Abstract
Rod cyclic nucleotide–gated (CNG) channels are modulated by changes in tyrosine phosphorylation catalyzed by protein tyrosine kinases (PTKs) and phosphatases (PTPs). We used genistein, a PTK inhibitor, to probe the interaction between the channel and PTKs. Previously, we found that in addition to inhibiting tyrosine phosphorylation of the rod CNG channel α-subunit (RETα), genistein triggers a noncatalytic inhibitory interaction between the PTK and the channel. These studies suggest that PTKs affects RETα channels in two ways: (1) by catalyzing phosphorylation of the channel protein, and (2) by allosterically regulating channel activation. Here, we study the mechanism of noncatalytic inhibition. We find that noncatalytic inhibition follows the same activity dependence pattern as catalytic modulation (phosphorylation): the efficacy and apparent affinity of genistein inhibition are much higher for closed than for fully activated channels. Association rates with the genistein–PTK complex were similar for closed and fully activated channels and independent of genistein concentration. Dissociation rates were 100 times slower for closed channels, which is consistent with a much higher affinity for genistein–PTK. Genistein–PTK affects channel gating, but not single channel conductance or the number of active channels. By analyzing single channel gating during genistein–PTK dissociation, we determined the maximal open probability for normal and genistein–PTK-bound channels. genistein–PTK decreases open probability by increasing the free energy required for opening, making opening dramatically less favorable. Ni2+, which potentiates RETα channel gating, partially relieves genistein inhibition, possibly by disrupting the association between the genistein–PTK and the channel. Studies on chimeric channels containing portions of RETα, which exhibits genistein inhibition, and the rat olfactory CNG channel α-subunit, which does not, reveals that a domain containing S6 and flanking regions is the crucial for genistein inhibition and may constitute the genistein–PTK binding site. Thus, genistein–PTK stabilizes the closed state of the channel by interacting with portions of the channel that participate in gating.
Keywords: cyclic GMP, protein kinase, ion channel gating, kinetics, rod photoreceptor
INTRODUCTION
Cyclic nucleotide–gated (CNG) channels are crucial for generating electrical signals during phototransduction in vertebrate photoreceptors. Light triggers a decrease in the cytoplasmic concentration of cGMP, causing CNG channels in the plasma membrane to close. CNG channels are not invariant reporters of the changes in cGMP concentration, but rather the sensitivity of CNG channels to cGMP can be modulated by intracellular and extracellular signals. Intracellular Ca2+ causes a decrease in cGMP sensitivity of rod CNG channels, mediated by direct binding of calmodulin and other Ca2+-binding proteins to the CNG channel (Hsu and Molday 1993; Chen et al. 1994; Gordon et al. 1995a). Rod CNG channels are modulated by transition metals (Zn2+ and Ni2+), which increase cGMP sensitivity (Ildefonse and Bennett 1991; Gordon and Zagotta 1995a; Karpen et al. 1993) and diacylglycerol and other phospholipid derivatives, which inhibit the channels (Gordon et al. 1995b; Womack et al. 2000). CNG channels can be modulated by changes in the phosphorylation state catalyzed by Ser/Thr kinases (Muller et al. 1998) and phosphatases (Gordon et al. 1992). We have recently shown that the sensitivity of rod CNG channels can be modulated by tyrosine dephosphorylation by protein tyrosine phosphatases (PTPs) and phosphorylation by protein tyrosine kinases (PTKs; Molokanova et al. 1997). Moreover, the target of tyrosine phosphorylation is the channel protein itself, with the crucial phosphorylation site located on the cyclic nucleotide binding site of the α-subunits of the rod CNG channel (Molokanova et al. 1999b). Modulation by tyrosine phosphorylation is dependent on the activation state of the channel. Thus, dephosphorylation, which favors channel opening, requires open channels, whereas phosphorylation, which promotes channel closing, requires closed channels.
Remarkably, it appears that PTKs can affect rod CNG channels not only by catalyzing phosphorylation, but also through allosteric regulation by a direct protein–protein interaction. This conclusion came to light while using genistein, a PTK inhibitor. Genistein competes with ATP binding to PTKs, but does not compete with protein substrates that bind to PTKs at a distinct site (Akiyama et al. 1987). We found that genistein not only prevents PTK from phosphorylating the channel in the presence of ATP, but also inhibits cGMP-activated current through CNG channel in the absence of ATP (Molokanova et al. 1999a). Moreover, structural and functional studies suggest that CNG channels do not have ATP binding sites to accommodate genistein. Therefore, it seemed possible that genistein does not bind directly, but rather acts indirectly, by binding to an accessory protein that subsequently binds to the CNG channel. The effect of genistein is inhibited by other PTK inhibitors that, by themselves, have no effect on the rod CNG channels. Thus, AMP-PNP, which competes with genistein for inhibition of PTKs, is a competitive inhibitor of genistein's action on CNG channels, whereas erbstatin, a noncompetitive inhibitor of PTKs, noncompetitively prevents genistein's effect on the channels. Taken together, these findings strongly suggest that genistein inhibition involves a noncatalytic, allosteric effect of the PTK on CNG channels.
Activation of CNG channels is a result of conformational changes in protein structure in response to ligand binding to the cytoplasmic cyclic nucleotide–binding domains. How does PTK affect channel activation? After exposure to genistein, the PTK might specifically reduce agonist binding affinity by altering the geometry of the cyclic nucleotide binding site. Alternately, the PTK might impose conformational constraints on the channel protein, hindering channel gating. The goal of this study is to distinguish between these possibilities and clarify the mechanism of genistein–PTK inhibition of rod CNG channels.
MATERIALS AND METHODS
Expression and Recording of CNG Currents
CNG channels were expressed in Xenopus laevis oocytes. Oocytes were injected with 50 nl containing either 1 ng/μl RNA (for single-channel experiments) or 50 ng/μl RNA (macroscopic currents) encoding the α-subunit of the bovine retinal rod CNG channel (RETα; Kaupp et al. 1989), α-subunit of the rat olfactory CNG channel (OLFα; Dhallan et al. 1990), and several chimeric channels (Gordon and Zagotta 1995a,Gordon and Zagotta 1995b). After 2–7 d, the vitelline membrane was removed, and the oocytes were placed in a chamber for patch-clamp recording with glass patch pipets (3–4 MΩ). Inside-out membrane patches usually containing 100–200 channels were studied in symmetrical control solution containing (in mM): 115 NaCl, 5 EGTA, 1 EDTA, and 5 HEPES, pH 7.5 with NaOH. cGMP and/or genistein were added to the intracellular control solution. EDTA and EGTA were excluded from Ni2+-containing solutions. After formation of a gigaohm seal, inside-out patches were excised and the patch pipet was quickly (<30 s) placed in the outlet of a 1-mm-diam tube for cGMP application. We used a perfusion manifold containing up to eight different solutions that is capable of solution changes within 50 ms. cGMP was obtained from Sigma-Aldrich, and genistein was obtained from LC Laboratories.
Data Acquisition and Analysis
Current responses through CNG channels were obtained with a patch-clamp (model Axopatch 200A; Axon Instruments), digitized, stored, and later analyzed on a Pentium PC using pClamp 6.0 software. Membrane potential was held at −75 mV. Current responses were normalized to the maximal CNG current (Imax), elicited by saturating (2 mM) cGMP. Normalized dose–response curves were fit to the Hill equation: I/Imax = 1/(1 + (K1/2/A)n), where A is the cGMP concentration and n is the Hill coefficient, using a nonlinear least squares fitting routine (Origin; Microcal Software, Inc.). To estimate the Ki for genistein, we used a modified Hill equation: Ib/Imax = (1− (Ib(max)/Imax))/(1 + (Ki/B)n) + Ib(max)/Imax, where B is the concentration of blocker, and Ib and Ib(max) are the currents activated by saturating cGMP in the presence of a given blocker concentration and a saturating blocker concentration, respectively. Variability is expressed as mean ± SEM.
Single Channels
Single CNG channels in membrane patches from mRNA-injected oocytes incubated at 18°C first appeared 12–18 h after mRNA injection. After this low level of expression was reached, the incubation temperature was reduced to 4°C to stop further expression. Single CNG channel currents were recorded from excised inside-out membrane patches using borosilicate glass pipets coated with Sylgard (Sigma-Aldrich) and fire-polished to resistance of 5–10 MΩ. The experiments were conducted at room temperature (20–22°C).
Membrane potential was held at −80 mV. Single channel events were sampled at 25 kHz and low-pass filtered at 5 kHz through an eight-pole Bessel filter. The opening and closing event was idealized by measuring the amplitude and dwell time, using the half-amplitude threshold detection technique (PClamp6; Axon Instruments). All-points amplitude histograms were constructed from at least 40 s of continuous data recordings and fit by the sum of two Gaussian functions, representing the closed and open states, and used to determine the amplitude of single-channel currents.
RESULTS
Genistein Inhibition of Closed and Open CNG Channels
CNG channels are normally activated within several milliseconds of application by direct binding of cyclic nucleotides. In excised patches, the limiting factor in activation is diffusion of the ligand to the binding sites. In the presence of genistein, channel activation is slowed dramatically and the steady-state cGMP-activated current is reduced.
To examine the interaction between closed channels and genistein–PTK, various concentration of genistein were preapplied on patches for 1 min followed by application of saturating cGMP (Fig. 1 A). At subsaturating genistein concentrations, a fraction of the total current, which we call “residual current,” activates over the normal rapid time course. The magnitude of the residual current is inversely proportional to genistein concentration, presumably reflecting the fraction of channels devoid of genistein (Molokanova et al. 1999a).
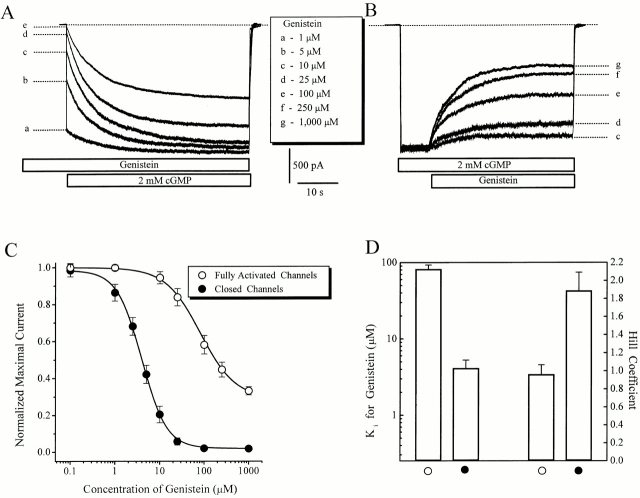
Figure 1.
Genistein is more potent in inhibiting closed than fully activated RETα channels. (A) Inhibition of closed channels by preexposure to various concentrations of genistein for 1 min. Closed channels were activated by application of saturating cGMP. Residual currents at five different genistein concentrations are indicated by dotted lines, and the genistein concentrations are indicated by letter to the right of each trace. In parts A and B the letter refers to the genistein concentration key. (B) Inhibition of steady-state CNG current, fully activated by saturating cGMP. The five different genistein concentrations are indicated by letter to the right of each trace. (C) Dose–inhibition curves of the effect of genistein on closed (residual current as in part A; n = 36) and fully activated channels (steady-state current, as in part B; n = 28). Continuous curves show fits of the data to the Hill equation. (D) Apparent affinity (Ki) and Hill coefficients derived from the Hill equation fits to dose–inhibition curves for closed and fully activated CNG channels ([open circles] closed channels; [filled circles] fully activated channels, as in Fig. 1 C).
Analyzing the interaction between genistein–PTK and open channels is more difficult because, even with saturating cGMP present, the rod CNG channels flicker between open and closed states. As an alternative to studying open channels, we studied fully activated channels, by applying various concentrations of genistein in the presence of saturating cGMP and recording steady-state currents (Fig. 1 B). For both closed and fully activated channels, genistein inhibition was proportional to genistein concentration, and the steady-state level of inhibition was the same regardless of the order of genistein and cGMP application.
Amplitudes of the residual currents (effect on closed channels) and steady-state currents (effect on fully activated channels) were used to generate dose–inhibition curves for genistein (Fig. 1 C). Comparison of closed versus fully activated channels reveals dramatic differences. First, the efficacy of genistein inhibition was higher for closed channels, with inhibition being nearly complete (98 ± 3%, n = 34) versus incomplete for fully activated channels (65 ± 6%, n = 28). Second, genistein had a much higher apparent affinity for closed channels, with Ki values of 4.3 ± 0.9 μM (n = 22) for closed channels and 84.1 ± 6.8 μM (n = 16) for fully activated channels. Thus, genistein inhibition of cGMP-activated currents exhibited activity-dependent pattern demonstrated previously for tyrosine phosphorylation of CNG channels (Molokanova et al. 1999b): closed channels are much more susceptible to both catalytic and noncatalytic actions of PTK than are fully activated channels.
We also observed a difference in the Hill coefficient of genistein inhibition of closed and fully activated channels. The Hill coefficient of genistein inhibition was 1.97 ± 0.08 (n = 22) for closed channels and 1.02 ± 0.03 (n = 16) for fully activated channels (Fig. 1 D). This observation suggests that closed channels require two genistein–PTK complexes for inhibition, whereas one is sufficient for inhibiting fully activated channels. However, even in the presence of saturating cGMP, a fraction of the channels will be in their closed state because the maximal open probability is <1.0. The resulting mixed population of open and closed channels with different apparent affinities for genistein could result in an underestimate of the actual numbers of genistein molecules required to inhibit open channels (Ruiz et al. 1999)
Kinetics of Genistein–PTK Inhibition
The observed difference in the apparent affinity of genistein–PTK for open versus closed channels must reflect state-dependent differences in association and/or dissociation between the complex and the channel. To characterize the kinetics of genistein–PTK association with closed channels, we recorded a series of residual cGMP-activated currents at various times after genistein pretreatment (Fig. 2 A). The genistein association time course was reconstructed by plotting residual current amplitude as a function of pretreatment duration. The association time course for closed channels could be fit with a single exponential function with time constants (τa) of 9.3 s. The time course of genistein inhibition of fully activated channels, determined by applying genistein after steady-state activation by saturating cGMP, was fit with a single exponential with τa = 13.1 s (Fig. 2 B).
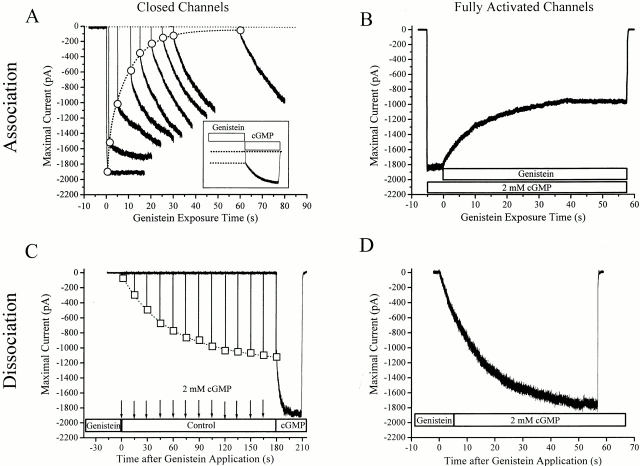
Figure 2.
Association/dissociation kinetics of genistein–PTK from closed (A and C) and fully activated (B and D) channels. (A) Association of genistein–PTK with closed channels. Currents activated by saturating cGMP obtained after preexposure to 100 μM genistein for various time intervals (1, 2, 5, 10, 15, 20, 25, 30, and 60 s). Open circles represent the amplitude of residual currents that remain uninhibited by genistein. Dashed curve shows single exponential fit of the data. Inset shows the application protocol. Note that genistein application time was variable. (B) Association of genistein–PTK with fully activated channels. (C) Dissociation of genistein–PTK from closed channels. After 1 min preexposure to genistein, saturating cGMP was puffed onto the patch every 15 s for 1 s (multiple arrows). Open squares indicate residual currents resulting from activation of genistein-free channels (D) Dissociation of genistein–PTK from fully activated channels. After 1 min preexposure to genistein, saturating cGMP alone was applied to record the recovery of activated channels from genistein inhibition.
The time required for genistein inhibition presumably reflects a multistep process, including the time necessary for genistein to find its target, PTK (which is dependent on genistein concentration) and the time required for PTK to affect CNG channels (which may be independent of genistein concentration). We found that the association time constant, for both closed and fully activated channels, is independent of genistein concentration. For closed channels, the τa values for 10, 25, and 100 μM genistein were 9.1 ± 0.4 (n = 3), 8.9 ± 0.6 (n = 3), and 8.6 ± 0.7 s (n = 6), respectively. For fully activated channels, the τa values for 25, 100, and 250 μM genistein were 12.1 ± 0.6 (n = 5), 12.6 ± 0.7 (n = 12), and 12.6 ± 0.9 s (n = 3), respectively. Therefore, our observation suggests that this second step, interaction of genistein–PTK with the channel, is the rate-limiting step of genistein inhibition.
The association rates of genistein are similar for closed and fully activated channels. To account for the dramatically different affinity of genistein–PTK for closed versus open channels, we expect that dissociation rates should exhibit more substantial differences. To test how channel opening affects the genistein–PTK dissociation rate, we used the following procedure. Genistein was preapplied for 1 min in the absence of cGMP, ensuring that all closed channels were genistein–PTK-bound. For analysis of dissociation from closed channels (Fig. 2 C), genistein was washed away and the level of remaining genistein inhibition was estimated by very briefly (1 s) applying saturating cGMP at 15-s intervals during 35–45 min of recording. For analysis of dissociation from fully activated channels, genistein was immediately replaced with saturating cGMP (Fig. 2 D). This procedure revealed a dramatic state-dependent difference in the apparent dissociation rate. For the fully activated channels, changes in the cGMP-activated current could be fit with a single exponential function with a time constant of 14.4 s. However, for closed channels, two exponentials were required with time constants of 66 and 808 s. Furthermore, the dissociation rates for closed channels were probably underestimated, because the estimated dissociation of genistein was speeded up by short cGMP application to make the changes in the inhibition level visible. Despite the fact that these channels were not continuously maintained in a closed state, genistein–PTK dissociation was still profoundly slower than that for fully activated channels. To ensure that CNG channels were still fully functional in these experiments, long applications of saturating cGMP opened the channels and accelerated genistein–PTK dissociation, resulting in full recovery of the cGMP-activated current with time constant of 2.4 s (Fig. 2 C).
Channels in the Open State Can Be Inhibited by Genistein
Genistein is much more potent at inhibiting closed than fully activated rod CNG channels. Are open channels affected by genistein, or is genistein inhibition in the presence of cGMP exclusively due to inhibition during brief closures that occur even with saturating cGMP? With saturating cGMP, rod CNG channels have a maximal open probability of ∼0.9, indicating that 10% of the channels are closed at any given moment. Since this fraction is subject to strong and very slowly reversible genistein inhibition, the number of inhibited closed channels would accumulate over time until association and dissociation of genistein–PTK from the channels reached equilibrium. Therefore, the apparent inhibition of open channels might be entirely a result of brief closures even in the presence of saturating cGMP.
To address whether channels in their open state can be inhibited by genistein–PTK, we simulated genistein inhibition (Fig. 3 A, dotted lines) using the dissociation and association rates determined in Fig. 2. Simulations were run assuming that only closed channels are subject to genistein inhibition, or that genistein inhibits open and closed channels equally. A comparison of the actual current recording in Fig. 3 A with the predicted current (assuming exclusive inhibition of closed channels) shows that genistein inhibition is faster and more complete than predicted by this model, suggesting that inhibition of open channels also contributes. However, the actual speed and extent of inhibition are smaller than the values predicted for equal effects on open and closed channels. Hence, although open channels appear to be susceptible to inhibition by genistein–PTK, the effects appear to be smaller than for closed channels.
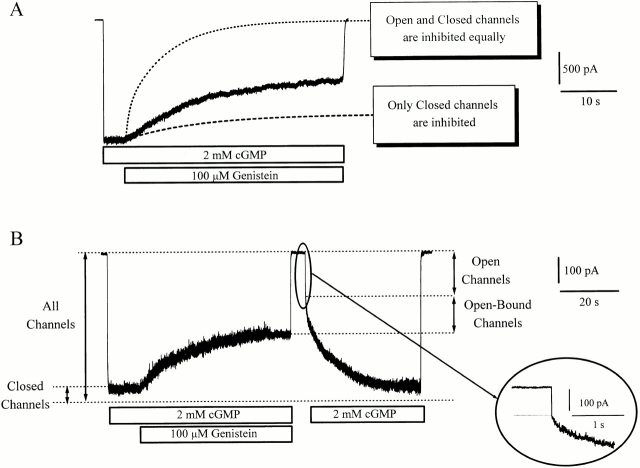
Figure 3.
Open channels participate in genistein inhibition. (A) Fully activated current inhibited by 100 μM genistein superimposed with simulations of models in which (1) only closed channels are inhibited, and (2) closed and open channels are inhibited equally. Note that inhibition of closed channels alone cannot account for observed current. (B) Dissection of open and open-bound states. Channels were first fully activated with saturating cGMP. Since maximal open probability is 0.9, ∼90% of the channels were open and 10% were closed. Genistein was added, cGMP was briefly removed, and then cGMP was reapplied without genistein. Channels that opened rapidly upon reapplication of cGMP (Open channels) were genistein–PTK-free (inset), whereas channels that opened sluggishly were genistein–PTK bound. Since the steady-state current in the presence of genistein was larger than the rapidly activating current upon cGMP reapplication, the difference between these currents represents open channels that were genistein–PTK-bound (Open-Bound).
To reveal the current flowing through open-bound channels, fully activated channels were first inhibited with genistein, and then briefly closed by removing cGMP and genistein for 4 s (Fig. 3 B). Since the dissociation rate of genistein–PTK from closed channels is very slow (>1 min; Fig. 2 C), very few of the closed channels should recover from genistein inhibition during this period. When the channels were reopened by subsequent application of saturating cGMP, there appeared a rapidly activating residual current, presumably reflecting channels without genistein–PTK bound (open channels). Application of various concentrations of cGMP showed that the residual current exhibits the same dose–response relationship for activation as do normal channels in the absence of genistein, further supporting the conclusion that the rapidly activating fraction of current is generated by channels devoid of genistein–PTK. Close inspection reveals that the residual current is smaller than the preceding steady-state current before genistein and cGMP had been washed away, indicating that a population of open-bound channels must have contributed to the steady-state current. In fact, at least 40% of the steady-state current flows through open channels that are genistein–PTK-bound, whereas <60% comes from channels devoid of genistein–PTK.
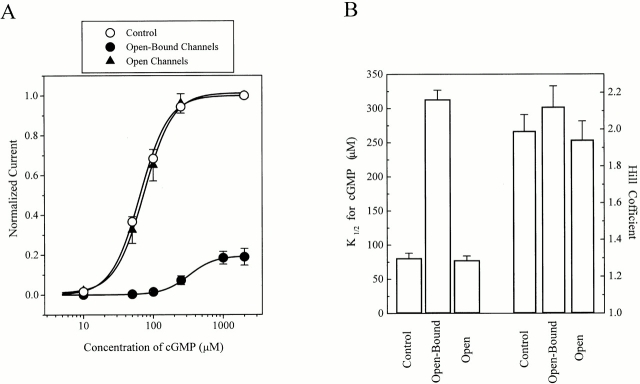
To determine the sensitivity of open-bound channels to cGMP, we performed the experiment illustrated in Fig. 3 B with different concentrations of cGMP, and measured the current flowing through open-bound channels as a fraction of the total control current, which was measured before genistein application (Fig. 4 A). As compared with control channels, open-bound channels exhibited a fourfold decrease in the K1/2 for cGMP, whereas the Hill coefficients were similar (Fig. 4 B). These results suggest that genistein does not affect the apparent stoichiometry or cooperativity of cGMP binding and agree with our previous studies (Molokanova et al. 1999a) showing that genistein is not a competitive inhibitor of cGMP activation.
Figure 4.
Genistein alters the apparent affinity of RETα channels for cGMP. (A) Dose–response curves for cGMP activation of CNG channels in the absence (control) and in the presence of 100 μM genistein (Open and Open-Bound channels). Continuous curves show fits to the Hill equation. (B) Apparent affinity (K1/2) for cGMP and Hill coefficients for control (n = 42), genistein-free (Open, n = 22), and genistein-inhibited (Open-Bound, n = 22) channels.
Effect of Genistein–PTK on Single Channels
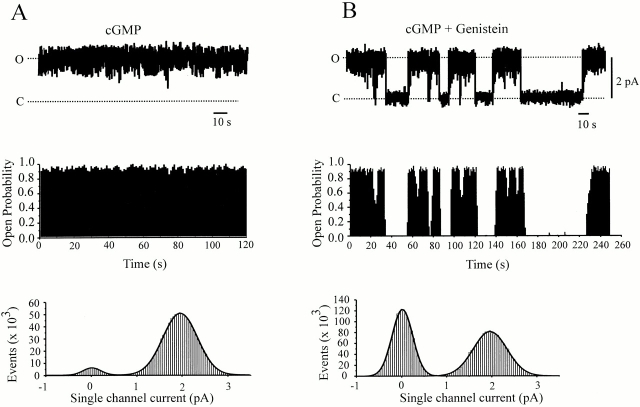
To further distinguish between kinetic states, we analyzed the single channel behavior of rod CNG channels with and without genistein present. Current carried by CNG channels can be described with the equation I = i × N × Po, where i is single-channel current, N is a number of active channels in the patch, and Po is the open probability. Our first goal was to determine which of these parameters is affected by genistein. Single-channel currents activated by saturating cGMP in the absence (Fig. 5 A) and in the presence (Fig. 5 B) of 100 μM genistein appeared to have the same average amplitudes, as confirmed by all-point histograms, indicating that single-channel current was unaffected by genistein. Likewise, in patches containing 2–3 channels, genistein did not cause an all-or-none dropout of individual channel activity, indicating that genistein does not affect the number of active channels. However, Fig. 5 shows that genistein dramatically alters the Po. The control activity pattern exhibited a nearly constant Po of ∼0.9, but after genistein application, long closures appeared, such that activity exhibited a “bursty” pattern with 15–30 s of high Po (0.4–0.9) interspersed with 10–80-s silent periods (Po = 0). In a total of eight patches without genistein present (>10 min of total recording time of single channel activity in saturating cGMP) long closures were never observed, whereas long closures were consistently observed in each of the six patches with genistein present (∼20 min total recording time). In these six patches, genistein caused a decrease in the average Po of 39 ± 6%, fully accounting for the 41% inhibition of macroscopic current induced by 100 μM genistein.
Figure 5.
Effects of genistein on single-channel activity. Representative single-channel currents (top), corresponding open probabilities (middle) sampled in 1-s bins, and all-point amplitude histograms of RETα channel activity activated by saturating cGMP (2 mM). Amplitude histograms were fitted by two Gaussians with variances σ2 c and σ2 o for closed and open distributions, respectively. All data were obtained at −80 mV. (A) Control single-channel currents. Parameters for Gaussians are as follows: σc = 0.19 pA, i =1.96 pA, σo = 0.39 pA, and Po = 0.94. (B) Single-channel current in the presence of genistein. Parameters for Gaussians are as follows: σc = 0.23 pA, i =1.97 pA, σo = 0.38 pA, and Po = 0.529.
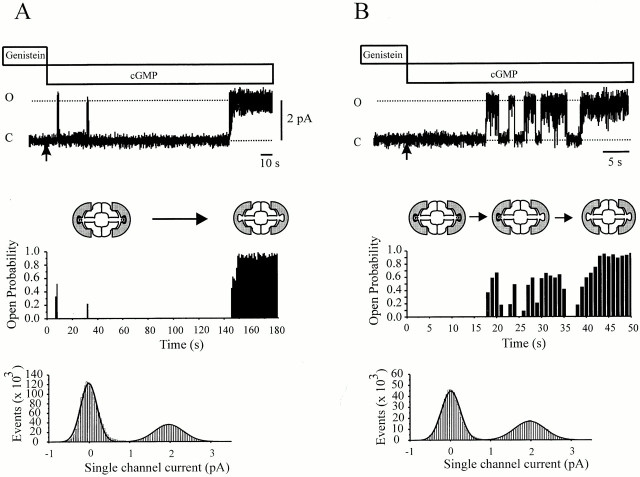
While the control gating pattern is relatively simple, in the presence of genistein, gating appears to fluctuate from one mode to another, each with distinct gating characteristics. These modes may correspond to genistein-bound and unbound states, but at steady state, we have no way to relate channel activity to specific kinetic states. Therefore, we examined the behavior of genistein-inhibited rod CNG channels immediately after removal of genistein. Naive channels that had not been preexposed to genistein activate rapidly with little delay (<1 s) upon exposure to saturating cGMP, and exhibit uniform gating with high Po. In contrast, channels preexposed to genistein exhibited a prolonged delay in activation upon application of cGMP, followed by bursts of channel openings, and eventually followed by a return to the normal (genistein-free) gating pattern (Fig. 6A and Fig. B). The transition from fully inhibited to normal gating appeared to involve discrete steps. Fig. 6 A shows an example of the activity pattern seen in three out of seven experiments, where the Po shifted in a relatively abrupt manner from a very low value (0.02 ± 0.01) to ∼0.9 with only a brief period (<3 s) of intermediate Po values. Fig. 6 B shows an example of channel behavior seen in the remaining four patches, where the Po was initially near zero and then shifted in two distinct steps. The first appeared as a burst of openings and had an average Po of ∼0.35, followed by an increase to 0.9. Analysis of all four patches that exhibited an intermediate gating mode indicated that the Po for this mode was 0.39 ± 0.08. The abrupt changes in the average Po were irreversible, such that channel activity increased in discrete steps, but never decreased again, as long as genistein was not reapplied. The irreversible jumps in open probability are consistent with the stepwise dissociation of genistein and/or PTK. The diagrams illustrate the channel configuration that we propose corresponds to each of the gating modes. As suggested previously (Molokanova et al. 2000), we propose that fully inhibited channels are associated with two PTKs, each bound to a genistein molecule. The bursty mode, which exhibits an intermediate Po, might reflect activity after one genistein has dissociated. Finally, the normal gating mode reflects activity after both genistein molecules have dissociated. Further studies are needed to confirm this proposal, but the existence of an intermediate gating mode is strong evidence that channels bound to genistein–PTK can open.
Figure 6.
Dissociation of genistein–PTK from single channels. (Top) Two examples of single-channel currents after preexposure to 100 μM genistein. Arrows in A and B show the moment (time 0) when superfusion with solution containing 2 mM cGMP genistein was applied, washing away the genistein-containing solution. (Middle) Corresponding plots of open probability. In A, note the very low open probability, followed by an abrupt increase 150 s later, as genistein unbinds. In B, note the two-step increase in open probability, possibly reflecting sequential dissociation of two genistein molecules. Diagrams illustrate tetrameric CNG channels (open symbols), PTK molecules (shaded symbols), and genistein (solid symbols). (Bottom) Amplitude histograms fit with Gaussian distributions. Parameters for Gaussians are as follows: (Α) σc = 0.21 pA, i = 1.97 pA, and σo = 0.36 pA; (B) σc = 0.24 pA, i = 1.96 pA, and σo = 0.38 pA.
Genistein Changes the Free Energy Associated with Channel Opening
To help clarify the mechanism of genistein inhibition, we used a model for channel activation that involves the cooperative binding of cyclic nucleotides, followed by an allosteric opening transition (Monod et al. 1965; Stryer 1987). The opening allosteric constant (L) can be related to open probability according to the equation L = Po/(1 − Po). Using two values for Po, reflecting one and two genistein molecules bound (Fig. 6), we find that as compared with control, L decreased 30–1,000-fold in the presence of genistein (Lcontrol = 17 vs. Lgenistein ( 1 ) = 0.61 and Lgenistein ( 2 ) = 0.02). The free energy associated with channel opening (ΔGopening) is related to L by the equation ΔGopening = −RT ln (1/L). According to this model, genistein–PTK changed ΔGopening from −1.65 to 0.24 kcal/mol (one genistein molecule) and 2.21 kcal/mol (two genistein molecules). Hence, genistein inhibits rod CNG channels by dramatically raising the ΔG for the allosteric opening transition, such that it becomes an energy-requiring rather than an energy-yielding reaction.
Interaction between Genistein and Ni2+ Effects on Rod CNG Channels
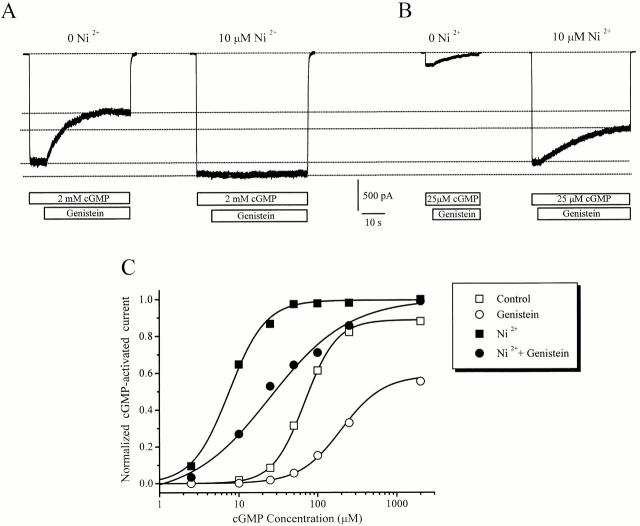
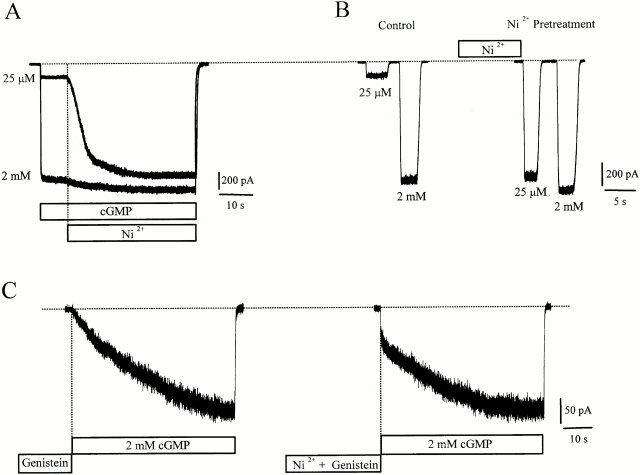
To further characterize the changes in the channel behavior elicited by genistein, we tested the effect of 10 μM Ni2+ on the channels whose gating was impaired by genistein. Ni2+ has been shown to promote rod CNG channel opening by binding to a specific histidine on the COOH terminus of the channel protein and stabilizing the open state (Gordon and Zagotta 1995a). Fig. 7 shows that the magnitude of Ni2+ potentiation at saturating cGMP (2 mM) is much smaller than at low cGMP (25 μM), presumably because at saturating cGMP, the open probability is already 0.9, which is very close to maximal (1.0). At steady state, we find that Ni2+ completely eliminates genistein inhibition of channels fully activated by saturating cGMP (Fig. 7 A), but only reduces genistein inhibition at subsaturating cGMP (Fig. 7 B). The effect of Ni2+ on genistein inhibition at a variety of cGMP concentrations is shown in Fig. 7 C. These effects were observed regardless of the application order of Ni2+ and genistein.
Figure 7.
Ni2+ attenuates genistein inhibition of RETα channels. (A) Effect of genistein on CNG current fully activated by saturating cGMP, in the absence and presence of 10 μM Ni2+. (B) Effect of genistein on CNG current, partially activated by 25 μM cGMP, in the absence and presence of 10 μM Ni2+. (C) Dose–response curves of channel activation in control, genistein, Ni2+, and genistein plus Ni2+.
We have considered two nonmutually exclusive explanations that might account for how Ni2+ reduces genistein inhibition. First, since Ni2+ dramatically increases the open probability, perhaps the attenuation of genistein inhibition results solely from the fact that genistein affects open channels less strongly than closed channels. However, we have shown that genistein has a finite ability to inhibit open channels. Therefore, the complete absence of genistein inhibition in the presence of Ni2+ suggests that an additional mechanism must be involved.
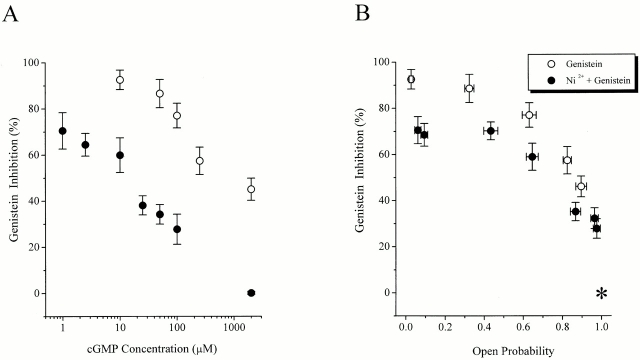
To determine the extent to which the effect of Ni2+ on genistein inhibition results from the increase in open probability, we calculated the extent of genistein inhibition and plotted it as a function of open probability. Curves relating cGMP concentration to the extent of genistein inhibition (Fig. 8 A) were transformed into curves relating open probabilities to the extent of genistein inhibition (Fig. 8 B) by assuming that with Ni2+ present, saturating cGMP leads to an open probability very close to 1.0. Hence, data were normalized with respect to the current elicited by 2 mM cGMP plus Ni2+ (Fig. 8 B, asterisk). If the channel open probability alone accounts for Ni2+ attenuation of genistein inhibition, then data sets with and without Ni2+ should be indistinguishable. However, at the same values of open probability, genistein inhibition is less for channels activated by cGMP with Ni2+ versus channels activated by cGMP alone. This deviation indicates that an additional effect must be involved in Ni2+ attenuation of genistein–PTK inhibition.
Figure 8.
Analysis of Ni2+ attenuation of genistein inhibition. (A) Genistein inhibition in the absence and presence of 10 μM Ni2+ at various cGMP concentrations. Genistein inhibition is defined as (control current − current with 100 μM genistein)/control current (n = 6–9 experiments for each point). (B) Genistein inhibition as a function of channel open probability, with and without Ni2+ present. Each data point, taken from the same experiments shown in A, represents mean ± SEM values for percent inhibition of CNG current by genistein, and mean ± SEM of open probability.
Therefore, we suggest that Ni2+ binding alters the physical association between the channel and the PTK, making it more difficult for genistein–PTK to affect channel behavior. We tested this hypothesis using closed channels. Ni2+ associates rather slowly (tens of seconds) with CNG channels, as demonstrated by the slow potentiation shown in Fig. 9 A) and by previous studies (Gordon and Zagotta 1995a). Pretreatment of closed channels with Ni2+ potentiates the initial current immediately upon application by cGMP, suggesting that Ni2+ had already bound to the closed channel (Fig. 9 B). If Ni2+ disrupts the association between the channel and the genistein–PTK complex when the channel is closed, then upon application of cGMP, one would expect a larger residual current, reflecting channels devoid of the genistein–PTK complex. We found that when the channels were pretreated with Ni2+ and genistein for 1 min, they exhibited a residual current that was 32.1 ± 3.4% (n = 8) of the maximal current, compared with channels pretreated with genistein alone, which exhibited a residual current 1.3 ± 0.8% (n = 22) as large as maximal (Fig. 9 C). The observation that relief of genistein inhibition is incomplete when Ni2+ is applied on closed channels, but is complete when Ni2+ is applied on open channels suggests that the genistein–PTK–channel complex is more stable when the channel is closed than when it is open.
Figure 9.
Ni2+ binds to closed channels and attenuates genistein inhibition. (A) Ni2+ slowly potentiates channels activated by 25 μM and 2 mM cGMP. (B) Preapplication of Ni2+ on closed channels potentiates subsequent activation by cGMP. (C) Genistein inhibition of closed channels is reduced by preapplication of Ni2+.
Structural Determinants of Genistein Inhibition of Rod CNG Channels
Which part of the rod CNG channel α-subunit (RETα) interacts with genistein–PTK and confers inhibition? To address the question, we took advantage of our previous finding that the rat olfactory CNG channel α-subunits (OLFα), which are highly homologous to the rod channel, do not exhibit genistein inhibition (Molokanova et al. 2000). To localize the channel region that confers the interaction between the RETα channel and genistein–PTK, we examined chimeric channels (Gordon and Zagotta 1995a,Gordon and Zagotta 1995b) containing portions of both channel sequences (Fig. 10). We used closed channels as an indicator of genistein inhibition because the gating kinetics of chimeric channels might influence the level of genistein inhibition of open channels, whereas closed channels present no complications associated with channel gating.
Figure 10.
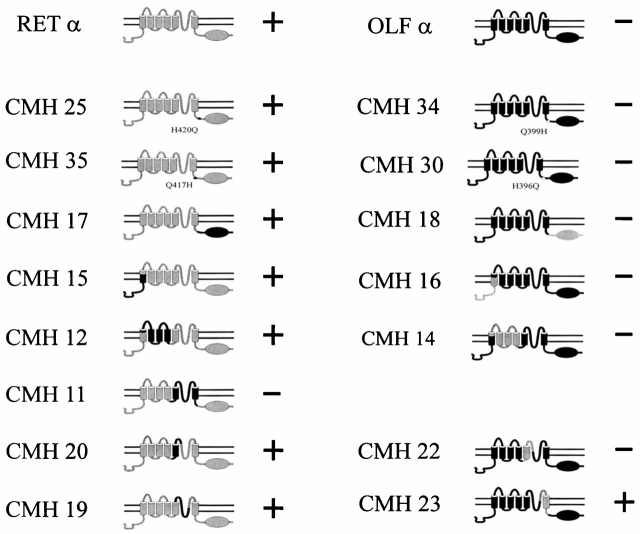
Structural determinants of genistein inhibition of RETα channels. (Left) RETα-based channels (shaded) with substituted regions containing OLFα sequences (black portions). (Right) OLFα-based channels (black) with substituted regions containing RETα sequences (shaded portions). Pluses and Minuses represent the presence or absence of genistein inhibition for each channel.
We first tested the role of specific histidine residues that were previously implicated in Ni2+ effects on CNG channels (Gordon and Zagotta 1995a,Gordon and Zagotta 1995b) by using chimeras in which amino acids at these positions were exchanged with the corresponding amino acids from OLFα. Substitution of H420 in RETα, which is necessary for Ni2+ potentiation, with an asparagine (CHM25) did not reduce genistein inhibition. Thus, CHM25, like its parent RETα channel, showed complete inhibition with genistein applied on closed channels. Likewise, substitution of Q417 with a histidine (CHM35), which is necessary for Ni2+ inhibition of OLFα channels, also did not reduce genistein inhibition. The converse chimeras (CHM34 and CHM 30) in which substitutions were made into the OLFα background also had no effect: neither substitution introduced genistein inhibition.
Exchange of the cyclic nucleotide–binding domains between RETα and OLFα channels did not alter genistein inhibition. Thus, RETα channels with the OLFα cyclic nucleotide–binding domain (CHM17) exhibited complete inhibition by genistein, whereas the OLFα channel with a RETα cyclic nucleotide–binding domain (CHM18) exhibited no inhibition. Switching the NH2-terminal domains through part of the first membrane-spanning segment (CHM 15 and CHM16) also had no effect; either chimera behaved like their respective RETα or OLFα parent channel. Substitution from mid-S1 through S4, including all of S2 and S4 also had no effect. Thus, CHM12, which consisted of RETα except for this region was fully inhibited by genistein, whereas CHM 14, which consisted of OLFα except for this region was not inhibited.
We were able to alter genistein inhibition of RETα channels by substituting “a pore module” derived from the OLFα channel, including S5, the pore-forming P region, S6, and the initial part of the C-linker flanking S6 (CHM11). In the open state, this chimera was completely insensitive up to 500 μM genistein; in the closed state, maximal inhibition caused by 500 μM genistein was only ∼15 vs. 98% in wild-type channel, suggesting that this region contains most, but not all, of the sites necessary for genistein–PTK interaction. We tested three parts of the S5-P-S6 region separately. Exchange of S5 domains between RETα and OLFα channels (CHM20 and CHM22) and introduction of the OLFα pore into RETα channel (CHM 19) did not alter genistein inhibition. However, introduction of a region including part of the pore, S6, and part of the C-linker from the RETα into the OLFα channel (CHM23) had a dramatic effect. Indeed, the resulting channel was susceptible to genistein inhibition to the same extent as RETα channels (99% inhibition of closed channels). Thus, introduction of the RETα S6 and flanking regions was the only change capable of conferring genistein inhibition onto the OLFα channel. These results suggest that the region in or around transmembrane domain S6 (namely, amino acids 343–421) is crucial for genistein inhibition, and may be the genistein–PTK binding site on the RETα channel.
DISCUSSION
Noncatalytic Inhibition of CNG Channels by PTK
PTKs can influence RETα channels in two ways: (1) by catalyzing phosphorylation, and (2) by noncatalytically inhibiting the channels in the presence of genistein (Molokanova et al. 1997, Molokanova et al. 1999a,Molokanova et al. 1999b). Noncatalytic genistein inhibition can be studied in isolation by recording channel activity from excised patches in the absence of ATP. Several key observations lead to the conclusion that noncatalytic inhibition by genistein is mediated by PTKs. First, PTK inhibitors that have no direct effect on the channel attenuate genistein inhibition. Second, the association and dissociation rates of genistein inhibition are orders of magnitude slower than predicted from the diffusion coefficient of genistein, which is inconsistent with a direct interaction between genistein and the channels. Third, for genistein concentrations of 1 μM and above, the on-rate of inhibition is independent of genistein concentration, which is also inconsistent with a direct interaction between genistein and the channels. Finally, the activity-dependent pattern observed for catalytic modulation by the PTK (tyrosine phosphorylation requires closed channels) matches the activity-dependence of noncatalytic genistein inhibition. In parallel with tyrosine phosphorylation, genistein is both more potent and more efficacious in inhibiting closed than open channels.
All of these observations lead to the proposal that genistein inhibits RETα channels by first binding to a PTK, which then impairs channel gating. The observation that the on-rate of genistein inhibition is independent of genistein concentration is consistent with the second step in this process being rate-limiting. It is unclear whether the PTK is stably associated with the channel, or whether the PTK can dissociate and associate with the channel. If the PTK is stably bound to the channel, then the slow kinetics of inhibition must reflect slow conformational changes induced by genistein. If the PTK can dissociate, the slow kinetics might reflect the diffusion of genistein–PTK and binding and unbinding of the complex and the channel.
Genistein is much more effective in inhibiting closed than open channels. While genistein inhibition of closed channels is dramatic and complete, we have shown that open channels can also bind genistein–PTK, causing a less pronounced degree of inhibition. The act of opening, which has been suggested to involve the S6 transmembrane domain, may decrease the stability of binding of channels to genistein–PTK. The association rate of genistein–PTK to either closed and open channels appear to be very similar, whereas dissociation of genistein–PTK occurs much more rapidly from open than from closed channels. Hence, opening channels with cGMP should cause a great acceleration of dissociation, ensuring more rapid recovery from genistein inhibition.
Noncatalytic genistein inhibition occurs not only in RETα channels exogenously expressed in oocytes, but also in native CNG channels from rod outer segments (Molokanova et al. 2000). This finding suggests that PTK(s) may be normal components of the phototransduction machinery that have important, but incompletely understood, roles in phototransduction. We have described the PTK as an inhibitory regulator of CNG channel function, such that genistein inhibits by allowing a negative association between the channel and the PTK. However, our results do not exclude the converse possibility that genistein disrupts a positive association between channel and the PTK. This second possibility would imply that the PTK has a permissive role in promoting channel gating, which is suppressed by genistein.
Structural Basis of Genistein Inhibition
The site in the RETα channel that confers genistein inhibition on OLFα channels is a region containing all of the S6 transmembrane domain and neighboring regions of the pore and part of the cytoplasmic C-linker (CHM 23). Genistein inhibition of RETα channels can be greatly reduced, but not completely eliminated, by substitution of a similar and overlapping region spanning from S4 to S6 from OLFα (CHM 11). No other parts of the channel appear crucial for allowing inhibition of RETα or conferring inhibition to OLFα channels. The simplest explanation for these mapping results is that the region in and around S6 is important, but other parts of the RETα channel protein also contribute to the binding site of genistein–PTK. Notably, the tyrosine phosphorylation site (Y498) responsible for catalytic modulation (i.e., phosphorylation) by PTKs (Molokanova et al. 1999b) is not required for genistein inhibition. To transfer a phosphate, PTKs must interact with the RETα channel at Y498. However, the binding site for the PTK must involve a much larger protein interface, apparently including a more distant region that may be primarily intramembranous (S6 and flanking regions).
Both genistein inhibition and the ability to modulate rod CNG channels by tyrosine phosphorylation are remarkably stable in membrane patches excised from Xenopus oocytes, persisting unabated for >30 min, even with continuous perfusion. Loosely associated membrane constituents, such as G-protein subunits, cytoskeletal components, and soluble kinases and phosphatases (Levitan 1999) are often stripped from membranes after cell dialysis or patch excision, resulting in wash-out of channel modulation or, in some cases, loss of channel activity itself. The observation that the catalytic and noncatalytic effects of the PTK are so stable is consistent with an integral membrane protein, rather than a loosely associated protein, being involved. The possible involvement of the S6 transmembrane domain as a binding interface for the PTK is consistent with this idea. Cysteine accessibility studies on voltage-gated K+ channels, which are closely homologous to CNG channels, show that the S6 domain plays a central role controlling intracellular access to the pore, suggesting that S6 constitutes part of the channel's gate (Liu et al. 1997). The important role of S6 in gating may provide a direct mechanism for how genistein–PTK inhibits channel opening. In addition, the C-linker also has been implicated in regulating CNG channel gating (Zong et al. 1998; Paoletti et al. 1999), providing an additional possible basis for genistein–PTK inhibition.
Interaction of Ni2+ Potentiation and Genistein Inhibition
Ni2+ increases current through RETα channels and increases the apparent affinity for cyclic nucleotides (Gordon and Zagotta 1995a). Ni2+ potentiation involves the coordination of histidines (H420) in adjacent subunits of the channel (Gordon and Zagotta 1995b). We have found that Ni2+ dramatically reduces genistein inhibition. Since Ni2+ increases open probability and genistein has a diminished effect on open channels, one would expect some relief of genistein inhibition in the presence of Ni2+. However, Ni2+ has an additional liberating effect on genistein inhibition, above and beyond that predicted by changes in open probability alone (Fig. 8 B). We propose that the conformational change triggered by Ni2+ affects the channel's ability to interact with genistein–PTK. The genistein–PTK binding region includes the specific binding site for Ni2+, but we have found that replacing the histidine with a glutamine (H420Q), fails to prevent genistein inhibition. Hence, even though Ni2+ and genistein–PTK have overlapping binding regions, mutation of the more focused binding site for Ni2+ has an insignificant effect on genistein inhibition. Since Ni2+ and genistein–PTK interact with the same part of the CNG channel, it is possible they affect the same underlying process (e.g., the same or similar conformational changes), albeit in opposite ways. PTKs involved in genistein inhibition are natural constituents of both oocytes and rods (Molokanova et al. 2000). Hence, it is possible that the Ni2+ potentiation observed in both of these systems is actually an indirect effect, involving relief of PTK inhibition. According to this idea, even though Ni2+ binds directly to the channel, its ability to potentiate gating results from an allosteric decrease in the binding of the channel to the PTK. In effect, Ni2+ potentiation may be mediated by PTK disinhibition.
How Does the Association between Channel and PTK Affect Channel Behavior?
Allosteric models involving concerted conformational changes between closed and open states, based on the classic model devised for hemoglobin (Monod et al. 1965), have been used to describe the activation of CNG channels (Stryer 1987; Goulding et al. 1993; Liu et al. 1998; Ruiz and Karpen 1999). According to these models, cyclic nucleotides preferentially bind and stabilize channels in their open states rather than binding to closed states and triggering channel opening. CNG channels open spontaneously without a ligand (Tibbs et al. 1997), indicating that conformation changes resulting in channel opening can occur separately from ligand binding and can be analyzed independently.
Application of genistein on fully activated channels results in a fourfold decrease in cGMP sensitivity. This could result from a decrease in the binding affinity for cyclic nucleotides or from a change in the energetics of channel opening. If genistein–PTK lowered the binding affinity for cGMP, one would expect that, in the presence of genistein, the gating pattern of single channels at high concentrations of cGMP would simply resemble the gating at low concentrations of cGMP. However, genistein–PTK induces a novel gating pattern, consisting of bursts of high open probability interspersed with silent periods, unlike the uniformly low open probability normally seen at low concentrations of cGMP. Hence, an effect on cGMP binding affinity alone cannot account for genistein inhibition. Therefore, at least some of genistein inhibition must result from a change in the process of channel gating.
To quantify the effect of genistein on channel gating, we measured the open probability of single genistein–PTK-bound channels by taking advantage of the slow dissociation rate of the complex from closed channels. At steady state, it is difficult to ascertain the number of genistein–PTK complexes bound to a channel at any given moment. However, by capturing channel gating as genistein–PTK is dissociating, we observed three sequential modes of channel gating, each with progressively higher open probability, as the channel returns to normal activity. At first, the maximal number of genistein–PTK complexes are still bound to the channels. The value of the Hill coefficient for genistein inhibition is 2, and our previous studies (Molokanova et al. 2000) suggest that closed RETα channels can bind up to two genistein–PTKs. Therefore, we suggest that the initial gating mode, exhibiting the lowest maximal open probability, corresponds to channels with two genistein–PTK complexes. Next, the channels exhibit an intermediate gating mode, corresponding to one genistein–PTK complex bound. Finally, the channel exhibits its normal high open probability, corresponding to complete dissociation of genistein–PTK from the channel. The energetics of channel gating progressively becomes more favorable as the channel sequentially sheds the two genistein–PTK complexes. With two genistein–PTK complexes bound, the ΔGopening is positive (+2.13 kcal/mol) and is very close to the value for normal channels with cAMP bound (+2.26 kcal/mol), a very poor partial agonist. Hence, we conclude that by binding to regions of the channel important for gating, genistein–PTK imposes conformational constraints on the channel protein, hindering the ability of the channel to open.
Acknowledgments
We thank Dr. William N. Zagotta for providing chemic channel constructs.
This work was supported by the Young Investigator Award from the National Alliance for Research on Schizophrenia and Affective Disorders to E. Molokanova and by grants from the National Institutes of Health (EY-11877 and EY-12608) to R.H. Kramer.
Footnotes
Address correspondence to Dr. Richard H. Kramer, 121 LSA, Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720. Fax: (510) 643-6791; E-mail: rhkramer@uclink4.berkeley.edu
Abbreviations used in this paper: CNG, cyclic nucleotide–gated; PTK, protein tyrosine kinase; PTP, protein tyrosine phosphatase.
References
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Chen T.Y., Illing M., Molday L.L., Hsu Y.T., Yau K.-W., Molday R.S. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca(2+)-calmodulin modulation. Proc. Natl. Acad. Sci. USA. 1994;91:11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhallan R.S., Yau K.-W., Schrader K.A., Reed R.R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Gordon S.E., Zagotta W.N. Localization of regions affecting an allosteric transition in the cyclic nucleotide-activated channels Neuron. 14 1995. 857 864a [DOI] [PubMed] [Google Scholar]
- Gordon S.E., Zagotta W.N. Subunit interaction in coordination of Ni2+ in cyclic-nucleotide-gated channels Proc. Natl. Acad. Sci. USA. 92 1995. 10222 10226b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.E., Brautigan D.L., Zimmerman A.L. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Gordon S.E., Downing-Park J., Zimmerman A.L. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor J. Physiol. 486 1995. 533 546a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.E., Downing-Park J., Tam B., Zimmerman A.L. Diacylglycerol analogs inhibit the rod cGMP-gated channel by a phosphorylation-independent mechanism Biophys. J. 69 1995. 409 417b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding E.H., Tibbs G.R., Liu D., Siegelbaum S.A. Role of H5 domain in determining pore diameter and ion permeation through cyclic nucleotide-gated channels. Nature. 1993;364:61–64. doi: 10.1038/364061a0. [DOI] [PubMed] [Google Scholar]
- Hsu Y.T., Molday R.S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Ildefonse M., Bennett N. Single-channel study of the cGMP-dependent conductance of retinal rods from incorporation of native vesicles into planar lipid bilayers. J. Membr. Biol. 1991;123:133–147. doi: 10.1007/BF01998084. [DOI] [PubMed] [Google Scholar]
- Karpen J.W., Brown R.L., Stryer L., Baylor D.A. Interactions between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods. J. Gen. Physiol. 1993;101:1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp B.U., Niidome T., Tanabe T., Terada S., Bonigk W., Stuhmer W., Cook N.J., Kangawa K., Matsuo H., Hirode T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cGMP-gated channel. Nature. 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Levitan I.B. Modulation of ion channels by protein phosphorylation. Adv. Second Messenger Phosphoprotein Res. 1999;33:3–22. doi: 10.1016/s1040-7952(99)80003-2. [DOI] [PubMed] [Google Scholar]
- Liu Y., Holmgren M., Jurman M.E., Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Liu D.T., Tibbs G.R., Paoletti P., Siegelbaum S.A. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron. 1998;21:235–248. doi: 10.1016/s0896-6273(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Molokanova E., Trivedi B., Savchenko A., Kramer R.H. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J. Neurosci. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E., Savchenko A., Kramer R.H. Noncatalytic inhibition of cyclic nucleotide–gated channels by tyrosine kinase induced by genistein J. Gen. Physiol 113 1999. 45 56a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E., Maddox F., Luetje C.W., Kramer R.H. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue J. Neurosci. 19 1999. 4786 4795b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E., Savchenko A., Kramer R.H. Interactions of cyclic nucleotide–gated channel subunits and protein tyrosine kinase probed with genistein. J. Gen. Physiol. 2000;115:685–696. doi: 10.1085/jgp.115.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J., Wyman J., Changeux J.P. On the nature of allosteric transitionsa plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Muller F., Bonigk W., Sesti F., Frings S. Phosphorylation of mammalian olfactory cyclic nucleotide-gated channels increases ligand sensitivity. J. Neurosci. 1998;18:164–173. doi: 10.1523/JNEUROSCI.18-01-00164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P., Young E.C., Siegelbaum S.A. C-linker of cyclic nucleotide–gated channels controls coupling of ligand binding to channel gating. J. Gen. Physiol. 1999;113:17–34. doi: 10.1085/jgp.113.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M., Karpen J.W. Opening mechanism of a cyclic nucleotide–gated channel based on analysis of single channels locked in each liganded state. J. Gen. Physiol. 1999;113:873–895. doi: 10.1085/jgp.113.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M., Brown R.L., He Y., Haley T.L., Karpen J.W. The single-channel dose-response relation is consistently steep for rod cyclic nucleotide-gated channelsimplications for the interpretation of macroscopic dose-response relations. Biochemistry. 1999;38:10642–10648. doi: 10.1021/bi990532w. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual transductiondesign and recurring motifs. Chem. Scr. 1987;27B:161–171. [Google Scholar]
- Tibbs G.R., Goulding E.H., Siegelbaum S.A. Allosteric activation and tuning of ligand efficacy in cyclic-nucleotide-gated channels. Nature. 1997;386:612–615. doi: 10.1038/386612a0. [DOI] [PubMed] [Google Scholar]
- Womack K.B., Gordon S.E., He F., Wensel T.G., Lu C.-C., Hilgemann D.W. Do phosphatidylinositides modulate vertebrate phototransduction? J. Neurosci. 2000;20:2792–2799. doi: 10.1523/JNEUROSCI.20-08-02792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X., Zucker H., Hofmann F., Biel M. Three amino acids in the C-linker are major determinants of gating in cyclic nucleotide-gated channels. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:353–362. doi: 10.1093/emboj/17.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]