30.Baumann MH, Char GU, de Costa BR, Rice KC, Rothman RB. GBR12909 attenuates cocaine-induced activation of mesolimbic dopamine neurons in the rat. J Pharmacol Exp Ther. 1994;271:1216–1222. [PubMed] [Google Scholar]
- 31.Glowa JR, Wojnicki FHE, Matecka D, Rice KC, Rothman RB. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: II: comparisons with other drugs and repeated administrations. Experimental and Clinical Psychopharmacology. 1995;3:232–239. [Google Scholar]
- 32.Kelley AE, Lang CG. Effects of GBR 12909, a selective dopamine uptake inhibitor, on motor activity and operant behavior in the rat. Eur J Pharmacol. 1989;167:385–395. doi: 10.1016/0014-2999(89)90447-0. [DOI] [PubMed] [Google Scholar]
- 33.Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology (Berl) 2000;148(3):299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- 34.Reith ME, Sershen H, Lajtha A. Binding of [3H]cocaine in mouse brain: kinetics and saturability. J Recept Res. 1981;2:233–243. doi: 10.3109/10799898109038802. [DOI] [PubMed] [Google Scholar]
- 35.Sögaard U, Michalow J, Butler B, Lund Laursen A, Ingersen SH, Skrumsager BK, Rafaelsen OJ. A tolerance study of single and multiple dosing of the selective dopamine uptake inhibitor GBR 12909 in healthy subjects. Int Clin Psychopharmacol. 1990;5:237–251. doi: 10.1097/00004850-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Howell LL, Czoty PW, Kuhar MJ, Carrol FI. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor RTI-113 in the squirrel monkey. J Pharmacol Exp Ther. 2001;292:521–59. [PubMed] [Google Scholar]
- 37.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14(6):375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 38.Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298(1):1–6. [PubMed] [Google Scholar]
- 39.Woolverton WL, Hecht GS, Agoston GE, Katz JL, Newman AH. Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology (Berl) 2001;154(4):375–82. doi: 10.1007/s002130000616. [DOI] [PubMed] [Google Scholar]
- 40.Roberts DC. Self-administration of GBR 12909 on a fixed ratio and progressive ratio schedule in rats. Psychopharmacology (Berl) 1993;111(2):202–6. doi: 10.1007/BF02245524. [DOI] [PubMed] [Google Scholar]
- 41.Skjoldager P, Winger G, Woods JH. Effects of GBR 12909 and cocaine on cocaine-maintained behavior in rhesus monkeys. Drug Alcohol Depend. 1993;33:31–39. doi: 10.1016/0376-8716(93)90031-k. [DOI] [PubMed] [Google Scholar]
- 42.Wojnicki FH, Glowa JR. Effects of drug history on the acquisition of responding maintained by GBR 12909 in rhesus monkeys. Psychopharmacology (Berl) 1996;123(1):34–41. doi: 10.1007/BF02246278. [DOI] [PubMed] [Google Scholar]
- 43.Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- 44.Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. Journal of Pharmacology & Experimental Therapeutics. 1990;254:312–317. [PubMed] [Google Scholar]
- 45.Cunningham KA, Callahan PM. Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat [published erratum appears in Psychopharmacology (Berl) 1991;104(4):552] Psychopharmacology. 1991;104:177–180. doi: 10.1007/BF02244175. [DOI] [PubMed] [Google Scholar]
- 46.Melia KF, Spealman RD. Pharmacological characterization of the discriminative-stimulus effects of GBR 12909. Journal of Pharmacology & Experimental Therapeutics. 1991;258:626–632. [PubMed] [Google Scholar]
- 47.Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. Journal of Pharmacology & Experimental Therapeutics. 1991;258:178–185. [PubMed] [Google Scholar]
- 48.Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- 49.Tella SR. Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav. 1995;51(4):687–92. doi: 10.1016/0091-3057(94)00438-o. [DOI] [PubMed] [Google Scholar]
- 50.Glowa JR, Wojnicki FHE, Matecka D, Bacher JJ, Mansbach RS, Balster RL, Rice KC. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: I. Dependence on unit dose of cocaine. Experimental and Clinical Psychopharmacology. 1995;3:219–231. [Google Scholar]
- 51.Stafford D, Rice KC, Lewis DB, Glowa JR. Response requirements and unit dose modify the effects of GBR 12909 on cocaine-maintained behavior. Exp Clin Psychopharmacol. 2000;8(4):539–48. doi: 10.1037//1064-1297.8.4.539. [DOI] [PubMed] [Google Scholar]
- 52.Villemagne V, Rothman RB, Yokoi F, Rice KC, Matecka D, Clough DJ, Dannals RF, Wong DF. Doses of GBR12909 which suppress cocaine self-administration in non-human primates substantially occupy DA transporters as measured by [11C]WIN35,428 PET scans. Synapse. 1999;32:44–50. doi: 10.1002/(SICI)1098-2396(199904)32:1<44::AID-SYN6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Dworkin SI, Lambert P, Sizemore GM, Carroll FI, Kuhar MJ. RTI-113 administration reduces cocaine self-administration at high occupancy of dopamine transporter. Synapse. 1998;30:49–55. doi: 10.1002/(SICI)1098-2396(199809)30:1<49::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 54.Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43(1):78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- 55.Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- 56.Tella SR, Ladenheim B, Andrews AM, Goldberg SR, Cadet JL. Differential reinforcing effects of cocaine and GBR-12909: biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. J Neurosci. 1996;16:7416–7427. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunko PM, Loeloff RJ, Izenwasser S. Chronic administration of the selective dopamine uptake inhibitor GBR 12,909, but not cocaine, produces marked decreases in dopamine transporter density. Naunyn Schmiedebergs Arch Pharmacol. 1997;356(5):562–9. doi: 10.1007/pl00005091. [DOI] [PubMed] [Google Scholar]
- 58.Izenwasser S, French D, Carroll FI, Kunko PM. Continuous infusion of selective dopamine uptake inhibitors or cocaine produces time-dependent changes in rat locomotor activity. Behav Brain Res. 1999;99(2):201–8. doi: 10.1016/s0166-4328(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 59.Glowa JR, Fantegrossi WE, Lewis DB, Matecka D, Rice KC, Rothman RB. Sustained decrease in cocaine-maintained responding in rhesus monkeys with 1-[2-]Bis(4-fluorophenyl)methoxy[ethyl]-4-(3-hydroxy-3-phenylprop yl)piperaziny] decanoate, a long-acting ester derivative of GBR 12909. J Med Chem. 1996;39:4689–4691. doi: 10.1021/jm960551t. [DOI] [PubMed] [Google Scholar]
- 60.Lewis DB, Matecka D, Zhang Y, Hsin L-W, Dersch CM, Stafford D, Glowa JR, Rothman RB, Rice KC. Oxygenated analogs of 1-[2-(diphenylmethoxy)ethyl]-and 1-[2-bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperaz ines ( GBR12935 and GBR12909) as potential extended-action cocaine-abuse therapeutic agents. J Med Chem. 1999;42:5029–5042. doi: 10.1021/jm990291q. [DOI] [PubMed] [Google Scholar]
- 61.Hsin LW, Dersch CM, Baumann MH, Stafford D, Glowa JR, Rothman RB, Jacobson AE, Rice KC. Development of Long-Acting Dopamine Transporter Ligands as Potential Cocaine-Abuse Therapeutic Agents: Chiral Hydroxyl-Containing Derivatives of 1-[2-[Bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine and 1-[2-(Diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine. J Med Chem. 2002;45(6):1321–1329. doi: 10.1021/jm010430f. [DOI] [PubMed] [Google Scholar]
- 62.Hsin LW, Prisinzano T, Wilkerson CR, Dersch CM, Horel R, Jacobson AE, Rothman RB, Rice KC. Synthesis and dopamine transporter affinity of chiral 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(2-hydroxypropyl)piperazines as potential cocaine abuse therapeutic agents. Bioorg Med Chem Lett. 2003;13(3):553–6. doi: 10.1016/s0960-894x(02)00940-x. [DOI] [PubMed] [Google Scholar]
- 63.Marder SR. Depot neuroleptics: side effects and safety. J Clin Psychopharmacol. 1986;6(1 Suppl):24S–29S. [PubMed] [Google Scholar]
- 64.Baumann MH, Ayestas MA, Sharpe LG, Lewis DB, Rice KC, Rothman RB. Persistent antagonism of methamphetamine-induced dopamine release in rats pretreated with GBR12909 decanoate. J Pharmacol Exp Ther. 2002;301(3):1190–7. doi: 10.1124/jpet.301.3.1190. [DOI] [PubMed] [Google Scholar]
- 65.Baumann MH, Phillips JM, Ayestas MA, Ali SF, Rice KC, Rothman RB. Preclinical evaluation of GBR12909 decanoate as a long-acting medication for methamphetamine dependence. Ann N Y Acad Sci. 2002;965:92–108. doi: 10.1111/j.1749-6632.2002.tb04154.x. [DOI] [PubMed] [Google Scholar]
- 66.Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162(8):1432–40. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- 67.Tang W, Kang J, Wu X, Rampe D, Wang L, Shen H, Li Z, Dunnington D, Garyantes T. Development and evaluation of high throughput functional assay methods for HERG potassium channel. J Biomol Screen. 2001;6(5):325–31. doi: 10.1177/108705710100600506. [DOI] [PubMed] [Google Scholar]
- 68.Horn AS, Coyle JT, Snyder SH. Catecholamine uptake by synaptosomes from rat brain. Structure-activity relationships of drugs with differential effects on dopamine and norepinephrine neurons. Mol Pharmacol. 1971;7(1):66–80. [PubMed] [Google Scholar]
- 69.Newman AH, Allen AC, Izenwasser S, Katz JL. Novel 3 alpha-(diphenylmethoxy)tropane analogs: potent dopamine uptake inhibitors without cocaine-like behavioral profiles. J Med Chem. 1994;37(15):2258–61. doi: 10.1021/jm00041a002. [DOI] [PubMed] [Google Scholar]
- 70.Newman AH, Agoston GE. Novel benztropine [3α-(diphenylmethoxy)tropane] analogs as probes for the dopamine transporter. Curr Med Chem. 1998;5(4):305–19. [PubMed] [Google Scholar]
- 71.Newman AH, Kline RH, Allen AC, Izenwasser S, George C, Katz JL. Novel 4′-substituted and 4′,4″-disubstituted 3 alpha-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. J Med Chem. 1995;38(20):3933–40. doi: 10.1021/jm00020a006. [DOI] [PubMed] [Google Scholar]
- 72.Kline RH, Izenwasser S, Katz JL, Joseph DB, Bowen WD, Newman AH. 3′-Chloro-3 alpha-(diphenylmethoxy)tropane but not 4′-chloro-3 alpha-(diphenylmethoxy)tropane produces a cocaine-like behavioral profile. J Med Chem. 1997;40(6):851–7. doi: 10.1021/jm950782k. [DOI] [PubMed] [Google Scholar]
- 73.Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther. 1999;288(1):302–15. [PubMed] [Google Scholar]
- 74.Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther. 2007;321(1):334–44. doi: 10.1124/jpet.106.118067. [DOI] [PubMed] [Google Scholar]
- 75.Kulkarni SS, Kopajtic TA, Katz JL, Newman AH. Comparative structure-activity relationships of benztropine analogues at the dopamine transporter and histamine H(1) receptors. Bioorg Med Chem. 2006;14(11):3625–34. doi: 10.1016/j.bmc.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell VC, Kopajtic TA, Newman AH, Katz JL. Assessment of the influence of histaminergic actions on cocaine-like effects of 3alpha-diphenylmethoxytropane analogs. J Pharmacol Exp Ther. 2005;315(2):631–40. doi: 10.1124/jpet.105.090829. [DOI] [PubMed] [Google Scholar]
- 77.Kulkarni SS, Grundt P, Kopajtic T, Katz JL, Newman AH. Structure-activity relationships at monoamine transporters for a series of N-substituted 3alpha-(bis[4-fluorophenyl]methoxy)tropanes: comparative molecular field analysis, synthesis, and pharmacological evaluation. J Med Chem. 2004;47(13):3388–98. doi: 10.1021/jm030646c. [DOI] [PubMed] [Google Scholar]
- 78.Newman AH, Robarge MJ, Howard IM, Wittkopp SL, George C, Kopajtic T, Izenwasser S, Katz JL. Structure-activity relationships at monoamine transporters and muscarinic receptors for N-substituted-3alpha-(3′-chloro-, 4′-chloro-, and 4′,4″-dichloro-substituted-diphenyl)methoxytropanes. J Med Chem. 2001;44(4):633–40. doi: 10.1021/jm000417f. [DOI] [PubMed] [Google Scholar]
- 79.Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem. 1997;40(26):4329–39. doi: 10.1021/jm970525a. [DOI] [PubMed] [Google Scholar]
- 80.Robarge MJ, Agoston GE, Izenwasser S, Kopajtic T, George C, Katz JL, Newman AH. Highly selective chiral N-substituted 3alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues for the dopamine transporter: synthesis and comparative molecular field analysis. J Med Chem. 2000;43(6):1085–93. doi: 10.1021/jm990265s. [DOI] [PubMed] [Google Scholar]
- 81.Zou MF, Cao J, Kopajtic T, Desai RI, Katz JL, Newman AH. Structure-activity relationship studies on a novel series of (S)-2beta-substituted 3alpha-[bis(4-fluoro-or 4-chlorophenyl)methoxy]tropane analogues for in vivo investigation. J Med Chem. 2006;49(21):6391–9. doi: 10.1021/jm060762q. [DOI] [PubMed] [Google Scholar]
- 82.Zou MF, Agoston GE, Belov Y, Kopajtic T, Katz JL, Newman AH. Enantioselective synthesis of S-(+)-2beta-carboalkoxy-3alpha-[bis(4-fluorophenyl)methoxy]tropanes as novel probes for the dopamine transporter. Bioorg Med Chem Lett. 2002;12(9):1249–52. doi: 10.1016/s0960-894x(02)00155-5. [DOI] [PubMed] [Google Scholar]
- 83.Zou MF, Kopajtic T, Katz JL, Newman AH. Structure-activity relationship comparison of (S)-2beta-substituted 3alpha-(bis[4-fluorophenyl]methoxy)tropanes and (R)-2beta-substituted 3beta-(3,4-dichlorophenyl)tropanes at the dopamine transporter. J Med Chem. 2003;46(14):2908–16. doi: 10.1021/jm0300375. [DOI] [PubMed] [Google Scholar]
- 84.Katz JL, Newman AH, Izenwasser S. Relations between heterogeneity of dopamine transporter binding and function and the behavioral pharmacology of cocaine. Pharmacol Biochem Behav. 1997;57(3):505–12. doi: 10.1016/s0091-3057(96)00441-8. [DOI] [PubMed] [Google Scholar]
- 85.Katz JL, Kopajtic TA, Agoston GE, Newman AH. Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther. 2004;309(2):650–60. doi: 10.1124/jpet.103.060525. [DOI] [PubMed] [Google Scholar]
- 86.Tanda G, Ebbs A, Newman AH, Katz JL. Effects of 4′-chloro-3 alpha-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther. 2005;313(2):613–20. doi: 10.1124/jpet.104.080465. [DOI] [PubMed] [Google Scholar]
- 87.Li SM, Newman AH, Katz JL. Place conditioning and locomotor effects of N-substituted, 4′,4″-difluorobenztropine analogs in rats. J Pharmacol Exp Ther. 2005;313(3):1223–30. doi: 10.1124/jpet.105.084541. [DOI] [PubMed] [Google Scholar]
- 88.Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. Relationship between in Vivo Occupancy at the Dopamine Transporter and Behavioral Effects of Cocaine, GBR 12909 [1-{2-[Bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and Benztropine Analogs. J Pharmacol Exp Ther. 2005;315(1):397–404. doi: 10.1124/jpet.105.091231. [DOI] [PubMed] [Google Scholar]
- 89.Ranaldi R, Woolverton WL. Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology (Berl) 2002;161(4):442–8. doi: 10.1007/s00213-002-1069-3. [DOI] [PubMed] [Google Scholar]
- 90.Woolverton WL, Rowlett JK, Wilcox KM, Paul IA, Kline RH, Newman AH, Katz JL. 3′- and 4′-chloro-substituted analogs of benztropine: intravenous self-administration and in vitro radioligand binding studies in rhesus monkeys. Psychopharmacology (Berl) 2000;147(4):426–35. doi: 10.1007/s002130050012. [DOI] [PubMed] [Google Scholar]
- 91.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 92.Raje S, Cao J, Newman AH, Gao H, Eddington ND. Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther. 2003;307(2):801–8. doi: 10.1124/jpet.103.053504. [DOI] [PubMed] [Google Scholar]
- 93.Rothman RB, Blough BE, Baumann MH. Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol Sci. 2006 doi: 10.1016/j.tips.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 94.Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48(11):3663–79. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]