Discovery of Single-File Pores
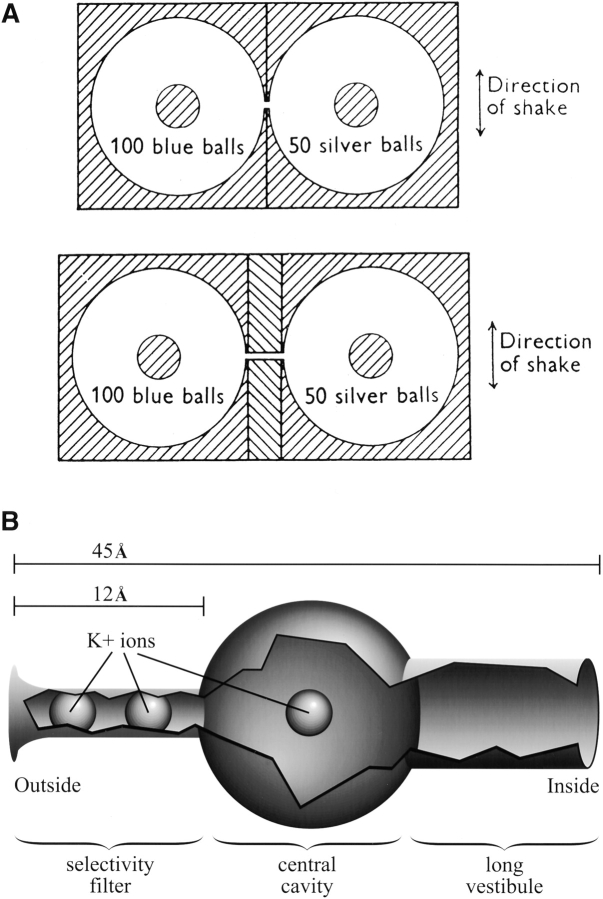
In 1955, Alan Hodgkin and Richard Keynes published a study whose intent was to answer a rather technical question, but whose result triggered the modern analysis of ion channel permeation (Hodgkin and Keynes, 1955). They aimed to determine whether the K+ fluxes in giant axons followed the “Ussing flux ratio criterion,” which was then considered to indicate whether flux through a membrane occurred passively through pores. The Ussing ratio states that passive unidirectional flux should be proportional to the activity of the compound on the side from which the flux occurs. Therefore, the ratio of inward:outward unidirectional K+ fluxes in the axon was expected to equal the ratio of external:internal K+ electrochemical activities. The K+ fluxes in axons clearly failed the Ussing test, as Hodgkin and Keynes noted that the ratio of fluxes varied with the activity ratio raised to the 2.5th power. However, they argued that this indicated a different kind of pore: a long one that could hold multiple ions traversing the pore in single file. To corroborate their mathematical arguments, they mimicked their observations with the mechanical model shown in Fig. 1 A.
Figure 1.
Multi-ion, single-file pores. (A) The mechanical model used by Hodgkin and Keynes (1955) to mimic their K+ flux data from squid axons. When a short pore separated the two chambers (top) and the device was shaken for a period, a little more than twice as many balls exited the 100-ball compartment than the 50 ball; thus, the flux ratio nearly equaled the concentration ratio, as predicted by the Ussing test. However, the flux ratio was 18× the concentration ratio when a long pore separated the chambers (bottom). This is because there were roughly three balls in the long pore at any time, so it took a series of four collisions for a ball to pass from one side to the other. When one side has twofold higher concentration than the other, it is 24-fold more likely to have four successive collisions. (B) The free space in the pore of the potassium channel crystallized and solved to 3.2 Å resolution by Doyle et al. (1998). The important features of this pore include: (a) the pore interacts with fully dehydrated ions over only 1/4 of the full length, (b) two ions sit within this “selectivity filter,” and (c) another ion sits outside the selectivity filter in a long vestibule that allows partial hydration and effectively diminishes the length of the pore that restricts flux the most.
Now, another dramatic paper (Doyle et al., 1998) has directly demonstrated multiple ions arranged in single file within a crystallized potassium channel pore (Fig. 1 B). A 12-Å length of this pore is so narrow that it provides a tight fit to a K+ that is stripped of water molecules. This must be the channel's selectivity filter. Two ions were found within the filter 7.5-Å apart. Thus, the multi-ion, single-file nature of the business region of the potassium channel pore is confirmed absolutely. The filter is electronegative, and the sources of the negativity are backbone carbonyl oxygens rather than amino acid side chains. An array of nearby α-helices appear poised to rigidly hold the selectivity filter structure so that it perfectly fits a K+ ion (∼3 Å), but cannot collapse to fit a Na+ ion (∼2 Å). This supports the classic “perfect fit” hypothesis for potassium channel selectivity (Mullins, 1959; Hille, 1973), which suggested that a 3–4-Å pore, deduced from permeation experiments, could fully solvate K+ ions, but fail to do so for the smaller Na+ ions. In contrast to potassium channels, permeation measurements of sodium channels (Hille, 1971) and calcium channels (McCleskey and Almers, 1985) suggest that their selectivity filters are far wider than their preferred ion. Sodium and calcium channels excel at distinguishing Na+ from Ca2+, two ions of identical diameter. These facts suggest that a rigid, perfect fit mechanism should not explain selectivity in sodium and calcium channels.
Four decades apart, Hodgkin and Keynes (1955) and Doyle et al. (1998) form fundamental pillars to the field of ion channel permeation. They unequivocally demonstrate multi-ion, single-file permeation. Theoretical work between these two landmarks has explored the functional consequences of this mechanism.
Modeling Single-File Pores
The crux of permeation and selectivity is to understand how channels can be highly selective and still pass millions of ions per second. Quantitative models of flux through multi-ion, single-file pores suggest possible solutions to this paradox.
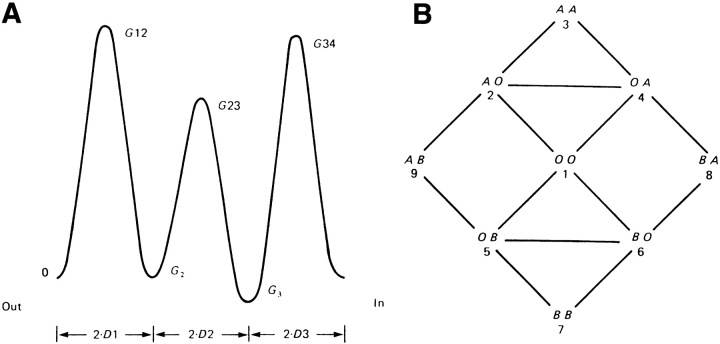
Fig. 2 illustrates the paradigm for calculating selective flux through a single-file pore using elementary chemical kinetics. Fig. 2 A represents the hills and valleys of potential energy experienced by one kind of ion as it traverses the pore. Fig. 2 B indicates the various ways that the two energy minima of the model might be occupied (or unoccupied, 0) by two different ions, A and B. The lines show the allowed transitions between occupancy states. For example, A0 (state 2) has ion A in the left site and nothing in the right; the transition to 0A (state 4) involves the ion moving from left to right. Two rules determine which transitions can occur: (a) an ion cannot enter a site that is already occupied by another, and (b) an ion cannot pass over a site without binding to it. Transitions between sites are considered to be instantaneous.
Figure 2.
Single-file permeation models (figure from Begenisich and Cahalan, 1980). (A) The free energy experienced by a particular ion as it passes through the pore when there is no membrane voltage. The Gs indicate the energy minima and maxima relative to the ion's energy in solution. Transit between energy minima follows rules of elementary chemical kinetics; for example, the rate constant for an ion passing from the innermost energy minima to the internal solution is: r = ν{exp[−(G 34 − G 3)/kT]}. The Ds indicate the fraction of the membrane voltage experienced in making a transition (physical distances are not defined in such models). If there is a membrane voltage, Vm, the energy to exit the innermost minima becomes (G 34 − G 3 + zD3Vm), where z is the ion's charge, and D3Vm is the voltage difference between the minima and the barrier. (B) The allowed states of occupancy by two different ions and the allowed transitions between states. For example, the state AB means that ion A occupies the outer site and ion B the inner site.
These rules—hopping without bumping or jumping— define single-file premeation models. The rules are analogous to the physics of ion movement in crystals and semiconductors, in which electrons hop between “holes” or vacancies in the crystal lattice. Ion flux in semiconductors has been modeled best with drift diffusion equations, which are the underpinning of the “PNP” description of ion channel permeation (see Perspective by Nonner et al.). Thus, single-file models and PNP models have the same basic process in mind, although the first uses discrete and the second continuous mathematics to describe the process. If the point is to debate discrete versus continuous descriptions of permeation, these Perspectives may lack perspective. Diffusion in homogeneous media is correctly described by both approaches (Einstein, 1908), and this must also be true for ion channel permeation. Debate about this fundamental fact arises only because present models of permeation, both discrete and continuous, are but crude approximations of real ion channels.
The rule that transitions are instantaneous is analogous to “collision theory,” the most simple-minded description of chemical reactions. Accordingly, flux of an ion over an energy barrier diminishes exponentially as the barrier height increases and is described by the rate constant: r = ν(exp[−G/kT]), where [G/kT] is the height of the energy barrier (G) relative to the atom's thermal energy (kT). The frequency factor, ν, is the maximum possible value of a rate constant, the value when there is no energy barrier. In single-file models, the frequency generally is set to kT/h (∼6 × 1012 s−1 at biological temperatures; k and h are the Boltzmann and Planck constants, respectively, and T is absolute temperature). The rationale for this and the difficulties with it are discussed below (Limitations of Single-File Models).
The touchstone of single-file modeling studies is Hille and Schwarz (1978). The paper successfully mimicked a variety of unintuitive potassium channel permeation properties, including the failure of unidirectional fluxes to obey the Ussing ratio, a minimum in conductance when the relative fractions of two permeant ions are varied (the “anomalous mole fraction effect”), and rectification due to blocking ions. Hille and Schwarz (1978) found that repulsion between intrapore ions had profound effects on the calculated flux and concluded that two properties of the model were necessary to reproduce physiological phenomena. First, external energy barriers had to be high compared with internal ones. This allows ions to equilibrate readily between intrapore sites—to rattle about the pore more easily than they can exit it. Second, the pore had to have at least three sites and contain at least two ions most of the time.
Now, Doyle et al. (1998) find two ions in the potassium channel selectivity filter sitting at opposite extremes of the filter's length. This image is consistent with repulsion pushing the two ions to the extremes of a pore from which exit is more difficult than is intrapore mobility. Thus, the image is consistent with the physical principles suggested by Hille and Schwarz (1978), as well as their number of ions. This is the value of single-file calculations. Although the models must be grossly simplistic representations of permeation through ion channels, they still provide a means to detect and test the basic principles involved. Other important single-file papers have described permeation and selectivity in gramicidin channels (Andersen and Procopio, 1980), sodium channels (Begenisich, 1987), and calcium channels (below).
Calcium Channel Models
Calcium channels have been useful for studying permeation for two reasons: (a) they are extraordinarily selective, and (b) they are the clearest case of a channel that selects its preferred ion through intrapore binding. The most elementary question about selectivity is whether a channel acts like a molecular sieve to reject certain ions, or whether it preferentially binds its chosen ion. Calcium channels select Ca2+ over Na+ at a ratio of 1,000:1, even though the two naked ions have identical diameters; no sieve could do this. Instead, the pore binds Ca2+ with an apparent K d of ∼1 μM. When the high affinity site is unoccupied by Ca2+, Na+ is freely permeant; when Ca2+ occupies the site, as it must at physiological Ca2+ concentrations, Na+ current through the channel is blocked. Thus, Ca2+ confers selectivity on calcium channels because it blocks permeation of competing ions. Similarly, Na+ current through potassium channels is blocked by K+ (Korn and Ikeda, 1995).
Discovery of high affinity binding in the calcium channel raised a fundamental issue. Since K d = k off/k on, and since the maximum k on is the diffusion limit of 109/M−1s−1 (Almers and McCleskey, 1984), the maximum off rate from a 1-μM binding site should be only 1,000/s. However, picoampere currents (millions of ions per second) pass through calcium channels. A goal of permeation models has been to suggest mechanisms for solving this 1,000-fold discrepancy between expected and observed flux.
This “sticky pore problem” is diagrammed in Fig. 3 A. Binding affinity is determined by the difference in energy between the outside environment and the well (arrow 1); flux is limited by the height of the greatest barrier to exit from the pore (arrow 2). In such a single site pore, tighter binding (a longer arrow 1) necessarily causes slower flux (a longer arrow 2); this makes selectivity by selective binding impossible. Models of multi-ion, single-file calcium channels have deduced two separate solutions to this problem, although the two mechanisms might well work together in a real pore. The repulsion model (Fig. 3 B) imagined two high affinity sites within the pore and allowed ions to repel each other when they occupied the pore simultaneously (Almers and McCleskey, 1984; Hess and Tsien, 1984). Foreign ions are blocked at micromolar Ca2+ concentrations when a single Ca2+ ion occupies the pore (left). When two Ca2+ ions occupy the pore, they repel each other, and this effectively diminishes the pore's affinity for Ca2+ (apparent K d changes from ∼1 μM to 10 mM, right). Once the pore is in this low affinity state, Ca2+ flux occurs readily. Recent mutation studies (see next section) cleanly disprove one aspect of this model because they demonstrate only a single high affinity site in the calcium channel. Nevertheless, the more fundamental point—that flux occurs because multiple occupancy lowers the affinity of the pore for Ca2+—remains reasonable.
Figure 3.
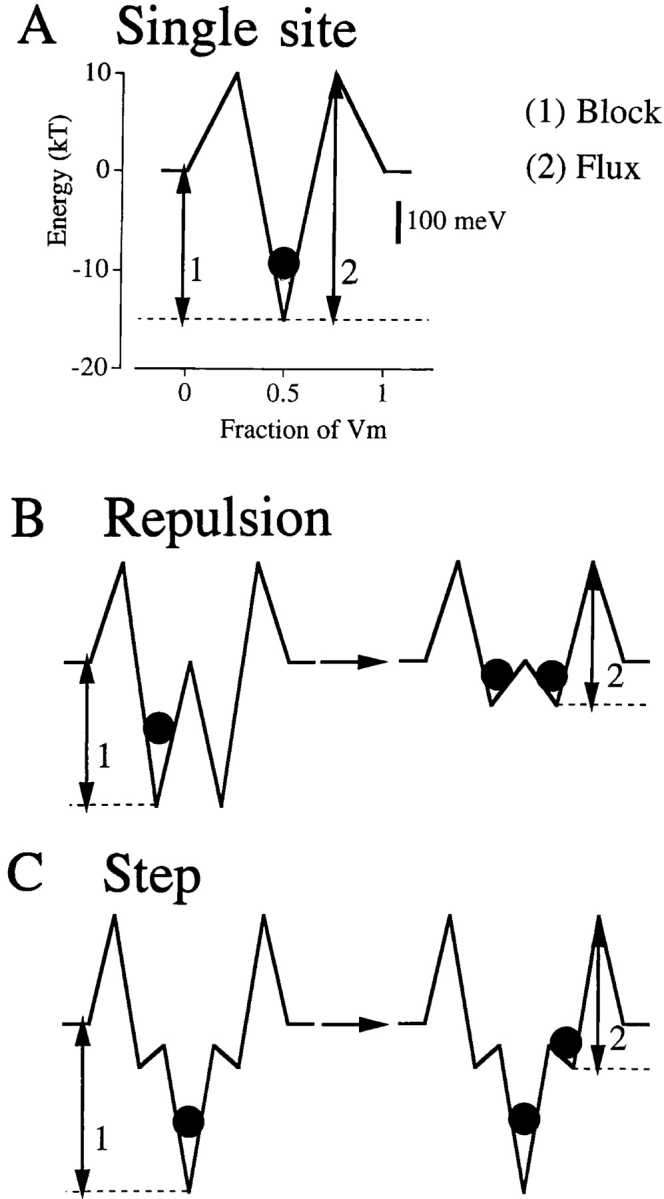
Ca2+ energies in three pore models (from Dang and McCleskey, 1998). The energies that determine Ca2+ block of the pore (1) and limit Ca2+ influx (2) are interlinked in the single site pore (A). Increasing one energy increases the other, so stronger binding must lead to lower flux. In contrast, the blocking and flux energies can be independently manipulated in the repulsion (B) and step (C) models. In the repulsion model, affinity for Ca2+ diminishes when multiple Ca2+ ions occupy the pore. The change in affinity might occur due to electrostatic repulsion or competition for intrapore binding ligands (see Fig. 4 B). High flux occurs from this low affinity condition. In the step model, multiple occupancy does not alter affinity of the pore. High flux occurs because low affinity states are part of the pore. The states might be caused by low affinity binding sites or by more subtle effects such as stepwise rehydration of the ion. Because arrows 1 and 2 are about the same length in the repulsion and step models, blocking concentration and flux amplitude are similar in each model.
The step model (Fig. 3 C) has just a single high affinity site, but this is flanked by low affinity sites on either side (Dang and McCleskey, 1998). Although the pore can be occupied by multiple ions, the pore's affinity for Ca2+ does not change with multiple occupancy; nevertheless, the model fits Ca2+ channel data almost identically to the repulsion model. High Ca2+ flux occurs because the flanking sites provide steps of potential energy. Stepwise conquest of potential energy barriers is a general mechanism for speeding chemical reactions; it is closely analogous to the way stairsteps increase the fraction of people who can surpass gravitational potential energy barriers. In essence, a Ca2+ ion takes the stairs to exit the deep binding well in the step model.
The PNP model of calcium channels (Nonner and Eisenberg, 1998) provides another major, and unexpected, insight. The model's pore is an electronegative tube that is 10-Å long and 6-Å wide. Despite the absence of an explicit high affinity binding site, the model exhibits high affinity Ca2+ block of Na+ current as well as high Ca2+ flux. This discovery is very timely: it shows that a rigid, diffusely negative tube (much like the crystal structure in Doyle et al., 1998) is sufficient to generate selectivity through high affinity binding. The pore binds Ca2+ without ligands that wrap around the ion, as is generally assumed necessary for high affinity binding; surely, this has great significance for ion channel permeation.
In an important sense, the differences between the single-file and PNP models have been overstated. The PNP/calcium channel demonstrates an unexpected mechanism for intrapore, high affinity binding. Single-file models do not, and cannot, address the mechanism or structures of binding (see Limitations of Single-File Modeling). Rather, they focus on the forces that allow rapid exit from the high affinity site. Two forces were described and here the models agree: both repulsion and potential energy steps contribute to rapid efflux from the PNP/calcium channel pore according to the authors. It is the mechanism of flux that seems so different in the two models, but this may largely be a matter of mathematical appearances. As discussed above, both types of models envision ion movement to be similar to that through crystals, but one describes this with discrete steps and the other with continuous functions. If the models are ever complete (and neither pretends to be), the two approaches must give identical results just as diffusion is described equally well through random walk or continuum equations (Einstein, 1908). For the time being, it is encouraging that such different approaches agree in the description of forces that underlay high flux out of high affinity pores.
Like every other model, the PNP/calcium channel comes with ad hoc assumptions. A critical one necessary to allow Ca2+ to block Na+ current is that the pore has an excess chemical potential that is negative for Ca2+ and positive for Na+; in other words, some undefined chemistry of the model pore is attractive for Ca2+ but repulsive for Na+. What chemistry could attract one positive ion while repelling another of the same size is unclear. Another issue, discussed below, is an explicit disagreement with observations made in mutation studies. Such failings confirm only that the PNP model is an incomplete approximation of the calcium channel. But, by definition, all models are incomplete. In my view, such specific inconsistencies should not detract from the more general discovery inherent in the PNP/ calcium channel: that selectivity by high affinity binding can occur in a rigid pore.
Calcium Channel Facts
The most fundamental facts known about the structural basis of Ca2+ selectivity had their birth in a sodium channel paper. Heinemann et al. (1992) noted four conspicuous glutamate residues in the structure of calcium channels. Though each is separated by ∼500 amino acids of sequence, these glutamates are in the putative pore-lining region of the four internally conserved repeats. In the analogous sodium channel sequence, two of the negative charges are present, but a neutral and a positive residue substitute at the other two sites. Mutation of these two residues to glutamates made the sodium channel behave like a calcium channel in that Ca2+ could block Na+ current at low concentrations. The four glutamate residues in calcium channels are called the EEEE locus.
Yang et al. (1993) mutated the EEEE locus in L-type calcium channels, and thereby demonstrated the role of these glutamates in high affinity Ca2+ binding (Fig. 4 A). Neutralizing any of the glutamates to a glutamine shifted the block of monovalent current to higher Ca2+ concentrations. The extent of the shift differed for glutamates in the different repeats, and neutralizing multiple residues shifted the blocking concentration by as much as 1,000-fold (Ellinor et al., 1995). Because a single point mutation can shift the dissociation constant for Ca2+, there must not be two independent high affinity sites in the pore. Moreover, pairwise mutations of the glutamates rigorously refute the two sites postulated by the repulsion model (Ellinor et al., 1995).
Figure 4.
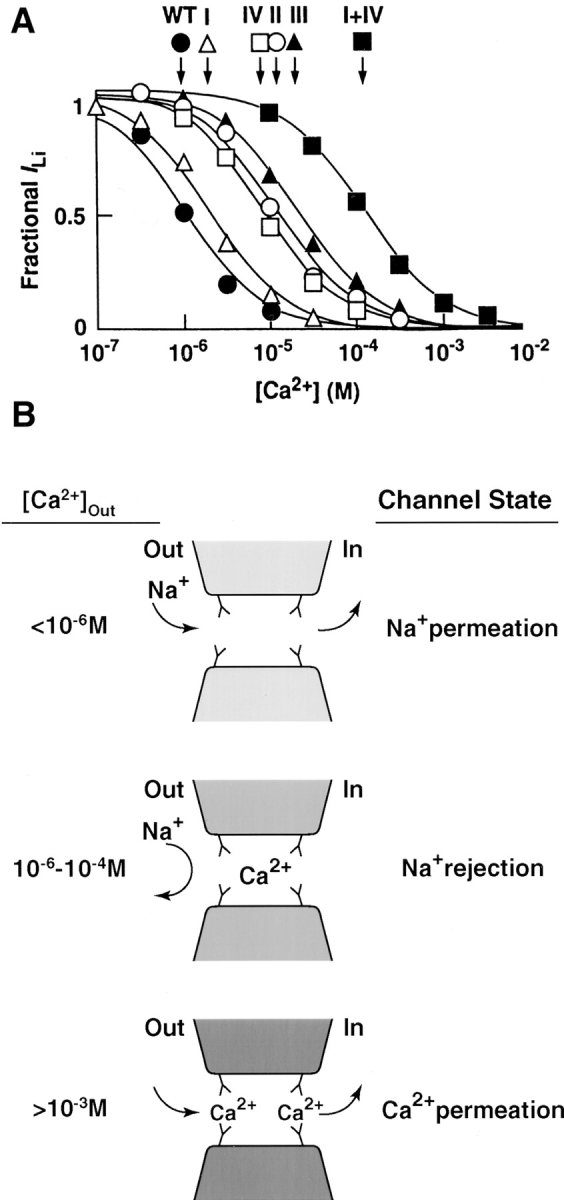
Evidence for an intrapore Ca2+ chelating site. (A) Data from Yang et al. (1993) showing block of monovalent current through calcium channels by various concentrations of Ca2+. Half block of the wild-type channel occurs at ∼1 μM Ca2+ (•). Single mutations of glutamate to glutamine in repeats I, II, III, or IV each shift block to higher Ca2+ concentrations (symbols indicated); the mutation in repeat III caused a 100-fold shift. A double mutation of glutamates in repeats I and IV shifted block further (▪, see also Ellinor et al., 1995). (B) An interpretation of the mutation data. The four glutamates each donate a carboxyl to a site that binds Ca2+ analogously to Ca2+ chelators. Below 1 μM Ca2+, the site is unoccupied and Na+ passes freely. Between 1 and 100 μM, Ca2+ blocks Na+ current but only rarely exits the high affinity site. Above millimolar concentrations, two Ca2+ ions likely occupy the pore. When the two compete for the carboxyl residues, affinity for Ca2+ decreases and Ca2+ flux ensues.
Yang et al. (1993) suggested that the four glutamate side chains form a binding site for Ca2+ in much the same way that the four carboxyls in EGTA wrap around Ca2+ (Fig. 4 B). When Ca2+ is absent from the site at low, submicromolar concentrations, Na+ can permeate. As its concentration rises above a micromolar, Ca2+ stably occupies the site and Na+ flux is blocked. When Ca2+ rises to millimolar concentrations, two Ca2+ ions likely occupy the pore simultaneously. The two compete with each other for the glutamate residues, thereby lowering the affinity of the pore for Ca2+ and allowing Ca2+ permeation. In an important sense, the idea is like the repulsion model because a decrease in binding affinity during multiple occupancy is what allows high Ca2+ flux. However, competition for binding ligands drives the affinity change rather than the electrostatic repulsion originally suggested by the model.
These mutation studies potently argue against the PNP model for the calcium channel. The PNP model pore contains four carboxyl groups that are meant to mimic the EEEE locus. However, there is no difference in the concentration at which Ca2+ blocks Na+ current in the PNP/calcium channel when the number of intrapore carboxyl residues is set to 1, 2, 3, or 4 (see Figure 6 A in Nonner and Eisenberg, 1998). Thus, the PNP model starkly fails to reproduce the data in Fig. 4 A. Whatever may be the actual structure of the EEEE locus, the four carboxyls in the PNP model do not mimic it.
Limitations of Single-File Modeling
The present goal of permeation studies is to understand how specific pore structures control flux and selectivity. In general, single-file models are unsuitable for this because they do not rely on known structure. More specifically, three problems diminish the value of the single-file approach to structure–function studies. First, the models do not define physical distances. The “electrical distance” along which the ion travels through these models is the fraction of the electric field experienced at each step; the relation between this and real distance is unaddressed and unaddressable in the single-file paradigm. Second, the relation between energy minima and protein structures is ambiguous. A minimum of energy is a place where the ion likely pauses longer than at adjacent spots, but this need not correspond to a specific binding site built into the protein. For example, an ion is relatively stable in the central cavity in the potassium channel of Doyle et al. (1998); this is likely not due to interaction of the ion with the protein, but to a more stable degree of hydration than in the narrower adjacent regions. The third problem is that the models are rigid, but proteins are not. In cases where dynamic protein structures have been described, substrates generally induce a fit of themselves into their binding sites, rather than fitting like a key in a rigid lock. Accumulating evidence argues the same for channels. For example, Immke et al. (1999) show that a K+ ion must be bound to the potassium channel selectivity filter for tetraethylammonium to effectively block the pore. Tetraethylammonium is about the size of a K+ ion with a single shell of attached water and its binding site is in the vestibule. Thus it appears that the presence of a naked K+ ion in the filter confers on the vestibule the shape appropriate for binding an incoming hydrated K+ ion. This agrees with earlier arguments that flexibility of the pore entrance is essential for rapid and efficient dehydration of entering ions (Eigen and Winkler, 1971; Andersen and Koeppe, 1992). Although clearly critical, such flexibility cannot be addressed with single-file models (or the PNP system). Ultimately, dynamic structure-based calculations (e.g., Karplus and Petsko, 1990) are necessary for describing ion permeation.
The Appendix in Nonner and Eisenberg (1998) takes a long step further in criticizing the single-file approach. It uses the framework of the PNP model to recalculate flux through the step model and finds that: (a) Ca2+ flux through this step-PNP model is many hundred-fold less than experimental observations, and (b) the pore fails to select Ca2+ over Na+. The results are not shown for other models, but it is stated that similar PNP recalculations of the repulsion models are “even less consistent” with experimental observations, and that “the reasons for failure are generic for rate theory models in the tradition of Hille and Schwarz (1978).” The problem, it says, is that single-file models use a frequency factor for calculating rate constants that is 10,000-fold too high. If so, all single-file models are incorrect by many orders of magnitude, implying that their insights are based on false physics and should be abandoned.
The primary problem with the Appendix in Nonner and Eisenberg (1998) is that it fails to recognize that frequency factors and barrier heights are not treated as separable entities in single-file models. For example, our models set the external barriers (the values that limit entry and exit of the pore and are therefore the most critical) so that they agree with experimental observation. Lansman et al. (1986) demonstrated that Ca2+ enters the pore at a rate of ∼109 M−1s−1, the value expected from diffusion-limited access. Assuming a prefactor of 6 × 1012 s−1, the external barriers are set no lower than 8.7 kT, thereby creating an entry rate of 109 M−1s−1 (Almers and McCleskey, 1984). If we had used a lower prefactor, we would have set the external barrier energies lower, always keeping the rate constant (the product of the prefactor and the exponential) consistent with experimental observations. If one diminishes the prefactor by 10,000 while keeping the barriers the same, the entry rate will be 10,000-fold less than experimental observation. In this trivial way, calculated flux would be unphysiologically low. This is the essence of the error in the Appendix in Nonner and Eisenberg (1998): it uses precise energies from one calculation scheme while applying assumptions from another.
Although the appendix's calculation is artificial (Nonner and Eisenberg, 1998), the point about frequency factors is important. To understand the flaw in setting the frequency factor, ν, equal to kT/h, one should understand its basis. kT/h is an atom's theoretical vibration frequency, obtained by assuming that all thermal energy (kT) becomes kinetic, or vibrational, energy (hν). The assumption confers a simple physical interpretation on the equation for a rate constant: the atom can attempt to surpass the reaction barrier each time that it vibrates, but the probability of a successful attempt is very low. The attempt rate is kT/h, about six times per picosecond, and the probability of success is exp[−G/kT]. The problem with this is nicely explained by Andersen and Koeppe (1992), and by Andersen's introduction to these Perspectives. In short, the frequency of vibration is only legitimate to use if the distance to surpass the barrier is similar to the length of a vibration, and this is just a small fraction of an angstrom. To traverse a selectivity filter that is many angstroms long, many such individual steps are necessary.
Assuming that movement within the filter is diffusive, these individual steps would have low energy barriers and transit would be fast compared with exit from the pore. Therefore, most single-file models simply lump these many intrapore steps into one or two individual steps that are not rate limiting. The models thereby focus on the high barriers that limit exit from the pore; these external barriers, because their size is determined by experimental measurements (see above), are handled appropriately by the models. However, there is no denying that lumping the internal steps together represents the pore in a physically inaccurate manner.
A perspective on the insights from single-file models arises from considering the ultimate aims of the permeation field. A major one is to construct an accurate map of the chemical potential energy of an ion as it traverses a pore of precisely known structure. This is analogous to converting photographs of a hillside into a topographic map, which describes your gravitational potential energy as you hike the hill. Single-file models have skipped directly to the topographic map without having the photographs. Unlinked to real structures, these energy diagrams are nothing more than quantitative cartoons, but the quantitation has been valuable. The models do not pretend to tell us about protein structure, but, by finding which forces allow calculated fluxes to mimic real experiments, they suggest general principles relevant to permeation. These principles include: the importance of having multiple ions moving in a coordinated fashion through a pore, that ions likely equilibrate within the pore far more easily than they exit it, that channels can select their ions through binding sites and still have high flux, and that exit from a tightly binding pore is promoted through either ion– ion interactions in a multiply occupied pore or by a series of lower affinity sites.
What Are the Real Questions?
First, there is a question that is not real: it is irrelevant to ask which of the earlier models is “correct.” Each provides insight, but each is incorrect. These models are crude approximations that cannot explicitly show how specific protein structures control permeation. However, their insights might help to guide the questions posed in future, structure-based modeling. Answers to the following three questions should provide a fundamental understanding of calcium channel permeation and selectivity.
(1) How can Ca2+ have diffusion-limited access to the pore? Three decades ago, Eigen and Winkler (1971) noted that ions enter carriers at the diffusion limit; Lansman et al. (1986) demonstrated the same for calcium channels. The high rate is unexpected: ions must completely dehydrate to enter pores and dehydration requires so much energy that it should occur only rarely. In addition to replacing an ion's hydrating waters with solvating intrapore ligands, channels and carriers must also rapidly catalyze the dehydration process. How is this done? Eigen and Winkler (1971) argue that rapid dehydration requires systematic, stepwise replacement of water molecules by substituting ligands, rather than a loss of all waters at once. Andersen and Koeppe (1992) note that this requires a pore entrance that is not rigid. What structures at the mouth of the pore are responsible for stepwise dehydration, and over how long a distance does this process occur?
(2) Is the strong binding of the selectivity filter of the calcium channel due to a flexible binding site analogous to Ca2+ chelators? The calcium channel mutation studies of Yang et al. (1993) and Ellinor et al. (1995) are most easily explained this way. However, the potassium channel selectivity filter described by Doyle et al. (1998) is a rigid pore and the PNP model for calcium channels (Nonner and Eisenberg, 1998) shows that high affinity binding can occur in such a structure. Is the selectivity filter of calcium channels rigid or flexible? Is it lined by backbone carbonyl oxygens or by amino acid side chains?
(3) What forces allow ions to exit this high affinity binding area so much faster than expected? Two have been suggested in various models: ion–ion interactions and stepwise increases in energy during exit. What might cause ion–ion interactions in the real pore— competition for a binding site and/or electrostatic repulsion? What might cause stairsteps of energy in the real pore—specific binding structures of successively lower affinity and/or stepwise rehydration? What is the relative importance of these two forces, and are there others that have not previously been considered?
references
- Almers W, McCleskey EW. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OS, Koeppe RE. Molecular determinants of channel function. Physiol Rev. 1992;72:S89–S158. doi: 10.1152/physrev.1992.72.suppl_4.S89. [DOI] [PubMed] [Google Scholar]
- Andersen OS, Procopio J. Ion movement through gramicidin A channels. Acta Physiol Scand (Suppl) 1980;481:27–35. [PubMed] [Google Scholar]
- Begenisich TB, Cahalan MD. Sodium channel permeation in squid axons. I. Reversal potential experiments. J Physiol. 1980;3007:217–242. doi: 10.1113/jphysiol.1980.sp013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich TB. Molecular properties of ion permeation through sodium channels. Annu Rev Biophys Biophys Chem. 1987;16:247–263. doi: 10.1146/annurev.bb.16.060187.001335. [DOI] [PubMed] [Google Scholar]
- Dang TX, McCleskey EW. Ion channel selectivity through stepwise changes in binding affinity. J Gen Physiol. 1998;111:185–193. doi: 10.1085/jgp.111.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+conduction and selectivity. Science. 1998;280:69–76. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Eigen M, Winkler R. Alkali ion carriers: specificity, architecture, and mechanisms. Neurosci Res Prog Bull. 1971;9:330–338. [PubMed] [Google Scholar]
- Einstein A. The elementary theory of Brownian motion. Z Electrochem. 1908;14:235–239. [Google Scholar]
- Ellinor PT, Yang J, Sather WA, Zhang J-F, Tsien RW. Ca2+ channel selectivity at a single locus for high-affinity Ca2+interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Heinemann S, Terlau H, Stühmer W, Imoto K, Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971;58:599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve: selective permeability to small cations. J Gen Physiol. 1973;61:669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978;72:409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Keynes RD. The potassium permeability of a giant nerve fibre. J Physiol. 1955;128:61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke D, Wood M, Kiss L, Korn SJ. Potassium- dependent changes in the conformation of the Kv2.1 potassium channel pore. J Gen Physiol. 1999;113:819–836. doi: 10.1085/jgp.113.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus M, Petsko GA. Molecular dynamics simulations in biology. Nature. 1990;347:631–639. doi: 10.1038/347631a0. [DOI] [PubMed] [Google Scholar]
- Korn SJ, Ikeda SR. Permeation selectivity by competition in a delayed rectifier potassium channel. Science. 1995;269:410–412. doi: 10.1126/science.7618108. [DOI] [PubMed] [Google Scholar]
- Lansman JB, Hess P, Tsien RW. Blockade of current through single Ca channels by Cd2+, Mg2+, and Ca2+: voltage and concentration dependence of Ca entry into the pore. J Gen Physiol. 1986;88:321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey EW, Almers W. The Ca2+channel in skeletal muscle is a large pore. Proc Natl Acad Sci USA. 1985;82:4328–4331. doi: 10.1073/pnas.82.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins LJ. The penetration of some cations into muscle. J Gen Physiol. 1959;42:817–829. doi: 10.1085/jgp.42.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W, Eisenberg B. Ion permeation and glutamate residues linked by Poisson-Nernst-Planck theory in L-type calcium channels. Biophys J. 1998;75:1287–1305. doi: 10.1016/S0006-3495(98)74048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ellinor PT, Sather WA, Zhang J-F, Tsien RW. Molecular determinants of Ca2+ channel selectivity and ion permeation in L-type Ca2+channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]