Summary
Dyskeratosis congenita (DC), an inherited bone marrow failure syndrome, is caused by defects in telomerase. Somatic cells from DC patients have shortened telomeres and clinical symptoms are most pronounced in organs with a high cell turnover, including those involved in hematopoiesis and skin function. We previously identified an autosomal dominant (AD) form of DC that is caused by mutations in the telomerase RNA component (TER). In this study, we evaluated whether retroviral expression of TER and/or telomerase reverse transcriptase (TERT), the catalytic component of telomerase, could extend telomere length and rescue AD DC cells from a phenotype characteristic of early senescence. Exogenous TER expression, without TERT, could not activate telomerase in AD DC skin fibroblasts. Transduction of TERT alone, however, provided AD DC cells with sufficient telomerase activity to extend average telomere length and proliferative capacity. Interestingly, we found that expression of TER and TERT together resulted in extension of lifespan and higher levels of telomerase and longer telomeres than expression of TERT alone in both AD DC and normal cells. Our results provide evidence that AD DC cells can be rescued from defects in telomere maintenance and proliferation, and that coexpression of TERT and TER together provides a more efficient means to elongate telomeres than expression of TERT alone. Similar strategies may be useful for ameliorating the detrimental effects of telomere shortening in AD DC and other diseases associated with telomerase or telomere defects.
Keywords: aging, aplastic anemia, dyskeratosis congenita, gene therapy, telomere, telomerase, TER, TERT
Introduction
Telomeres consist of hexameric tandem repeats (TTAGGG) of DNA located at the chromosome ends and are necessary for maintaining chromosome integrity, function, and replication (Moyzis et al., 1988; Greider, 1991). Telomeres normally shorten with each cell division and it is thought that this eventually results in cellular dysfunction, aging, and genetic instability (Vaziri et al., 1994; Allsopp et al., 1995; Blasco, 2005). Shortening of telomeres is primarily due to what has been termed ‘the end replication problem’, which simply means that DNA polymerase cannot completely replicate the 5′ end of newly synthesized linear DNA strands because it requires a 3′ primer (Levy et al., 1992). In addition, a number of other mechanisms for telomere loss have been described (Lansdorp, 2005). Telomeres can be maintained by telomerase, an enzyme complex consisting of an RNA component called TER that acts as a template for a reverse transcriptase component TERT (telomerase reverse transcriptase) to catalyze the addition of telomere repeats to the telomere ends (Greider & Blackburn, 1987; Feng et al., 1995; Autexier et al., 1996; Meyerson et al., 1997; Nakamura et al., 1997; Weinrich et al., 1997). Telomerase is active in human germline cells and most cancers (Kim et al., 1994; Wright et al., 1996). Most normal human somatic cells, such as fibroblasts, express TER but have low to undetectable levels of TERT and almost undetectable levels of telomerase activity. Some highly proliferative cells such as activated B and T lymphocytes also have active telomerase, although in these cells it is thought to be tightly regulated (Broccoli et al., 1995; Counter et al., 1995; Harle-Bachor & Boukamp, 1996). Definitive proof that telomere shortening is involved in cellular senescence was provided in a seminal study in which it was demonstrated that expression of TERT could activate telomerase, maintain telomeres, and extend the lifespan of human cells without any other apparent changes (Bodnar et al., 1998; Jiang et al., 1999).
Dyskeratosis congenita (DC), inherited in both an X-linked and an autosomal dominant (AD) manner, is a premature aging syndrome characterized by bone marrow failure, leukoplakia, abnormal skin pigmentation, and nail dystrophy (Dokal, 2000; Collins & Mitchell, 2002; Dokal & Vulliamy, 2003). A variety of somatic abnormalities normally seen in aged individuals have also been reported in DC, including aplastic anemia, hair loss, gray hair, osteoporosis, cancer, and pulmonary and hepatic fibrosis. DC patients also display an increased risk for malignancy, although bone marrow failure is the main cause of early mortality. The X-linked version of the disease is caused by mutations in dyskerin, a protein that interacts with TER, and is required for TER accumulation (Heiss et al., 1998; Mitchell et al.,1999a; Collins & Mitchell, 2002; Fu & Collins, 2003). Measurement of telomeres from the somatic cells from patients of the X-linked form of the disease revealed that they were shorter than normal (Mitchell et al., 1999b; Vulliamy et al., 2001a). The AD form of DC (AD DC), a more rare form of DC, is generally caused by mutations in TER (Vulliamy et al., 2001b; Yamaguchi et al., 2003), although a recent article reported a three-generation AD DC kindred that was associated with a mutation in TERT (Armanios et al., 2005). Other studies have shown that certain forms of aplastic anemia, without overt signs of DC, are also associated with mutations in TER and TERT (Yamaguchi et al., 2003, 2005; Ly et al., 2005; Vulliamy et al., 2005; Xin et al., 2006). These latter results suggest that certain hematologic disorders besides DC may also be associated with defects in telomere length maintenance.
We have recently identified a large three-generation kindred of AD DC individuals with a TER mutation that display all of the typical features associated with DC (Vulliamy et al., 2001a). The mutation in this family creates a 3′ truncation of the last 74 bases of TER. Interestingly, this region has been shown to be important for binding to dyskerin, thus potentially linking the X-linked and autosomal forms of the disease (Mitchell et al., 1999a). It has been speculated that some TER mutations may act in a dominant negative fashion, interfering with the normal TER expressed from the other allele (Prescott & Blackburn, 1997; Ren et al., 2003). This does not appear to be the case for the TER mutant found in our family as it expressed at low to undetectable levels, and reconstitution experiments indicate that it has no dominant negative effect (Marrone et al., 2004). A recent study suggests that some naturally derived TER mutants can act in a dominant negative fashion, although none of these mutants had a large-scale deletion (Xin et al., 2006). Average telomere lengths in lymphocytes from AD DC patients are shorter than normal (Vulliamy et al., 2001a,b; Baerlocher et al., 2002; Goldman et al., 2005), and AD DC lymphocytes exhibit proliferative and functional defects (Knudson et al., 2005). Telomere length analysis of individual chromosomes in AD DC cells failed to detect a bimodal distribution of telomere lengths, but instead demonstrated that cells had shortened telomeres overall, suggesting that shortened telomeres inherited from the affected parent may be preferentially elongated during development at the expense of longer telomeres from the unaffected parent (Goldman et al., 2005).
Reconstitution of telomerase components and telomere length in DC cells could be considered as a potential means to prevent premature senescence and cell dysfunction in vitro and in vivo. A recent report demonstrated that exogenous expression of TERT in X-linked DC fibroblasts was able to extend lifespan but was insufficient to elongate telomeres without coexpression of TER (Wong & Collins, 2006). To our knowledge, no definitive experiments have been performed to determine whether telomerase activation in cells from patients with AD DC can restore telomere length. In the present study, we have transduced AD DC fibroblasts with different combinations of TERT and TER vectors and demonstrate that telomerase activation can restore telomere length and rescue cells from senescence. Our studies indicate that coexpression of TERT and TER together may provide a more efficient means to restore and elongate telomere length than expression of TERT alone.
Results
Shortened telomeres and lifespan in AD DC fibroblasts
AD DC fibroblasts proliferated at rates similar to normal cells at early passage. However, the proliferative lifespan of the AD DC cells was about half that of their normal counterparts (Fig. 1A). To quantitatively assess telomere signal between the AD DC and normal cells and in the cells as they were passaged in culture, we used an established real-time polymerase chain reaction (PCR) methodology that measures relative levels of telomere repeats as compared to a single gene copy control (Cawthon, 2002). Using this method, we found that telomere signal in the early passage AD DC cells was significantly less than that observed in early passage normal cells (~three-fold less) and even less than that observed in normal cells at the point of senescence (Fig. 1B). This is similar to data obtained previously on lymphocytes from third-generation patients from this family (Knudson et al., 2005). Thus, somatic cells from AD DC patients start out with extremely short telomeres and a proliferative lifespan in vitro that essentially mirrors proliferative defects in vivo.
Fig. 1.
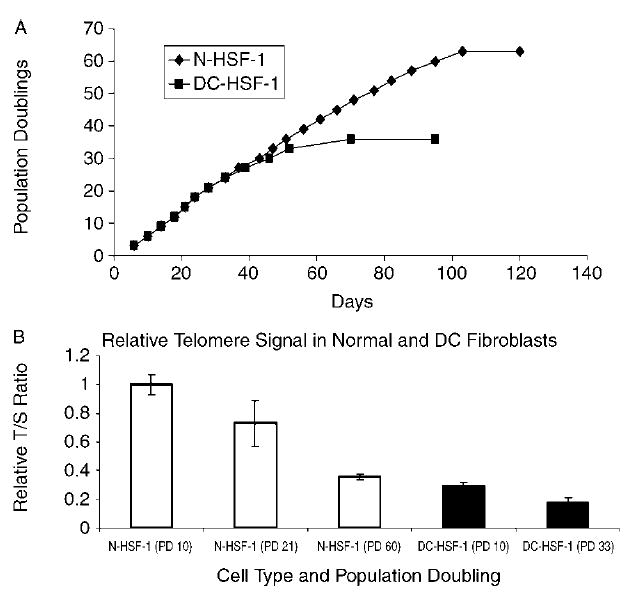
Premature senescence and short telomeres in AD DC (DC-HSF-1) as compared to normal (N-HSF-1) fibroblasts. (A) DC-HSF-1 cells proliferated for approximately half the lifespan of normal cells. (B) Quantification of telomere signal in normal and AD DC fibroblasts at early passage and senescence. Telomere signal was ascertained by real-time PCR methodology as described in the Experimental procedures. The T : S ratio represents the ratio of telomere signal over that of a single gene copy control (all relative to the T : S ratio of N-HSF-1 at early passage). All error bars represent standard error of the mean from three replicate assays.
Reconstitution of TER, telomerase, and telomere length in AD DC fibroblasts
To determine whether telomerase and telomere length could be reconstituted in AD DC cells, we generated a replication defective feline immunodeficiency virus (FIV) vector that coexpresses TER and enhanced green fluorescent protein (eGFP) from separate promoters (Fig. 2A). In this construct, the TER gene contains a large portion (515 bp) of the endogenous 3′ end, which we reasoned would allow for proper processing of the RNA into the mature form (Kim et al., 2001; Fu & Collins, 2003). As has been described previously (Fu & Collins, 2003), a snoRNA U3 promoter was used to drive TER expression, whereas eGFP was expressed from a cytomegalovirus (CMV) promoter. This vector, designated FIV TER/eGFP, was used to generate vesicular stomatitis virus G (VSV-G) pseudotyped virus and was tested for functionality in telomerase negative VA13 cells. VA13, which utilizes an alternative lengthening of telomeres (ALT) mechanism of telomere elongation, is negative for expression of both TER and TERT, but a variant was generated that expresses TERT but still remains telomerase negative (Fu & Collins, 2003). Infection of TERT VA13 cells with our vector resulted in high telomerase activity, demonstrating that the vector was efficient and functional at expressing TER (Fig. 2B). Northern analysis also verified that cells transduced with the TER/eGFP construct expressed a mature TER RNA of approximately 450 bases in length (Fig. 2C).
Fig. 2.
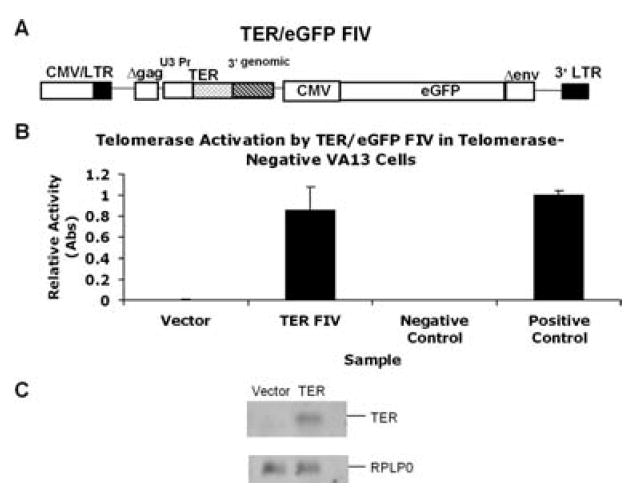
Reconstitution of telomerase in TER negative cells. (A) The TER/eGFP FIV lentiviral construct. The TER gene along with a U3 small nucleolar (sno) RNA polymerase II promoter was inserted into the replication defective eGFP FIV construct. (B) The TER/eGFP FIV and eGFP vector alone were pseudotyped with VSV-G and were transduced into TERT expressing, TER negative VA13 cells. Transduction resulted in high telomerase activity in these cells, indicating functionality of the introduced TER gene. (C) Northern blot showing the accumulation of mature TER of approximately 450 bases in cells transduced with the TER/eGFP FIV as compared to eGFP FIV alone. RPLP0 is an internal control for RNA loading.
We then performed experiments to determine whether the FIV TER/eGFP vector could reconstitute TER expression and telomerase activity in AD DC cells. As it is known that normal fibroblasts express barely detectable levels of TERT, we reasoned that activation of telomerase in AD DC and normal fibroblasts would require exogenous expression of TERT. AD DC and normal fibroblasts were therefore transduced with different combinations of vectors to express GFP alone, TER alone, TERT alone, or TER and TERT together. Approximately 90% transduction efficiency was obtained using the FIV constructs at a multiplicit of infection (MOI) of 5, as assessed by GFP expression (data not shown). Cells that had been successfully transduced with the TERT retroviral vectors were selected in G418, whereas cells that were transduced with the lentiviral vector were followed for eGFP expression. Cells were passaged as pools or individual colonies and were ring cloned and subcultured. Analysis of TER levels in the vector only cells by quantitative reverse transcriptase PCR (RT-PCR) demonstrated that AD DC cells had approximately half as much TER transcript as normal cells, as would be expected due to one defective copy of TER in AD DC cells (Fig. 3A). Based on this result, it was not surprising that expression of TERT alone activated telomerase in AD DC fibroblasts but to a lower level (~1/2) than that observed when TERT alone was expressed in normal fibroblasts (Fig. 3B). Interestingly, expression of TERT alone, in both AD DC and normal fibroblasts, resulted in higher levels of TER, suggesting that TERT can stabilize TER as has been previously proposed (Fig. 3A) (Wong & Collins, 2006). As expected, both AD DC and normal fibroblasts transduced with the TER vector alone had higher levels of TER than untransduced cells, but TER expression alone did not activate telomerase in either cell type. Expression of TER and TERT together in AD DC fibroblasts, on the other hand, brought the level of telomerase to approximately the same as what was observed in normal fibroblasts that expressed TERT alone. Coexpression of TER and TERT in normal fibroblasts caused telomerase activity that was higher than that observed with TERT alone, indicating that TER levels were a limiting component in cells that overexpressed TERT.
Fig. 3.
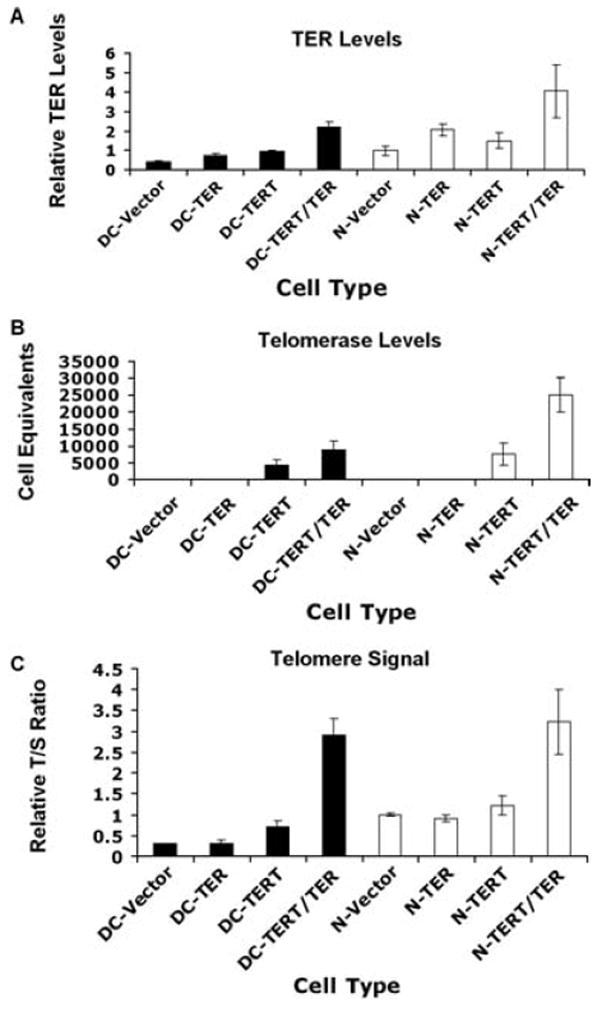
Reconstitution of TER, telomerase, and telomere length in AD DC fibroblasts. Cells were transduced with vector, TER alone, TERT alone, or TER and TERT together. AD DC cells are in black and normal cells in white. (A) TER levels in transduced cells as measured by QRT-PCR. Values are relative to vector transduced normal cells (N-Vector). (B) Telomerase activity as measured by a quantitative PCA TRAP assay [in cell equivalents as compared to the activity of a standard E6/E7 immortalized human skin keratinocytes (HSK) cell line]. (C) Relative telomere length in transduced cells. Quantification of telomere signal was ascertained by real-time PCR methodology. The T : S ratio represents the ratio of telomere signal over that of a single gene copy control (all relative to the T : S ratio of N-Vector). All error bars represent standard error of the mean from three replicate assays.
We next assessed telomere signal, as a measure of telomere repeats, in the cells by qPCR. Telomere signal corresponded well with telomerase activity (Fig. 3B,C). Transduction of vector or TER alone did not activate telomerase or elongate telomeres in either AD DC or normal cells, and TERT AD DC cells had approximately half the telomere signal of TERT normal cells (Fig. 3C). Telomere signal was clearly much greater in AD DC or normal cells that expressed TER and TERT together. Thus, our results indicate that AD DC cells were partially deficient for telomere elongation upon TERT expression alone but that coexpression of TERT and TER together resulted in robust telomerase activity and telomere elongation in both AD DC and normal cells.
Extension of proliferative capacity and maintenance of telomeres in AD DC cells
Experiments were next undertaken to determine the fate of transduced cells over long-term culture. To do so, we cultured three clones each of TERT and TERT/TER expressing cells for an additional 20 passages (~60 pd). Comparison of cells at approximately 15 pd postcloning (E) to cells at approximately 75 pd postcloning (L) demonstrated that AD DC fibroblasts clones expressing TERT alone maintained shorter telomeres than normal fibroblasts expressing TERT alone, again reflecting haplo-insufficiency of TER in the AD DC cells (Fig. 4A). None of the TERT DC clones exhibited elongation of telomeres beyond that observed at early passage and at least one of these clones exhibited telomere shortening over time. However, both the normal and AD DC clones expressing TERT and TER together generally had longer telomeres at later passage, with some clones exhibiting extremely long telomeres. Measurement of telomerase activity in early and later passage transduced DC cells indicated heterogeneity among different clones, and several of the TERT and TERT/TER clones exhibited a decrease in telomerase activity over time (Fig. 4B). The reason for this loss is unknown. Not surprisingly, the one TERT/TER DC clone, DC-TERT/TER-C, with the longest telomeres at later passage also maintained the highest levels of telomerase at later passage (Fig. 4).
Fig. 4.
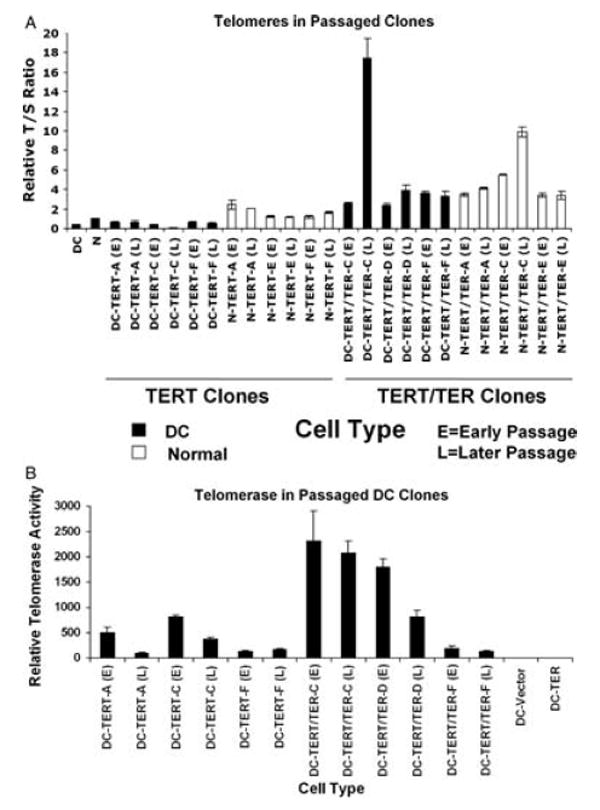
(A) Telomere length over long-term passaging in TERT and TERT/TER transduced AD DC (DC) and normal (N) fibroblast clones. E, early passage (P5, ~15 pd postcloning); L, later passage (P25, ~75 pd postcloning). Three clones of each cell type were analyzed. Telomere length was assessed as in Fig. 3 with a quantitative PCR assay. Relative T : S ratio is the ratio of telomere over single gene signal made relative to early passage normal (N) fibroblasts. All values represent the average of three replicate assays. Error bars are standard error of the mean. (B) Telomerase activity in early and late passages of TERT and TERT/TER transduced DC cells. ‘E’ and ‘L’ designations are similar to those described in 4A. Error bars represent standard error of the mean for three replicate assays.
Despite differences in telomerase and telomere signal, all AD DC normal clones expressing TERT alone or TERT and TER together had a significantly extended lifespan, which was greater than three times that of vector alone (> 75 pd vs. ~20 pd post-subcloning), and these cells are apparently immortal. Visual examination of the transduced AD DC fibroblasts demonstrated that expression of TERT alone or TERT and TER together caused a ‘rejuvenated’ phenotype in that they exhibited a morphology that was similar to early passage normal fibroblasts (Fig. 5). No consistent differences in cell growth or morphology were observed between cells that expressed TERT alone and cells that expressed TERT and TER together. In contrast, cells expressing TER alone did not appear to be phenotypically different than vector alone, indicating that expression of TER alone has no profound consequences in AD DC or normal fibroblasts.
Fig. 5.
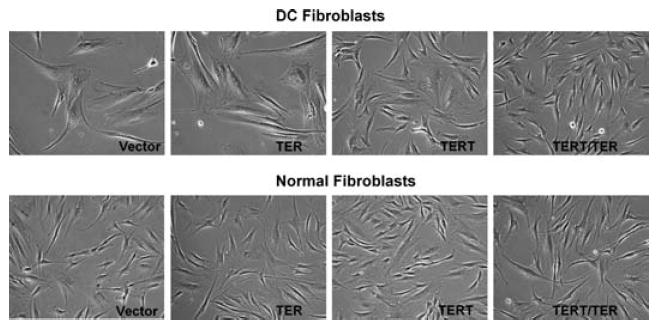
Telomerase reconstitution ‘rejuvenates’ AD DC fibroblasts. Normal and DC fibroblasts were transduced with vector, TER, TERT, or TERT/TER as described in the Experimental procedures. Photographs were taken within three passages after selection (×100 magnification).
To further characterize how TER and TERT expression affected telomere length in AD DC and normal fibroblasts, telomere specific quantitative fluorescence in situ hybridization (Q-FISH) was performed on a subset of transduced cells. Using Q-FISH, it was demonstrated that vector AD DC fibroblasts had telomere lengths that were approximately half that of normal (6.7 kb vs. 11.2 kb) (Fig. 6A,B). Expression of TERT alone resulted in intermediate telomere lengths (11.2 kb for TERT DC and 24.4 kb for TERT normal) whereas coexpression of TERT and TER together resulted in long telomere lengths (32 kb for TERT/TER DC and 51 kb for TERT/TER normal). Interestingly, the TERT/TER DC clone at early passage exhibited two subpopulations that resulted in an apparent bimodal distribution of telomere length (Fig. 6B). This pattern was not observed in DC cells transduced with vector or TERT alone, indicating that DC cells do not start with a bimodal distribution. Furthermore, two distinct subpopulations of cells with different telomere lengths were apparent in the early passage DC-TERT/TER-C clone (data not shown). Assessment of a later passage of this particular clone demonstrated even more extensive elongation (average telomere length of ~114 kb) and a more normal distribution of length (Fig. 6C), suggesting that the subpopulation with longer telomeres outgrew the population with shorter telomeres. Interestingly, all the transduced cells at early and later passage retained an apparently normal diploid karyotype (data not shown). Overall, these results indicate that TERT alone can activate telomerase and, for the most part, maintain telomeres in AD DC cells, but that TERT and TER together leads to higher telomerase activity and longer telomeres.
Fig. 6.
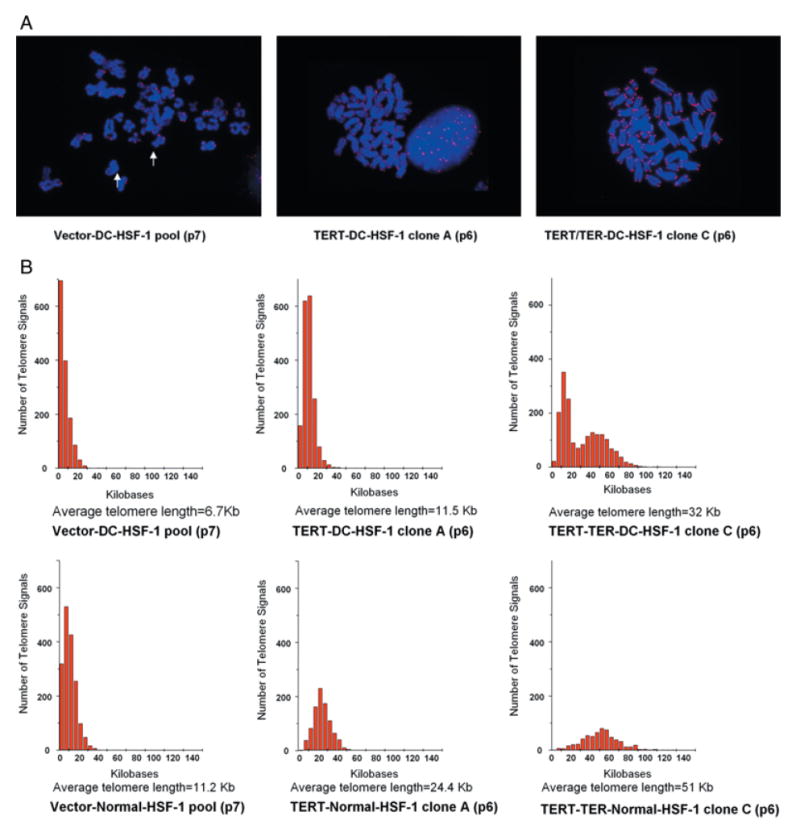
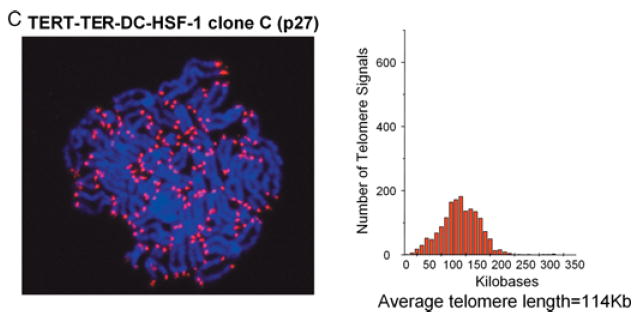
Telomere length as assessed by Q-FISH. (A) Representative metaphases of vector only AD DC fibroblasts (Vector-DC-HSF-1), TERT expressing AD DC fibroblast clone at early passage (TERT-DC-HSF-1 clone A), and TERT-TER expressing AD DC fibroblast clone at early passage (TERT-TER-DC-HSF-1 clone C). Q-FISH was performed using a PNA telomere-specific probe (see Experimental procedures). (B) Histogram representation of telomere signals from > 400 telomeres per cell type of transduced early passage AD DC fibroblasts including Vector-DC-HSF-1, TERT-DC-HSF-1 clone A, and TERT-TER-DC-HSF-1 clone C (top three panels) and transduced early passage normal (N-HSF-1) fibroblasts Vector-N-HSF-1, TERT-N-HSF-1 clone A, and TERT-TER-N-HSF-1 clone C (bottom three panels). The Y-axis on each graph represents frequency of events while the X-axis represents telomere length (as ascertained by calibration with controls). Average telomere lengths are shown beneath each graph. (C) Extensive elongation and a normal distribution of telomere length in later passage TERT-TER DC cells.
Telomerase acts on shortest telomeres in AD DC cells
To examine the effects of telomerase reactivation in AD DC fibroblasts, we measured telomere length on individual chromosomes through Q-FISH and karyotyping. Most chromosomes in AD DC cells that expressed vector alone or TER alone had short telomeres with some chromosomes, such as chromosome 2, having extremely short telomeres (Fig. 7). Upon TERT expression, overall telomere length on all the chromosomes increased and, interestingly, even very short telomeres were restored to a length that was similar to other chromosomes. Our results indicate that telomerase acts on the shortest telomeres, preferentially, and that all telomeres are brought to a new baseline length. These results nicely validate studies on lymphocytes from AD DC individuals and unaffected children of an AD DC parent, which suggested that telomerase acts on the shortest telomeres during development (Goldman et al., 2005).
Fig. 7.
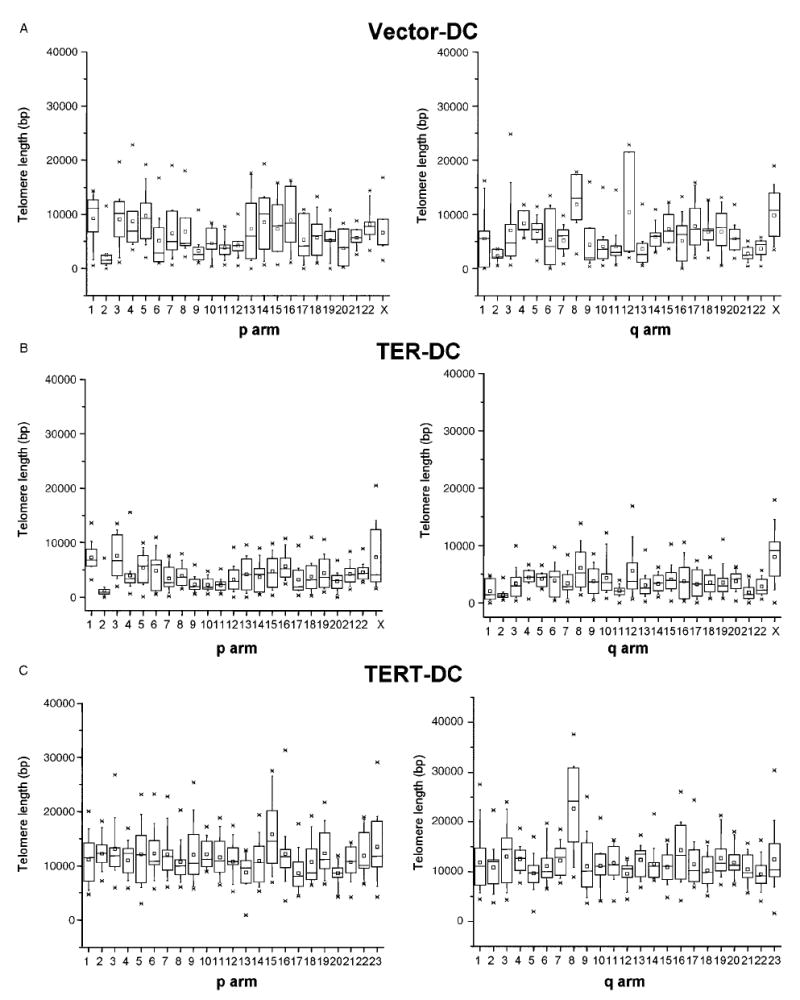
Effects of telomerase on telomere length at individual chromosome arms. Graphs represent telomere length on the ‘p’ and ‘q’ arms from individual chromosomes of early passage cells expressing eGFP only (A), TER (B), or TERT (C). The distribution of telomere length at individual chromosome arms in 7–20 metaphase cells is expressed in box plots. In each box plot, the stars represent the first and 99th percentile of the telomere length values, the lines represent the 10th and 90th percentile, and the boxes represent the 25th and 75th percentile. Median values are given by the small box and the 50th percentile of the telomere length distribution by the horizontal bar in the box.
Discussion
In this study, we have demonstrated that telomerase reconstitution in AD DC cells restores telomere length and significantly extends cellular lifespan. Expression of TERT alone, although sufficient to extend the lifespan of AD DC cells, did not result in telomeres that were as long as TERT expressing normal cells, although our results indicate that even limited telomerase activity acts on the shortest telomeres in the AD DC cells. Coexpression of TERT and TER together caused high levels of telomerase activity and greatly extended telomere length in both AD DC and normal fibroblasts. Our results provide important insights into how TERT and TER synergize in human cells to restore telomeres and point to possible strategies to extend telomere length in hematopoietic stem cells or other cells that are refractory to telomere elongation by expression of TERT alone.
Clearly, telomerase is tightly regulated in normal cells and levels are critical for determining whether telomeres are maintained or not. In AD DC families with mutations in TER, it is the level of TER that is rate limiting for telomerase activity. Telomerase haplo-insufficiency during development is thought to result in telomere shortening and telomeres become even shorter in dividing cells from tissues of highly proliferative organs such as the hematopoietic system and the skin. Mouse knockout studies support this conclusion in that TER or TERT heterozygotes exhibit telomere shortening over time and with increasing generations (Hathcock et al., 2002; Armanios et al., 2005; Hao et al., 2005). In our experiments, overexpression of TERT resulted in telomerase levels that were approximately half that of TERT expressing normal cells, reflecting the expected haplo-insufficiency for TER in AD DC cells. Our finding that TERT expression alone in AD DC cells led to an overall increase in average telomere length as compared to cells with vector alone indicates that limiting levels of telomerase act on the shortest telomeres. This result is in line with mouse studies demonstrating that crosses between telomerase knockout mice with short telomeres and mice with wild-type telomerase and long telomeres results in progeny with an intermediate telomere length equilibrium characterized by elongation of the shortest telomeres and preferential maintenance of short telomeres (Hathcock et al., 2002; Liu et al., 2002). Our findings also complement results showing that unaffected homozygous wild-type children from affected AD DC patients have shorter average telomere length overall but no specific set of chromosomes that have extremely short length (Armanios et al., 2005; Goldman et al., 2005).
Recent studies indicate that coexpression of TER and TERT together results in higher telomerase and longer telomeres than TERT expression alone, providing further evidence that TER levels can limit telomere maintenance (Cristofari & Lingner, 2006; Wong & Collins, 2006). In our studies, exogenous expression of TER and TERT together also resulted in robust telomerase activity and greatly extended telomeres in some clones. Although our experiments indicated that TERT alone resulted in extended lifespan and longer telomeres than cells transduced with vector alone, we did not observe extensive telomere elongation over time in these cells and, in at least one case, we observed telomere shortening. This latter result is similar to a recent study by Wong and Collins showing that TERT expression alone in fibroblasts from X-linked DC patients resulted in higher telomerase and significantly extended lifespan but not telomere elongation (Wong & Collins, 2006). Any differences in telomere length maintenance upon expression of TERT alone between X-linked and AD DC cells could be due to a variety of factors including expression levels of dyskerin in the different cells.
Whether transduction of TER alone could activate telomerase in other somatic cells from AD DC patients is unknown, but may in part be dependent on the level of endogenous TERT, which is nearly undetectable in fibroblasts. Some cells such as hematopoietic stem cells appear to be somewhat refractory to immortalization by TERT, and TERT expression alone did little in terms of maintaining telomeres (Wang et al., 2005). Coexpression of TERT and TER together might provide a more efficient means to activate telomerase, restore telomeres, and extend proliferative lifespan in these cell types. In our studies, coexpression of TERT and TER sometimes led to telomeres that were incredibly long. Such long telomeres are not generally observed in diploid human cells and it is possible that these cells have lost mechanisms to regulate normal telomere length. In this regard, it will be of interest to ascertain levels of proteins known to be involved in telomere length regulation (e.g., TRF1, TRF2, and POT1) in cells with such long telomeres (de Lange, 2005).
Because there is the potential of upregulating telomerase to ameliorate problems associated with telomere shortening and poor cell proliferation in DC patients (and even the elderly), it will be important to determine whether AD DC cells with restored telomeres retain normal cellular function. TERT has been shown to have functions that go beyond its ability to elongate telomeres and these functions could interfere with normal cell physiology (Dong et al., 2005). Extensive telomere elongation may also disrupt normal cell responses. For example, there is evidence that telomere length plays a significant role in regulating response to DNA damaging agents and oxidative stress (Goytisolo et al., 2000; Wong et al., 2000; Gonzalez-Suarez et al., 2003). The cells that we have generated should be of significant value in determining whether restored telomere length, particularly extremely long telomeres, alters responses to these agents. Exogenous expression of TERT may also result in cellular transformation and the consequences of telomerase activation and telomere elongation with regard to the carcinogenic process in AD DC cells is unknown. It should be noted that telomere length is not the only factor that regulates senescence in human cells. Several studies, including our own, indicate that telomere-independent stress-related pathways such as p16INK4a upregulation are important for inducing a senescence response even in the presence of high telomerase activity and long telomeres (Kiyono et al., 1998; Dickson et al., 2000; Noble et al., 2004; Darbro et al., 2006; Janzen et al., 2006). It is interesting to note that in our experiments, normal fibroblasts senesced with a telomere length that was longer than that observed in AD DC fibroblasts, indicating that other factors may come into play in determining when senescence in culture occurs.
Although still speculative, it is possible that problems associated with telomerase mutations and telomere shortening could be ‘fixed’ in adult stem cell populations by transduction of telomerase component genes. One could envision that such a strategy might be useful for treating certain patients with aplastic anemia, including DC patients, where there is a demonstrated association between telomere shortening and disease. Even if one could safely extend telomeres in hematopoietic stem cells to extend their lifespan, thus potentially alleviating bone marrow failure, other tissue in the affected individuals could still fail due to premature telomere shortening and senescence in the cells of these tissues. Nevertheless, our studies are proof of principle that it is possible to restore telomeres and proliferative capacity in cells from AD DC patients and provide an entry point to develop strategies to alleviate telomere shortening in vivo.
Experimental procedures
Cell culture
The University of Iowa Internal Review Board approved this study and all subjects gave informed consent. Fibroblasts were obtained from 4 mm skin punch biopsies of a third-generation AD DC-affected patient (Knudson et al., 2005) and a normal age-matched control. Briefly, the epidermis was removed by overnight dispase treatment at 4°C. The underlying dermis was incubated in collagenase overnight at 37 °C and dissociated by pipetting. Cells were spun down and replated in culture dishes in 10% FBS/DMEM with Fungizone, penicillin, and streptomycin for the first passages, with removal of Fungizone for subsequent passages. The cells were split 1 : 8 when they were 90% confluent. TERT expressing VA-13 cells were kindly provided by Kathy Collins (Berkeley) and grown in 10% FBS/DMEM with penicillin and streptomycin.
Retroviral constructs, and transduction of cells
A FIV vector that coexpresses TER and eGFP from separate promoters was generated by inserting the TER gene with a 5′ upstream U3 promoter and the TER endogenous 3′ end (a gift from Kathy Collins, Berkeley) into the Mre11 site of eGFP pVETL (kindly provided by Beverly Davidson, University of Iowa). The TER gene contains a large portion of the endogenous 3′ end (515 bp), which allows processing of the RNA into the mature form, resulting in a normal 451 base product (Kim et al., 2001; Fu & Collins, 2003). FIV constructs were pseudotyped with VSV-G at the Gene Vector Core at the University of Iowa using previously described methods (Wang et al., 1999). The TERT-pBABE-neo vector (Dickson et al., 2000) was obtained from Robert Weinberg (Whitehead Institute, Cambridge, MA, USA) and the pLXSN vector from Denise Galloway (Fred Hutchinson Cancer Research Center, Seattle, WA, USA). These latter murine leukemia virus (MLV)-based vectors were packaged in Phoenix Amphotropic packaging lines according to previously published protocols (Darbro et al., 2006). All retroviral infections were carried out overnight in the presence of polybrene at 8 μg mL−1 All cells were dually infected with combinations of an FIV or MLV-based vector for consistency. Infection with eGFP FIV or TER eGFP FIV vectors were performed at ~2 MOI and infection was monitored by visualizing green fluorescence after 48 h. Cells infected with TERT-pBABE-neo or pLXSN were selected in 1 mg mL−1 G418 for 10 days. Cells were passaged as pools or plated at high dilutions and ring cloned after approximately 10 days growth. Clones were selected on the basis of green fluorescent expression (presence of eGFP FIV or TER eGFP FIV).
Real-time PCR to quantify telomere signal
Real-time quantitative PCR was performed based on the protocol from Cawthon et al. (2003) (Cawthon, 2002; Epel et al., 2004). Genomic DNA was isolated from one 100 mm confluent plate by trypsinizing and pelleting the cells and using the Qiagen (Qiagen, Valencia, CA, USA) blood/tissue DNA kit according to manufacturer’s instructions. DNA was eluted in 100 μL volumes. Thirty-five nanogram of DNA was amplified using two different sets of primers, one for telomere sequences, and the other for the house-keeping gene 36B4. The composition of the master mixes was identical except for the primer pairs. PCR reactions contained 1× 6-ROX reference dye (Invitrogen, Carlsbad, CA, USA), 0.2×Syber Green (Roche, Indianapolis, IN, USA), 15 mm Tris-HCl pH 8.0, 50 mm KCl, 2 mm MgCl2, 0.2 mm each dNTP, 5 mm DTT, 1% DMSO, and 1.25 U AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA). Telomere primer concentrations were: tel1, 270 nm (5′-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT-3′) and tel2, 900 nm (5′-TCCCGACTATCCCTATCCCTATCCCTATCCCCTATCCCTA-3′). The 36B4 primer concentrations were: 36B4u, 300 nm (5′-CAGCAAGTGGGAAGGTGTAATCC-3′) and 36B4d, 500 nm (5′-CCCATTCTATCATCAACGGGTACAA-3′). The PCR profile for both primer sets was 95 °C for 10 min, followed by 30 cycles at 95 °C for 15 s, and 54 °C for 2 min. Samples were run in triplicate in a 25-μL volume. The T : S ratio (telomere signal over single gene signal) was calculated as [2Ct(telomere)/2Ct(36B4)]−1 or 2−ΔCt Once the T/S ratio was calculated, it was made relative to one sample (usually normal fibroblasts or vector control) as indicated and replicate values were averaged. For error bars, standard error of the mean was calculated from the three independent replicates.
Q-FISH and karyotyping
Q-FISH with Cy-3-labeled (CCCTAA)3 peptide nucleic acid (PNA) and subsequent analysis of digital images were performed as described (Poon et al., 1999; Baerlocher et al., 2002). Briefly, we hybridized a PNA probe (CCCTAA)3 specific for mammalian telomeres (Applied Biosystems) for 2 h at room temperature to methanol/acetic acid-fixed cells followed by heat denaturation at 72 °C for 3 min. To remove nonhybridized PNA probes, slides were washed with 0.05% Tween-20-containing PBS at 56 °C for 15 min and were visualized by using a Nikon Eclipse E800 fluorescence microscope. For each cell line, at least 400 chromosomes were analyzed and the mean fluorescence intensity was correlated to telomere length as ascertained by utilizing plasmids with fixed numbers of telomere repeats (Poon et al., 1999). Image acquisition and analysis software (TFL-Telo, developed in the Lansdorp laboratory and available from http://www.flintbox.com/technology.asp?sID=7BDFF89E0F704B879F6EBFB3C841A343&page=535) was used for analysis and display of data. For analysis of telomere length from individual chromosomes, both Q-FISH and karyotyping was performed as previously described (Martens et al., 1998).
Measurement of TER levels
Total RNA was collected from cultured primary fibroblast samples using TRI Reagent followed by chloroform extraction and ethanol precipitation as per the manufacturer’s instructions followed by purification using RNeasy (Qiagen) according to manufacturer’s protocol. cDNA was constructed from 1.8 μg of isolated RNA using the RETROscript reagents and protocols (Ambion, Austin, TX, USA). TER RNA expression levels were analyzed using real-time PCR. Briefly, cDNA was amplified in triplicate using TER primers1 (forward: 5′-TCTAACCCTAACTGAGAAGGGCGTAG-3′; reverse: 5′-GTTTGCTCTAGAATGAACGGTGGAAG-3′) in conjunction with SYBR-GREEN PCR master mix (Applied Biosystems) and was analyzed on an ABI PRISM 7000 Sequence Detection System. Primers amplifying 18 s RNA (forward: 5′-CCT TGGATGTGGTAGCCCGTTT-3′; reverse: 5′-AACTTTCGATGGTAGTCGCCG-3′) were run in parallel to normalize RNA levels among fibroblast samples and ΔCt was calculated for each sample by the equation: ΔCt = CtTER − Ct18S. Fold differences in TER mRNA levels were calculated relative to normal fibroblasts infected with empty vector (Vector N-HSF-1) by using the equation: 2(ΔΔCt) where ΔΔCt = ΔCt(Vector N-HSF-1) − ΔCt(Cell Type).
Quantitative telomerase assay
Real-time quantitative telomeric repeat amplification protocol (RTQ-TRAP) assays were done based on a protocol from Hou et al. (2001). Briefly, a 50-μL reaction mixture containing 1× SYBER Green buffer (Applied Biosystems), 2.5 mm each dNTP, 1.5 mm MgCl2, 1 mm EGTA, 1 μg T4 Gene 32 protein (NEB), 0.1 μg each of primers TS (5′-AATCCGTCGAGCAGAGTT-3′) and ACX [5′-GCGCGG(CTTACC)3CTAACC-3′], one unit AmpliTaq Gold polymerase, and 1 μL (1000 cell equivalent) of protein lysate. Reactions were performed in the ABI PRISM 7700 thermal cycler (Applied Biosystems). The PCR mixture was incubated at room temperature for 30 min followed by 40 cycles (95 °C for 10 min, 95 °C for 15 s, and 60 °C for 1 min). Triplicate threshold values (Ct) were compared with standard curves derived from serial dilutions of telomerase-immortalized E6 LXSN E7 LXSH keratinocytes (5000, 1000, 200, 40 cells).
Northern analysis
DIG Northern analysis was conducted using a DIG Northern Kit as described in the manufacturer’s protocol (Roche Applied Science). Total RNA of 2 μg was separated on a 2% denaturing agarose gel and was transferred to Hybond-N membrane (Amersham, Arlington Heights, IL, USA). DIG-labeled hTER antisense RNA was synthesized using T7 polymerase and SfoI cut pBSV3hTR500 (hTER plasmid from Kathy Collins) as a template. Antisense DIG-labeled RPLP0 (Ribosomal Protein Large P0, aka 36B4) from a SalI digested pGEM5 plasmid clone of RPLP0 was used as a loading control. After hybridization and washes, chemiluminescent detection was performed with anti-DIG-AP antibody and CDP-Star (Roche) followed by exposure in a FujiImager.
Acknowledgments
We thank Kathy Collins for the TER construct and the VA13 cells, Beverly Davidson for the FIV vector, and Robert Weinberg for the TERT-pBABE-neo vector. The authors also wish to acknowledge the Gene Transfer Vector Core Facility of the University of Iowa Center for Gene Therapy of Cystic Fibrosis and Other Genetic Diseases for their assistance with FIV production and the University of Iowa DNA Core for help with quantitative PCR. This study was supported by NIH Grant AI29524 (P.M.L.), a pilot award from University of Iowa Cancer Aging Program Grant 5P20 CA103672 (A.J.K.), and a grant from the Aiming for a Cure Foundation (F.D.G.).
References
- Allsopp RC, Chang E, Kashefiaazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci USA. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autexier C, Pruzan R, Funk WD, Greider CW. Reconstitution of human telomerase activity and identification of a minimal functional region of the human telomerase RNA. EMBO J. 1996;15:5928–5935. [PMC free article] [PubMed] [Google Scholar]
- Baerlocher GM, Mak J, Tien T, Lansdorp PM. Telomere length measurement by fluorescence in situ hybridization and flow cytometry: tips and pitfalls. Cytometry. 2002;47:89–99. doi: 10.1002/cyto.10053. [DOI] [PubMed] [Google Scholar]
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- Counter CM, Gupta J, Harley CB, Leber B, Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995;85:2315–2320. [PubMed] [Google Scholar]
- Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006;25:565–574. doi: 10.1038/sj.emboj.7600952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbro BW, Lee KM, Nguyen NK, Domann FE, Klingelhutz AJ. Methylation of the p16(INK4a) promoter region in telomerase immortalized human keratinocytes co-cultured with feeder cells. Oncogene. 2006;25:7421–7433. doi: 10.1038/sj.onc.1209729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- Dokal I, Vulliamy T. Dyskeratosis congenita: its link to telomerase and aplastic anaemia. Blood Rev. 2003;17:217–225. doi: 10.1016/s0268-960x(03)00020-1. [DOI] [PubMed] [Google Scholar]
- Dong CK, Masutomi K, Hahn WC. Telomerase: regulation, function and transformation. Crit Rev Oncol Hematol. 2005;54:85–93. doi: 10.1016/j.critrevonc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu JH, Le SY, West MD, Harley CB, Andrews WH, Greider CW, Villeponteau B. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Fu D, Collins K. Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol Cell. 2003;11:1361–1372. doi: 10.1016/s1097-2765(03)00196-5. [DOI] [PubMed] [Google Scholar]
- Goldman F, Bouarich R, Kulkarni S, Freeman S, Du HY, Harrington L, Mason PJ, Londono-Vallejo A, Bessler M. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci USA. 2005;102:17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Goytisolo FA, Flores JM, Blasco MA. Telomere dysfunction results in enhanced organismal sensitivity to the alkylating agent N-methyl-N-nitrosourea. Cancer Res. 2003;63:7047–7050. [PubMed] [Google Scholar]
- Goytisolo FA, Samper E, Martin-Caballero J, Finnon P, Herrera E, Flores JM, Bouffler SD, Blasco MA. Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J Exp Med. 2000;192:1625–1636. doi: 10.1084/jem.192.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW. Telomeres. Curr Opin Cell Biol. 1991;3:444–451. doi: 10.1016/0955-0674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci USA. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock KS, Hemann MT, Opperman KK, Strong MA, Greider CW, Hodes RJ. Haploinsufficiency of mTR results in defects in telomere elongation. Proc Natl Acad Sci USA. 2002;99:3591–3596. doi: 10.1073/pnas.012549799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- Hou M, Xu D, Bjorkholm M, Gruber A. Real-time quantitative telomeric repeat amplification protocol assay for the detection of telomerase activity. Clin Chem. 2001;47:519–524. [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PLC, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kim MM, Rivera MA, Botchkina IL, Shalaby R, Thor AD, Blackburn EH. A low threshold level of expression of mutant-template telomerase RNA inhibits human tumor cell proliferation. Proc Natl Acad Sci USA. 2001;98:7982–7987. doi: 10.1073/pnas.131211098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Knudson M, Kulkarni S, Ballas Z, Bessler M, Goldman F. Association of immune abnormalities with telomere shortening in autosomal dominant dyskeratosis congenita. Blood. 2005;105:682–688. doi: 10.1182/blood-2004-04-1673. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Lansdorp PM. Major cutbacks at chromosome ends. Trends Biochem Sci. 2005;30:388–395. doi: 10.1016/j.tibs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kha H, Ungrin M, Robinson MO, Harrington L. Preferential maintenance of critically short telomeres in mammalian cells heterozygous for mTert. Proc Natl Acad Sci USA. 2002;99:3597–3602. doi: 10.1073/pnas.062549199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly H, Calado RT, Allard P, Baerlocher GM, Lansdorp PM, Young NS, Parslow TG. Functional characterization of telomerase RNA variants found in patients with hematologic disorders. Blood. 2005;105:2332–2339. doi: 10.1182/blood-2004-09-3659. [DOI] [PubMed] [Google Scholar]
- Marrone A, Stevens D, Vulliamy T, Dokal I, Mason PJ. Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood. 2004;104:3936–3942. doi: 10.1182/blood-2004-05-1829. [DOI] [PubMed] [Google Scholar]
- Martens UM, Zijlmans JM, Poon SS, Dragowska W, Yui J, Chavez EA, Ward RK, Lansdorp PM. Short telomeres on human chromosome 17p. Nat Genet. 1998;18:76–80. doi: 10.1038/ng0198-018. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999a;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999b;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Noble JR, Zhong ZH, Neumann AA, Melki JR, Clark SJ, Reddel RR. Alterations in the p16(INK4a) and p53 tumor suppressor genes of hTERT-immortalized human fibroblasts. Oncogene. 2004;23:3116–3121. doi: 10.1038/sj.onc.1207440. [DOI] [PubMed] [Google Scholar]
- Poon SS, Martens UM, Ward RK, Lansdorp PM. Telomere length measurements using digital fluorescence microscopy. Cytometry. 1999;36:267–278. doi: 10.1002/(sici)1097-0320(19990801)36:4<267::aid-cyto1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Prescott J, Blackburn EH. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 1997;11:2790–2800. doi: 10.1101/gad.11.21.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Gavory G, Li H, Ying L, Klenerman D, Balasubramanian S. Identification of a new RNA.RNA interaction site for human telomerase RNA (hTR): structural implications for hTR accumulation and a dyskeratosis congenita point mutation. Nucleic Acids Res. 2003;31:6509–6515. doi: 10.1093/nar/gkg871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001a;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001b;27:353–357. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis. 2005;34:257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Wang G, Slepushkin V, Zabner J, Keshavjee S, Johnston JC, Sauter SL, Jolly DJ, Dubensky TW, Jr, Davidson BL, McCray PB., Jr Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest. 1999;104:R55–R62. doi: 10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Warner JK, Erdmann N, Lansdorp PM, Harrington L, Dick JE. Dissociation of telomerase activity and telomere length maintenance in primitive human hematopoietic cells. Proc Natl Acad Sci USA. 2005;102:14398–14403. doi: 10.1073/pnas.0504161102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, Taylor RD, Carlos R, Andrews WH, Wright WE, Shay JW, Harley CB, Morin GB. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- Wong KK, Chang S, Weiler SR, Ganesan S, Chaudhuri J, Zhu C, Artandi SE, Rudolph KL, Gottlieb GJ, Chin L, Alt FW, DePinho RA. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat Genet. 2000;26:85–88. doi: 10.1038/79232. [DOI] [PubMed] [Google Scholar]
- Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Xin ZT, Beauchamp AD, Calado RT, Bradford JW, Regal JA, Shenoy A, Liang Y, Lansdorp PM, Young NS, Ly H. Functional characterization of natural telomerase mutations found in patients with hematological disorders. Blood. 2006;109:524–532. doi: 10.1182/blood-2006-07-035089. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]


