Abstract
A transferred-DNA insertion mutant of Arabidopsis that lacks AKT1 inward-rectifying K+ channel activity in root cells was obtained previously by a reverse-genetic strategy, enabling a dissection of the K+-uptake apparatus of the root into AKT1 and non-AKT1 components. Membrane potential measurements in root cells demonstrated that the AKT1 component of the wild-type K+ permeability was between 55 and 63% when external [K+] was between 10 and 1,000 μM, and NH4 + was absent. NH4 + specifically inhibited the non-AKT1 component, apparently by competing for K+ binding sites on the transporter(s). This inhibition by NH4 + had significant consequences for akt1 plants: K+ permeability, 86Rb+ fluxes into roots, seed germination, and seedling growth rate of the mutant were each similarly inhibited by NH4 +. Wild-type plants were much more resistant to NH4 +. Thus, AKT1 channels conduct the K+ influx necessary for the growth of Arabidopsis embryos and seedlings in conditions that block the non-AKT1 mechanism. In contrast to the effects of NH4 +, Na+ and H+ significantly stimulated the non-AKT1 portion of the K+ permeability. Stimulation of akt1 growth rate by Na+, a predicted consequence of the previous result, was observed when external [K+] was 10 μM. Collectively, these results indicate that the AKT1 channel is an important component of the K+ uptake apparatus supporting growth, even in the “high-affinity” range of K+ concentrations. In the absence of AKT1 channel activity, an NH4 +-sensitive, Na+/H+-stimulated mechanism can suffice.
Keywords: Arabidopsis, plant nutrition, root, transferred-DNA insertion mutant
introduction
It has been known since the work of Knop and Sachs over 130 yr ago that plants cannot grow in the absence of potassium (Pfeffer, 1900). It is their most abundant inorganic constituent, contributing importantly to the osmotic potential and electrolytic character of cytoplasm. The plasma membrane is typically more permeable to K+ than to other ions, so the difference in its concentration across the membrane has a large influence on the membrane potential and, hence, cell physiology. Another reason for its essentiality is that some enzymes require K+ as a cofactor.
The mechanism by which cells concentrate K+ from dilute extracellular sources such as soil has received considerable attention because plant growth depends directly on it. Early kinetic studies by Epstein et al. (1963) gave evidence of two distinct uptake mechanisms: a high affinity system operating over micromolar concentration ranges and a low affinity system that predominates when [K+]ext is in the millimolar range. Recent measurements of K+ electrochemical potential gradients were incorporated into this classical model to create the widely held view that active transport is necessary when [K+]ext is less than ∼300 μM, but that a passive mechanism suffices at higher values of [K+]ext (Maathuis and Sanders, 1993, 1994, 1997; Walker et al., 1996a). This important thermodynamic information was readily integrated with ground-breaking molecular advances occurring at about the same time. Genes encoding passive K+ channels and active K+ cotransporters were cloned by complementation of yeast mutants, functionally characterized after heterologous or ectopic expression, and demonstrated to be expressed in roots (reviewed in Smart et al., 1996; de Boer, 1999). These advances collectively gave rise to the dominant view that transporters such as HKT1 (Schachtman and Schroeder, 1994; Rubio et al., 1995; Gassmann et al., 1996; Wang et al., 1998) and the KUP family (Quintero and Blatt, 1997; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998) are responsible for “high affinity” K+ uptake and that inward-rectifying K+ channels such as AKT1 (Sentenac et al., 1992; Basset et al., 1995; Lagarde et al., 1996) mediated uptake when K+ was more concentrated than ∼300 μM.
This paradigm was shown to require modification when an Arabidopsis mutant lacking detectable AKT1 channel activity (akt1) was found to be defective in K+ uptake and growth on solutions as dilute as 10 μM K+, a concentration previously thought to be well outside the realm of possibilities for channels (Hirsch et al., 1998). However, measurements of membrane potentials more negative than −230 mV in Arabidopsis roots demonstrated that uptake of K+ from 10 μM solutions by channels was indeed energetically feasible, at least in cells near the root apex (Hirsch et al., 1998). Now it seems reasonable to view inward-rectifying K+ channels as passive uptake mechanisms capable of conducting growth-supporting K+ fluxes in the high-affinity concentration range, provided that the K+ electrochemical potential gradient is inward.
The existence of a mutant lacking inward-rectifying K+ channels in the root provides an opportunity to dissect genetically the channel-mediated contribution to K+ uptake from that of other transporters, and to determine the significance of each under various ionic conditions a plant may encounter. A condition meriting close attention in this respect is the presence of NH4 +, as Hirsch et al. (1998) found it must be present to observe the akt1 phenotype (poor growth relative to wild type on [K+]ext < 0.1 mM). In the absence of NH4 +, mutant and wild type grow similarly. This would be expected if NH4 + inhibited a K+ transport mechanism that operates in parallel with AKT1 and is necessary for growth when AKT1 activity is lacking. There is much support in the literature for this possibility. Inhibitory effects of NH4 + on K+ uptake have been noted (Rufty, et al., 1982; Van Beusichem, 1988) and, in a study of maize roots, Vale et al. (1987) found that K+ uptake was comprised of NH4 +-sensitive and NH4 +-insensitive components. Smith and Epstein (1964) presented evidence that NH4 + inhibited K+ uptake by competing for a binding site on the transporter in maize leaves. However, the converse (K+ inhibition of NH4 + uptake) does not seem to occur, a result that at least one authority considered “quite surprising” (Marschner, 1995). The present work takes advantage of the akt1 mutation to produce an explanation of this relationship between K+, NH4 +, and growth.
A related and somewhat controversial topic is the role of Na+ in K+ uptake (Maathuis et al. 1996; Rubio et al., 1996; Walker et al. 1996b). The renewal of interest in Na+–K+ relationships is due to the finding that the HKT1 transporter of barley functions as a Na+-coupled K+ symporter (Rubio et al., 1995; Gassmann et al., 1996), and to genetic advances in understanding the relationship between the ability of a plant to resist Na+ stress and K+ nutritional status (Zhu et al., 1998). The akt1 mutant was used here in studies that shed light on how the uptake mechanisms responsible for growth-sustaining K+ fluxes are importantly influenced by NH4 + and Na+.
materials and methods
Electrical and Flux Measurements
Measurements of membrane potential (Vm) in apical root cells were made with an intracellular microelectrode as described in Hirsch et al. (1998) in order to assess the permeability of the membrane to K+. Eq. 1 is a simplified description of the ionic basis of Vm in plant cells:
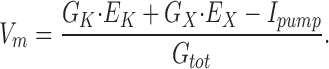 |
1 |
G K and E K are the conductance and equilibrium potential for K+, G X and E X represent the conductance and equilibrium potential for all other ions lumped together, and I pump is the current created by an electrogenic pump (the H+-ATPase in the case of plants). G tot is the total conductance of the membrane.
Shifts in extracellular KCl concentration ([KCl]ext) were imposed on the root while Vm was recorded continuously. The change in Vm resulting from shifts in [KCl]ext is described by:
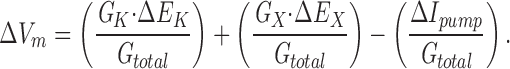 |
2 |
Assuming that an imposed shift in [KCl]ext affects only the K+ and Cl− components, Eq. 2 simplifies to:
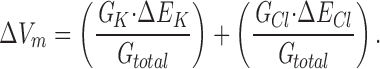 |
3 |
Increasing [KCl]ext caused positive shifts in Vm (see Fig. 1), demonstrating that the membrane was more permeable to K+ than the counterion Cl−, as is typical of plant cells. In the extreme case of a negligible Cl− conductance, Eq. 3 reduces to:
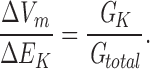 |
4 |
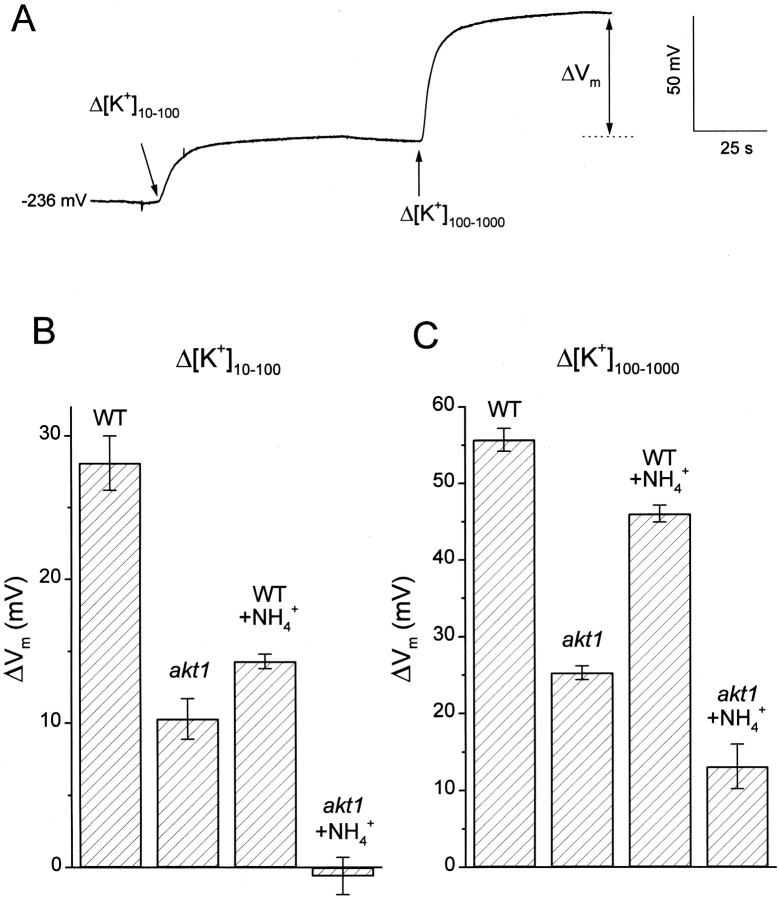
Figure 1.
Changes in membrane potential resulting from changes in K+ concentration of the solution bathing wild-type and akt1 roots. (A) Recording of Vm in a cell ∼150 μm from the apex of an Arabidopsis root. From an initial membrane potential of −236 mV in this example, the membrane depolarized in response to increasing [K+]ext from 10 to 100, and then to 1,000 μM. (B) The magnitude of the change in steady state Vm in response to changing [K+]ext from 10 to 100 μM in wild-type and akt1 roots, in the presence and absence of 2 mM NH4 +. (C) Same as in B, except the change in [K+]ext was from 100 to 1,000 μM. All values are the means of 6–10 independent experiments ± SEM.
For the purposes of determining the effects of the akt1 mutation and various ionic treatments, the assumptions implicit in Eq. 4 were adopted. Thus, the magnitude of the ΔVm resulting from shifts in [KCl]ext is interpreted here as a measure of the relative K+ permeability of the membrane.
The solutions used to bathe the roots were exactly the same solutions used for the growth experiments (below), except agarose was omitted. For experiments that tested the effects of NH4 +, Na+, and H+, the mounted seedlings were bathed in the test solution for ∼2 h before impalement. Rb+ fluxes were also performed exactly as described by Hirsch et al. (1998). Percent inhibition by NH4 + was calculated so that the results of independent trials involving different specific activities could be averaged.
Plant Growth
24 surface-sterilized seeds of either akt1 or the Wassilewskija wild type were sown with equal spacing across square Petri plates containing media (described below) solidified with 0.8% agarose. They were maintained in darkness at 4°C for 48 h before being placed in a growth chamber set to deliver 16 h days and 8 h nights at 21°C. Germination was assayed after 72 h when [K+]ext was 10 or 100 μM (see Fig. 4, A and B), but after only 48 h when [K+]ext was 1,000 μM (Fig. 4 C) because of the faster embryo growth in this condition. A seed was considered to have germinated if emergence of the radicle from the seed coat could be detected with the aid of a 40× dissecting scope. After 4 d of growth, the fresh weight of the group of seedlings was determined to the nearest 0.1 mg, and at 8 d the harvesting/weighing procedure was repeated with a separate plate of seedlings. The difference in mass between the two time points was divided by the number of intervening days to obtain an average growth rate for the group of seedlings between days 4 and 8. Experiments spanning 12 d of growth produced similar results. All data shown are the averages of at least three independent trials.
Figure 4.
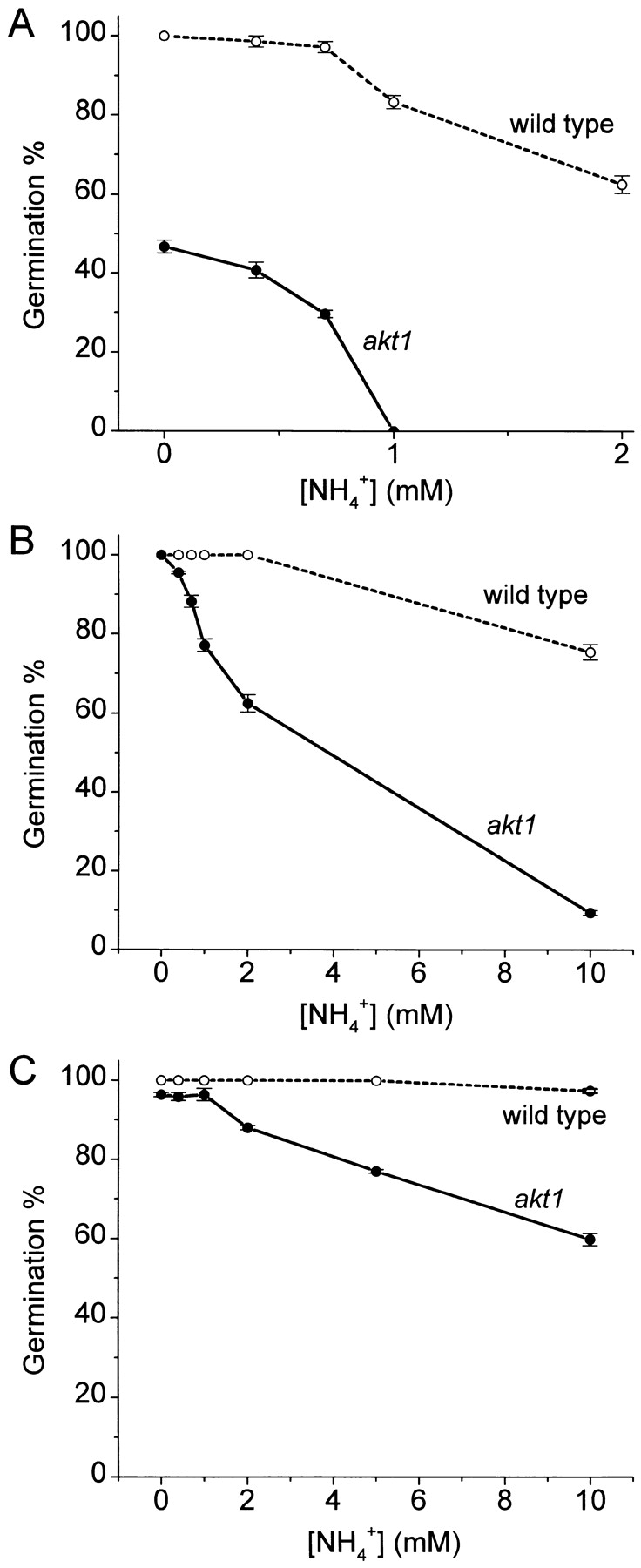
Concentration dependence of the NH4 + inhibition of wild-type and akt1 germination rate (embryo growth). Each datum is the mean ± SEM of at least three independent trials, with each trial involving 24 seeds. (A) [K+]ext = 10 μM; (B) [K+]ext = 100 μM; (C) [K+]ext = 1,000 μM. ○, wild-type; •, akt1.
Solutions and Media
The following base solution was used for studying the effects of NH4 + (see Figs. 1–5): 2.5 mM NaNO3, 2.5 mM Ca(NO3)2, 2 mM MgSO4, 0.1 mM NaFeEDTA, 80 μM Ca(H2PO4)2, 25 μM CaCl2, 25 μM H3BO3, 2 μM ZnSO4, 2 μM MnSO4, 0.5 μM CuSO4, 0.5 μM Na2MoO4, 0.01 μM CoCl2, 0.5% sucrose, and 2.5 mM Mes. NH4 + was added as NH4H2PO4 to achieve the desired amount and 1 mM Ca(H2PO4)2 was added to the 0 NH4 + solution to balance the phosphate concentrations. K+ was added as KCl. The pH of the mixture was adjusted to 5.7 with NaOH and autoclaved for 10 min. Longer autoclaving frequently produced a crystalline precipitate that probably contained NH4 + because it formed copiously in solutions containing >1 mM NH4 +, and not at all in its absence. Also, the normal inhibitory effect of NH4 + on growth was not observed when solutions containing the precipitate were used in experiments. This is a very important technical detail.
Figure 5.
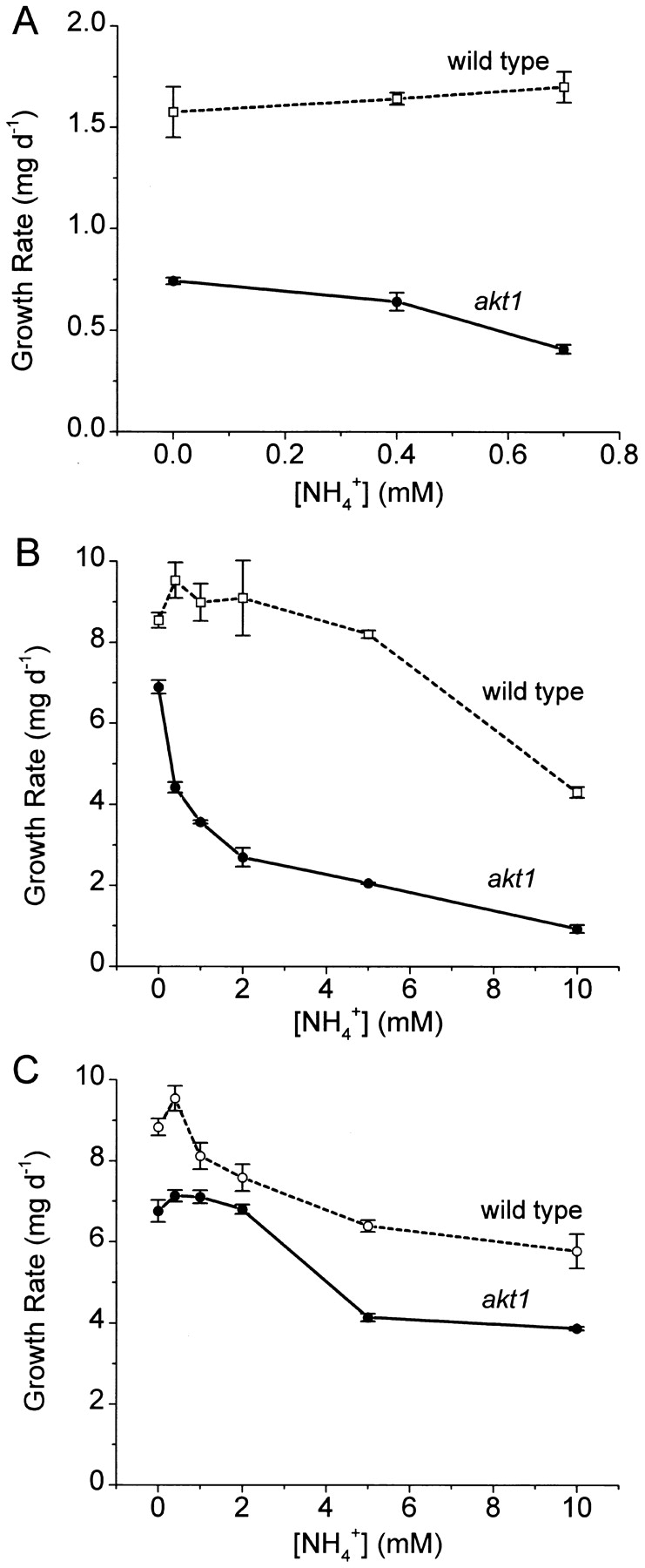
Concentration dependence of the inhibition of wild-type and akt1 growth rate by NH4 +. (A) [K+]ext = 10 μM; (B) [K+]ext = 100 μM; (C) [K+]ext = 1, 000 μM. ○, wild-type; •, akt1. The rates at which the fresh weight of 24 seedlings increased between days 4 and 8 were determined from three independent trials and were plotted as means ± SEM.
The following base solution was used for studying the effects of Na+ (see Figs. 6 and 7): 2.5 mM Ca(NO3)2, 2 mM MgSO4, 0.1 mM EDTA, 0.1 mM FeCl2, 80 μM Ca(H2PO4)2, 25 μM CaCl2, 25 μM H3BO3, 2 μM ZnSO4, 2 μM MnSO4, 0.5 μM CuSO4, 0.5 μM Na2MoO4, 0.01 μM CoCl2, 0.5% sucrose, and 2.5 mM Mes or 2.5 mM HEPES when the intended pH was basic. K+ was added as KCl and Na+ was added as NaCl. The pH was adjusted to 5.7 for growth experiments or otherwise to the indicated value with BTP. Note that the nominally 0 Na+ treatment has 1 μM Na+ from the Na2MoO4 and any contaminating Na+ in the water or chemicals. Also noteworthy, growth was inhibited when BTP {1,3-bis [tris(hydroxymethyl)methylamino]propane} was used instead of NaOH to adjust the pH of media containing specific Na+ concentrations. Experiments demonstrated that this amount of BTP, all else equal, inhibited growth rate by 50%. This is another important technical detail.
Figure 6.
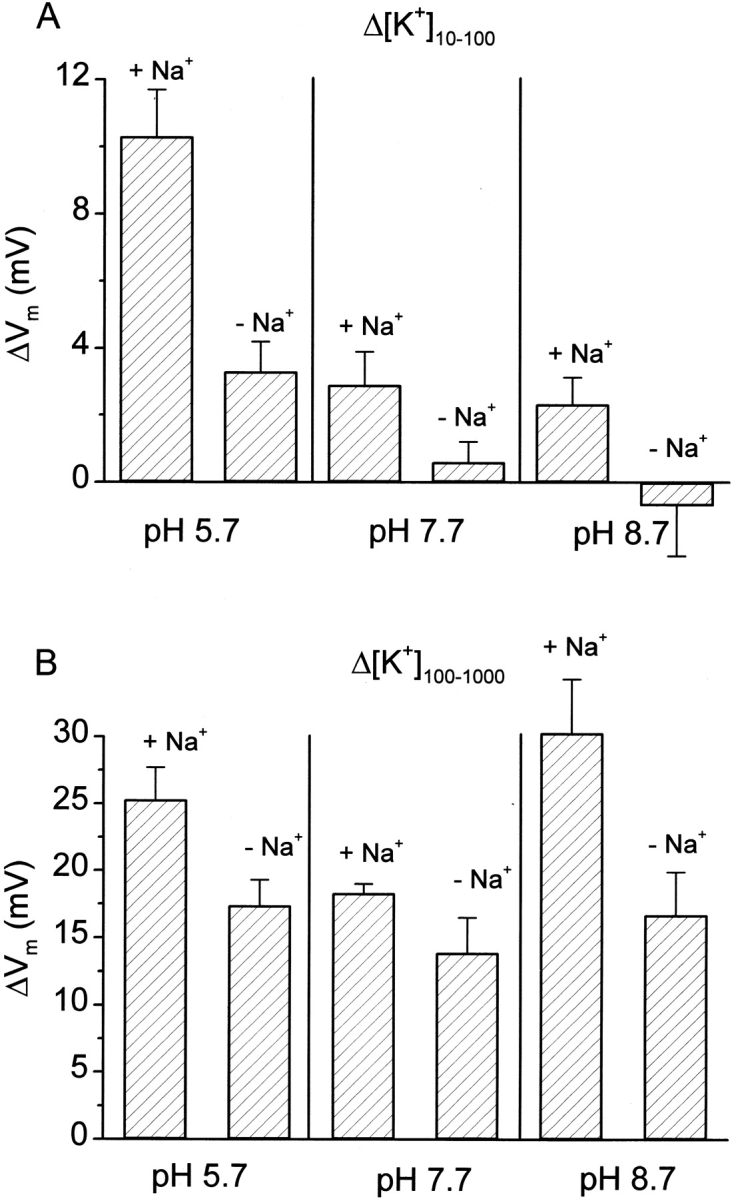
Enhancement of the K+ permeability of the akt1 root plasma membrane by Na+ and H+. Roots were preincubated and maintained in the indicated combinations of Na+ and H+ while K+ permeability was assessed by measuring changes in Vm induced by shifts in [K+]ext. Each value is the mean ± SEM of three to eight independent measurements.
Figure 7.
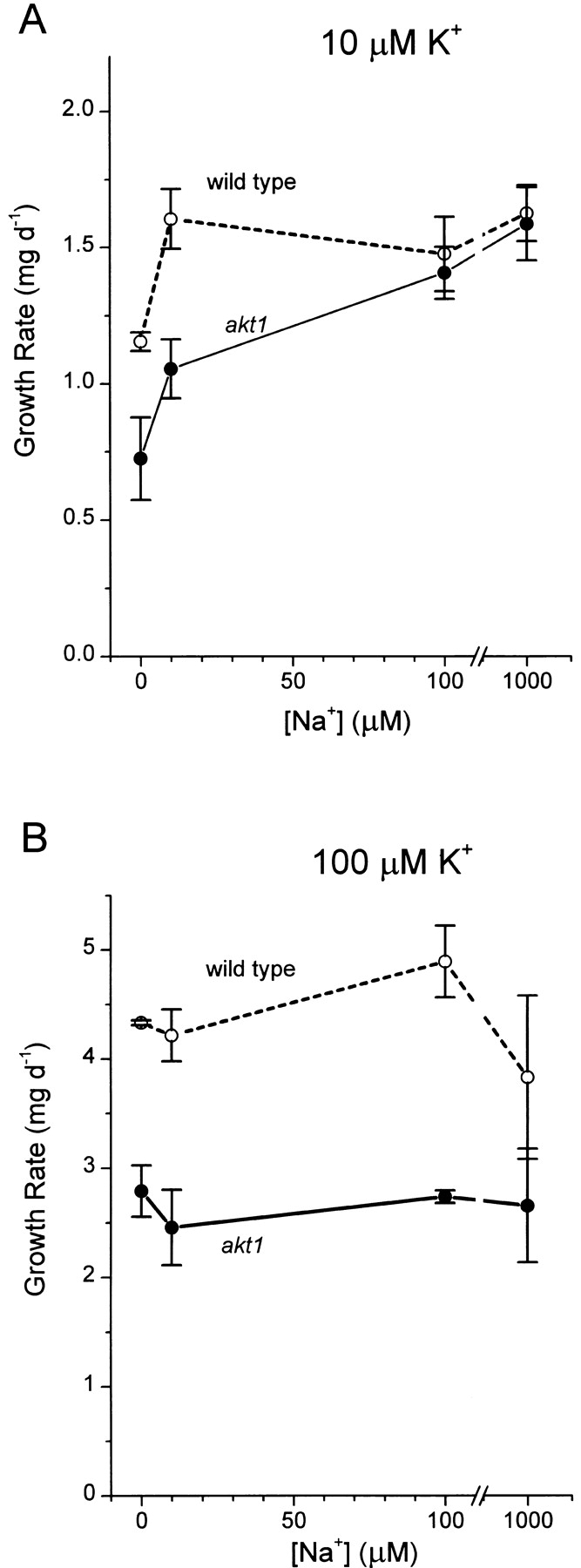
Stimulation of akt1 and wild-type growth rate by Na+. The rates of fresh-weight increase for 24 seedlings between days 4 and 8 were determined at each Na+ concentration in three to five independent trials and were plotted as means ± SEM.
results
Effects of NH4 + on K+ Permeability
The first goal was to compare the effect of NH4 + on the K+ permeability of the plasma membrane in wild-type and akt1 root cells. Fig. 1 A shows a typical recording of Vm made by impaling a root cell ∼150 μm from the apex of the cap with a microelectrode. After the voltage stabilized at −236 mV, the continuously flowing bathing solution containing 10 μM K+ was switched to one containing 100 μM K+, a treatment referred to as Δ[K+]10–100, and then subsequently to 1,000 μM K+ (Δ[K+]100–1000). The change in steady state Vm that occurred in response to these shifts (ΔVm) is related to the K+ permeability of the plasma membrane as discussed in materials and methods. The permeability detected by this method in akt1 mutant roots may be attributed to non-AKT1 activities because of the evidence that the mutant allele is functionally a null, despite the transferred-DNA being inserted in what might appear to be a dispensable cytoplasmic tail (Hirsch et al., 1998). The contribution of AKT1 channel activity to the K+ permeability of wild-type roots may be inferred by subtracting the ΔVm measured in akt1 roots from the wild-type ΔVm. Following this reasoning, Fig. 1 B shows that the wild-type K+ permeability, in the absence of NH4 +, was ∼63% due to AKT1 channel activity and 37% due to non-AKT1 activities when the shift was Δ[K+]10–100. When assayed at the higher concentration (Δ[K+]100–1,000 shift), the AKT1 component was a similar 55% of the now larger wild-type K+ permeability (Fig. 1 C). Such accounting of the membrane's K+ permeability permitted an examination of which components were affected by NH4 +. Approximately 50% of the wild-type ΔVm resulting from a Δ[K+]10–100 shift was inhibited by 2 mM NH4 +. The NH4 +-sensitive component of the wild-type response was very similar in magnitude to the minus-NH4 + akt1 response, which was completely blocked by 2 mM NH4 +. Thus, the ΔVm in wild-type roots, a parameter related to K+ permeability, behaves as the quantitative sum of a NH4 +-insensitive AKT1 component and a NH4 +-sensitive non-AKT1 component. This simple quantitative relationship did not persist when [K+]ext was increased from 100 to 1,000 μM (Fig. 1 C). Instead, it appeared that 2 mM NH4 + inhibited only 50% of the non-AKT1 component, as opposed to 100% at the lower [K+]ext (compare akt1 responses ± NH4 + in Fig. 1, B and C). The actual steady state value of Vm for each genotype in each condition is shown in Table 1.
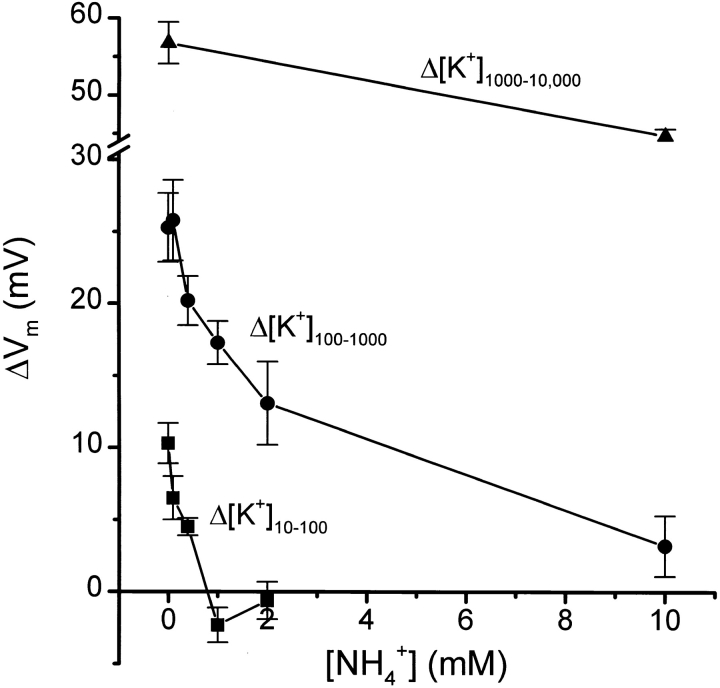
The finding that the degree of inhibition by 2 mM NH4 + depended on [K+]ext prompted a more detailed investigation of the K+ and NH4 + concentration interdependence of the phenomenon. Fig. 2 demonstrates that 2 mM NH4 + inhibited ∼50% of the ΔVm caused by Δ[K+]100–1,000 in akt1 roots, consistent with the data in Fig. 1 C. Only 0.5 mM NH4 + was needed to inhibit 50% of the smaller response to Δ[K+]10–100 and 2 mM was completely inhibitory, consistent with Fig. 1 B. The large ΔVm response to shifting [K+]ext from 1 to 10 mM was much less sensitive to this range of [NH4 +]ext (Fig. 2). Taken together, the results in Figs. 1 and 2 indicate that AKT1 channel activity accounts for 50–60% of the K+ permeability of the root plasma membrane, with the remainder resulting from one or more NH4 +-sensitive transporters. Furthermore, the data in Fig. 2 may be taken as evidence that the non-AKT1 transporter has a K+-binding site to which NH4 + may competitively bind when it is in large excess, preventing K+ transport. The 50% block of the response to Δ[K+]100–1,000 by 2 mM NH4 + may be taken as evidence that the K+-binding site of the non-AKT1 mechanism has a 50% probability of being occupied by NH4 + under those particular conditions. This occupancy by NH4 + increased to 100% when [K+]ext was 10-fold lower, and it decreased to near negligible levels when [K+]ext was in the millimolar range.
Figure 2.
Concentration dependence of the NH4 + inhibition of the K+ permeability of the akt1 root plasma membrane. Changes in steady state Vm produced by three different shifts in [K+]ext are plotted versus the NH4 + concentration present before and during the experiment. Note the axis break and change of scale for the 1,000–10,000 μM shift in [K+]ext. All values are the means of 6–10 independent experiments ± SEM.
Effects of NH4 + on K+ Uptake and Growth
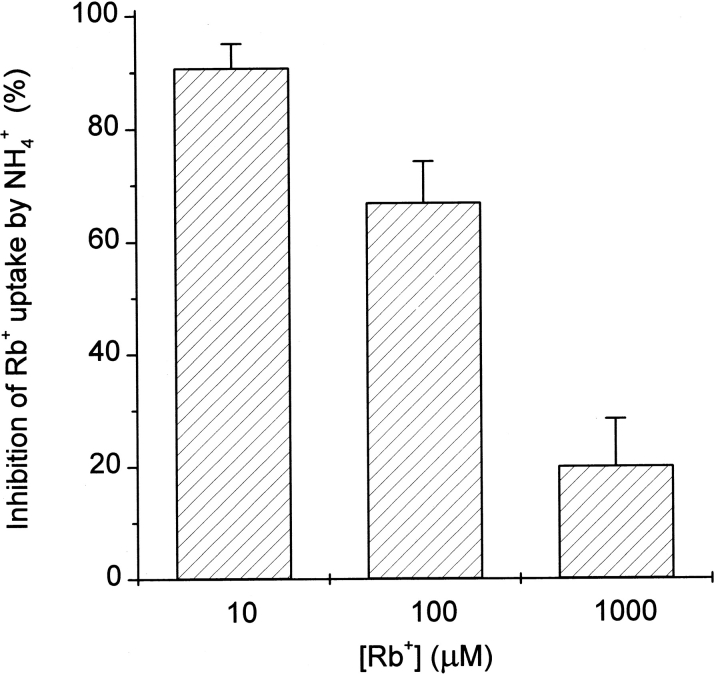
Experiments were performed to determine if the decrease in K+ permeability caused by NH4 + characterized in Fig. 2 actually resulted in decreased K+(86Rb+) uptake at the whole-root level. Fig. 3 demonstrates that ∼90% of Rb+ uptake into akt1 roots from 10 μM solutions was inhibited by treatment with 4 mM NH4 +. This inhibition by NH4 + was [Rb+]ext dependent, being only 20% at 1,000 μM Rb+. Thus, fluxes at the organ level (Fig. 3) and electrical changes at the membrane (Figs. 1 and 2) both indicate that NH4 + competitively inhibits one or more important K+-transport mechanisms detectable in the absence of AKT1 channel activity. The data presented thus far indicate that the wild-type root employs at least two K+-uptake mechanisms operating in parallel, each contributing significantly to the total flux even from 10 μM external solutions. One of these “high affinity” transporters is the passive AKT1 channel and the other is an NH4 +-sensitive transporter of unknown molecular identity.
Figure 3.
Inhibition by NH4 + of Rb+ fluxes into akt1 roots as a function of [Rb+]. Uptake of 86Rb+ by akt1 roots in the presence and absence of 4 mM NH4 + was used to determine the mean amount of inhibition, which is plotted ± SEM.
Most important to the field of plant mineral nutrition is whether both of these K+-transport activities mediate fluxes of sufficient magnitude to be relevant to growth. If so, akt1 plants should grow more slowly than wild-type when K+ is limiting, and their growth should be NH4 + sensitive in a manner similar to the membrane permeability and fluxes presented in Figs. 1–3. This idea was tested by measuring the growth of mutant and wild-type plants at various concentrations of K+ and NH4 + and at two stages of plant development— germination and seedling establishment. Germination is a consequence of, among other processes, the rapid expansion of cells already present in the mature embryo. Fig. 4 shows that at 10 μM K+, germination of akt1 seeds was strongly inhibited by increasing [NH4 +]ext compared with wild type. In the presence of 1 mM NH4 +, no akt1 seeds had germinated 72 h after stratification, compared with 80% of the wild type. Half of the maximal akt1 germination was inhibited by 0.76 mM NH4 +. The lower germination rate of akt1 seeds provided with 10 μM K+ in the absence of NH4 + (47 vs. 100% of wild-type seeds) is evidence that post-imbibition embryo growth depends upon AKT1-mediated K+ uptake when [K+]ext is low. Note that these germination percentages were determined at one point in time. Not shown is that nearly all seeds eventually germinated except those in the most inhibitory conditions (low K+ with high NH4 +). When 100 μM K+ was present and NH4 + absent, 100% of both akt1 and wild-type seeds germinated within 72 h. This indicates that AKT1 activity is not required in this situation and that non-AKT1 activities were sufficient to meet the demands imposed by embryo growth. Increasing the [NH4 +] of this higher K+ medium significantly inhibited akt1 germination while only modestly affecting the wild type. Germination of akt1 seeds was 50% inhibited by 3.8 mM NH4 +, and nearly complete inhibition of akt1 germination was achieved by 10 mM NH4 +. Increasing [K+]ext from 100 to 1,000 μM further protected germination rates from inhibition by NH4 +. Thus, as with the membrane permeability assays in Fig. 2 and the fluxes in Fig. 3, increasing [K+]ext lessened the inhibitory effect of NH4 + on this earliest stage of akt1 plant growth.
Growth rates of seedlings were also determined under the same conditions. Fig. 5 A demonstrates that in the absence of NH4 +, akt1 seedlings grew more slowly than wild type on 10 μM K+, as was the case with the embryo growth responsible for germination (Fig. 4 A). This is evidence that the K+ flux conducted by AKT1 channels contributed significantly to growth even when [K+]ext was 10 μM. Submillimolar NH4 + added to the 10 μM K+ medium inhibited the growth rate of akt1 seedlings, which was too low to measure reliably at concentrations >700 μM. The faster wild-type growth was not inhibited by NH4 + in this concentration range. Embryo growth, assayed as germination rate, behaved similarly with respect to inhibition by NH4 + (Fig. 4 A). When [K+]ext was increased to 100 μM (Fig. 5 B), wild-type and akt1 seedlings grew several times faster than at 10 μM K+, and similar to each other in the absence of NH4 + (as was also the case for embryos). Increasing [NH4 +]ext from 0 to 2 mM strongly inhibited the growth rate of akt1 seedlings without affecting the wild-type rate. This inhibition of akt1 growth rate by NH4 + displayed a concentration dependence very similar to the NH4 + inhibition of membrane K+-permeability assayed by Δ[K+]100–1,000 (Fig. 2). This result, along with those in Figs. 2 and 3, supports the idea that NH4 + inhibits growth of akt1 seedlings by inhibiting K+ permeability and fluxes mediated by one or more non-AKT1 transporters. Increasing [K+]ext to 1,000 μM markedly reduced the amount of inhibition caused by NH4 + (Fig. 5 C). Thus, protection against NH4 + inhibition by increasing K+ was observed for seedling growth as it was with K+ permeability, Rb+ fluxes, and embryo growth. This is consistent with the notion that the K+ transport activity supporting growth in the absence of AKT1 channel activity employs at least one substrate (K+) binding site for which NH4 + can compete under physiologically relevant conditions.
Transport Characteristics of the Non-AKT1 Activity
The lack of inward-rectifying channel activity in akt1 roots was exploited in experiments designed to reveal information about what energizes the parallel, NH4 +-sensitive, non-AKT1 activity. The approach was to measure Vm in cells of akt1 roots in the absence of NH4 + and administer shifts in [K+]ext. Specifically, the hypothesis to be tested was whether the non-AKT1 K+-transport activity behaved as a coupled transporter, such as a H+-K+ cotransporter (Rodriguez-Navarro et al., 1986; Newman et al., 1987; Maathuis and Sanders, 1994) or a Na+-K+ cotransporter (Schachtman and Schroeder, 1994; Rubio et al., 1995; Gassmann et al., 1996; Wang et al., 1998). Fig. 6 demonstrates that the presence of 2 mM Na+ more than doubled the ΔVm induced by Δ[K+]10–100 when the pH of the medium was buffered at 5.7. Decreasing the proton concentration to pH 7.7 significantly reduced the magnitude of the ΔVm (K+ permeability) of akt1 roots, but Na+ stimulation was still observed. Reducing the proton concentration further (pH 8.7) essentially eliminated the response to Δ[K+]10–100 in the absence of Na+, though a measurable ΔVm could be observed in the presence of Na+. At higher K+ concentrations (Δ[K+]100–1,000), a significant pH dependence of K+ permeability was not detected. The Na+ effect was relatively weaker than observed in the lower K+ conditions, and not significant at the P = 0.05 level. These results are consistent with the non-AKT1 K+ transport occurring by a symport mechanism that is energized by the electrochemical potential gradient of Na+ and H+. Perhaps separate Na+-K+ and H+-K+ symporters function in parallel to actively transport K+. If so, the substrate-binding sites of both must have an affinity for NH4 +. Alternatively, a single K+ symporter may be capable of using electrochemical potential gradients of either Na+ or H+ as an energy source. It is also possible that the non-AKT1 transporter has an obligate requirement for both Na+ and H+ to actively transport K+, as our nominally 0 Na+ conditions contain trace amounts (see materials and methods).
Stimulation of Growth by Na+
The results in Fig. 6 formed the basis of another test of the hypothesis that the K+ permeability detected electrophysiologically in the absence of AKT1 channels (Figs. 1 and 2) represents the uptake pathway upon which growth of akt1 plants depends. Na+ should stimulate growth of akt1 plants if the K+ required for growth is taken up by this Na+-stimulated, NH4 +-sensitive, non-AKT1 activity. Furthermore, the growth rate of wild-type plants should be less Na+ dependent, given that a significant portion (50–60%) of wild-type K+ permeability was attributed to AKT1 channels (Fig. 2). Fig. 7 A demonstrates that both of these predicted results were observed when seedlings were grown on 10 μM K+. The growth rate of akt1 plants increased by 119% as [Na+]ext was increased to 1,000 μM. Wild-type seedlings also benefited from increasing [Na+]ext, though not to the same relative extent. At stressful levels of Na+ (50– 100 mM), the growth rates of wild-type and akt1 plants were relatively equally inhibited (data not shown), indicating that the akt1 phenotype is distinct from that of the salt overly-sensitive mutants (Wu et al., 1996; Zhu et al., 1998). The growth rate of akt1 plants was not stimulated by Na+ when [K+]ext was 100 μM. This is consistent with the relatively weaker stimulatory effect of Na+ on K+ permeability when assayed at this higher [K+]ext (Fig. 6 B) and the evidence that, when >100 μM, [K+] is not limiting growth rate (0 NH4 + points in Fig. 5, B and C are similar).
discussion
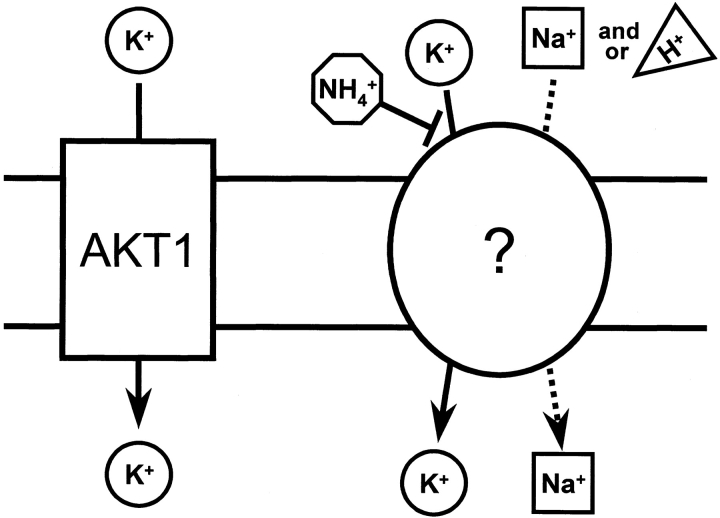
Our interpretation of the results presented here is that AKT1 channels mediate K+ uptake across the plasma membrane of root cells in parallel with one or more genetically distinct K+ transporters that are inhibited by NH4 + (Fig. 8). The concentration of NH4 + that forces growth to depend on AKT1-channel activity depends on the K+ status of the soil solution, and is in agreement with the roughly millimolar levels found to follow fertilizer application (Barraclough, 1989). It seems reasonable to suppose that other soil conditions encountered by plants may impair AKT1 function, shifting the bulk of K+-uptake activity to the non-AKT1 mechanism.
Figure 8.
Model of K+ uptake by parallel AKT1 and non-AKT1 mechanisms showing how NH4 +, Na+, and H+ may exert their effects. Transport by the AKT1 channel is passive and, therefore, the possibility of K+ uptake by this mechanism depends strictly on the K+ electrochemical potential gradient. NH4 + inhibits the non-AKT1 transporter(s), apparently by competing with the substrate K+ for binding. Na+ and H+ may stimulate the one or more non-AKT1 transporters by serving as an energy source, in which case they would be cotransported substrates. Na+ and H+ may instead, or also, act as modulators of transport kinetics to increase the K+ permeability of the plasma membrane, which would not require their cotransport with K+. When the AKT1 mechanism is made inoperative by mutation, plants must depend on the non-AKT1 activity for K+ uptake. This can explain why the growth of akt1 plants is inhibited by NH4 + and stimulated by Na+.
The conclusion that AKT1 and non-AKT1 mechanisms mediate K+ uptake in substantially overlapping concentration ranges seems inescapable, though different than the conclusions of Maathuis and Sanders (1997), which were based on studies performed before a null mutant was available to exploit. Both AKT1 and non-AKT1 mechanisms clearly contribute in the absence of NH4 + when [K+]ext is 10 or 100 μM (Fig. 1). This is somewhat surprising, given that the enhancement of K+ permeability by Na+ and H+ at low [K+]ext suggests that the non-AKT1 mechanism is an active symporter with K+ transport coupled to the electrochemical potential gradient of one or both of those ions. It would be surprising, though not a violation of any thermodynamic law, if a cotransport mechanism contributed significantly to fluxes that could be conducted by passive channels. It is possible that the non-AKT1 mechanism is also passive and the enhancement of ΔVm by Na+ and H+ (Fig. 6) is due to a faster transport cycle, higher open probability, or the recruitment of more transporters into action. Interestingly consistent with this notion is the demonstration that Na+ positively modulates the kinetics of AKT1 without permeating the channel (Bertl et al., 1997). Rigorous voltage-clamp studies and Na+ flux measurements are needed to distinguish whether Na+ affects the kinetics or thermodynamics of the non-AKT1 transport mechanism(s). Such a study may reveal that Na+ affects both because the two possibilities are not mutually exclusive.
Regardless of how the non-AKT1 transport activity is energized, its inhibition by NH4 + and stimulation by Na+ were mirrored in most conditions by the effects of these ions on the growth of akt1 and, to a much lesser extent, wild-type plants. These close positive and negative correlations constitute evidence that the K+ permeability detected electrically in akt1 roots is due to an activity that supports growth when the AKT1 mechanism is inoperative. The results also indicate that the relative contributions to plant growth of genetically distinct K+ transport systems depend on ionic variables of the sort and magnitude encountered in soils. This finding may be relevant to the agronomic practice of managing plant nutrients. There is every reason to believe that continuing the combined electrophysiological and reverse-genetic approach will lead to a more complete and useful molecular-level accounting of the K+-transport activities supporting growth.
The reverse-genetic approach to studying the non-AKT1 contributor requires knowing beforehand what gene or genes to eliminate. Therefore, it is now very important to consider what genes may be responsible for the non-AKT1 transport activity characterized physiologically by the present work. The recent impressive isolation and characterization of plant genes encoding proteins that perform K+ transport has produced two strong candidates. The stimulation by Na+ (Fig. 6) brings the HKT1 transporter originally found in wheat to the forefront as a candidate for the non-AKT1 activity. HKT1 is believed to function as a K+-Na+ symporter (Rubio et al., 1995; Gassmann et al., 1996). The earlier report of H+ gradients serving as an energy source for HKT1-mediated K+ transport (Schachtman and Schroeder, 1998) also can be accommodated by the pH dependence of the non-AKT1 activity (Fig. 6). Unfortunately, the present literature on HKT1 does not contain tests of NH4 + as an inhibitor. Ideally, an Arabidopsis mutant with a disruption in an HKT1 homologue will be isolated and provide for a combined genetic and physiological test of the idea that AKT1 and HKT1 together conduct the K+ fluxes needed for growth.
The increase in K+ permeability due to the presence of Na+ was greater when assayed by Δ[K+]10–100 shifts than Δ[K+]100-1,000 shifts (Fig. 6, A vs. B). The same trend was observed in akt1 seedling growth rate: Na+ more than doubled the growth rate at 10 μM K+, but was without effect when [K+]ext was 100 μM (Fig. 7, A vs. B). Perhaps the non-AKT1 mechanism is more Na+ coupled when the electrochemical potential gradient for K+ is great, but less so when the energetics permit a passive mode of operation. Previous work has attributed a passive conductance to HKT1 that is separate from its Na+-K+ symport activity (Gassmann et al., 1996), indicating that cotransporters can display such complexity of mechanism. Also, the growth rate of seedlings was limited by something other than K+ at concentrations above 100 μM (Fig. 5), so Na+ may have stimulated K+ uptake from 100-μM solutions, but limitations in some other factor prevented growth rate from responding.
Another possible contributor to the non-AKT1 transport activity is one or more of the KUP family of K+ transporters recently identified in Arabidopsis and barley. These transporters can complement K+-uptake deficiencies in mutants of Escherichia coli and yeast and can confer enhanced K+ uptake into cultured Arabidopsis cells when overexpressed (Quintero and Blatt, 1997; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998). A member of this family from barley is inhibited by NH4 +, similar to the non-AKT1 activity studied here in planta (Santa-Maria et al., 1997). Arabidopsis KUP-mediated K+ transport is also inhibited by NH4 + (E. Kim and J.I. Schroeder, personal communication), though it is not stimulated by Na+ (Fu and Luan, 1998). Thus, the Na+ data (Figs. 6 and 7) currently favor HKT1, while the NH4 + data (Figs. 1–5) favor KUP as the molecule(s) responsible for the non-AKT1 component of the root K+-uptake apparatus. It is also possible that the non-AKT1 activity is due to a combination of KUP and HKT1 activities insofar as both are inhibited by NH4 +.
The last point to make is that the competition between NH4 + and K+ for a binding site on the non-AKT1 transporter (Figs. 2–5) explains the previously observed inhibition of K+ transport by NH4 + in corn roots (Vale et al., 1987). The fact that plants have a specific NH4 + transporter that is not blocked by K+ (Ninneman et al., 1994) explains why the converse (block of NH4 + uptake by K+) is typically not observed. Thus, the result that surprised Marschner (1995) receives a molecular-level explanation as a result of the present work.
Table I.
Steady State Membrane Potentials
| [K+]ext | Wild type | akt1 | Wild type + NH4 + | akt1 + NH4 + | ||||
|---|---|---|---|---|---|---|---|---|
| μM | mV | mV | mV | mV | ||||
| 10 | −219 ± 4 | −218 ± 9 | −209 ± 5 | −226 ± 8 | ||||
| 100 | −192 ± 5 | −211 ± 10 | −194 ± 5 | −227 ± 8 | ||||
| 1000 | −136 ± 5 | −188 ± 9 | −151 ± 5 | −214 ± 8 |
Steady state values of membrane potential (Vm) measured in apical root cells sequentially bathed in flowing medium containing 10, 100, and 1,000 μM K+ with or without 2 mM NH4 +. Values are averages ± SEM of 6–10 separate plants.
Footnotes
Rebecca E. Hirsch's present address is Department of Zoology, University of Wisconsin, Madison, WI 53706. Bryan D. Lewis' present address is Department of Biology, Clarke College, Dubuque, IA 52001.
references
- Barraclough PB. Root growth, macronutrient uptake dynamics and soil fertility requirements of a high-yielding winter oilseed rape crop. Plant Soil. 1989;119:59–70. [Google Scholar]
- Basset M, Conejero G, Lepetit M, Fourcroy P, Sentenac H. Organization and expression of the gene coding for the potassium transport system AKT1 of Arabidopsis thaliana. . Plant Mol Biol. 1995;29:947–958. doi: 10.1007/BF00014968. [DOI] [PubMed] [Google Scholar]
- Bertl A, Reid JD, Sentenac H, Slayman CL. Functional comparison of plant inward-rectifier channels expressed in yeast. J Exp Bot. 1997;48:405–413. doi: 10.1093/jxb/48.Special_Issue.405. [DOI] [PubMed] [Google Scholar]
- de Boer AH. Potassium translocation into the root xylem. Plant Biol. 1999;1:36–46. [Google Scholar]
- Epstein E, Rains DW, Elzam OE. Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA. 1963;49:684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H-H, Luan S. AtKUP1: a dual-affinity K+ transporter from Arabidopsis. . Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996;10:869–882. doi: 10.1046/j.1365-313x.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Kim EI, Myong J, Kwak, Uozumi N, Schroeder JI. AtKUP1: an Arabidopsisgene encoding high-affinity potassium transport activity. Plant Cell. 1998;10:51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Gaymard F, Astruc S, Grignon C. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+nutrition. Plant J. 1996;9:195–203. doi: 10.1046/j.1365-313x.1996.09020195.x. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Energization of potassium uptake in Arabidopsis thaliana. . Planta (Heidelb) 1993;191:302–307. [Google Scholar]
- Maathuis FJM, Sanders D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. . Proc Natl Acad Sci USA. 1994;91:9272–9276. doi: 10.1073/pnas.91.20.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Regulation of K+ absorption in plant root cells by external K+: interplay of different plasma membrane K+transporters. J Exp Bot. 1997;48:451–458. doi: 10.1093/jxb/48.Special_Issue.451. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Verlin D, Smith FA, Sanders D, Fernández JA, Walker NA. The physiological relevance of Na+-coupled K+-transport. Plant Physiol. 1996;112:1609–1616. doi: 10.1104/pp.112.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner, H. 1995. Mineral Nutrition of Higher Plants. 2nd edition. Academic Press, Inc., New York. 889 pp.
- Newman IA, Kochian LV, Grusak MA, Lucas WJ. Fluxes of H+ and K+in corn roots: characterization and stoichiometries using ion-selective microelectrodes. Plant Physiol. 1987;84:1177–1184. doi: 10.1104/pp.84.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninneman O, Jauniaux J-C, Frommer WB. Identification of a high affinity NH4 +transporter from plants. EMBO (Eur Mol Biol Organ) J. 1994;13:3464–3471. doi: 10.1002/j.1460-2075.1994.tb06652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, W. 1900. The Physiology of Plants. 2nd edition. Vol. 1. Oxford University Press, Oxford, UK. 632 pp.
- Quintero FJ, Blatt MR. A new family of K+ transporters from Arabidopsisthat are conserved across phyla. FEBS Lett. 1997;415:206–211. doi: 10.1016/s0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A, Blatt MR, Slayman CL. A potassium-proton symport in Neurospora crassa. . J Gen Physiol. 1986;87:649–674. doi: 10.1085/jgp.87.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Technical comment. Science. 1996;273:978–979. doi: 10.1126/science.273.5277.978. [DOI] [PubMed] [Google Scholar]
- Rufty TW, Jr, Jackson WA, Raper CD. Inhibition of nitrate assimilation in roots in the presence of ammonium: the moderating influence of potassium. J Exp Bot. 1982;33:1122–1137. [Google Scholar]
- Santa-Maria GE, Rubio F, Dubcovsky J, Rodriguez-Navarro A. The HAK1gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Smart CJ, Garvin DF, Prince JP, Lucas WJ, Kochian LV. The molecular basis of potassium nutrition in plants. Plant Soil. 1996;187:81–89. [Google Scholar]
- Smith RC, Epstein E. Ion absorption by shoot tissue: kinetics of potassium and rubidium absorption by corn leaf tissue. Plant Physiol. 1964;39:992–996. doi: 10.1104/pp.39.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale FR, Jackson WA, Volk RJ. Potassium influx into maize root systems: influence of root potassium concentration and ambient ammonium. Plant Physiol. 1987;84:1416–1420. doi: 10.1104/pp.84.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beusichem ML, Kirkby EA, Baas R. Influence of nitrate and ammonium nutrition on the uptake, assimilation, and distribution of nutrients in Ricinus communis. . Plant Physiol. 1988;86:914–921. doi: 10.1104/pp.86.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ. Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA. 1996a;93:10510–10514. doi: 10.1073/pnas.93.19.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NA, Sanders D, Maathuis FJM. Technical comment. Science. 1996b;273:977–978. doi: 10.1126/science.273.5277.977. [DOI] [PubMed] [Google Scholar]
- Wang T-B, Gassmann W, Rubio F, Schroeder JI, Glass ADM. Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol. 1998;118:651–659. doi: 10.1104/pp.118.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-J, Ding L, Zhu J-K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell. 1996;8:617–627. doi: 10.1105/tpc.8.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K, Liu J, Xiong L. Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition. Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]