Abstract
From the carbolithiation of 6--dimethylamino fulvene (3) and 2,4[bis(-dimethylamino)methyl]--methylpyrrolyl lithium (2a), -(-dimethylaminomethyl)benzimidazolyl lithium (2b), or -(-dimethylamino)methylphenyl lithium (2c), the corresponding lithium cyclopentadienide intermediate (4a–c) was formed. These three lithiated intermediates underwent a transmetallation reaction with TiCl4' resulting in -dimethylamino-functionalised titanocenes 5a–c. When these titanocenes were tested against a pig kidney epithelial cell line (LLC-PK), the values obtained were of 23, and 52 M for titanocenes 5a and 5b, respectively. The most cytotoxic titanocene in this paper, 5c with an value of 13 M, was found to be approximately two times less cytotoxic than its analogue Titanocene C ( M) and almost four times less cytotoxic than cisplatin, which showed an value of 3.3 M when tested on the LLC-PK cell line.
1. INTRODUCTION
Titanium-based reagents have significant potential against solid tumors. Budotitane ([cis-diethoxybis(1-phenylbutane-1,3-dionato)titanium(IV)]) looked very promising during its preclinical evaluation, but did not go beyond Phase I clinical trials, although a Cremophor EL-based formulation was found for this rapidly hydrolysing molecule [1]. Much more robust in this aspect of hydrolysis is titanocene dichloride (Cp2TiCl2), which shows medium antiproliferative activity in vitro but promising results in vivo [2, 3]. Titanocene dichloride reached clinical trials, but the efficacy of Cp2TiCl2 in Phase II clinical trials in patients with metastatic renal cell carcinoma [4] or metastatic breast cancer [5] was too low to be pursued.
More recently, novel methods starting from fulvenes [6–17] and other precursors [18–20] allow direct access to highly substituted titanocenes via reductive dimerisation, carbolithiation, or hydridolithiation of the fulvene followed by transmetallation in the last two cases.
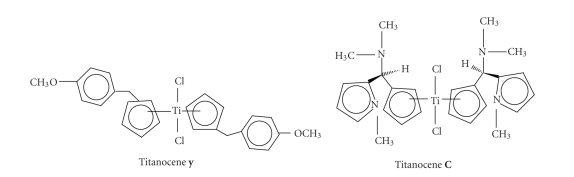
Titanocene Y was obtained using hydridolithiation of fulvene, and it showed an IC50 value of 21 μM [12]. The antiproliferative activity of Titanocene Y has been studied in 36 human tumor cell lines [21] and in explanted human tumors [22]. These in vitro and ex vivo experiments showed that prostate, cervix, and renal cell cancers are prime targets for these novel classes of titanocenes, whereas the IC50 values for the breast cancer cell lines were very promising as well. These results were underlined by first mechanistic studies concerning the effect of these titanocenes on apoptosis and the apoptotic pathway in prostate cancer cells [23]. Furthermore, first animal studies have been published recently, reporting the successful treatment of xenografted Ehrlich's ascites tumor in mice with an ansa-titanocene [24] and xenografted Caki-1 tumors with Titanocene Y [25], showing that the effect of Titanocene Y against xenografted Caki-1 tumors in mice was superior to cisplatin. The structure of Titanocene Y is shown in Figure 1.
Figure 1.
Structure of Titanocenes Y and C.
So far, our most cytotoxic titanocene, Titanocene C (bis-(-dimethylamino-2(-methylpyrrolyl)methylcyclo pentadienyl) titanium (IV) dichloride, was obtained through carbolithiation of fulvenes, which has been published recently [26]. It has an IC50 value of 5.5 μM when tested on the LLC-PK cell line. Its structure is shown in Figure 1. This meant significant progress, since Cp2TiCl2 exhibits an IC50 value of only 2000 μM against LLC-PK, which explains partly the failed Phase II clinical trials against renal cell carcinoma. The main idea behind the research presented in this paper was to improve the cytotoxicity of Titanocene C by adding extra dimethylamino groups using the well-established Mannich reaction. Within this paper, we present a new series of chiral titanocenes, their synthesis, and preliminary cytotoxicity studies.
2. EXPERIMENTAL
2.1. General conditions
Titanium tetrachloride (1.0 M solution in toluene) and butyl lithium (2.0 M solution in pentane) were obtained commercially from Aldrich Chemical Co. (Wis, USA). THF was dried over Na and benzophenone, and it was freshly distilled and collected under an atmosphere of argon prior to use. Manipulations of air and moisture sensitive compounds were done using standard Schlenk techniques under an argon atmosphere. NMR spectra were measured on either a Varian 300 or a 400 MHz spectrometer. Chemical shifts are reported in ppm and are referenced to TMS. IR spectra were recorded on a Perkin Elmer Paragon 1000 FT-IR Spectrometer employing a KBr disk. UV/Vis spectra were recorded on a Unicam UV4 Spectrometer, while CHN analysis was done with an Exeter Analytical CE-440 Elemental Analyser.
2.2. Synthesis
6-(-dimethylamino) fulvene (3) was synthesised according to the already published procedure [27].
Synthesis of bis-(3-[2,4-di(-dimethylamino)methyl]- -methylpyrrolyl-(-dimethylamino)-methyl-cyclopentadienyl) titanium (IV) dichloride, {η 5–C5H4–CH[N(CH3)2][C4H2(CH2–N(CH3)2)2N(CH3)]}2TiCl2 (5a) —
To a Schlenk flask with 2,4[bis(-dimethylamino) methyl]--methyl pyrrole (1.61 g, 8.25 mmol), 20 ml of THF were added until a transparent solution was formed, while stirring at room temperature. The solution was cooled down to −78°C for 15 minutes and 4.8 mL (8.25 mmol) of butyl lithium were added. The solution was allowed to warm up to 0°C for 20 minutes, resulting in the formation of the yellow lithium intermediate.
In a second Schlenk flask, 6-(-dimethylamino) fulvene (1.00 g, 8.25 mmol) was dissolved in THF, and the resultant orange solution was added via cannula at −78°C to the Schlenk flask containing the lithiated intermediate. The reaction mixture was then allowed to warm up to room temperature and left stirring for 40 minutes. Titanium tetrachloride (4.1 ml, 4.13 mmol) was added afterwards in situ at room temperature, and the mixture was refluxed for 20 hours. Subsequently, the solvent was removed under vacuum, resulting in the formation of a dark brown oil that was dissolved in dichloromethane and filtered through Celite to remove the LiCl. The black filtrate was filtered additionally twice by gravity filtration. The solvent was removed under reduced pressure forming a shiny dark red solid, which was washed with 20 ml of pentane and then dried in vacuo (1.44 g, 1.93 mmol, 46.8% yield).
1H NMR (δ ppm CDCl3, 400 MHz): 6.36 (m, 8 H, C5 H 4); 6.05 (s, 2 H, [C4 H(CH2–N(CH3)2)2N(CH3)]); 3.8–2.6 (m, 14 H, [C4H(CH2–N(CH 3)2)2N(CH 3)], [C4H(CH2–N(CH3)2)2N(CH 3)], [C4H(CH 2–N(CH3)2)2N(CH 3)]); 2.36 (s, 26 H, C4 H(CH2–N(CH 3)2)2N(CH3)).
13C NMR (δ ppm CDCl3, 125 MHz, proton decoupled): 138, 135, 132, 126, 121, 119, 108 [C 5H4 and C 4H(CH2–N(CH3)2)2N(CH3)]; 52 [C5H4–CH–(N(CH3)2)(C4H(CH2–N(CH3)2)2N(CH3)]; 34, 32 [N(CH3)2 and C4H(CH2–N(CH3)2)2N(CH3)].
IR absorptions (cm−1 KBr): 3414, 2917, 2769, 1620, 1466, 1382, 1018.
Anal. Calc. for C38H62N8TiCl2: C, 60.87; H, 8.35; N, 14.95; Cl, 9.46; Found: C, 59.80; H, 8.29; N, 14.18; Cl, 9.45.
UV-Vis (CH2Cl2): λ 244 nm ( 10 833), λ 330 nm ( 12 996), 510 nm (weak).
Synthesis of bis-[(-dimethylaminomethyl- 2-benzimidazolyl)(dimethylamino) methylcyclopentadienyl]titanium (IV) dichloride, {η 5–C5H4–CH[N(CH3)2][C10H12N3]}2TiCl2 (5b) —
To a Schlenk flask with -(-dimethylaminomethyl) benzimidazol (1.45 g, 8.25 mmol), 20 ml of THF were added until a transparent solution was formed, while stirring at room temperature. The solution was cooled down to −78°C for 15 minutes and 14.0 ml (8.25 mmol, 1.7 M) of butyl lithium were added. The solution was allowed to warm up to 0°C for 20 minutes, resulting in the formation of the yellow lithium intermediate.
In a second Schlenk flask, 1.00 g (8.25 mmol) of 6-,-dimethylamino fulvene was dissolved in THF, and the resultant red solution was added via cannula at −78°C to the Schlenk flask containing the lithiated intermediate. The reaction mixture was then allowed to warm up to room temperature and left stirring for 40 minutes. Titanium tetrachloride (4.1 ml, 4.13 mmol) was added afterwards in situ at room temperature and the mixture was refluxed for 20 h. Subsequently, the solvent was removed under vacuum, resulting in the formation of a dark green oil that was dissolved in dichloromethane and filtered through Celite to remove the LiCl. The black filtrate was filtered additionally twice by gravity filtration. The solvent was removed under reduced pressure forming a shiny black solid, which was washed with 20 ml of pentane and then dried in vacuo (1.97 g, 2.77 mmol, 67.3% yield).
1H NMR (δ ppm CDCl3, 400 MHz): 7.80–7.82 [m, 4H, C5H4–CH[N(CH3)2 ]–C–N–C–CH–CH–CH–CH–C–N (CH2N(CH3)2)]; 7.49–7.51 [m, 4 H, C5H4 –CH[N(CH3)2]–C–N–C–CH–CH–CH–CH–C–N(CH2N(CH3)2]; 6.42–6.71 [m, 8H, C5 H 4–CH[N(CH3)2]–C7H4NN–CH2-N (CH3)2]; 4.85 [s, 4H, C5H4–CH[N(CH3)2]–C7H4NN–CH 2-N (CH3)2]; 4.00 [m, 2H, C5H4–CH[N(CH3)2]–C7H5N2–CH2-N–(CH3)2]; 2.16, 2.23, 2.34 [s, 24 H, C5H4–CH [N(CH 3)2]–C7H5N2–CH2-N–(CH 3)2].
13C NMR (δ ppm CDCl3, 125 MHz, proton decoupled): 144, 142, 136, 135, 132, 126, 123, 122, 121, 120, 119, 112[C 5H4–CH[N(CH3)2]–C 7H5N2–CH2-N–(CH3)2]; 69, 68,64,44,43,36,34,32,23,28,19,14,13[C5H4–CH[N(CH3)2]–C7H5N2–CH2-N–(CH3)2].
IR absorptions (cm−1 KBr): 3429, 2926, 1635, 1456, 1270, 1039, 861, 743.
Anal. Calc. for C36H46N8TiCl2: C, 60.93; H, 6.53; N, 15.79; Cl, 9.99 Found: C, 59.99; H, 6.52; N, 15.72; Cl, 9.99.
UV-Vis (CH2Cl2): λ 230 nm ( 22 770), λ 402 nm ( 2020), λ 499 nm ( 210), 523 nm (weak).
Bis-(-dimethylamino)--(-dimethyl- amino)methylphenylcyclopentadienyl) titanium (IV) dichloride, {η 5-C5H4–CH[N(CH3)2][C6H4CH2N(CH3)2]}2TiCl2 (5c) —
To a Schlenk flask with 0.37 g (1.73 mmol) -(-dimethylamino)methylphenylbromide, 14 ml of THF were added until a transparent solution was formed, while stirring at room temperature. The solution was cooled down to −78°C and 1.02 ml (1.73 mmol, 1.7 M) of -butyl lithium were added. The solution was allowed to warm up to 0°C for 20 minutes, resulting in the formation of the yellow lithium intermediate.
In a second Schlenk flask, 0.21 g (1.73 mmol) of 6-(-dimethylamino) fulvene was dissolved in THF, and the resultant red solution was added via cannula at −78°C to the Schlenk flask containing the lithiated intermediate. The reaction mixture was then allowed to warm up to room temperature and left stirring for 40 minutes. Titanium tetrachloride (0.86 ml, 0.86 mmol) was added afterwards in situ at room temperature and the mixture was refluxed for 20 h. Subsequently, the solvent was removed under vacuum, resulting in the formation of a dark brown oil that was dissolved in dichloromethane and filtered through Celite to remove the LiCl. The black filtrate was filtered additionally twice by gravity filtration. The solvent was removed under reduced pressure forming a shiny dark brown solid, which was washed with 150 ml of pentane and then dried in vacuo (0.45 g, 0.71 mmol, 82.7% yield).
1H NMR (δ ppm CDCl3, 300 MHz): 7.73–7.43 [m, 8H, C6 H 4CH2N(CH3)2]; 6.70–6.40 [m, 8H, C5 H 4]; 3.14 [s, 2H, C5H4–CH–(N(CH3)2(C6H4CH2N(CH3)2)]; 4.25–4.21 [s, 4H, C5H4–CH–(N(CH3)2(C6H4CH 2N(CH3)2)]; 2.84–2.81 [s, 12H, C5H4–CH–(N(CH 3)2(C6H4CH2N(CH3)2)]; 2.81–2.77 [s, 12H, C5H4–CH–(N(CH3)2(C6H4CH2N(CH 3)2)].
13C NMR (δ ppm CDCl3, 125 MHz, proton decoupled): 146, 138, 136, 132, 131, 129, 127, 124, 120 [(C 6H4 CH2N(CH3)2) and (C 5H4)]; 61 [C5H4–CH–(N(CH3)2 (C6H4CH2N(CH3)2))]; 60 [(C6H4 CH2N(CH3)2)]; 42 [C5H4–CH–(N(CH3)2(C6H4CH2N(CH3)2))].
IR absorptions (cm−1 KBr): 3444, 3391, 2958, 2670, 2470, 1621, 1467, 1411, 1261, 1164, 1071, 1013, 943, 798.
Anal. Calc. for C34H46N4TiCl2: C, 64.90; H, 7.37; N, 8.91; Cl, 11.27 Found: C, 64.88; H, 7.36; N, 8.90; Cl, 11.27.
UV-Vis (CH2Cl2): λ 263 nm (ɛ 86 000), λ 275 nm (ɛ 94 000), λ 298 nm (ɛ 98 000), λ 320 nm (ɛ 108 000), λ 340 nm (ɛ 72 000), λ 390 nm (ɛ 35 000), 455 nm (weak).
3. RESULTS AND DISCUSSION
3.1. Synthesis
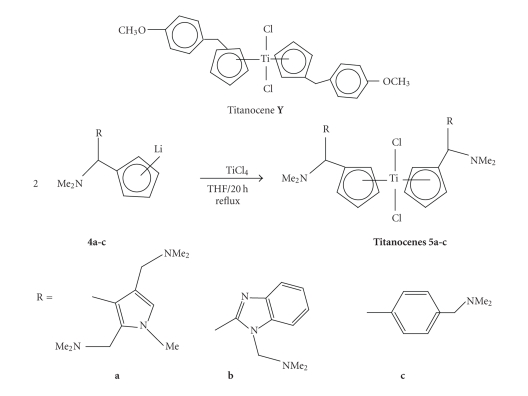
6-(-dimethylamino) fulvene (3) was synthesised according to the already published procedure [27], and its structure is shown in Scheme 1.
Scheme 1.
Synthesis of Titanocenes 5a–c.
The use of aryl lithium in the synthesis of other metallocenes is well known [28–30], and it has recently been used for the synthesis of chiral titanocene dichlorides [26].
This time, the carbolithiation method led to the synthesis of a new group of titanocenes that contain stereo centres (5a–c).
The first step of the reaction consists of the formation of the functionalised lithium intermediates (2a–c) by reacting the corresponding heterocycles (1a–c) with tert-butyl lithium. Side reactions were avoided by cooling the reaction down to −78°C during the addition of tert-butyl lithium, and subsequent warming up to 0°C.
This step was followed by a nucleophilic addition of the lithiated intermediate to the double bond of 6--dimethylamino fulvene at −78°C. Then the reaction mixture was allowed to warm up to 0°C, resulting in the formation of the appropriately substituted lithium cyclopentadienyl intermediates 4a–c. This reaction occurs with no stereo-selectivity, and the intermediates 4a–c already contain a stereogenic carbon.
After stirring the reaction mixture for 40 minutes, two molar equivalents of 4a, 4b or 4c underwent a transmetallation reaction when reacted with TiCl4 under reflux over 20 h in THF to give titanocenes 5a–c.
The compounds obtained are shiny dark red solids. The synthesis of these compounds is shown in Scheme 1.
All three titanocenes shown in this paper have different isomers as seen in Figure 1. As a result of this, three different signals should be seen for every proton and carbon in the 1H and 13C NMR spectra. The R,R and S,S isomers are enantiomers and thus give identical NMR spectra, whereas for protons or carbons corresponding to the R,S (same as S,R) isomer, two signals can be observed as the environment of the two cyclopentadienyl rings is different. A relation of 2 : 1 : 1 for S,S and R,R, and the two signals for the S,R (or R,S) isomers can be observed in the integration pattern.
3.2. Cytotoxicity studies
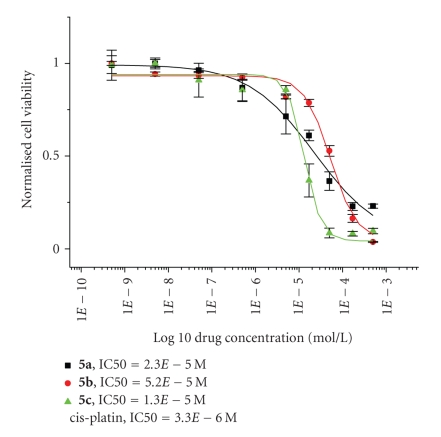
Preliminaryin vitro cell tests were performed on LLC-PK cells in order to compare the cytotoxicity of the compounds presented in this paper. This cell line was chosen based on their long-lasting growth behavior, similar to the one shown in carcinoma cells. It was obtained from the ATCC (american tissue cell culture collection) and maintained in Dulbecco's modified Eagle medium containing 10% (v/v) FCS (foetal calf serum), 1% (v/v) penicillin streptomycin, and 1% (v/v) L-glutamine. Cells were seeded in 96-well plates containing 200 μl microtiter wells at a density of 5,000-cells/200 μl of medium and were incubated at 37°C for 24 h to allow for exponential growth. Then the compounds used for the testing were dissolved in the minimal amount of DMSO (dimethylsulfoxide) possible and diluted with medium to obtain stock solutions of −4 M in concentration and less than 0.7% of DMSO. The cells were then treated with varying concentrations of the compounds and incubated for 48 hours at 37°C. Then the solutions were removed from the wells, the cells were washed with PBS (phosphate buffer solution), and fresh medium was added to the wells. Following a recovery period of 24 h incubation at 37°C, individual wells were treated with a 200 μl of a solution of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) in medium. The solution consisted of 30 mg of MTT in 30 ml of medium. The cells were incubated for 3 hours at 37°C. The medium was then removed and the purple formazan crystals were dissolved in 200 μL DMSO per well. Absorbance was then measured at 540 nm by a Wallac Victor (Multilabel HTS Counter) Plate Reader. Cell viability was expressed as a percentage of the absorbance recorded for control wells. The values used for the dose response curves of Figure 2 represent the values obtained from four consistent MTT-based assays for each compound tested.
Figure 2.
Cytotoxicity studies of Titanocenes 5a–c against LLC-PK cells.
As seen in Figure 2, Titanocenes 5a–c showed an IC50 value of 23, 52, and 13 μM, respectively.
When compared to unsubstituted titanocene dichloride (IC50 value = 2000 μM), titanocene 5c shows a major decrease in magnitude in terms of the IC50 value, and approximately a fourfold increase in magnitude with respect to cisplatin itself (IC50 value = 3.3 μM). However, titanocene 5c shows a decrease in cytotoxicity with respect to Titanocene C. The increased polarity of the new titanocenes together with an increase in size might be the cause of the decrease in cytotoxicity shown.
3.3. Structural DFT discussion
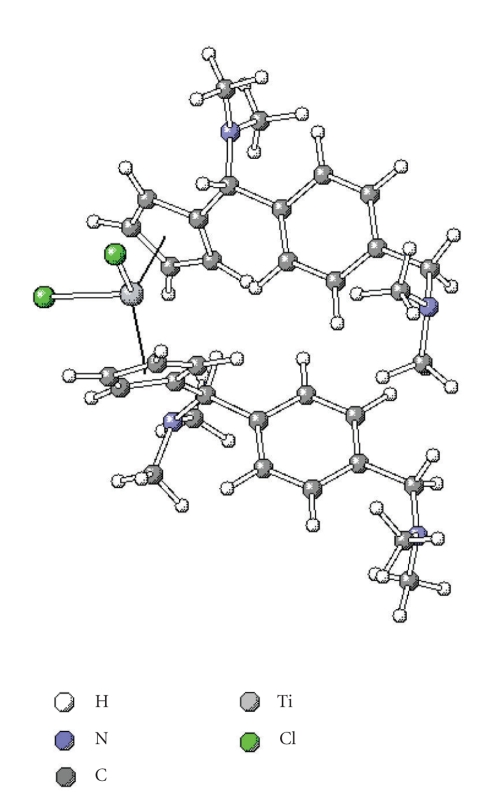
Despite our efforts to crystallise these three titanocenes, no crystal structures were obtained. This might be explained by the existence of different isomers in the racemic mixture. In order to overcome this problem, density functional theory (DFT) calculations were carried out for titanocene 5c at the B3LYP level using the 6-31G** basis set [31], and compared with that of Titanocene C [26].
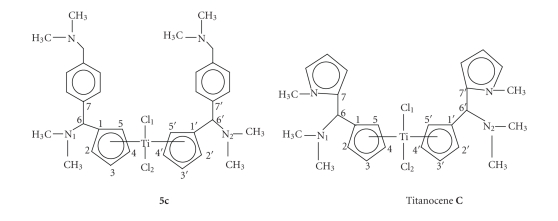
Selected bond lengths of the optimised structure of titanocenes 5a and C are listed in Table 1 (for atom numbering, see Scheme 2). The calculated structure of 5c is presented in Figure 3.
Table 1.
Selected bond lengths from the DFT-calculated structure of 5c and DFT-calculated structure of Titanocene C.
| DFT structure (5c) | DFT structure Titanocene C | |
|---|---|---|
| Bond length (pm) | Bond length (pm) | |
| Ti−C1 | 251.3 | 250.4 |
| Ti−C2 | 242.9 | 242.8 |
| Ti−C3 | 244.2 | 240.0 |
| Ti−C4 | 240.4 | 237.4 |
| Ti−C5 | 243.2 | 242.9 |
| Ti−C1′ | 248.5 | 247.8 |
| Ti−C2′ | 239.1 | 239.0 |
| Ti−C3′ | 234.2 | 233.1 |
| Ti−C4′ | 245.6 | 243.7 |
| Ti−C5′ | 250.2 | 249.3 |
| C1−C2 | 143.4 | 143.2 |
| C2−C3 | 141.6 | 141.5 |
| C3−C4 | 141.2 | 141.3 |
| C4−C5 | 142.3 | 142.3 |
| C5−C1 | 141.4 | 141.4 |
| C1′−C2′ | 141.4 | 141.4 |
| C2′−C3′ | 142.2 | 142.4 |
| C3′−C4′ | 142.3 | 142.2 |
| C4′−C5′ | 140.2 | 140.2 |
| C5′−C1′ | 143.0 | 143.0 |
| C1−C6 | 152.2 | 152.2 |
| C1′−C6′ | 152.2 | 152.0 |
| C6−C6′ | 559.5 | 559.5 |
| C6−C7 | 151.5 | 152.0 |
| C6′−C7′ | 152.0 | 151.5 |
| C6−N1 | 152.0 | 148.3 |
| C6′−N2 | 149.7 | 148.4 |
| Ti−Cl1 | 240.5 | 234.9 |
| Ti−Cl2 | 234.1 | 236.1 |
Scheme 2.
Numbering scheme of 5c and Titanocene C for the structural DFT discussion of 5c.
Figure 3.
DFT calculated structure of 5c.
The length of the bond between the metal centre and the cyclopentadienyl carbons is slightly different for the different Cp rings (251.3 and 248.5 pm, resp.). The same applies for the carbon-carbon bonds of the cyclopentadienyl rings with bond lengths between 140.2 and 143.4 pm.
The bond length between the methylic carbon centre and the carbon centre of the Cp group is of 152.2 and 152.0 pm, respectively. As well, the length of the bond between the methylic carbon and the nitrogen of the dimethylamino group is almost identical in all cases, and between 152.0 and 148.2 pm, respectively. The steric impediment of the dimethylamino groups attached to the methylic carbons causes a lengthening of the bond, in order to relieve the resultant steric strain.
The ClTiCl angle was calculated to be 95.1°. The angle formed between C1 and C1′, the respective methylic carbons (C6 or C6′), and C7 or C7′, respectively, was of 11.42° in both cases, and almost identical to the one formed between each nitrogen of the dimethylamino group, C6 or C6′, and C1 and C1′, respectively.
The DFT calculated structure of 5a was then compared to the calculated structure of its mono--methylpyrrolyl-substituted analogue, Titanocene C [26]. In this complex, the length of the bond between the titanium centre and the two Cl atoms appeared to differ in only 1 pm approximately from the one found for 5c, and of 234.9 and 236.1 pm, respectively. The same applies to the bond length between the N1 or N2 and C6 or C6′, respectively, and to the length of the bond between the Cp carbon atoms and the titanium centre.
The ClTiCl angle in Titanocene C is very similar to the one calculated for 5c, and of 94.9°, and so is the angle formed between the titanium centre and the centre of the Cp rings (with a difference of 0.3°).
Selected bond lengths from the calculated DFT structure of Titanocene C are listed in Table 1, while atom numbering can be seen in Scheme 2.
The cartesian coordinates for the DFT optimised structure of titanocene 5C can be observed in the supplementary material, where the energy of optimisation is also found.
4. CONCLUSIONS AND OUTLOOK
The carbolithiation of 6-(-dimethylamino) fulvene with Mannich-functionalised lithiated species followed by transmetallation offers a general way into the synthesis of new chiral -dimethylamino-functionalised metallocenes. Derivative 5c exhibits an impressive cytotoxicity with an IC50 value of 13 μM against LLC-PK cells, but is slightly less active with respect to Titanocene C that shows the highest cytotoxicity for a published titanocene so far.
ACKNOWLEDGMENTS
The authors thank the Higher Education Authority (HEA), the Centre for Synthesis and Chemical Biology (CSCB), UCD, and COST D39 for funding.
References
- 1.Schilling T, Keppler KB, Heim ME, et al. Clinical phase I and pharmacokinetic trial of the new titanium complex budotitane. Investigational New Drugs. 1995;13(4):327–332. doi: 10.1007/BF00873139. [DOI] [PubMed] [Google Scholar]
- 2.Meléndez E. Titanium complexes in cancer treatment. Critical Reviews in Oncology/Hematology. 2002;42(3):309–315. doi: 10.1016/s1040-8428(01)00224-4. [DOI] [PubMed] [Google Scholar]
- 3.Caruso F, Rossi M. Antitumor titanium compounds and related metallocenes. Metal Ions in Biological Systems. 2004;42:353–384. [PubMed] [Google Scholar]
- 4.Lümmen G, Sperling H, Luboldt H, Otto T, Rübben H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemotherapy and Pharmacology. 1998;42(5):415–417. doi: 10.1007/s002800050838. [DOI] [PubMed] [Google Scholar]
- 5.Kröger N, Kleeberg UR, Mross K, Edler L, Saß G, Hossfeld D. Phase II clinical trial of titanocene dichloride in patients with metastatic breast cancer. Onkologie. 2000;23(1):60–62. [Google Scholar]
- 6.Eisch J, Shi X, Owuor F. Novel synthesis of ansa-metallocenes via the reductive dimerization of fulvenes with group 4 metal divalent halides. Organometallics. 1998;17(24):5219–5221. [Google Scholar]
- 7.Fox S, Dunne J, Tacke M, Gallagher JF. Novel derivatives of ansa-titanocenes procured from 6-phenylfulvene: a combined experimental and theoretical study. Inorganica Chimica Acta. 2004;357(1):225–234. [Google Scholar]
- 8.Tacke M, Allen L, Cuffe L, et al. Novel titanocene anti-cancer drugs derived from fulvenes and titanium dichloride. Journal of Organometallic Chemistry. 2004;689(13):2242–2249. [Google Scholar]
- 9.Rehmann F-J, Cuffe L, Mendoza O, et al. Heteroaryl substituted ansa-titanocene anti-cancer drugs derived from fulvenes and titanium dichloride. Applied Organometallic Chemistry. 2005;19(3):293–300. [Google Scholar]
- 10.Tacke M, Cuffe L, Gallagher W, et al. Methoxy-phenyl substituted ansa-titanocenes as potential anti-cancer drugs derived from fulvenes and titanium dichloride. Journal of Inorganic Biochemistry. 2004;98(12):1987–1994. doi: 10.1016/j.jinorgbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Rehmann F-J, Rous A, Mendoza O, et al. A trimethoxyphenyl substituted ansa-titanocene: a possible anti-cancer drug. Polyhedron. 2005;24(11):1250–1255. [Google Scholar]
- 12.Sweeney N, Mendoza O, Müller-Bunz H, et al. Novel benzyl substituted titanocene anti-cancer drugs. Journal of Organometallic Chemistry. 2005;690(21-22):4537–4544. [Google Scholar]
- 13.Pampillón C, Mendoza O, Sweeney N, Strohfeldt K, Tacke M. Diarylmethyl substituted titanocenes: promising anti-cancer drugs. Polyhedron. 2006;25(10):2101–2108. [Google Scholar]
- 14.Pampillón C, Sweeney N, Strohfeldt K, Tacke M. Diheteroarylmethyl substituted titanocenes: a novel class of possible anti-cancer drugs. Inorganica Chimica Acta. 2006;359(12):3969–3975. [Google Scholar]
- 15.Sweeney N, Gallagher WM, Müller-Bunz H, Pampillón C, Strohfeldt K, Tacke M. Heteroaryl substituted titanocenes as potential anti-cancer drugs. Journal of Inorganic Biochemistry. 2006;100(9):1479–1486. doi: 10.1016/j.jinorgbio.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Strohfeldt K, Müller-Bunz H, Pampillón C, Sweeney N, Tacke M. Glycol methyl ether and glycol amine substituted titanocenes as antitumor agents. European Journal of Inorganic Chemistry. 2006;2006(22):4621–4628. [Google Scholar]
- 17.Sweeney N, Claffey J, Müller-Bunz H, Pampillón C, Strohfeldt K, Tacke M. The synthesis and cytotoxic evaluation of a series of benzodioxole substituted titanocenes. Applied Organometallic Chemistry. 2007;21(2):57–65. [Google Scholar]
- 18.Allen O, Croll L, Gott A, Knox R, McGowan P. Functionalized cyclopentadienyl titanium organometallic compounds as new antitumor drugs. Organometallics. 2004;23(2):288–292. [Google Scholar]
- 19.Causey P, Baird M, Cole SPC. Synthesis, characterization, and assessment of cytotoxic properties of a series of titanocene dichloride derivatives. Organometallics. 2004;23(19):4486–4494. [Google Scholar]
- 20.Meyer R, Brink S, van Rensburg C, Jooné G, Görls H, Lotz S. Synthesis, characterization and antitumor properties of titanocene derivatives with thiophene containing ligands. Journal of Organometallic Chemistry. 2005;690(1):117–125. [Google Scholar]
- 21.Kelter G, Sweeney N, Strohfeldt K, Fiebig H-H, Tacke M. In-vitro anti-tumor activity studies of bridged and unbridged benzyl-substituted titanocenes. Anti-Cancer Drugs. 2005;16(10):1091–1098. doi: 10.1097/00001813-200511000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Oberschmidt O, Hanauske A-R, Rehmann F-J, Strohfeldt K, Sweeney N, Tacke M. Activity of [1,2-di(cyclopentadienyl)-1,2-di(--dimethylaminophenyl)-ethanediyl] titanium dichloride against tumor colony-forming units. Anti-Cancer Drugs. 2005;16(10):1071–1073. doi: 10.1097/00001813-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor K, Gill C, Tacke M, et al. Novel titanocene anti-cancer drugs and their effect on apoptosis and the apoptotic pathway in prostate cancer cells. Apoptosis. 2006;11(7):1205–1214. doi: 10.1007/s10495-006-6796-1. [DOI] [PubMed] [Google Scholar]
- 24.Valadares M, Ramos A, Rehmann F-J, et al. Antitumour activity of [1,2-di(cyclopentadienyl)-1,2-di(--dimethylaminophenyl)-ethanediyl] titanium dichloride in xenografted Ehrlich's ascites tumour. European Journal of Pharmacology. 2006;534(1–3):264–270. doi: 10.1016/j.ejphar.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 25.Fichtner I, Pampillón C, Sweeney N, Strohfeldt K, Tacke M. Anti-tumor activity of Titanocene Y in xenografted Caki-1 tumors in mice. Anti-Cancer Drugs. 2006;17(3):333–336. doi: 10.1097/00001813-200603000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Pampillón C, Sweeney N, Strohfeldt K, Tacke M. Synthesis and cytotoxicity studies of new dimethylamino-functionalised and heteroaryl-substituted titanocene anti-cancer drugs. Journal of Organic Chemistry. 2007;692(11):2153–2159. [Google Scholar]
- 27.Suzuka T, Ogasawa M, Hayashi T. Asymmetric synthesis of metallocenes through enantioselective addition of organolithium reagents to 6-(dimethylamino)fulvene. Journal of Organic Chemistry. 2002;67(10):3355–3359. doi: 10.1021/jo0111199. [DOI] [PubMed] [Google Scholar]
- 28.Qian Y, Huang J, Yang J, et al. Syntheses, structures and reactions of some new benzyl-substituted cyclopentadienyl titanium complexes. Journal of Organometallic Chemistry. 1997;547(2):263–279. [Google Scholar]
- 29.Horáček M, Štěpnička P, Gentil S, et al. Syntheses and properties of some exo,exo-bis(isodicyclopentadienyl)titanium low-valent complexes. Journal of Organometallic Chemistry. 2002;656(1-2):81–88. [Google Scholar]
- 30.Knüppel S, Wang C, Kehr G, Fröhlich R, Erker G. Bis(enamino-Cp) Group 4 metal complex chemistry: developing a Mannich-type carbon-carbon coupling reaction at the bent metallocene famework. Journal of Organometallic Chemistry. 2005;690(1):14–32. [Google Scholar]
- 31.Gaussian '03 . Revision C.02. Wallingford, Conn, USA: Gaussian; 2004. [Google Scholar]