Abstract
Purpose
To identify and compare the phosphorylated lipids in normal and dry eye rabbit tears using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS).
Methods
MALDI-TOF MS studies were performed on tear samples from normal and dry eyes of female New Zealand White rabbits. Experimental dry eye was induced by complete removal of the main and accessory lacrimal glands and nictitating membranes. A solid ionic crystal MALDI matrix of paranitroaniline and butyric acid was used to enhance the mass spectral responses of the phospholipids. In addition, a novel lipid isolation, preconcentration, and clean-up method using pipettes containing immobilized metal ion affinity chromatography (IMAC) medium was used.
Results
The polar phospholipids present in the normal and dry eye rabbit tears showed both similarities and differences. Species related to platelet-activating factor (PAF) and/or lysophosphatidylcholine (lyso-PC), phosphatidylcholine (PC), and sphingomyelin (SM) were found in both the normal and dry eye rabbit tears. However, the number of types and the concentrations of SM molecules were markedly greater in the dry eye tears than in the normal tears. In addition, phosphatidylserine (PS) species that were readily detectable in dry eye tears were not found in normal tears.
Conclusions
The combination of immobilized metal ion affinity chromatography and the solid ionic crystal matrix for MALDI enabled the detection and study of phosphorylated lipids in the tears. Specific differences between phospholipid levels in normal and dry eye tears were observable with this methodology. The appearance of various SM species only in the dry eye tears may provide markers for this disease state in the future.
The development of dry eye syndrome in people is associated with a number of physiological and environmental conditions,1 including aging,2 hormonal imbalance,3 disease,4 surgery,5,6 smoke, wind, heat, and/or low humidity1,7,8 which alter the composition of the tear film. However, the ability to classify patients with dry eye within specific etiological categories, except for those with specific diseases such as Sjögren’s syndrome,4 is difficult because the exact effect of the various causative conditions on the compositional structure of the tear film is unknown.
The normal tear fluid volume is approximately 7 μL in both rabbits and humans. The structure of the tear fluid is generally divided into three layers: the innermost epithelial-glycocalyx layer, an intermediate aqueous-mucin layer,9,10 and an outermost lipid layer.11 The components of the tear film are supplied by several ocular tissues. The innermost glycocalyx layer is secreted by the stratified squamous cells of the conjunctiva and cornea. Its primary functions are to protect the ocular surface cells from bacterial binding and smooth out any small surface irregularities. The aqueous-mucin layer, composed of mucins, proteins, and electrolytes, is secreted by the main and accessory lacrimal glands and the conjunctival goblet cells. The various proteins found in the aqueous layer perform a myriad of functions vital to maintaining the health of the ocular surface, regulating surface cell function, and maintaining the tear film structure.12 The outer lipid layer is secreted by the meibomian glands, which are located in the eyelids; its main function is to retard the evaporation of the aqueous layer.13,14 Because the cells of the ocular surface function within a narrow range of pH, osmolarity, and ionic compositions, it is vital that the tears maintain the appropriate concentrations of constituents in each of the three layers. Instability of the outer lipid layer of the tear film caused by changes in the polar lipid concentration has been identified as a potential factor in the development of dry eye syndrome.15,16
Most of the polar lipids are supplied to the tear film by the meibomian glands. The chemical composition of the human meibomian gland secretions is a complex mixture of cholesterol and wax esters, triacylglycerols, free fatty acids, diesters, free cholesterol, hydrocarbons, and polar lipids.17-21 The polar lipids found in meibomian gland secretion contain 70% phospholipids and 30% sphingolipids; the phospholipid portion consists of 38% phosphatidylcholine (PC), 18% phosphatidylethanolamine (PE), 7% sphingomyelin (SM), and 39% unknowns.17 Polar lipids make up approximately 6% to 16% of the compounds found in the liquid phase of the tear film.17,22,23 Based on these data, the percentages of phosphorylated lipids in the lipid phase of the tear film would be 1.6% to 4% PC, 0.8% to 2% PE, and <1% SM. Analysis of the rabbit meibomian gland cells and secretions by Greiner et al.22,23 has shown the polar lipid content to be similar to that of humans, with PC and PE making up the majority (∼60%) of the phospholipids secreted. The ability to identify specific phospholipid changes in tears of known dry eye origin would enhance our knowledge of dry eye development and potential treatments.
However, previous investigations into molecular changes in tear film structure have been hampered by the need to pool tears to make up a sample large enough to allow detection by analytical methods such as one- or two-dimensional electrophoresis.24-26 Another problem has been the requirement that the tear components be derivatized before they are subjected to gas chromatography (GC), mass spectrometry (MS), or both.17,27 The chemical composition of the human meibomian gland liquid secretions has been characterized by high-pressure liquid chromatography (HPLC),28 pico-nuclear magnetic resonance (p-NMR),22 and thin-layer chromatography (TLC),29 and fatty acid fragment profiles have been obtained by HPLC-MS.30 During the past several years, the analysis of phospholipids in general has been reported by HPLC31 and micellar electrokinetic chromatography (MEKC),31,32 capillary electrophoresis-electrospray-mass spectrometry (CE-ES-MS),33 electrospray tandem mass spectrometry (ES-MS/MS),34-41 and to a lesser extent matrix-assisted laser desorption/ionization-Fourier-transform ion cyclotron resonance (MALDI-FTICR)42 and MALDI-time-of-flight (TOF) MS.43-47 With the advent of soft ionization techniques such as ES-MS and MALDI-MS, the derivatization of volatile lipids is no longer essential.48 In this article, we report a MALDI-TOF MS study of the phosphorylated lipids of normal and dry eye rabbit tears using an immobilized metal ion affinity chromatography (IMAC; ZipTipMC Millipore, Bedford, MA) clean-up method coupled with a newly synthesized solid ionic crystal MALDI matrix to detect nanogram-to-picogram quantities of phosphorylated lipids in small-volume tear samples. Comparison of the types and relative quantities of the polar phospholipids in normal and dry eye rabbit tears should provide insight into the effect of polar lipid changes on the stability of the tear film.
Materials and Methods
Animals
Six female New Zealand White rabbits (7-8 lb. body weight; Harlan, Indianapolis, IN) were used. All animal studies were conducted in accordance with the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research.
Rabbit Tear Samples
A nonevaporative rabbit dry eye model was created in female New Zealand White rabbits by two sequential surgical procedures. First, the main lacrimal gland was removed from the experimental eye; the contralateral eye was used as the normal control. Approximately 2 weeks later, the accessory lacrimal gland and nictitating membrane were removed from the experimental eye. Because the aqueous phase of the mammalian tear film is produced by the combined activity of the main and accessory lacrimal glands,1,12 removal of these glands reduces the total tear volume and protein content. The resultant dry eye condition is qualitatively different from an “evaporative” dry eye, in which the decreased concentration of the lipids and/or the instability of the lipid layer allows for enhanced evaporation of the tear film.15 In our rabbit dry eye model, the meibomian gland remained intact and was thus believed to be responsive to the normal physiological stimulus for lipid expression. The harderian gland, which is present in rabbits but not in humans, also provides lipids to the tears. Although the gland itself was not removed in our model, the ducts from this gland, which traverse the nictitating membrane, were removed completely along with the membrane, thereby eliminating any tear lipid contribution from this source. Thus, only lipids produced by the meibomian glands were analyzed.
Corneal and ocular health was determined by slit-lamp evaluation on a weekly basis after surgery. Tear breakup time (TBUT) was measured daily (Tearscope; Keeler Instruments, Broomall, PA). When the TBUT of the surgical eye was half or less that of the control eye, the surgical eye was considered to be dry. Baseline tears (representing tears produced under nonirritating conditions) were collected daily from the normal and surgically induced dry eyes with 5-μL silanized microcapillary pipettes.29 The tear samples were immediately placed in tubes (Eppendorf, Fremont, CA) and stored at -70°C.
Chemicals
Lyso-1-palmitoylcholine, dimyristoyl phosphatidylcholine (DMPC), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, 1-palmitoyl-2-hydroxysn-glycero-3-phosphocholine, and SM were purchased as standards (Avanti Polar Lipids, Alabaster, AL). Methanol and chloroform solvents of HPLC/spectroscopy grade were purchased from EM Science (Darm-stadt, Germany). All other chemicals were of analytical-reagent grade. All standards and chemicals were used as received without further purification. Distilled and deionized (dd) water (18 MΩ, Milli-Q Water Purification System; Millipore) was used throughout standard and sample preparations.
Extraction of Nonpolar and Polar Lipids
The nonpolar and polar lipid fractions were extracted from tear samples with a two-phase (chloroform, water) extraction technique. The resultant aqueous phase contained the tear proteins, and the chloroform phase contained the lipids.49,50 Briefly, tear samples collected from a single eye over a 1-week period were pooled and diluted to 300 μL with ddH2O. The aqueous tear solution was then extracted three times with 500 μL of chloroform. The chloroform extracts were combined and evaporated to near dryness. At least three sets of samples from each eye were analyzed using the MALDI-TOF method described later in the article (ZipTipMC; Millipore).
To extract the phosphorylated lipids from the lacrimal glands, the gland was homogenized with 100 mL of chloroform. The extract was filtered, and the volume of the solution was reduced to approximately 20 mL using low heat and a gentle stream of nitrogen. Aliquots were diluted 1:10 with matrix solution (see description of MALDI-TOF MS later) and spotted directly onto the MALDI target for analysis. At least three aliquots per sample type were analyzed by MALDI-TOF.
Cleanup and Preconcentration of Polar Lipids
A modified phosphopeptide enrichment method, developed in this laboratory51 using pipette tips (ZipTipMC; Millipore) containing an IMAC medium (iminodiacetic acid, IDA resin; Millipore), was used to concentrate the phosphorylated lipids. The method suggested by the manufacturer (Millipore) was followed, except for two key solutions: The binding solution was changed to 0.1% acetic acid in 1:1 methanol-acetonitrile, and the elution solution was changed to 0.3 N ammonium hydroxide in 1:1 methanol-acetonitrile. The IMAC medium was found to be soluble in chloroform; therefore, care was taken to remove all chloroform from the extracted lipids before application of the IMAC medium. The lipids were reconstituted in 5 μL of the binding solution, aspirated through the matrix for binding, washed, and eluted with 3 μL of elution solution.
MALDI-TOF MS
MALDI-TOF mass spectra were acquired with a MALDI-rTOF mass spectrometer with delayed extraction (Voyager Elite; Applied Biosystems, Inc. [ABI], Framingham, MA). A UV light, 337 nm emission wavelength, nitrogen laser was used for irradiation. A typical spectrum was collected at an extraction voltage of 20 kV. Delayed (175 ns) extraction mode was used for all acquisitions. The laser power was adjusted to a level just above the threshold for signal appearance to minimize head group loss. Each phospholipid mass spectrum was collected by averaging data from 250 laser shots. For sample plate spotting, 3 μL of IMAC-eluted phospholipids was mixed with 3 μL of matrix, deposited onto the MALDI plate, and allowed to air dry. The MALDI matrix used in all studies was a recently developed solid ionic crystal matrix consisting of 20 mg of paranitroaniline (PNA) and butyric acid in a 1:2 molar ratio dissolved in ethanol.51
All spectra were collected using a two-point calibration of protonated lyso-PC at a mass/charge ratio (m/z) of 496.34 and protonated DMPC at m/z 678.51. Phospholipids in the biological samples were assigned according to their molecular weights, as derived from either the monoisotopic protonated molecules, monoisotopic sodium adducts, and/or postsource decay (PSD) product ions in MALDI-TOF mass spectra.
Results
Rabbit Eyes and Tear Samples
All the rabbit eyes remained healthy throughout the study period; the corneas and conjunctivas of both the normal and dry eyes were healthy and showed no epithelial staining. The experimental dry eyes had TBUTs approximately one third those of the corresponding contralateral control eyes. The usual tear volume obtained from a single normal eye was 3 μL, whereas only 1 μL was typically obtained from a dry eye.
MALDI-TOF MS Analysis of Tear Phospholipids
By inducing dry eye in only one eye of each rabbit, we were able to compare lipid expression in dry eye and normal tear samples from the same animal, thereby reducing or eliminating the effect of physiological variation from rabbit to rabbit. The polar phospholipids present in the normal and dry eye rabbit tears showed both similarities and differences.
Figure 1 shows composites of normal (Fig. 1A) and dry eye (Fig. 1B) tear mass spectra obtained by our method. In general, the normal eye tear spectra showed six major peaks (m/z 494, 522, 550, 577, 610, and 638) and seven minor peaks (m/z 637, 642, 659, 678, 695, 866, and 936). The dry eye tear spectra showed 10 major peaks (m/z 494, 522, 550, 577, 605, 610, 621, 637, 642, and 659) and four minor peaks (m/z 678, 695, 828, and 886) assignable within their mass spectra. Five of the major peaks (m/z 494, 522, 550, 577, and 610) and two of the minor peaks (m/z 678 and 695) were consistently found in the same relative abundance in both types of tears.
Figure 1.
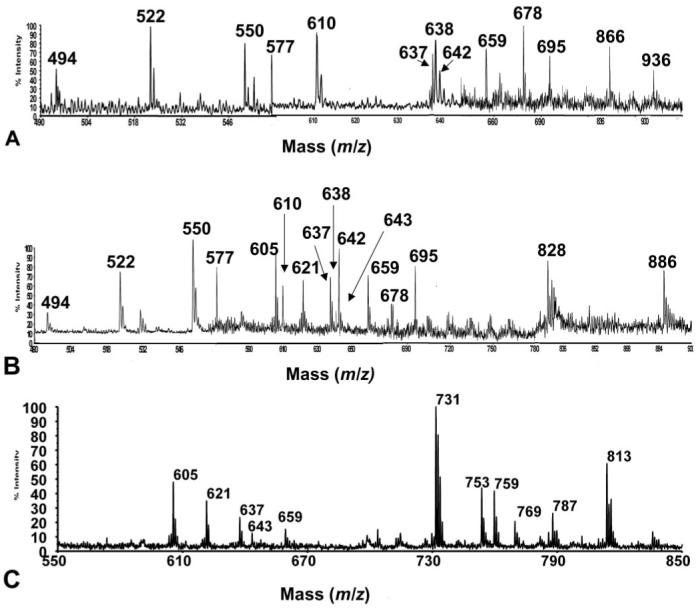
(A) Composite spectra of normal eye tear lipid extracts. (B) Composite spectra of dry eye tear lipid extracts. (C) SM standard.
Assignment of specific proteins to peaks was made using PSD spectra, m/z number, and molecular weight. Table 1 lists the phosphorylated lipid species observed in the tear extracts and their relative abundances in normal versus dry eye tears. Peaks without reliable PSD spectra for specific identification are listed as unidentifiable and require further study. The major peak assignments corresponded to types of platelet-activating factor (PAF)-, lyso-PC-, and SM-related compounds, with minor peaks corresponding to PC and phosphatidylserine (PS).
Table 1.
Major Phosphorylated Analyte Peaks Observed in the MALDI-TOF Mass Spectra of Normal and Dry Eye Rabbit Tears with Tentative Assignments
| Lipid in Tear Extract | Molecular Ion (MI) | Normal Eye* | Dry Eye* |
|---|---|---|---|
| C14:1-2:0 PAF or C16:1 Lyso-PC | [M + H]+, m/z 494 | Major | Major |
| M/z 522 (unidentified) | [M + H]+ | Major | Major |
| M/z 550 (unidentified) | [M + H]+ | Major | Major |
| Pyrophosphate SM C22H46N2O11P2 | [M + H]+, m/z 577 | Major | Major |
| Pyrophosphate SM C24H50N2O11P2 | [M + H]+, m/z 605 | Major | |
| Pyrophosphate SM C24H50N2O12P2 | [M + H]+, m/z 621 | Major | |
| Pyrophosphate SM C24H50N2O13P2 | [M + H]+, m/z 637 | Minor | Major |
| Pyrophosphate SM C24H50N2O13P2Na | [M + Na]+, m/z 659 | Minor | Major |
| M/z 610 (unidentified) | [M + H]+ | Major | Major |
| M/z 638 (unidentified) | [M + H]+ | Major | |
| M/z 642 (unidentified) | [M + H]+ | Minor | Major |
| C14:0-14:0 PC | [M + H]+, m/z 678 | Minor | Minor |
| M/z 695 (unidentified) | [M + H]+ | Minor | Minor |
| 16:0-20:4 PS C42H73NO10PNa | [M + 2Na-H]+, m/z 828 | Minor | |
| M/z 866 (unidentified) | [M + H]+ | Minor | |
| M/z 886 (unidentified) | [M + 2Na-H]+ | Minor | |
| M/z 936 (unidentified) | [M + H]+ | Minor |
The assignment of a major or minor presence is qualitative and is derived from the relative intensity abundance of the peak in the mass spectra.
The m/z 494, 522, and 550 species in the tear mass spectra did not have reliable PSD spectra for their specific identification. However, it is likely that they are phosphorylated and contain one nitrogen. For the m/z 494 species, possible assignments, based on molecular weight alone, could be a C14:1-2:0 PAF or a C16:1 lyso-PC, both with a molecular formula of C24H48NO7P. The m/z 522 and 550 species appear to be related to the m/z 494 species by the addition of one and two (-CH2CH2-) groups, respectively.
The m/z 577, 605, 621, 637, and 659 species (Fig. 1B) exhibited affinities to the IMAC resin, indicating that they are phosphorylated lipids. The odd numbered m/z values pointed to an even number of nitrogen atoms (e.g., two) in each of the compounds. The PSD spectra of the m/z 577, 605, and 621 species indicated an m/z 184 head group. These findings support the hypothesis that the five compounds are related to SM. Notably, the peaks at m/z 605, 621, 637, and 659 appeared as impurities in a C18:1 SM standard (Fig. 1C). PSD was performed on the m/z 605 species from the SM standard (Fig. 2). Informative product ion peaks include the PC head group at m/z 184, a pyrophosphate PC head group at m/z 280, neutral loss of the long chain substituent at m/z 335 (M+H-C17H34O2)+, and loss of the epoxide oxygen at m/z 589 (M+H-O)+. From the derived molecular weights and PSD spectra, tentative assignments of these compounds, based on SM structures, can be made (Fig. 3). The m/z 605, 621, and 637 species are proposed to be related structures, where the m/z 605 species is a protonated, oxidized, pyrophosphate-SM-related compound with a molecular formula of C24H50N2O11P2, probably resulting from epoxidation of the unsaturation on the long chain substituent. The m/z 621 species may represent a further oxidized form of the same molecule, resulting in peroxide formation with a molecular formula of C24H50N2O12P2. The m/z 637 species is a further oxidized form of m/z 605 via formation of an epoxide of the m/z 621 species, with a molecular formula of C24H50N2O13P2. Finally, the m/z 659 species (structure not shown) could represent the sodium adduct form of the m/z 637 protonated species, with the former having a molecular formula of C24H50N2O13P2Na. It does not seem unreasonable that these same assignments could apply to the dry eye tear extract spectrum shown in Figure 1B.
Figure 2.
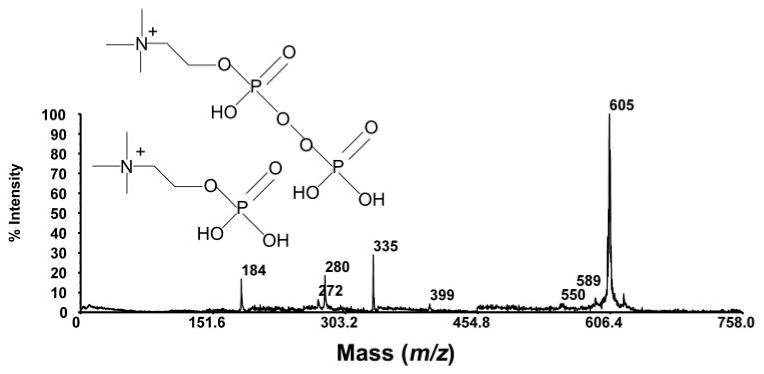
PSD spectrum of m/z 605 species in the SM standard.
Figure 3.
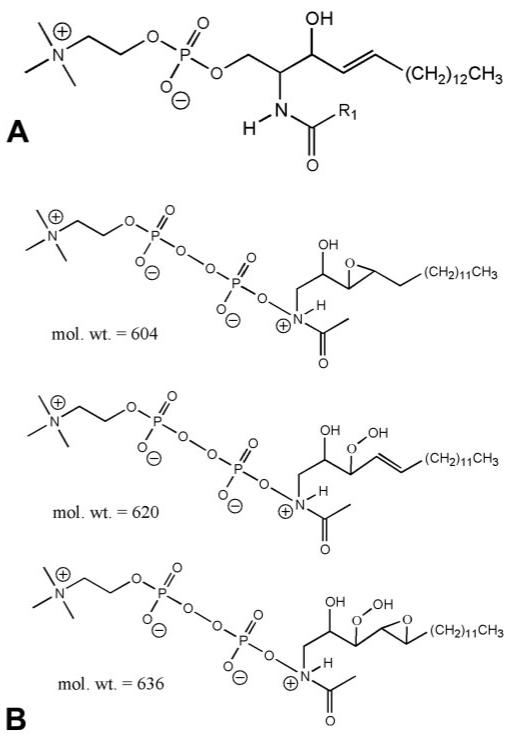
Proposed structures of (A) the SM standard and (B) pyrophosphate SMs.
The PSD spectra for the m/z 610 and 638 species (Fig. 4) indicate a relationship between the two, but these compounds remain unassigned. M/z 678 (Fig. 5A) has been assigned as C14:0-14:0 PC based on its molecular weight and the PSD spectrum, which illustrates the m/z 184 product ion indicative of the presence of a PC head group. The PSD spectrum of the m/z 828 species (Fig. 5B) indicates that it may be a PS, as supported by a diagnostic neutral loss of 57 Da producing a peak at m/z 741. The m/z 695, 866, 886, and 936 species are unidentified.
Figure 4.
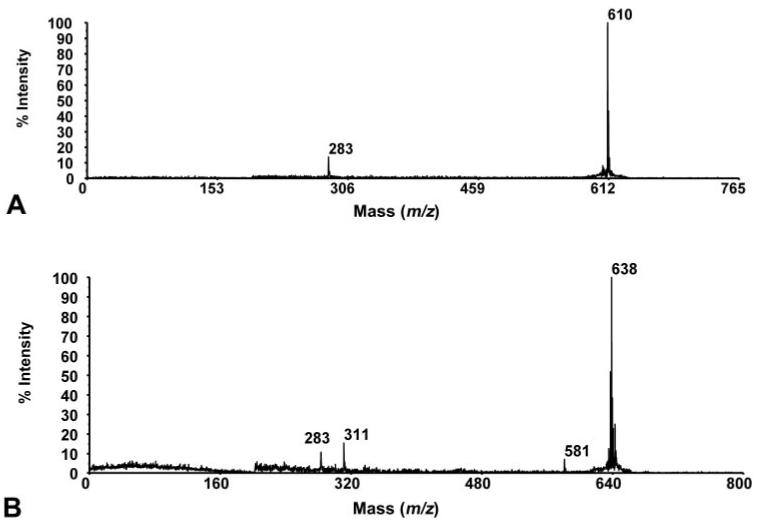
PSD spectrum of species found in MALDI-TOF mass spectra of tears. (A) PSD spectrum of m/z 610 species found as a major component in both normal and dry eye tears. (B) PSD spectrum of m/z 638 species, found as a major component only in normal tears.
Figure 5.
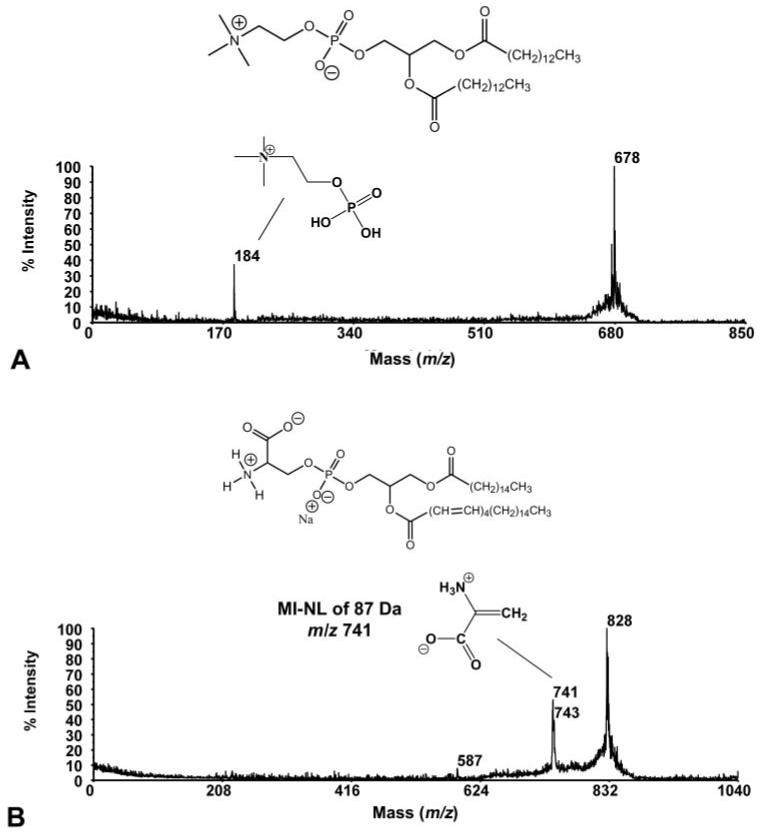
PSD spectra and molecular structures of species found in MALDI-TOF mass spectra of tears. (A) PSD spectrum and molecular structure of m/z 678 species (PC), found as a minor component in both normal and dry eye tears. (B) PSD spectrum and molecular structure of m/z 828 species (PS), found as a minor component in dry eye tears only.
Major differences in the normal and dry eye tear mass spectra were found in the peaks corresponding to SM and PS molecules. Two major SM peaks not found in the normal tears were present in the dry eye tears, and two minor SM peaks found in the normal tears were major peaks in the dry eye tears. A minor peak corresponding to PS was identifiable in the dry eye tears, but was not seen in the normal tears. In addition, a minor peak in the normal tears possibly corresponding to an oxidized form of PC was seen as a major peak in dry eye tears, and two of the minor, unidentifiable peaks in the normal tears were not present in the mass spectra of dry eye tears.
MALDI-TOF MS Analysis of Extracted Lacrimal Glands
Figure 6 is a representative MALDI-TOF MS spectrum of the extracted lipids from the main lacrimal glands of the rabbits. The predominant set of phosphorylated lipids, which is located in the region between m/z 700 and m/z 850, consists mainly of PCs, with some phosphatidylethanolamines (PEs). Table 2 lists the major peaks observed in the lacrimal gland extracts and their assignments. In addition to PC and PE molecules, peaks corresponding to PAF or lyso-PC (m/z 496) and pyrophosphate SM (m/z 577) were found. No peaks corresponding to PS were identified.
Figure 6.
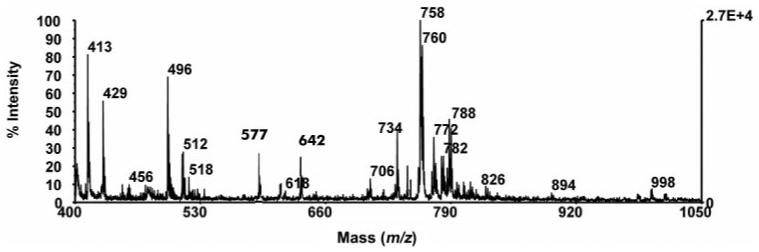
MALDI-TOF spectrum of phosphorylated lipids from rabbit lacrimal gland.
Table 2.
Assignment of MALDI-TOF Mass Spectra Peaks from Rabbit Main Lacrimal Gland
| Spectral Peak | Compound Assignment | Spectral Peak | Compound Assignment |
|---|---|---|---|
| M/z 413 | Unidentified | M/z 756 | 16:0-18:3 PC or 16:1-18:2 PC |
| M/z 429 | Unidentified | M/z 758 | 16:0-18:2 PC |
| M/z 496 | C14:0-2:0 PAF or C16:1 Lyso-PC | M/z 760 | 16:0-18:1 PC |
| M/z 511 | Unidentified | M/z 762 | 16:0-18:0 PC |
| M/z 512 | Unidentified | M/z 770 | 18:0-20:3 PE |
| M/z 518 | Unidentified | M/z 772 | 18:0-20:2 PE |
| M/z 577 | Pyrophosphate SM C22H46N2O11P2 | M/z 774 | 18:0-20:1 PE |
| M/z 610 | Unidentified | M/z 780 | 16:1-20:4 PC |
| M/z 642 | Unidentified | M/z 782 | 16:0-20:4 PC |
| M/z 706 | 14:0-16:0 PC | M/z 786 | 18:0-18:2 PC |
| M/z 720 | 16:0-18:0 PE | M/z 788 | 18:0-18:1 PC |
| M/z 734 | 14:0-18:0 PC | ||
| M/z 744 | 18:0-18:2 PE | M/z 810 | 18:0-20:4 PC |
| M/z 746 | 18:0-18:1 PE |
Discussion
Current tear film models suggest that a chemically stable, lamellar layer of polar phospholipids lies anterior to the aqueous fluid and binds the nonpolar meibomian oil to the aqueous layer.11,23,52,53 The presence of this polar phospholipid interface is thought to be critical to the spreading of the nonpolar lipid film over the aqueous layer. Shine and McCulley16 found that specific changes in the content of the polar phospholipid interface correlate directly with the presence of dry eye in patients with chronic blepharitis. However, in that the causes of most kinds of dry eye are unknown, conclusions regarding the origin of tear content differences are speculative. We used a nonevaporative dry eye rabbit model with specific glandular differences to determine the efficiency of our analysis method to identify tear content differences.
Although the meibomian gland supplies most of the lipids to the tear film, the lacrimal gland also provides some lipids, as well as most of the tear proteins. An early study (Stuchell RN, et al. IOVS 1984;25:ARVO Abstract 2) reported that the lipid composition of lacrimal gland secretions consisted of 55.0% glycolipids, 44.1% other neutral lipids, and 0.9% phospholipids. However, the types of phospholipids were not reported. In our study, we found that rabbit lacrimal glands had significant amounts of the same polar phospholipids found in the human tear fluid, although the degree to which these phospholipids are excreted into the tears is unknown.
In addition to the meibomian and main and accessory lacrimal glands, the rabbit has a harderian gland that contributes to the tear film components.27,54 The harderian gland lies in the nasal region of the orbit with its secretions flowing through ducts within the nictitating membrane. Analysis of the harderian gland has shown that tear proteins, mucins, and lipids are produced within the gland, although the degree to which these molecules are actively secreted is unknown.21,54-56 In our dry eye model, both the main and accessory lacrimal glands were removed, and the ducts from the harderian gland were severed and cauterized. Only the meibomian glands remained intact to supply lipids to the tear film.
Analysis of the rabbit meibomian gland phospholipids by Greiner et al.22,23 using NMR techniques revealed a higher concentration of zwitterionic and neutral phospholipids compared with anionic phospholipids. Rabbit meibomian oil was determined to contain 40% PC, 18% PE, 9% SM, 9% ethanolamine plasmalogen (EPLAS), 7% PS, and 6% dihydrosphingo-myelin (DHSM), with less than 3% other minor phospholipids.22,23 Our analysis of rabbit tear phospholipids also found a predominance of zwitterionic and neutral phospholipids, although the specific composition of phospholipids was slightly different. However, the ratio of zwitterionic and neutral phospholipids to anionic phospholipids was closer to that found for tarsal and meibomian gland tissue versus that found in expressed meibomian oil.23 Rabbit tears were found to contain high concentrations of SM, PAF, and lyso-PC with minor amounts of PC and PS.
Some strong similarities were found between the polar phospholipids of the normal and dry eye tears, using our novel analytical method. Phospholipid species related to PAF and/or lyso-PC, PC, and SM were found in similar quantities in both the normal and dry eye rabbit tears. Although specific identification as to which phospholipid (PAF, lyso-PC, or both) is represented by the m/z 486 peak remains to be determined, it would not be unreasonable to find either species, or both, present in the tears. PAF is a tear lipoprotein component known to be involved in the human lipid-mediated inflammatory response and such specific ocular conditions as allergic conjunctivitis57 and contact lens-induced acute red eye (CLARE).58 Lyso-PC, a species of PC with only one long carbon chain on the glycerol backbone, is generally associated with cell membrane stabilization, but has also been identified in meibomian gland secretions and may be present in the lipid layer of the tear film as a stabilizing factor. PAF and lyso-PC are also major cellular phospholipid components and could indicate possible cellular contamination in the tear samples. However, cell counts of tear samples revealed that, whereas only very small numbers of cells (<50 per sample) were present in the tear samples, there were almost three times as many cells in the dry eye tears as in the control tears. If the contribution from the cell components was significant, we would expect to see a larger amount of PAF and/or lyso-PC in the dry eye samples. Thus, the similar degree of expression of the peaks associated with PAF and lyso-PC may indicate that neither an inflammatory response nor an increased release of cell membrane phospholipids was associated with our dry eye model.
In addition, some significant differences in polar phospholipids were found in normal and dry eye rabbit tears using our novel analytical method. The number of types and the concentrations of SM molecules were significantly greater in the dry eye tears than in the normal tears. The normal rabbit tears showed the presence of three types of SM, with one major type present in a markedly higher concentration than the other two. The dry eye tears showed the same three SMs as in the normal tears, plus two additional SMs, all in substantially higher concentrations. SMs are zwitterionic phospholipids that have been suggested to play a significant role in maintaining the interfacial layer between the aqueous and lipid layers of the tear film. The polar head groups of zwitterionic and neutral phospholipids are known to align themselves head-to-tail, creating an interfacial layer between meibomian oil nonpolar lipids and aqueous tears that is chemically stable and resistant to mechanical rupture.11,23 A decrease in the percentage of SMs in the tear film has been correlated with the presence of dry eye in patients with chronic blepharitis, although the different SM species within the tear film were not identified.16 The presence of different SM species in the dry eye tears may indicate a compensation mechanism provided by the rabbit meibomian glands to stabilize the tear film in the absence of lacrimal and harderian gland secretions.
Previous work has shown that PE is one of the major phospholipids found in meibomian gland secretions, both human and rabbit, and may play a significant role as a zwitterionic phospholipid in the maintenance of tear stability.16,23 PE was not found in either the normal or dry eye rabbit tears examined. However, PS, a precursor in PE synthesis in mammalian cells, was found in significant quantities in the dry eye tears. PS is an anionic phospholipid and, as such, aids in the spreading of the meibomian oil film within the lipid interfacial layer through the mutual repulsion of negative charges.
The nonevaporative dry eye rabbit model used in this study allows us to analyze the physiological changes in the tear film when there is little or no lacrimal gland secretion. Many tear proteins produced by the lacrimal glands, such as tear lipocalin, play important roles in maintaining the continuity of the aqueous/lipid tear film layers. Changes in the phospholipid content may reflect attempts by the ocular system to stimulate meibomian gland secretions to stabilize the aqueous-protein-deficient tear film. Specifically, the presence of more precursor-like molecules, such as the oxidative SM species and PS found in this study, could indicate the overstimulation of the meibomian glands and premature release of cellular phospholipid reaction products.
The total chloroform extract of the tear layer constitutes a complex mixture of biological compounds that includes nonpolar and polar lipids and some lipoproteins. The polar lipid components make up a very small fraction of the total extract, rendering their analysis difficult by mass spectrometry alone. Combining the use of IMAC with the optimized solid ionic crystal MALDI matrix, paranitroaniline/butyric acid, enabled the detection and comparison of the lipoprotein and phosphorylated lipid components of normal versus dry eye rabbit tears.
Our analytical method not only allowed the assignment of the types of polar phospholipids present in the tear film, but also allowed assignment of specific molecular compositions within the phospholipid groups. Comparative analysis of polar phospholipids in rabbit tears from normal and dry eyes points to the importance of SM and PS in stabilizing the tear film in the rabbit dry eye model. Further development and application of this technique to human tears may allow identification of individual tear components and precise diagnosis of dry eye conditions.
Acknowledgments
Supported by Louisiana Board of Regents Grant LEQSF (2001-04)-RD-B-11 (RBC); National Eye Institute R03 EY014021 (JTJ) and P30 EY002377 (LSU Eye Center Core grant); and an unrestricted challenge grant from Research to Prevent Blindness, Inc. (LSU Eye Center).
Footnotes
Disclosure: B.M. Ham, None; R.B. Cole, None; J.T. Jacob, None
References
- 1.Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 2.Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly: a population-based perspective. Ophthalmology. 1997;104:1395–1401. doi: 10.1016/s0161-6420(97)30125-0. [DOI] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee JB, Ryu CH, Kim J-H, Kim EK, Kim HB. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:1326–1331. doi: 10.1016/s0886-3350(00)00566-6. [DOI] [PubMed] [Google Scholar]
- 6.De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004;137:109–115. doi: 10.1016/s0002-9394(03)00897-3. [DOI] [PubMed] [Google Scholar]
- 7.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 8.Ousler GW, 3rd, Abelson MB, Nally LA, Welch D, Casavant JS. Evaluation of the time to “natural compensation ” in normal and dry eye subject populations during exposure to a controlled adverse environment. Adv Exp Med Biol. 2002;506:1057–1063. doi: 10.1007/978-1-4615-0717-8_150. [DOI] [PubMed] [Google Scholar]
- 9.Moore JC, Tiffany JM. Human ocular mucus. Chemical studies. Exp Eye Res. 1981;33:203–212. doi: 10.1016/s0014-4835(81)80069-3. [DOI] [PubMed] [Google Scholar]
- 10.Carrington SD, Hicks SJ, Corfield AP, et al. Structural analysis of secreted ocular mucins in canine dry eyes. Adv Exp Med Biol. 1998;438:253–263. doi: 10.1007/978-1-4615-5359-5_37. [DOI] [PubMed] [Google Scholar]
- 11.McCulley JP, Shine WE. The lipid layer: the outer surface of the ocular surface tear film. Biosci Rep. 2001;21:407–418. doi: 10.1023/a:1017987608937. [DOI] [PubMed] [Google Scholar]
- 12.Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196. doi: 10.1016/s0074-7696(03)31004-6. [DOI] [PubMed] [Google Scholar]
- 13.Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004;78:389–394. doi: 10.1016/s0014-4835(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 14.Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- 16.Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852. doi: 10.1001/archopht.116.7.849. [DOI] [PubMed] [Google Scholar]
- 17.McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolaides N, Santos EC. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 1985;20:454–467. doi: 10.1007/BF02534237. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 20.Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360. doi: 10.1007/978-1-4615-5359-5_50. [DOI] [PubMed] [Google Scholar]
- 21.Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- 22.Greiner JV, Glonek T, Korb DR, Booth R, Leahy CD. Phospholipids in meibomian gland secretion. Ophthalmic Res. 1996;28:44–49. doi: 10.1159/000267872. [DOI] [PubMed] [Google Scholar]
- 23.Greiner JV, Glonek T, Korb DR, Leahy CD. Meibomian gland phospholipids. Curr Eye Res. 1996;15:371–375. doi: 10.3109/02713689608995827. [DOI] [PubMed] [Google Scholar]
- 24.Hemsley S, Cole N, Canfield P, Willcox MDP. Protein microanalysis of animal tears. Res Vet Sci. 2000;68:207–209. doi: 10.1053/rvsc.1999.0358. [DOI] [PubMed] [Google Scholar]
- 25.Herber S, Grus F, Sabuncuo P, Augustin AJ. Two-dimensional analysis of tear protein patterns of diabetic patients. Electrophoresis. 2001;22:1838–1844. doi: 10.1002/1522-2683(200105)22:9<1838::AID-ELPS1838>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Dartt DA, Matkin C, Gray K. Comparison of proteins in lacrimal gland fluid secreted in response to different stimuli. Invest Ophthalmol Vis Sci. 1988;29:991–995. [PubMed] [Google Scholar]
- 27.Millar TJ, Pearson M. The effects of dietary and pharmacological manipulation on lipid production in the meibomian and harderian glands of the rabbit. Adv Exp Med Biol. 2002;506:431–440. doi: 10.1007/978-1-4615-0717-8_61. [DOI] [PubMed] [Google Scholar]
- 28.Ohyama T, Matsubara C, Takamura K. Sensitive densitometry for the determination of platelet-activating factor and other phospholipids in human tears. Analyst. 1996;21:1943–1947. doi: 10.1039/an9962101943. [DOI] [PubMed] [Google Scholar]
- 29.Wollensak G, Mur E, Mayr A, Baier G, Gottinger W, Stoffler G. Effective methods for the investigation of human tear film proteins and lipids. Graefes Arch Clin Exp Ophthalmol. 1990;228:78–82. doi: 10.1007/BF02764296. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85:4866–4873. doi: 10.1210/jcem.85.12.7066. [DOI] [PubMed] [Google Scholar]
- 31.Szucs R, Verleysen K, Duchateau GSMJE, Sandra P, Vandeginste BGM. Analysis of phospholipids in lecithins comparison between micellar electrokinetic chromatography and high-performance liquid chromatography. J Chromatogr A. 1996;738:25–29. [Google Scholar]
- 32.Verleysen K, Sandra P. Analysis of phospholipids in lecithins: separation according to hydrophobicity by lowering the temperature. J High Resolution Chromatogr. 1997;20:337–339. [Google Scholar]
- 33.Raith K, Wolf R, Wagner J, Neubert RHH. Separation of phospholipids by nonaqueous capillary electrophoresis with electrospray ionization mass spectrometry. J Chromatogr A. 1998;802:185–188. [Google Scholar]
- 34.Han X, Gross RW. Structural determination of lysophospholipid regioisomers by electrospray ionization tandem mass spectrometry. J Am Chem Soc. 1996;118:451–457. [Google Scholar]
- 35.Hoischen C, Ihn W, Gura K, Gumpert J. Structural characterization of molecular phospholipid species in cytoplasmic membranes of the cell wall-less Streptomyces hygroscopicus L form by use of electrospray ionization coupled with collision-induced dissociation mass spectrometry. J Bacteriol. 1997;179:3437–3442. doi: 10.1128/jb.179.11.3437-3442.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu FF, Bohrer A, Turk J. Formation of lithiated adducts of glycerophosphocholine lipids facilitates their identification by electrospray ionization tandem mass spectrometry. J Am Soc Mass Spectrom. 1998;9:516–526. doi: 10.1016/S1044-0305(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 37.Khaselev N, Murphy RC. Electrospray ionization mass spectrometry of lysoglycerophosphocholine lipid subclasses. J Am Soc Mass Spectrom. 2000;11:283–291. doi: 10.1016/s1044-0305(99)00158-0. [DOI] [PubMed] [Google Scholar]
- 38.Hsu FF, Turk J. Characterization of phosphatidylethanolamine as a lithiated adduct by triple quadrupole tandem mass spectrometry with electrospray ionization. J Mass Spectrom. 2000;35:596–606. doi: 10.1002/(SICI)1096-9888(200005)35:5<595::AID-JMS965>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 39.Liebisch G, Drobnik W, Lieser B, Schmitz G. High-throughput quantitation of lysophosphatidylcholine by electrospray ionization tandem mass spectrometry. Clin Chem. 2002;48:2217–2224. [PubMed] [Google Scholar]
- 40.Ho YP, Huang PC, Deng KH. Metal ion complexes in the structural analysis of phospholipids by electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:114–121. doi: 10.1002/rcm.880. [DOI] [PubMed] [Google Scholar]
- 41.Hsu FF, Turk J, Shi Y, Groisman EA. Characterization of acylphosphatidylglycerols from Salmonella typhimurium by tandem mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2004;15:1–11. doi: 10.1016/j.jasms.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Marto JA, White FM, Seldomridge S, Marshall AG. Structural characterization of phospholipids by matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem. 1995;67:3979–3984. doi: 10.1021/ac00117a025. [DOI] [PubMed] [Google Scholar]
- 43.Schiller J, Arnhold J, Benard S, Muller M, Reichl S, Arnold K. Lipid analysis by matrix-assisted laser desorption and ionization mass spectrometry: a methodological approach. Anal Biochem. 1999;267:46–56. doi: 10.1006/abio.1998.3001. [DOI] [PubMed] [Google Scholar]
- 44.Ishida Y, Nakanishi O, Hirao S, et al. Direct analysis of lipids in single zooplankter individuals by matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 2003;75:4514–4518. doi: 10.1021/ac030072j. [DOI] [PubMed] [Google Scholar]
- 45.Al-Saad KA, Zabrouskov V, Siems WF, Knowles NR, Hannan RM, Hill HH., Jr. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of lipids: ionization and prompt fragmentation patterns. Rapid Commun Mass Spectrom. 2003;17:87–96. doi: 10.1002/rcm.858. [DOI] [PubMed] [Google Scholar]
- 46.Rujoi M, Estrada R, Yappert MC. In situ MALDI-TOF MS regional analysis of neutral phospholipids in lens tissue. Anal Chem. 2004;76:1657–1663. doi: 10.1021/ac0349680. [DOI] [PubMed] [Google Scholar]
- 47.Woods AS, Ugarov M, Egan T, et al. Lipid/peptide/nucleotide separation with MALDI-ion mobility-TOF MS. Anal Chem. 2004;76:2187–2195. doi: 10.1021/ac035376k. [DOI] [PubMed] [Google Scholar]
- 48.Murphy RC, Fiedler J, Hevko J. Analysis of nonvolatile lipids by mass spectrometry. Chem Rev. 2001;101:479–526. doi: 10.1021/cr9900883. [DOI] [PubMed] [Google Scholar]
- 49.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 50.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 51.Ham BM, Jacob JT, Cole RB. MALDI-TOF MS of phosphorylated lipids in biological fluids using immobilized metal affinity chromatography and a solid ionic crystal matrix. Analyt Chem. 2005;77:4439–4447. doi: 10.1021/ac058000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62. doi: 10.1016/b978-0-12-024922-0.50005-9. [DOI] [PubMed] [Google Scholar]
- 53.Tiffany JM. Composition and biophysical properties of the tear film: knowledge and uncertainty. Adv Exp Med Biol. 1994;350:231–238. doi: 10.1007/978-1-4615-2417-5_40. [DOI] [PubMed] [Google Scholar]
- 54.Seyama Y, Kasama T, Yasugi E, Park SH, Kano K. Lipids in harderian glands and their significance. In: Webb SM, Hoffman RA, Puig-Domingo ML, Reier RJ, editors. Harderian Glands: Porphyrin, Metabolism, Behavioral and Endocrine Effects. Springer-Verlag; Berlin: 1992. pp. 195–217. [Google Scholar]
- 55.Millar TJ, Herok G, Koutavas H, Martin DK, Anderton PJ. Immunohistochemical and histochemical characterization of epithelial cells of the rabbit lacrimal glands in tissue sections and cell cultures. Tissue Cell. 1996;28:301–312. doi: 10.1016/s0040-8166(96)80017-6. [DOI] [PubMed] [Google Scholar]
- 56.Dartt D, Knox I, Palau A, Bothelho SY. Proteins in fluids from individual orbital glands and in tears. Invest Ophthalmol Vis Sci. 1980;19:1342–1347. [PubMed] [Google Scholar]
- 57.Kato M, Mano H, Ota A, Konomi K, Nakata K. Platelet activating factor degradation in tear fluid from guinea pigs with allergic conjunctivitis. J Ocul Pharmacol Ther. 2001;17:83–91. doi: 10.1089/108076801750125757. [DOI] [PubMed] [Google Scholar]
- 58.Thakur A, Willcox MDP. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp Eye Res. 1998;67:9–19. doi: 10.1006/exer.1998.0480. [DOI] [PubMed] [Google Scholar]


