Abstract
Xylitol is promoted in caries-preventive strategies, yet its effective dose range is unclear. This study determined the dose-response of mutans streptococci in plaque and unstimulated saliva to xylitol gum. Participants (n = 132) were randomized: controls (G1) (sorbitol/maltitol), or combinations giving xylitol 3.44 g/day (G2), 6.88 g/day (G3), or 10.32 g/day (G4). Groups chewed 3 pellets/4 times/d. Samples were taken at baseline, 5 wks, and 6 mos, and were cultured on modified Mitis Salivarius agar for mutans streptococci and on blood agar for total culturable flora. At 5 wks, mutans streptococci levels in plaque were 10x lower than baseline in G3 and G4 (P = 0.007/0.003). There were no differences in saliva. At 6 mos, mutans streptococci in plaque for G3 and G4 remained 10x lower than baseline (P = 0.007/0.04). Saliva for G3 and G4 was lower than baseline by 8 to 9x (P = 0.011/0.038). Xylitol at 6.44 g/day and 10.32 g/day reduces mutans streptococci in plaque at 5 wks, and in plaque and unstimulated saliva at 6 mos. A plateau effect is suggested between 6.44 g and 10.32 g xylitol/day.
Keywords: dental plaque, mutans streptococci, xylitol, clinical trials
INTRODUCTION
The substitution of xylitol for sucrose and fructose, or the addition of xylitol to the diet, has been shown to reduce dental caries (Tanzer, 1995). Xylitol is being promoted as a preventive measure and cariostatic agent. However, to be cost-effective, recommendations of agents for use in health improvement should be based on the minimum concentration and frequency required for clinical benefit to be attained. Two studies have directly or indirectly suggested a concentration-dependent response (Wennerholm et al., 1994; Makinen et al., 1995). However, no dose-response study has been done. While there is an impressive body of literature on xylitol, the mechanism of action is uncertain. Some studies suggest that xylitol reduces the ability of S. mutans to adhere, making it more easily removed from plaque (Söderling et al., 1987; Sato et al., 2000). Other studies showed that xylitol reduces S. mutans and hence a decrease in the amount of acid produced (Loesche et al., 1984; Makinen et al., 1989; Miyasawa et al., 2003). Still others report that acid production from other sugars is inhibited in the presence of xylitol (Vadeboncoeur et al., 1983; Assev and Rølla, 1986). Several studies have been published showing xylitol's effect on S. mutans in saliva and plaque. Söderling et al. (1989) showed that, among 19- to 35-year-olds, consumption of 10.9 g xylitol/day for 14 days resulted in reductions of plaque and salivary S. mutans, as well as a decrease in the amount of plaque by 29.4%, and enhanced resistance to pH drops induced by sucrose rinse. Isotupa et al. (1995), in a study of 11- to 15-year-old children wearing fixed orthodontic appliances and who chewed gum with a maximum of 10.5 g xylitol/day for 28 days, reported a 17 to 20% reduction in S. mutans in plaque and saliva. Loesche et al. (1984) previously found that children given 5 g xylitol/day for 4 wks had reduced saliva and plaque S. mutans levels compared with baseline. Other long-term clinical studies did not find reductions in plaque S. mutans (Makinen et al., 1989, 1996; Söderling et al., 1991).
The aim of this study was to determine the relationship between dose and effects on mutans streptococci for adults chewing xylitol gum. For this study, mutans streptococci included both S. mutans and S. sobrinus species. This paper investigates the following hypotheses: (1) The level of mutans streptococci will decrease, compared with baseline, in both plaque and unstimulated saliva in response to increased concentrations of xylitol delivered via gum pellets over time; and (2) the total cultivable facultative flora, as defined by growth on blood agar (BA), will not change in response to increased concentrations of xylitol.
MATERIALS & METHODS
Participants
Potential subjects from Seattle, WA, USA (n = 422), were interviewed for conditions that would preclude participation. Individuals were excluded if they had taken antibiotics during the preceding 4 wks or anticipated doing so during the study. Subjects with a history of GI problems or phenylalanine intolerance were excluded. Screening plaque samples were taken for mutans streptococci enumeration from potential subjects who met the inclusion criteria (n = 405). Plaque was placed into 1 mL of sterile pre-reduced saline, and subjects with ≥ 104 CFU mutans streptococci/mL in their sample were invited to participate (n = 189). From this group, 132 (70%) subjects completed all baseline procedures and were enrolled.
The 132 participants had a mean age of 35 yrs (range, 18-73), and 68% were women. Participants were 70%, 19%, and 8% Caucasian, Asian, and African American, respectively. Less than 2% were either Hispanic, Native American, or of mixed race/ethnicity. The University of Washington Institutional Review Board approved this study, and informed consent was obtained from the participants.
Study Design
This prospective controlled, double-blind clinical trial had a four-group design. Each group chewed 3 gum pellets, 4 times/day. Subjects were randomly assigned to groups by a blocked randomization procedure and were blinded to group assignment, as were investigators. Block randomization ensured similar proportions of participants in each group.
The 3 active groups received a mixture of control gum and/or xylitol gum, resulting in a total daily xylitol dose of 3.44 g (G2), 6.88 g (G3), or 10.32 g (G4). The controls received 9.83 g of sorbitol and 0.702 g of maltitol per day, but no xylitol. Plaque and unstimulated saliva samples were taken at baseline, 5 wks, and 6 mos of exposure. The study was carried out at the University of Washington Regional Clinical Dental Research Center.
Gum
Each xylitol pellet contained 0.858 g xylitol plus gum base, peppermint, menthol, gum Arabic, glycerol, soy lecithin, and glazing agents. Each pellet of the control gum contained the same non-active ingredients plus 0.819 g sorbitol, 0.0585 g maltitol, and 0.0015 g Acesulfame K. All gum pellets were formulated (Fennobon-Oy, Karkkila, Finland) to be similar in size, consistency, color, and sweetness.
Sample Size
We determined that the sample size provided sufficient power to test the primary hypothesis that mutans streptococci in plaque and saliva will decrease in response to increasing doses of xylitol. For dose-effect analysis, log10 transformation of mutans streptococci counts and linear regression model were used. A sample size of n = 26 subjects per group provided 81% power (two-sided, α = 0.05), where a difference in total mutans streptococci counts between the lowest and highest groups was assumed to be log0.75 = 5.6-fold. Assuming 10-20% attrition, 33 subjects per group were necessary.
Adherence
Participants were coached on development of a daily routine for gum chewing and asked to chew the gum for ≥ 5 min. Daily gum packets were distributed on a weekly basis for the first 3 wks, then bi-weekly to promote and monitor compliance.
Unstimulated Saliva and Plaque Sampling
Plaque samples were first collected from the cervical third of the buccal surfaces of all teeth, with 1 sterile Kerr applicator used per arch, and placed in 5-mL tubes containing glass beads and 1 mL of pre-reduced saline. Then, subjects were instructed to let saliva collect without swallowing for at least 1 min, and then to expectorate into the collection tube. This process was repeated as necessary until the minimum 1 mL of saliva was collected. The tubes were immediately carried to the laboratory, where they were stored at room temperature until processed, generally within 3 hrs. However, samples after 3:30 p.m. were not processed until the following morning (15-17 hrs) after delivery. Staff was trained to conduct plaque and saliva samplings. No differences in total counts were observed between samples processed the same day and those processed the following morning.
Culture of Mutans Streptococci
Samples were taken from study participants at baseline, 5 wks, and 6 mos after enrollment. Plaque was prepared in pre-reduced saline, and 10-fold dilutions of plaque and saliva were prepared separately as described above. For each sample, Brucella agar (Difco Laboratories, Becton Dickinson, Franklin Lakes, NJ, USA) supplemented with 0.05 μg/mL vitamin K, 5 μg/mL hemin, and 5% sheep blood (BA) was used for the enumeration of total cultivable facultative/aerobic bacteria, and a modified Mitis-salivarius agar (Difco Laboratories) supplemented with 500 μg/mL kanamycin, 1% potassium tellurite solution, and 50 U/mL bacitracin (MSKB) was used for the enumeration of mutans streptococci. The MSKB medium was more specific than MSB for mutans streptococci isolation (Kimmel and Tinanoff, 1991; Roberts et al., 2002), but did not distinguish between S. mutans and S. sobrinus. Thus, mutans streptococci counts from MSKB plates in this report included both of these species. Only freshly prepared plates were used.
The plaque samples were vortexed to break up the plaque. Then, plaque and saliva samples were diluted. The 10-0 to 10-3 dilutions were plated on BA and MSKB media and incubated in 5% CO2 at 36.5°C for 2-3 days or 7 days, respectively, prior to enumeration. In addition, dilutions (10-0- 10-3) of plaque and saliva samples were plated onto MSKB plates supplemented with 5%, 10%, or 15% (w/v) xylitol and incubated for 7 days as above (Roberts et al., 2002). This provided the number of mutans streptococci able to grow at various xylitol concentrations.
Verification of Mutans Streptococci Counts
To verify that only mutans streptococci colonies were counted, we randomly selected 1400 colonies from the MSKB media and hybridized them with DNA probes (SSP001 and SM002/SM010) specific for the rRNA variable region of S. mutans and S. sobrinus (Roberts et al., 2002). Greater than 90% were positive, indicating that they were either S. mutans or S. sobrinus.
Statistical Procedures
Using the SPSS (v.11.5), and SAS (v.8e) software packages, we determined the significance levels between means at baseline vs. follow-ups and evaluated linear regression models for dose-response. Where appropriate, descriptive statistics, t test, and analysis of variance (ANOVA) were used to determine differences among the groups at baseline and between baseline and levels at 5 wks and 6 mos.
RESULTS
Each of the 4 groups had 33 participants (n = 132), with females constituting from 64% to 73% in the groups. Racial distributions were similar except in G4, which had more Caucasian (88% vs. 64-70% for other groups). The mean (range) ages were also similar—34.45 (19, 70), 34.12 (19, 64), 37.22 (19, 73), and 34.15 (18, 60)—for groups G1 through G4, respectively.
At the five-week follow-up, 91% (n = 120) of participants came in for plaque and saliva samples (G1 = 30, G2 = 31, G3 = 30, G4 = 28). At 6 mos, 79% (n = 104) completed the study (G1 = 27, G2 = 25, G3 = 28, G4 = 24). Dropouts were similar across groups, and excluding them from analysis did not change the mean for any group at baseline.
Baseline
Baseline log10 mutans streptococci levels on MSKB and total cultivable facultative/aerobic flora levels on BA plates for each group are given in Table 1. There were no differences (P > 0.05) in mutans streptococci levels in MSKB or BA plates among the groups where 69% (87/126) of subjects had mutans streptococci growing on MSKB media with 5% xylitol, while 18% had growth on MSKB media with 10% xylitol.
Table 1.
Mean Log10 CFU Mutans Streptococci/mL, Standard Deviation (SD), and P-value (ANOVA) in Plaque and Unstimulated Saliva by Xylitol Dose at Baseline, 5 Wks, and 6 Mos
| Log CFU Mutans Streptococci/mL | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | 5 Wks | 6 Mos | |||||
| Mean (SD) | P-value | Mean (SD) | P-value | Mean (SD) | P-value | ||
| MSKB Plaque | G1 - Control | 5.0 (1.7) | 5.7 (1.1) | 5.3 (1.6) | |||
| G2 - Xylitol 3.44 g/d | 5.5 (1.1) | 0.24 | 5.4 (1.1) | 0.43 | 4.9 (1.9) | 0.32 | |
| G3 - Xylitol 6.88 g/d | 5.2 (1.6) | 0.65 | 4.8 (1.5) | 0.01 | 4.1 (1.4) | 0.00 | |
| G4 - Xylitol 10.32 g/d | 5.3 (1.3) | 0.45 | 4.6 (1.4) | 0.00 | 4.3 (1.8) | 0.04 | |
| MSKB Unstimulated Saliva | G1 - Control | 4.7 (1.4) | 5.3 (1.1) | 5.2 (1.3) | |||
| G2 - Xylitol 3.44 g/d | 4.9 (1.4) | 0.57 | 5.0 (1.1) | 0.37 | 4.8 (1.7) | 0.24 | |
| G3 - Xylitol 6.88 g/d | 4.7 (1.3) | 0.84 | 4.7 (1.1) | 0.05 | 4.3 (1.2) | 0.01 | |
| G4 - Xylitol 10.32 g/d | 5.1 (1.0) | 0.27 | 4.7 (1.4) | 0.10 | 5.4 (1.2) | 0.04 | |
| BA Plaque | G1 - Control | 7.6 (0.7) | 7.4 (0.7) | 7.5 (0.6) | |||
| G2 - Xylitol 3.44 g/d | 7.8 (0.8) | 0.32 | 7.5 (0.6) | 0.56 | 7.2 (0.7) | 0.12 | |
| G3 - Xylitol 6.88 g/d | 7.6 (0.5) | 0.78 | 7.4 (0.7) | 0.88 | 7.3 (0.8) | 0.30 | |
| G4 - Xylitol 10.32 g/d | 7.5 (0.6) | 0.73 | 7.3 (0.5) | 0.62 | 7.4 (0.7) | 0.52 | |
| BA Unstimulated Saliva | G1 - Control | 7.9 (0.7) | 7.7 (0.9) | 8.0 (0.5) | |||
| G2 - Xylitol 3.44 g/d | 8.1 (0.6) | 0.21 | 7.9 (0.6) | 0.42 | 7.9 (0.8) | 0.92 | |
| G3 - Xylitol 6.88 g/d | 7.9 (0.8) | 0.87 | 7.7 (0.9) | 0.82 | 7.9 (0.6) | 0.64 | |
| G4 - Xylitol 10.32 g/d | 8.0 (0.7) | 0.46 | 8.0 (0.6) | 0.19 | 8.1 (0.8) | 0.65 | |
Hypothesis Testing
Mutans streptococci levels in plaque decreased with increasing exposure to xylitol, and there appeared to be a plateau effect between 6.88 g and 10.32 g (Fig.). This trend persisted over 6 mos (Table 1, Fig.). Compared with baseline, plaque mutans streptococci levels at 5 wks and 6 mos were 10-fold lower in the groups exposed to 6.88 g/day and 10.32 g/day (Table 1). At 5 wks, salivary mutans streptococci levels did not differ among groups compared with baseline. However, the salivary levels were lower in the groups exposed to 6.44 g/day and 10.32 g/day in the six-month samples, though the levels of reduction were less in saliva (based on the unstandardized beta [not shown]) than in plaque. Similar to plaque, the reduction of mutans streptococci in saliva suggested a dose plateau effect (Fig.). The five-week samples showed a trend toward a greater decrease in plaque mutans streptococci levels than in the saliva levels, such that a ratio of plaque mutans streptococci divided by saliva mutans streptococci levels changed from baseline samples to 5 wks (Table 2). This trend was also suggested at 6 mos. However, neither the trend at 5 wks nor that at 6 mos was significant (P > 0.05). The group taking the 3.44 g/day did not show a reduction in mutans streptococci levels in plaque or saliva at any point. Total cultivable facultative/aerobic flora remained consistent throughout the study and among the 4 groups (Table 1).
Figure.
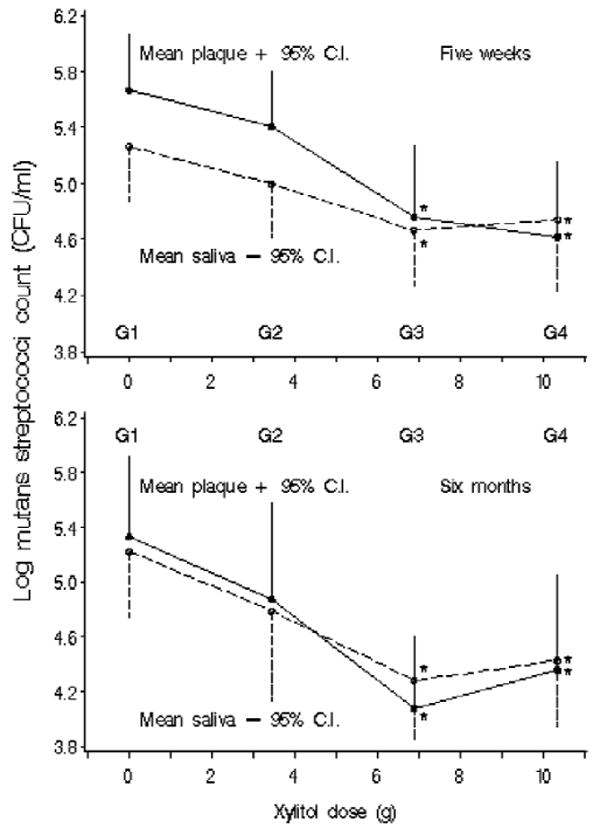
Mean log10 CFU mutans streptococci/mL in plaque and unstimulated saliva by xylitol dose at 5 wks and at 6 mos. *Significant-difference group compared with placebo (G1) in least-significant-difference multiple comparisons.
Table 2.
Mean Log10 CFU Mutans Streptococci/mL and P-value (ANOVA) of Plaque/Unstimulated Saliva Ratio by Xylitol Dose at Baseline, 5 Wks, and 6 Mos
| Log Mutans Streptococci Plaque/Unstimulated Saliva Ratio | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | 5 Wks | 6 Mos | |||||
| Mean | P-value | Mean | P-value | Mean | P-value | ||
| MSKB | G1 - Control | 0.29 | 0.40 | 0.09 | |||
| G2 - Xylitol 3.44 g/d | 0.53 | 0.27 | 0.41 | 0.98 | 0.05 | 0.98 | |
| G3 - Xylitol 6.88 g/d | 0.48 | 0.37 | 0.11 | 0.23 | -0.21 | 0.39 | |
| G4 - Xylitol 10.32 g/d | 0.22 | 0.76 | -0.12 | 0.04 | -0.08 | 0.63 | |
| BA | G1 - Control | -0.27 | -0.34 | -0.45 | |||
| G2 - Xylitol 3.44 g/d | -0.32 | 0.78 | -0.40 | 0.79 | -0.74 | 0.15 | |
| G3 - Xylitol 6.88 g/d | -0.32 | 0.78 | -0.27 | 0.77 | -0.56 | 0.58 | |
| G4 - Xylitol 10.32 g/d | -0.44 | 0.35 | -0.71 | 0.12 | -0.67 | 0.27 | |
DISCUSSION
This study is part of a series to determine the minimum level (g/day) and frequency (exposures/day) required for gum containing xylitol to be useful in public health interventions. The design controls for the potential impact of salivary stimulation, since all groups chewed 3 pellets of gum 4 times/day. Mutans streptococci counts included both S. mutans and S. sobrinus species. Both species have been implicated in the development of caries. This study demonstrated a relationship between the level of xylitol used and the changes in mutans streptococci levels in plaque and saliva. Reductions in mutans streptococci levels were found at 5 wks in plaque and in both plaque and saliva at 6 mos. The earlier reduction in plaque mutans streptococci levels is important, because other studies have used saliva rather than plaque samples (Tenovuo et al., 1997). This may account for some of the inconsistency in the literature.
Even though there were changes in mutans streptococci levels at xylitol 6.44 g/day (G3) and 10.32 g/day (G4), none of the groups showed changes in cultivable facultative/aerobic flora. This verifies that xylitol differentially affects mutans streptococci without impact on the overall normal facultative/aerobic oral flora.
Mutans streptococci levels for subjects receiving xylitol 6.44 g/day and 10.32 g/day were reduced over time compared with the levels in the controls. The group receiving 3.44 g/day was no different from the control group, suggesting that this level of xylitol was not effective in changing the mutans streptococci levels in either plaque or saliva. Stecksen-Blicks et al. (2004) recently reported that there were no changes in S. mutans counts in plaque or saliva after the use of xylitol lozenges at doses of 1.7 g/day and 3.4 g/day for 18 wks.
Furthermore, the results suggested a plateau effect in both plaque and saliva. Previous studies have used doses from 1.7 g/day to > 12 g/day, with various results. Our finding is significant in suggesting that xylitol exceeding 10.32 g/day is not likely to increase effectiveness, while a dose of 3.44 g/day or less is not likely to show changes in mutans streptococci levels. In contrast, the absence of detectable effect at 3.44 g/day may be because two-thirds of the participants had mutans streptococci, at baseline, capable of growing on media supplemented with 5% xylitol. This was interpreted to mean that two-thirds of the subjects had been exposed to low levels of xylitol in their diet. This pre-exposure could have masked any effect that the 3.44 g/day might have had in a population with nominally no exposure to xylitol. Pre-exposure to xylitol needs to be considered in the design of intervention protocols. Chewing gum 4 times/day is cumbersome. Thus, future studies will use xylitol 10.32 g/day taken at 2, 3, or 4 times/day to determine mutans streptococci responses to frequency.
This study had several limitations. Plaque collection was standardized not by plaque weight, but rather by sampling the arches of teeth. This may be viewed as qualitative, and may affect the quantitative analysis. However, the effect is minimized by standardization of sampling and by training of staff. Furthermore, plaque samples at screening, compared with baseline and among controls throughout the study, did not yield differences in mutans streptococci levels. The study used unstimulated saliva to control for mechanical dislodging of mutans streptococci from plaque, and falsely or inconsistently increasing mutans streptococci levels.
Acknowledgments
The authors acknowledge the consultation of Drs. Brian Leroux, Jason M. Tanzer, and Eva Söderling in the design of the studies. This work was supported by Grant No. U54DE14254 from the National Institute of Dental and Craniofacial Research and the National Center for Minority Health and Health Disparities, NIH, Bethesda, MD, USA.
References
- Assev S, Rølla G. Further studies on the growth inhibition of Streptococcus mutans OMZ 176 by xylitol. Acta Pathol Microbiol Immunol Scand [B] 1986;94:97–102. doi: 10.1111/j.1699-0463.1986.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Isotupa KP, Gunn S, Chen CY, Lopatin D, Makinen KK. Effect of polyol gums on dental plaque in orthodontic patients. Am J Orthod Dentofacial Orthop. 1995;107:497–504. doi: 10.1016/s0889-5406(95)70117-6. [DOI] [PubMed] [Google Scholar]
- Kimmel L, Tinanoff N. A modified mitis salivarius medium for a caries diagnostic test. Oral Microbiol Immunol. 1991;6:275–279. doi: 10.1111/j.1399-302x.1991.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Grossman NS, Earnest R, Corpron R. The effect of chewing xylitol gum on the plaque and saliva levels of Streptococcus mutans. J Am Dent Assoc. 1984;108:587–592. doi: 10.14219/jada.archive.1984.0390. [DOI] [PubMed] [Google Scholar]
- Makinen KK, Söderling E, Isokangas P, Tenovuo J, Tiekso J. Oral biochemical status and depression of Streptococcus mutans in children during 24- to 36-month use of xylitol chewing gum. Caries Res. 1989;23:261–267. doi: 10.1159/000261189. [DOI] [PubMed] [Google Scholar]
- Makinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HR, Jr, et al. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995;74:1904–1913. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- Makinen KK, Chen CY, Makinen PL, Bennett CA, Isokangas PJ, Isotupa KP, et al. Properties of whole saliva and dental plaque in relation to 40-month consumption of chewing gums containing xylitol, sorbitol or sucrose. Caries Res. 1996;30:180–188. doi: 10.1159/000262157. [DOI] [PubMed] [Google Scholar]
- Miyasawa H, Iwami Y, Mayanagi H, Takahashi N. Xylitol inhibition of anaerobic acid production by Streptococcus mutans at various pH levels. Oral Microbiol Immunol. 2003;18:215–219. doi: 10.1034/j.1399-302x.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- Roberts MC, Riedy CA, Coldwell SE, Nagahama S, Judge K, Lam M, et al. How xylitol-containing products affect cariogenic bacteria. J Am Dent Assoc. 2002;133:435–441. doi: 10.14219/jada.archive.2002.0201. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yamamoto Y, Kizaki H. Xylitol-induced elevated expression of the gbpC gene in a population of Streptococcus mutans cells. Eur J Oral Sci. 2000;108:538–545. doi: 10.1034/j.1600-0722.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Söderling E, Alaraisanen L, Scheinin A, Makinen KK. Effect of xylitol and sorbitol on polysaccharide production by and adhesive properties of Streptococcus mutans. Caries Res. 1987;21:109–116. doi: 10.1159/000261011. [DOI] [PubMed] [Google Scholar]
- Söderling E, Makinen KK, Chen CY, Pape HR, Jr, Loesche W, Makinen PL. Effect of sorbitol, xylitol, and xylitol/sorbitol chewing gums on dental plaque. Caries Res. 1989;23:378–384. doi: 10.1159/000261212. [DOI] [PubMed] [Google Scholar]
- Söderling E, Isokangas P, Tenovuo J, Mustakallio S, Makinen KK. Long-term xylitol consumption and mutans streptococci in plaque and saliva. Caries Res. 1991;25:153–157. doi: 10.1159/000261359. [DOI] [PubMed] [Google Scholar]
- Stecksen-Blicks C, Holgerson PL, Olsson M, Bylund B, Sjostrom I, Skold-Larsson K, et al. Effect of xylitol on mutans streptococci and lactic acid formation in saliva and plaque from adolescents and young adults with fixed orthodontic appliances. Eur J Oral Sci. 2004;112:244–248. doi: 10.1111/j.1600-0722.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Tanzer JM. Xylitol chewing gum and dental caries. Int Dent J. 1995;45(1) 1:65–76. [PubMed] [Google Scholar]
- Tenovuo J, Hurme T, Ahola A, Svedberg C, Ostela I, Lenander-Lumikari M, et al. Release of cariostatic agents from a new buffering fluoride- and xylitol-containing lozenge to human whole saliva in vivo. J Oral Rehabil. 1997;24:325–331. doi: 10.1046/j.1365-2842.1997.00519.x. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C, Trahan L, Mouton C, Mayrand D. Effect of xylitol on the growth and glycolysis of acidogenic oral bacteria. J Dent Res. 1983;62:882–884. doi: 10.1177/00220345830620080601. [DOI] [PubMed] [Google Scholar]
- Wennerholm K, Arends J, Birkhed D, Ruben J, Emilson CG, Dijkman AG. Effect of xylitol and sorbitol in chewing-gums on mutans streptococci, plaque pH and mineral loss of enamel. Caries Res. 1994;28:48–54. doi: 10.1159/000261620. [DOI] [PubMed] [Google Scholar]


