Abstract
CD73 (ecto-5′-nucleotidase) on human gingival fibroblasts plays a role in the regulation of intracellular cAMP levels through the generation of adenosine, which subsequently activates adenosine receptors. In this study, we examined the involvement of ecto-adenosine deaminase, which can be anchored to CD26 on human gingival fibroblasts, in metabolizing adenosine generated by CD73, and thus attenuating adenosine receptor activation. Ecto-adenosine deaminase expression on fibroblasts could be increased by pre-treatment with a lysate of Jurkat cells, a cell line rich in cytoplasmic adenosine deaminase. Interestingly, the cAMP response to adenosine generated from 5′-AMP via CD73 and the ability of 5′-AMP to induce hyaluronan synthase 1 mRNA were significantly decreased by the pre-treatment of fibroblasts with Jurkat cell lysate. This inhibitory effect was reversed by the specific adenosine deaminase inhibitor. These results suggest that ecto-adenosine deaminase metabolizes CD73-generated adenosine and regulates adenosine receptor activation.
Keywords: adenosine receptor, CD73, ecto-adenosine deaminase
INTRODUCTION
Adenosine is an immunomodulator with anti-inflammatory properties, such as the promotion of endothelial barrier function (Lennon et al., 1998) and the regulation of cytokine production by macrophages (Bouma et al., 1994; Hasko et al., 1996), superoxide production by neutrophils (Cronstein et al., 1986), and mediator release by mast cells (Ramkumar et al., 1993). The effects of adenosine are mediated by seven-transmembrane-spanning G-protein-coupled adenosine receptors that modulate intracellular cAMP levels.
CD73 is a widely distributed membrane-bound glycosyl phosphatidylinositol (GPI)-anchored protein (Frick and Lowenstein, 1978; Darvish et al., 1996). It is involved in transmitting activation signals to T-cells (Thompson et al., 1989), binding to fibronectin and laminin (Stochaj et al., 1989), and the adhesion of lymphocytes to endothelial cells (Airas et al., 1997; Arvilommi et al., 1997). CD73 catalyzes the dephosphorylation of AMP and other nucleoside monophosphates and is a dominant contributor to the generation of extracellular adenosine (Zimmermann, 1992). Adenosine can be metabolized by two enzymes, adenosine deaminase and adenosine kinase. While adenosine kinase rephosphorylates adenosine to AMP, adenosine deaminase deaminates adenosine to inosine, the first step in its conversion to uric acid. Adenosine deaminase is distributed in virtually all human tissues and is expressed in the lymphoid system at high levels. Although adenosine deaminase is found mainly in the cytoplasm, in humans it also appears on the cell surface as an ecto-enzyme, anchored to CD26 (Kameoka et al., 1993).
We previously demonstrated that CD73 is responsible for the formation of extracellular adenosine (Hashikawa et al., 2003). Since adenosine is rapidly metabolized, it is likely that adenosine generated from 5′-AMP by CD73 can subsequently interact only with adenosine receptors proximal to CD73 (Matsuoka et al., 2002; Hashikawa et al., 2003). Interestingly, membrane-bound adenosine deaminase might also be located close to adenosine receptors (Hashikawa et al., 2004). If so, this would further confine adenosine receptor activation to the microenvironmental site of adenosine generation through CD73. However, the role of ecto-adenosine deaminase in regulating the extracellular concentration of adenosine and subsequent adenosine receptor activation, and especially in adenosine receptor activation mediated by CD73-generated adenosine, remains to be clarified. In this study, to clarify the mechanisms by which adenosine action is regulated, we investigated the expression of CD26 and ecto-adenosine deaminase in human gingival fibroblasts, and the involvement of ecto-adenosine deaminase in CD73-dependent adenosine receptor stimulation.
MATERIALS & METHODS
Reagents
Adenosine, adenosine 5′-monophosphate (5′-AMP), xanthine amine congener (XAC), and 4-(3-butoxy-4-methoxy-benzyl) imidazolidin-2-one were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The specific adenosine deaminase inhibitor, 2′-deoxycoformycin (dCF), was a gift from SuperGen Inc. (San Ramon, CA, USA).
Human Gingival Fibroblasts
All human subjects participating in this study provided informed consent to a protocol that was reviewed and approved by the Institutional Review Board of the Osaka University Graduate School of Dentistry. Human gingival fibroblasts obtained from biopsies of healthy gingiva from six healthy volunteers (four males and two females, from 12 to 18 yrs old) were explanted into α-modified Eagle's medium (α-MEM, Nikken Biomedical Laboratory, Kyoto, Japan) supplemented with 300 μg/mL kanamycin sulfate and 2.5 μg/mL fungizone, and then cultured with α-MEM supplemented with 10% fetal calf serum (FCS, JRH Biosciences, Lenexa, KS, USA) at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells detached from the explants with 0.05% trypsin-0.02% EDTA (Life Technologies, Grand Island, NY, USA) in PBS (Nikken Biomedical laboratory) were subcultured in plastic flasks (Corning, Corning, NY, USA). Fibroblasts were passaged after trypsinization and used for experiments at passages 4-10.
Preparation of Jurkat Lysate as a Source of Adenosine Deaminase
Jurkat cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in RPMI-1640 (Nikken Biomedical laboratory) supplemented with 10% FCS and 60 μg/mL kanamycin sulfate. Logarithmically grown Jurkat cells were collected by centrifugation, re-suspended in culture medium at a concentration of 1 × 108/mL, and subject to lysis by being frozen and thawed 3 times. Debris was removed by centrifugation (15,000 rpm in a microfuge for 10 min). The enzyme activity of adenosine deaminase from the Jurkat lysate was 219 μmols/hr/mL. To increase ecto-adenosine deaminase expression in fibroblasts, we incubated fibroblasts in 100 μL of the above-mentioned Jurkat lysate for 30 min at 37°C, and then washed them twice before further experiments.
Flow Cytometric Analysis
Cell-surface antigens were detected by flow cytometry with a Becton-Dickinson FACSCalibur. Cells were stained as previously described (Murakami et al., 1993), with an isotype-matched murine myeloma protein (Mouse IgG1, MOPC-21) or normal goat IgG as controls. The following antibodies were used: PE-anti-human CD26 (Pharmingen, San Diego, CA, USA), goat anti-adenosine deaminase (Hashikawa et al., 2004), and FITC-donkey anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Data were collected on 10,000 cells for single-color staining and were analyzed with CellQuest software (Becton Dickinson, Mountain View, CA, USA).
Measurements of Cyclic Adenosine Monophosphate (cAMP) Responses
Monolayers of fibroblasts grown to confluence in six-well plates (Corning) were washed twice with HBSS. Cells were pre-incubated for 10 min at 37°C in the medium containing the cAMP phosphodiesterase inhibitor, 4-(3-butoxy-4-methoxy-benzyl) imidazolidin-2-one, at 10 μM. Cells were then incubated in the same plates for 5 min at 37°C in 5% CO2 with the following reagents: media alone, and adenosine (20 μM) or AMP (20 μM), with or without dCF (5 μM). Each of the doses induced an unsaturated cAMP response and hyaluronan synthase 1 mRNA expression. cAMP levels were determined with the use of a cAMP enzyme immunoassay (EIA) kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) according to the manufacturer's instructions. All assays were performed in duplicate with 50 μL of cell extracts. cAMP levels in unstimulated cells were subtracted from the values recorded.
Detection of Hyaluronan Synthase 1-3 (HAS1, HAS2, and HAS3) mRNAs by RT-PCR
Total RNA was isolated from fibroblasts with an RNA-Bee kit (TEL-TEST, Inc., Friendswood, TX, USA) according to the manufacturer's instructions. The precipitated RNA was re-suspended in 0.1% diethylpyrocarbonate-treated distilled water. cDNA was synthesized and amplified via PCR as described previously (Murakami et al., 2001). Oligonucleotide PCR primers specific for hyaluronan synthase (HAS) 1, 2, 3, and HPRT mRNA were synthesized by Clontech (Palo Alto, CA, USA). The primer sequences were as follows: HPRT (hypoxanthine phosphoribosyl transferase), 5′-CGAGATGTGATGAAGGAGATGGG-3′ (forward), 5′-GCCTGACCAAGGAAAGCAAAGTC-3′ (reverse); HAS1, 5′-TGCGATACTGGGTAGCCTTCAATG-3′ (forward), 5′-CGTTGTACAGCCACTCACGGAAGTA-3′ (reverse); HAS2, 5′-TCTGGGAATGTACAGAAACTC-3′ (forward), 5′-AGACATGAAGACCATGACGAT-3′ (reverse); and HAS3, 5′-TTGGGCATGTACCGCAACA-3′ (forward), 5′-GGGACATGAAGATCATCTCTGC-3′ (reverse). The density of each band in the agarose gels was quantified with the use of Quantity One software (BIO-RAD Laboratories, Inc., Hercules, CA, USA).
Statistical Analysis
Statistical analyses of the results were performed by one-way ANOVA and Fisher's PLSD tests.
RESULTS
Surface Expression of Ecto-adenosine Deaminase on Human Gingival Fibroblasts
Since adenosine deaminase can localize on the cell surface through its interaction with CD26 (Kameoka et al., 1993; Hashikawa et al., 2004), the expression of ecto-adenosine deaminase and CD26 on fibroblasts was examined. Little expression of ecto-adenosine deaminase was observed on in vitro-maintained fibroblasts (Fig. 1A), although the expression of CD26 was detected constitutively (Fig. 1B). Fibroblasts were then treated with Jurkat cell lysate as a source of exogenous adenosine deaminase (Hashikawa et al., 2004). As expected, treatment with Jurkat lysate resulted in an increase in the expression of ecto-adenosine deaminase on the fibroblasts (Fig. 1A).
Figure 1.
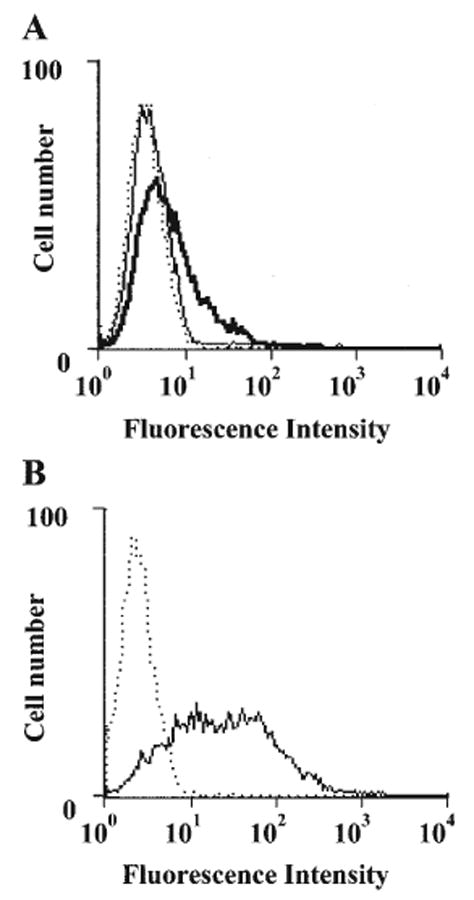
FACS analysis of surface expression of CD26 and ecto-ADA on human gingival fibroblasts. (A) Human gingival fibroblasts (1 × 106 cells) were incubated with (bold line) or without (solid line) Jurkat cell lysate for 30 min at 37°C, washed twice, and stained with anti-ADA plus FITC-donkey anti-goat IgG. Normal goat IgG was utilized as a control (dotted line). (B) Untreated human gingival fibroblasts were stained with PE-anti-human CD26 mAb (solid line) or isotype-matched murine myeloma protein (dotted line). The data shown are representative of 3 separate experiments.
Regulation of cAMP Responses to Adenosine by Ecto-adenosine Deaminase
We then investigated the regulation of adenosine receptor (AdoR) engagement by ecto-adenosine deaminase on fibroblasts. We first maximized ecto-adenosine deaminase levels on fibroblasts by saturating CD26 with ecto-adenosine deaminase through pre-incubation of fibroblasts with Jurkat cell lysate. Then, we examined the adenosine-induced cAMP responses in the pre-incubated fibroblasts in the presence or absence of dCF. The adenosine-induced cAMP response was significantly inhibited (39%) by Jurkat cell lysate treatment, and the inhibitory effect was reversed by the addition of dCF (Fig. 2A). In the presence of dCF, the cAMP response to adenosine after cell lysate treatment was higher than that without lysate treatment (Fig. 2A).
Figure 2.
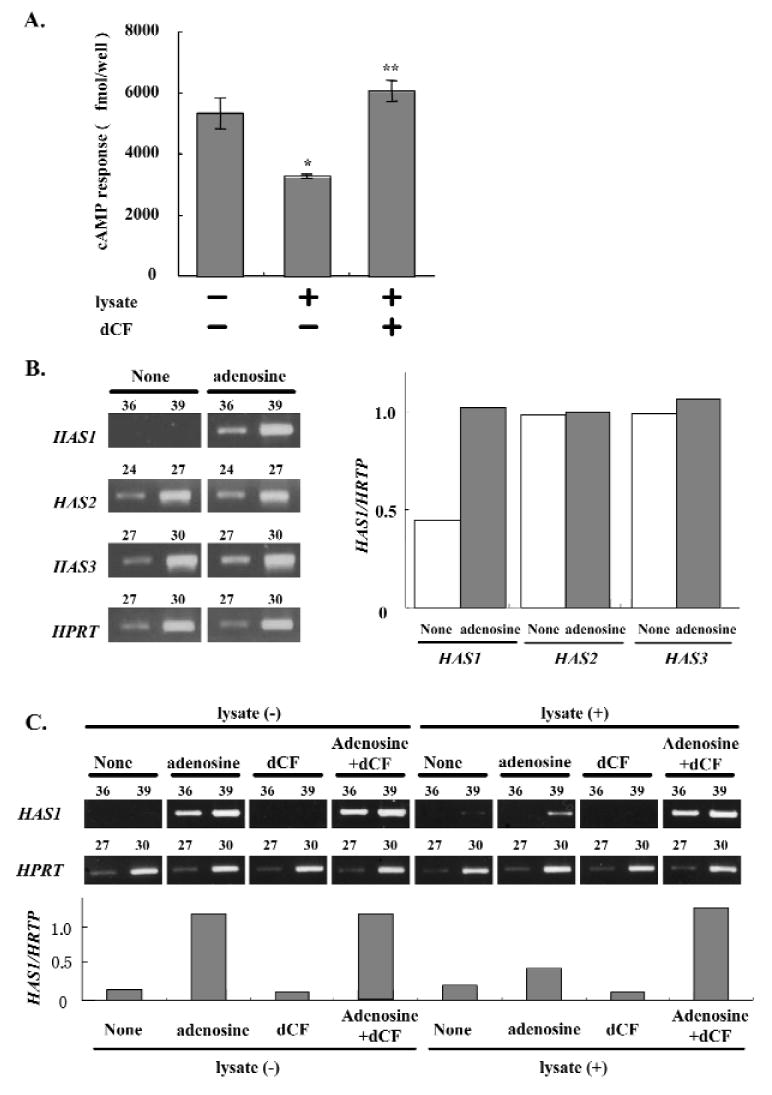
Regulation of adenosine receptor engagement by ecto-ADA. (A) Regulation of cAMP responses by ecto-ADA. Human gingival fibroblasts (5.0 × 105 cells/well) were incubated with or without Jurkat cell lysate for 30 min at 37°C and stimulated with 20 μM adenosine for 5 min at 37°C in the presence or absence of 5 μM dCF. The data shown are the mean ± SD of 4 cAMP determinations and are representative of 3 separate experiments. *p < 0.05 compared with the lysate (-) dCF (-) group. **p < 0.05 compared with the lysate (+) dCF (-) group. (B) Effects of adenosine (Ado) on HAS mRNA expression in human gingival fibroblasts. Human gingival fibroblasts were cultured with or without Ado (20 μM) for 3 hrs, and then RT-PCR was carried out for the detection of HAS1, HAS2, and HAS3 mRNA expression. The results shown are representative of 3 separate experiments. The number of PCR cycles is shown above each lane. Each band was standardized against the amount of HPRT. (C) Effects of ecto-ADA on adenosine (Ado)-induced HAS1 mRNA expression in human gingival fibroblasts. Human gingival fibroblasts were pre-incubated with or without Jurkat cell lysate for 30 min at 37°C, and were treated with Ado (20 μM) with or without dCF (5 μM) for 2.5 hrs. HAS1 mRNA expression was examined by RT-PCR. Results of 1 representative experiment from among 3 identical experiments are shown. The number of PCR cycles is shown above each lane. Each band was standardized against the amount of HPRT.
Regulation of HAS 1 mRNA Expression by Ecto-adenosine Deaminase
Production of extracellular matrices is an important function of human gingival fibroblasts. Among the extracellular matrices, hyaluronan (HA) plays multi-functional roles in the growth and differentiation of cells during the course of inflammatory reactions and the process of wound-healing and regeneration in periodontal tissues (Shimabukuro et al., 2005). We previously reported that adenosine enhanced HAS mRNA expression in HGF, and that the expression was markedly abrogated by the AdoR antagonist XAC (Murakami et al., 2001). This also indicates that intracellular signaling via AdoR can be monitored by HAS mRNA expression in gingival fibroblasts. Of the three different isoforms of HAS (HAS1, HAS2, and HAS3), we found that HAS1 mRNA was specifically induced by adenosine treatment of fibroblasts (Fig. 2B). In contrast, adenosine treatment did not affect the expression of either HAS2 or HAS3 mRNA (Fig. 2B). To evaluate further the ability of ecto-adenosine deaminase to regulate AdoR engagement, we examined the influence of dCF on adenosine-induced HAS1 mRNA expression by fibroblasts. Fibroblasts pre-incubated with or without Jurkat cell lysate were examined for adenosine-induced HAS1 mRNA expression in the presence and absence of dCF, by RT-PCR. Pre-treatment with Jurkat cell lysate decreased not only adenosine-induced cAMP (Fig. 2A), but also HAS1 mRNA expression (Fig. 2C), and the effect of the pre-treatment was also reversed by the addition of dCF (Fig. 2C). The fact that the intensity of HAS1 mRNA expression with dCF was unaffected by Jurkat lysate treatment indicates that the increase in HAS1 mRNA expression was not caused by dCF alone.
Regulation of cAMP Response and HAS1 mRNA Expression in Human Gingival Fibroblasts by Ecto-adenosine Deaminase and CD73
We recently found that CD73 is expressed in fibroblasts and is involved in the production of adenosine (Hashikawa et al., 2003). Our next question was whether ecto-adenosine deaminase could regulate the interaction of adenosine generated from AMP via CD73, with AdoRs. Fibroblasts pre-incubated with or without Jurkat lysate were examined for AMP-induced cAMP responses in the presence or absence of dCF. Interestingly, pre-treatment with Jurkat lysate significantly inhibited the cAMP response (27%), and the inhibitory effect was reversed by the addition of dCF (Fig. 3). To investigate further the regulation of AdoR engagement by ecto-adenosine deaminase and CD73, we examined AMP-induced HAS1 mRNA expression in fibroblasts. Treatment with 5′-AMP also increased HAS1 mRNA expression in fibroblasts (Fig. 4A), and the increase was completely inhibited by the AdoR antagonist, XAC (Fig. 4A). Furthermore, pre-treatment of fibroblasts with Jurkat cell lysate decreased AMP-induced HAS1 mRNA expression (Fig. 4B), and the effect of the pre-treatment was reversed by dCF (Fig. 4B), consistent with the findings shown in Fig. 3.
Figure 3.
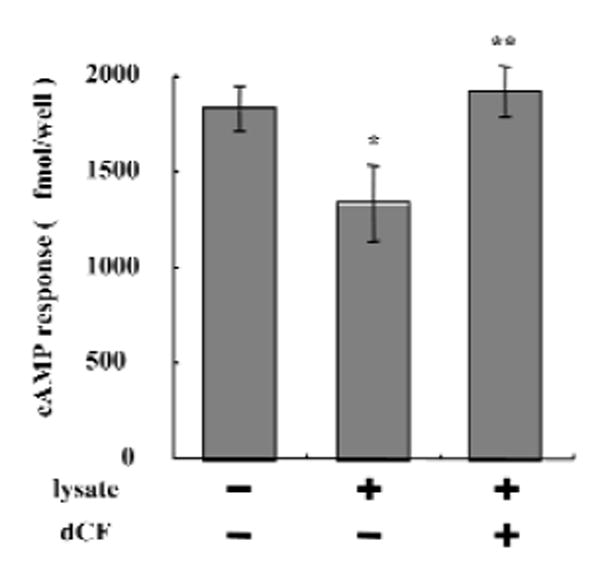
Regulation of cAMP responses by ecto-ADA and CD73. Human gingival fibroblasts (5.0 × 105 cells/well) were incubated with or without Jurkat lysate for 30 min at 37°C and stimulated with 20 μM AMP for 5 min at 37°C in the presence or absence of 5 μM dCF. The data shown are the mean ± SD of 4 cAMP determinations and are representative of 3 separate experiments. *p < 0.05 compared with the lysate (-) dCF (-) group. **p < 0.05 compared with the lysate (+) dCF (-) group.
Figure 4.
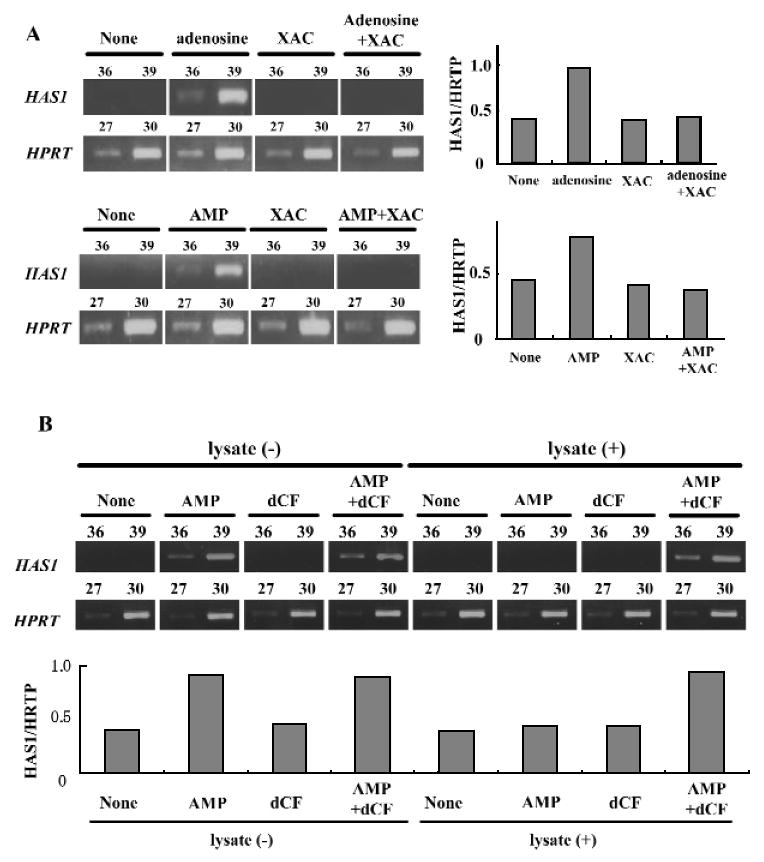
Regulation of adenosine receptor engagement by ecto-ADA and CD73. (A) Effect of XAC on adenosine (Ado) or AMP-induced HAS1 mRNA expression in human gingival fibroblasts. Human gingival fibroblasts (5.0 × 105 cells/well) were treated with Ado (20 μM) or AMP (20 μM) for 2.5 hrs in the presence or absence of XAC (5 μM). HAS1 mRNA expression in human gingival fibroblasts was examined by RT-PCR. The results shown are representative of 3 separate experiments. The number of PCR cycles is shown above each lane. Each band was standardized against the amount of HPRT. (B) Effects of ecto-ADA on AMP-induced HAS1 mRNA expression in human gingival fibroblasts. Human gingival fibroblasts were pre-incubated with or without Jurkat lysate for 30 min at 37°C, and treated with AMP (20 μM) in the presence and absence of dCF (5 μM) for 2.5 hrs. HAS1 mRNA expression in human gingival fibroblasts was examined by RT-PCR. The results shown are representative of 3 separate experiments. The number of PCR cycles is shown above each lane. Each band was standardized against the amount of HPRT.
DISCUSSION
In this study, we first clarified the involvement of fibroblast ecto-adenosine deaminase, which is likely anchored on CD26, in regulating AdoR engagement by extracellular adenosine, in CD73-dependent adenosine receptor stimulation.
Adenosine deaminase is found mainly in the cytosol, but it has been shown to be anchored to CD26 on cell surfaces as well (Aran et al., 1991; Darvish et al., 1996). Pre-treatment of fibroblasts with an exogenous source of adenosine deaminase, a cell lysate of Jurkat, which includes abundant cytosolic adenosine deaminase, resulted in an increase in ecto-adenosine deaminase expression. Although this increase in ecto-adenosine deaminase expression on fibroblasts led to some metabolism of extracellular adenosine added to the culture medium, the reduction in adenosine concentration was not statistically significant (data not shown). In contrast, pre-treatment of fibroblasts with Jurkat cell lysate led to a significant suppression of adenosine-induced cAMP and HAS1 mRNA responses. These decreases were abrogated by dCF. These results are consistent with our findings with CD26-transfected lymphoid cells and recombinant adenosine deaminase (Hashikawa et al., 2004). Our previous findings suggested that although adenosine deaminase anchored on the cell surface of fibroblasts cannot markedly diminish overall extracellular adenosine levels in culture media (data not shown), it can regulate local concentrations of adenosine in the vicinity of AdoRs and, consequently, AdoR activation. Since a high concentration of adenosine can be cytotoxic, this micro-environmental regulation of adenosine concentration is extremely beneficial to cells and tissues. It is likely that lysed cells in inflammatory lesions can be a source of adenosine deaminase, which subsequently binds to CD26. In addition, CD26 expression on fibroblasts can be induced by inflammatory cytokines such as IL-1β, leading to an increased capacity for adenosine deaminase binding (data not shown).
In contrast, CD73 (ecto-5′-nucleotidase) on fibroblasts contributes to the generation of adenosine and, in turn, the activation of adenosine receptors (Hashikawa et al., 2003). Thus, both CD73 and ecto-adenosine deaminase on fibroblasts play key roles in the regulation of the adenosine concentration in the vicinity of AdoR and subsequent adenosine receptor activation. Furthermore, the expression level of CD73 and its enzymatic activity are also modulated by several inflammatory mediators, such as IL-1β (Savic et al., 1990), NO (Obata et al., 1998), PGE2 (Savic et al., 1991), and TNFα (Savic et al., 1990; Kalsi et al., 2002). Thus, the regulation of AdoR activation by CD73 and CD26-anchored adenosine deaminase on fibroblasts can be modulated by various mediators in inflamed periodontal lesions.
The anti-inflammatory effects of adenosine are well-documented (Cronstein, 1994; Ohta and Sitkovsky, 2001). We recently found, by RT-PCR, that both CD73 and adenosine deaminase gene expression are enhanced in inflamed human gingival tissues, compared with control healthy gingival tissues (data not shown), although the specific cells expressing CD73 and adenosine deaminase, and the mechanisms by which the expression of these molecules is modulated in inflamed tissue, remain to be clarified. Our findings suggest that the enzymatic activities of both molecules should be increased in periodontitis lesions. Thus, enhancing the biological effects of adenosine at inflamed sites, possibly by an activator of CD73 or an inhibitor of adenosine deaminase, might be a new therapeutic approach to periodontitis. Further studies will be required to address the molecular mechanisms by which adenosine regulates inflammatory reactions in periodontitis.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (the Japan Society for the Promotion of Science, No. 15649498 and No. 16791311) and by the 21st Century COE entitled “Origination of Frontier BioDentistry”, Osaka University Graduate School of Dentistry, supported by the Ministry of Education, Culture, Sports, Science and Technology. LFT is supported by Grant AI18220 from the National Institutes of Health and holds the Putnam City Schools Chair in Cancer Research.
References
- Airas L, Niemela J, Salmi M, Puurunen T, Smith DJ, Jalkanen S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kD adhesion molecule, on lymphocytes and endothelial cells. J Cell Biol. 1997;136:421–431. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran JM, Colomer D, Matutes E, Vives-Corrons JL, Franco R. Presence of adenosine deaminase on the surface of mononuclear blood cells: immunochemical localization using light and electron microscopy. J Histochem Cytochem. 1991;39:1001–1008. doi: 10.1177/39.8.1856451. [DOI] [PubMed] [Google Scholar]
- Arvilommi AM, Salmi M, Airas L, Kalimo K, Jalkanen S. CD73 mediates lymphocyte binding to vascular endothelium in inflamed human skin. Eur J Immunol. 1997;27:248–254. doi: 10.1002/eji.1830270137. [DOI] [PubMed] [Google Scholar]
- Bouma MG, Stad RK, van den Wildenberg FA, Buurman WA. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994;153:4159–4168. [PubMed] [Google Scholar]
- Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Levin RI, Belanoff J, Weissmann G, Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986;78:760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvish A, Pomerantz RW, Zografides PG, Metting PJ. Contribution of cytosolic and membrane-bound 5′-nucleotidases to cardiac adenosine production. Am J Physiol. 1996;271:H2162–H2167. doi: 10.1152/ajpheart.1996.271.5.H2162. [DOI] [PubMed] [Google Scholar]
- Frick GP, Lowenstein JM. Vectorial production of adenosine by 5′-nucleotidase in the perfused rat heart. J Biol Chem. 1978;253:1240–1244. [PubMed] [Google Scholar]
- Hashikawa T, Takedachi M, Terakura M, Saho T, Yamada S, Thompson LF, et al. Involvement of CD73 (ecto-5′-nucleotidase) in adenosine generation by human gingival fibroblasts. J Dent Res. 2003;82:888–892. doi: 10.1177/154405910308201108. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Hooker SW, Maj JG, Knott-Craig CJ, Takedachi M, Murakami S, et al. Regulation of adenosine receptor engagement by ecto-adenosine deaminase. FASEB J. 2004;18:131–133. doi: 10.1096/fj.03-0011fje. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- Kalsi K, Lawson C, Dominguez M, Taylor P, Yacoub MH, Smolenski RT. Regulation of ecto-5′-nucleotidase by TNF-alpha in human endothelial cells. Mol Cell Biochem. 2002;232:113–119. doi: 10.1023/a:1014806916844. [DOI] [PubMed] [Google Scholar]
- Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka I, Ohkubo S, Kimura J, Uezono Y. Adenine nucleotide-induced activation of adenosine A(2B) receptors expressed in Xenopus laevis oocytes: involvement of a rapid and localized adenosine formation by ectonucleotidases. Mol Pharmacol. 2002;61:606–613. doi: 10.1124/mol.61.3.606. [DOI] [PubMed] [Google Scholar]
- Murakami S, Saho T, Shimabukuro Y, Isoda R, Miki Y, Okada H. Very late antigen integrins are involved in the adhesive interaction of lymphoid cells to human gingival fibroblasts. Immunology. 1993;79:425–433. [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Hashikawa T, Saho T, Takedachi M, Nozaki T, Shimabukuro Y, et al. Adenosine regulates the IL-1 beta-induced cellular functions of human gingival fibroblasts. Int Immunol. 2001;13:1533–1540. doi: 10.1093/intimm/13.12.1533. [DOI] [PubMed] [Google Scholar]
- Obata T, Sato T, Yamanaka Y, Arita M. NO and cGMP facilitate adenosine production in rat hearts via activation of ecto-5′-nucleotidase. Pflugers Arch. 1998;436:984–990. doi: 10.1007/s004240050733. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- Savic V, Stefanovic V, Ardaillou N, Ardaillou R. Induction of ecto-5′-nucleotidase of rat cultured mesangial cells by interleukin-1 beta and tumour necrosis factor-alpha. Immunology. 1990;70:321–326. [PMC free article] [PubMed] [Google Scholar]
- Savic V, Blanchard A, Vlahovic P, Stefanovic V, Ardaillou N, Ardaillou R. Cyclic adenosine monophosphate-stimulating agents induce ecto-5′-nucleotidase activity and inhibit DNA synthesis in rat cultured mesangial cells. Arch Biochem Biophys. 1991;290:202–206. doi: 10.1016/0003-9861(91)90609-m. [DOI] [PubMed] [Google Scholar]
- Shimabukuro Y, Ichikawa T, Takayama S, Yamada S, Takedachi M, Terakura M, et al. Fibroblast growth factor-2 regulates the synthesis of hyaluronan by human periodontal ligament cells. J Cell Physiol. 2005;203:557–563. doi: 10.1002/jcp.20256. [DOI] [PubMed] [Google Scholar]
- Stochaj U, Dieckhoff J, Mollenhauer J, Cramer M, Mannherz HG. Evidence for the direct interaction of chicken gizzard 5′-nucleotidase with laminin and fibronectin. Biochim Biophys Acta. 1989;992:385–392. doi: 10.1016/0304-4165(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Thompson LF, Ruedi JM, Glass A, Low MG, Lucas AH. Antibodies to 5′-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J Immunol. 1989;143:1815–1821. [PubMed] [Google Scholar]
- Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]


