Abstract
AMPA-type glutamate receptors in the nucleus tractus solitarii (NTS) are necessary for the baroreceptor reflex, a primary mechanism for homeostatic regulation of blood pressure. Within NTS, the GluR1 subunit of the AMPA receptor is found primarily in dendritic spines. We previously showed that both GluR1 and dendritic spine density are increased in NTS of spontaneously hypertensive rats (SHRs). We hypothesize that both receptor and synaptic plasticity are induced by a sustained elevation in arterial pressure. To test the general nature of this hypothesis, we examined whether similar changes in GluR1 density are found in a renovascular model of hypertension, the DOCA-salt rat, and if these changes are preventable by normalizing blood pressure with hydralazine, a peripherally acting vasodilator. Using immunoperoxidase detection, GluR1 appears as small puncta at the light microscopic level, and is found in dendritic spines at the ultrastructural level. Following the development of hypertension, GluR1 spine and puncta counts were significantly greater in DOCA-salt rats than controls. Hydralazine treatment (4 - 5 weeks) prevented the development of hypertension in DOCA-salt rats and reduced blood pressure of SHRs to normotensive levels. The density of GluR1 puncta in the NTS was significantly reduced by hydralazine treatment in the SHR model. These results show that hypertension alters dendritic spines containing AMPA-type glutamate receptors within NTS, suggesting that adjustments in GluR1 expression within NTS are part of the synaptic adaptations to the hypertensive state.
Keywords: blood pressure, DOCA-salt, SHR, glutamate receptor, immunocytochemistry, electron microscopy
1. Introduction
The baroreceptor reflex is a primary mechanism for homeostatic regulation of blood pressure (Andresen and Kunze, 1994). The central initiation of this reflex begins with baroreceptive afferent release of glutamate to activate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors on second-order neurons in the nucleus tractus solitarii (NTS) (Andresen and Yang, 1990; Botsford et al., 1999; Gordon and Leone, 1991; Yen et al., 1999). The AMPA receptor is a tetramer composed of four possible subunits, GluR1-4, with each subunit conferring specific attributes to the generally heteromeric receptors (Hollmann and Heinemann, 1994). Most notably, AMPA receptors lacking the GluR2 subunit display permeability to calcium (Dingledine et al., 1999). All four AMPA receptor subunits are present within NTS, with GluR1-3 being found in neurons (Aicher et al., 2002; Botsford et al., 1999; Kessler and Baude, 1999), and GluR4 predominantly within astrocytic glia (Petralia and Wenthold, 1992; Saha et al., 2004).
Unlike N-methyl-D-aspartic acid (NMDA) receptors which are stable synaptic components, AMPA receptors have been demonstrated to dynamically cycle on and off the synapse in relation to neuronal activity (Tomita et al., 2001). Activity-dependent alterations in the number of AMPA receptors and/or their subunit composition alter the sensitivity of postsynaptic neurons to released glutamate (Wierenga et al., 2005) as well as mediate neuroplastic processes such as long-term potentiation and long-term depression (Malinow and Malenka, 2002). AMPA receptors on NTS neurons of hypertensive animals may be undergoing similar alterations. Increased afferent input (Jones and Thoren, 1977) during chronic hypertension may lead to activity-dependent synaptic or cellular remodeling of NTS neurons. Alterations in central nervous system function of baroreceptor reflexes during hypertension are well documented, but the mechanisms are unclear.
We previously showed an increase in the density of GluR1 AMPA receptor subunits in the NTS of spontaneously hypertensive rats (SHRs) (Aicher et al., 2003). We hypothesized that this change was related to the sustained increase in blood pressure, and that altered AMPA receptors may be located at the synapse between primary afferents and second-order neurons within NTS. Therefore, the first goal of the present study was to examine the normal distribution of the GluR1 subunit at these synapses. This was determined by ultrastructural analysis of the dendritic targets of vagal afferents, as well as examination of GluR1 localization in functionally identified second-order neurons (Doyle and Andresen, 2001).
Next, we examined whether the increase in GluR1 in NTS seen in SHRs could be extended to DOCA-salt rats; a non-genetic, renovascular model of hypertension (Watts and Fink, 1999). Quantification of GluR1-ir at the light and ultrastructural levels of hypertensive animals supported the previous findings within SHRs, that GluR1-ir and spine density within NTS are related to increases in arterial pressure. Finally, we evaluated GluR1-ir in DOCA-salt rats as well as SHRs treated with hydralazine, a peripherally acting vasodilator (Pang and Sutter, 1980), that returned the blood pressure of experimental animals to normotensive levels. Thus, we determined that the increase in arterial pressure was responsible for the structural, and presumably functional, changes seen in NTS.
2. Results
We hypothesized that the GluR1 subunit of the AMPA-type glutamate receptor mediates synaptic remodeling of primary afferent synapses within NTS during the development of hypertension. While the AMPA receptor has been shown pharmacologically to be required for electrophysiological activation of the first synapse in NTS, it has not been shown anatomically to be located at this site. To demonstrate this, we examined whether the receptor was in the dendritic targets of vagal afferent fibers and whether it is found in functionally identified second-order NTS neurons.
2.1 GluR1 is postsynaptic to vagal afferent terminals in NTS
Vagal afferents were identified by anterograde tracing from the nodose ganglion and the GluR1 subunit of the AMPA receptor was detected using an immunogold method (Aicher et al., 1999). Similar to our previous studies, the anterograde tracer BDA was found in axons and axon terminals throughout medial NTS. In addition, some BDA-labeled axon terminals formed asymmetric synapses with GluR1-ir dendrites in medial NTS (Fig. 1). GluR1-immunogold was found both within the cytoplasm of dendrites and along the plasma membrane. GluR1-ir membrane labeling was not confined to sites of synaptic input seen in the plane of section, but was also seen at extrasynaptic sites. Intense GluR1-ir was seen in smaller dendritic targets of vagal afferents (Fig. 1C), while GluR1-ir was also seen in larger dendritic targets (Fig. 1A). These findings are consistent with the our previous studies showing that GluR1-ir is found primarily in small dendrites and dendritic spines in NTS (Aicher et al., 2002; Aicher et al., 2003).
Figure 1.
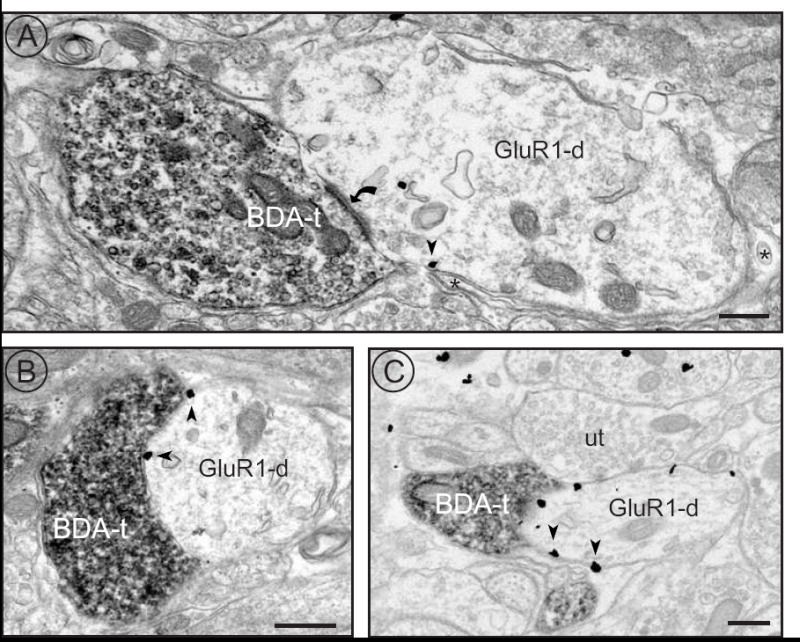
Anterogradely labeled vagal afferents contact dendrites within NTS containing immunogold labeled GluR1. A) A BDA-labeled terminal (BDA-t) forms an asymmetric synapse (curved arrow) with a GluR1-ir dendrite (GluR1-d). Arrowheads indicate GluR1-immunogold labeling associated with the membrane. Astrocytic glia surround the dendrite (asterisks). B) A BDA-labeled terminal forms an apposition with an immunogold labeled dendrite (GluR1-d). C) A BDA-labeled terminal is apposed to an immunogold labeled dendrite (GluR1-d) that is also contacted by an unlabeled terminal (ut). Immunogold particles are associated with non-synaptic portions of the plasma membrane. Scale bars equal 500 nm.
2.2 GluR1 is found in spines of second-order NTS neurons
To verify that GluR1-ir is found in functionally identified second-order NTS neurons (Doyle and Andresen, 2001) we conducted immunocytochemical studies on second-order neurons that had been filled with an intracellular dye (Fig. 2). Confocal analyses demonstrated that functionally defined second-order NTS neurons possess dendritic spines (Fig. 2A), and that some of these spines contain GluR1-ir (Fig. 2B-E). The approximate maximum width of the dendritic spines was 1.0 μm, a value later used as the maximum cross-sectional diameter for dendritic spines in our ultrastructural analyses of GluR1-ir (see below). These second-order neurons showed excitatory responses to electrical stimulation of the solitary tract. The EPSCs showed low jitter and few synaptic failures (Fig. 2F,G), criteria that are used to distinguish second-order neurons from other neurons that receive polysynaptic input (Bailey et al., 2006). These findings demonstrate that GluR1-ir is localized to dendritic targets of cranial primary visceral afferents.
Figure 2.
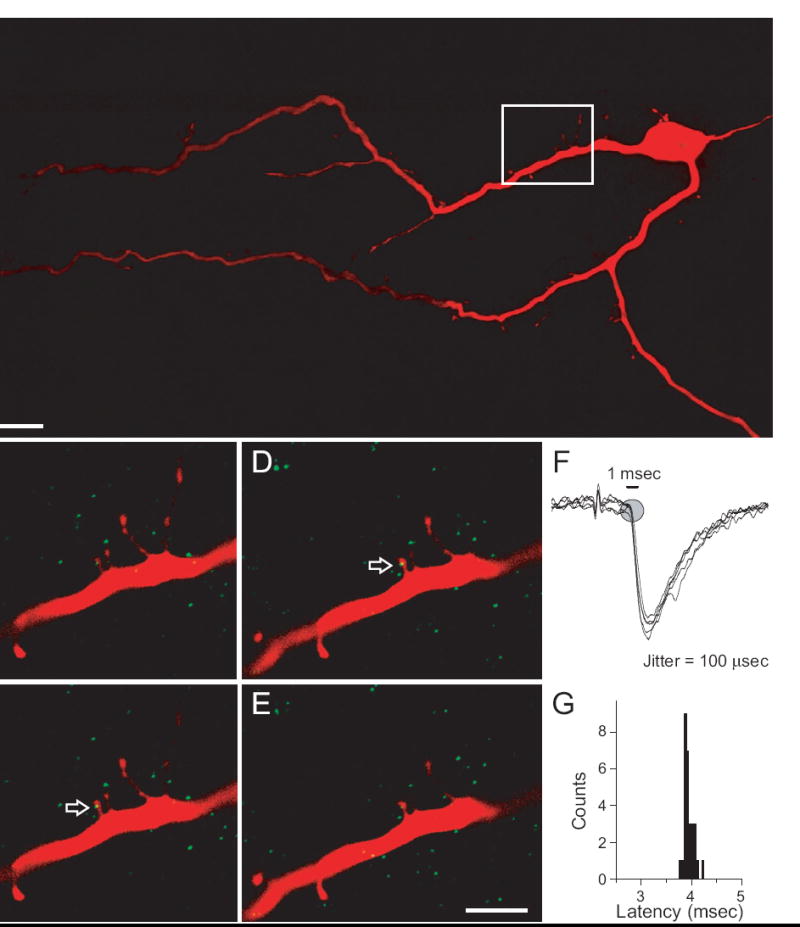
Confocal images of a biocytin-filled second-order NTS neuron. A) A projection (31 um) of images through the Z-axis illustrates the dendritic branching of a biocytin-filled second order neuron (red). The rectangle indicates the area enlarged in panels B-E. Scale bar = 25 um. B - E) Serial confocal images through the Z-axis show that second-order neurons possess dendritic spines and contain the GluR1 subunit of the AMPA receptor (green). Arrows point to GluR1-ir in a dendritic spine. Each panel represents a single optical section (0.4 μm). Scale bar equals 5 μm. F) Solitary tract (ST) afferent evoked synaptic currents identify second-order NTS neurons brainstem slices. Six overlaid current traces from a representative, biocytin-labeled second-order neuron. Suprathreshold electrical shocks (50 Hz) of the solitary tract evoked postsynaptic EPSCs with low jitter and low rates of synaptic failure (<0.2%). G) Histogram of ST-evoked EPSC latencies with an average latency of 3.8 msec and a very narrow range of individual latencies consistent with low jitter, monosynaptic afferent synapses.
2.3 GluR1-ir within NTS: puncta by light microscopy, spines by electron microscopy
By light microscopy, intense GluR1-ir is seen within area postrema at the level of medial NTS. Staining within NTS corresponds to small, dark puncta and less intensely stained cell bodies (Fig. 3A, B). Electron microscopy revealed that the small, dark structures seen by light microscopy correspond to small dendrites and dendritic spines. Consistent with previous reports (Aicher et al., 2002), the vast majority of GluR1-ir profiles in NTS were dendritic spines that often received asymmetric synaptic contacts from unlabeled axon terminals (Fig. 3C, D). Next, we sought to determine if hypertension could induce changes in the distribution of this receptor in NTS.
Figure 3.
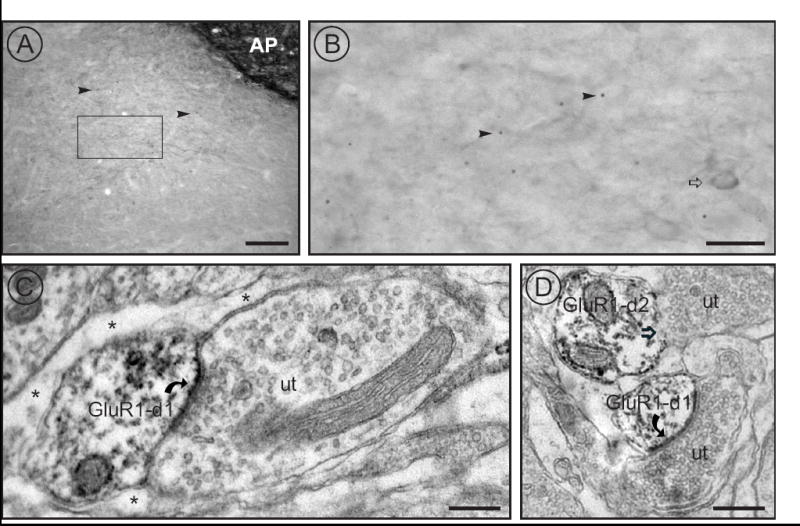
GluR1 localization in NTS. A) Immunocytochemistry for GluR1 resulted in intense labeling within area postrema (AP) and in discrete punctate labeling (arrowheads) within NTS. The box indicates the region shown at higher magnification in panel B. Scale bar equals 100 μm. B) Higher magnification of the rectangle (representing the area sampled) in panel A reveals occasional somatic labeling (open arrow) as well as discrete puncta (arrowheads). Scale bar equals 25 μm. C) and D) Ultrastructural analyses revealed that GluR1 was predominantly located in small dendritic spines. Peroxidase labeled GluR1 dendrites (GluR1-d1), (C,D) receive asymmetric synapses (curved arrows) from unlabeled terminals (ut). Astrocytic glia (asterisks) surround the dendrite. D) Another GluR1 labeled dendrite (GluR-d2) receives an apposition (open arrow) from an unlabeled terminal (ut). Scale bars equal 500 nm.
2.4 Increased blood pressure and GluR1-ir puncta in DOCA rats
DOCA-salt treatment induced hypertension in rats over the five-week treatment course (Fig. 4A). Systolic blood pressure was elevated in DOCA rats (n=7) within two weeks following initiation of the treatment protocol and remained elevated for the duration of the experiment (Fig. 4A). After five weeks of treatment, systolic blood pressure of control (143.6 ± 8.4 mm Hg) and DOCA rats (189.5 ± 6.1 mm Hg) were significantly different (paired t-test, t = -4.4, p<0.001; Fig. 4B).
Figure 4.
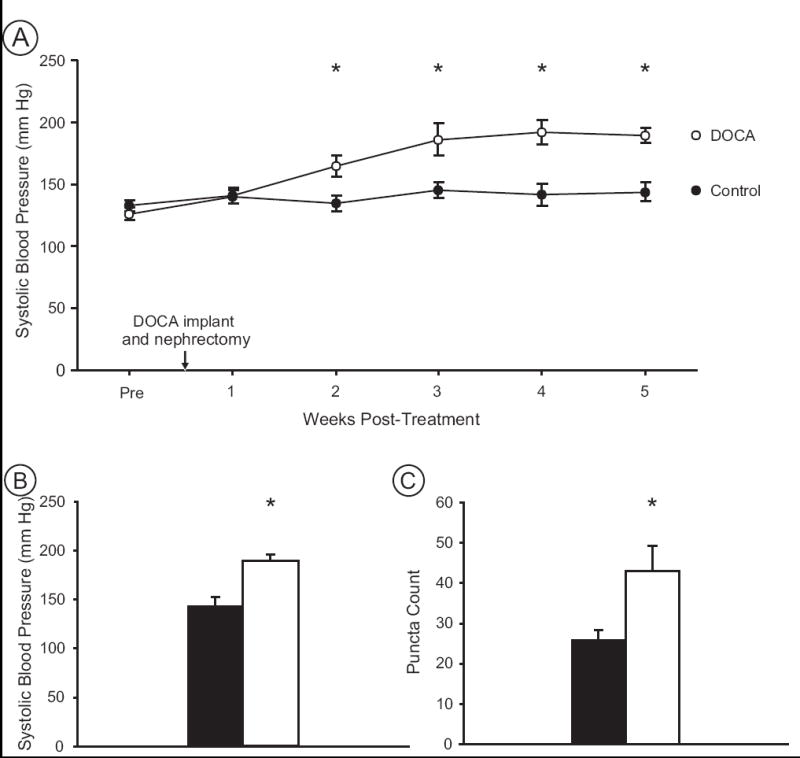
Blood pressure and GluR1-ir puncta are increased in NTS of DOCA rats. A) The timecourse of blood pressure changes shows that beginning at 2 weeks post-implant, DOCA rats (n=7) were significantly hypertensive compared to controls (n=7). Baseline values (Pre) prior to surgery (DOCA implant and nephrectomy) were not different between groups. B) Average systolic blood pressure at 5 weeks post-implant illustrates that DOCA rats (open bars) were hypertensive compared to normotensive controls (black bars). C) Hypertensive DOCA rats exhibited a significantly greater density of GluR1 puncta than normotensive controls within a fixed region of NTS. Asterisks indicate significant differences between groups (see text).
The density of GluR1-ir was examined in the NTS of DOCA-salt hypertensive rats compared to normotensive controls. Using immunocytochemical detection of GluR1, punctate GluR1-ir profiles were counted in medial NTS (Fig. 3A). Medial NTS was chosen as the area of interest based on prior research in SHRs that demonstrated structural changes following hypertension were specific to this region of the NTS, and were not seen in rostral NTS (Aicher et al., 2003). These counts revealed a significant increase (paired t-test, t = -2.5, p = 0.027) in the average number of GluR1-ir puncta within medial NTS of DOCA rats (43.0 ± 6.2) compared to control rats (26.3 ± 2.4; Fig. 4C). These results support our hypothesis that hypertension increases AMPA receptors in the primary destination of arterial baroreceptors within NTS.
Ultrastructural analyses were conducted to confirm the light microscopic puncta counts and to determine if the increase in GluR1-ir is due to an increase in the density of dendritic spines in NTS or an increased trafficking of GluR1 to pre-existing dendritic spines. Both GluR1-ir labeled and unlabeled dendrites and spines were counted from a fixed area of tissue. Dendrites with a cross-sectional diameter up to 1.0 μm that received asymmetric synaptic input were classified as dendritic spines, while dendrites with a cross-sectional diameter greater than 1.0 μm were classified as large dendrites regardless of synaptic input. The number of GluR1-ir dendritic spines was greater in DOCA rats compared to controls (Fig. 5, left side), with no corresponding loss of unlabeled spines from the same area (Fig. 5, right side) (Chi-square = 5.06, p = 0.024). This suggests that hypertension leads to both a greater number of dendritic spines in NTS, as well as an increase in the proportion of those spines that contain GluR1-ir. These results parallel our previous findings from SHRs (Aicher et al., 2003). Since large dendrites in DOCA and control rats had similar distributions of GluR1-ir in large dendrites in NTS (Chi-square = 0.011, p = 0.91), BP increases appear to specifically increase GluR1 in dendritic spines.
Figure 5.
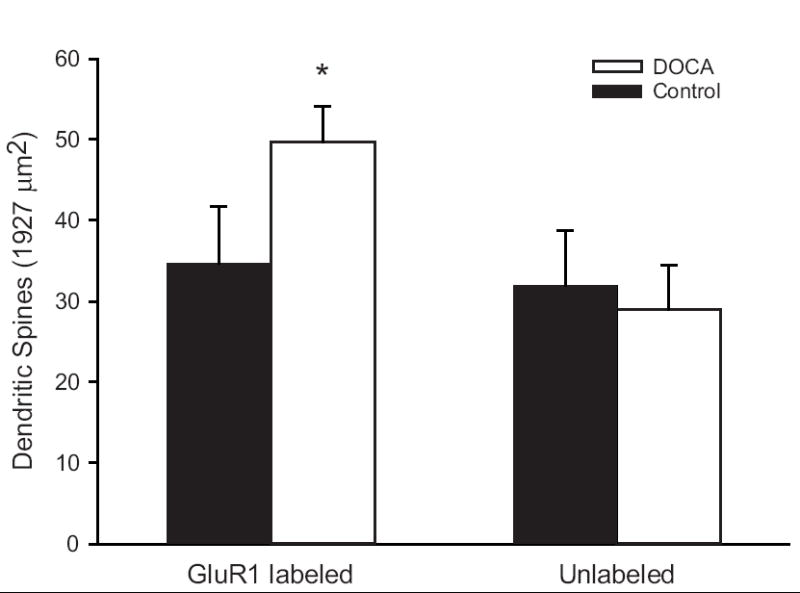
Ultrastructural analyses of GluR1-ir and unlabeled spines in the NTS of DOCA-salt rats (open bars) compared to controls (filled bars). Left panel, DOCA rats had more GluR1-ir dendrites within NTS compared to normotensive controls. Right panel, the number of unlabeled dendrites is similar in hypertensive DOCA rats and normotensive controls, suggesting that GluR1 is up-regulated and possibly preferentially trafficked to small dendrites during hypertension. Asterisks indicate significant differences between groups (see text) (n=7 animals per treatment group).
2.5 Hydralazine treatment, systolic pressure and GluR1-ir in NTS of DOCA rats
To determine if the changes in GluR1-ir within NTS are related to arterial pressure, we examined whether preventing the development of hypertension in DOCA rats would also prevent the increase in GluR1-ir in NTS. Systolic pressure was elevated in DOCA rats within three weeks following initiation of the protocol and remained elevated for the duration of the experiment (Fig. 6A). At five weeks, systolic pressure of control rats (133.1 ± 5.8 mm Hg) and DOCA-hydralazine rats (134.3 ± 5.5 mm Hg) was significantly lower than systolic pressure of DOCA rats (197.8 ± 6.5 mm Hg) (one-way ANOVA, p<0.001; Tukey test pair-wise comparisons, DOCA versus control and DOCA versus DOCA-hydralazine, p<0.001; Fig. 6B).
Figure 6.
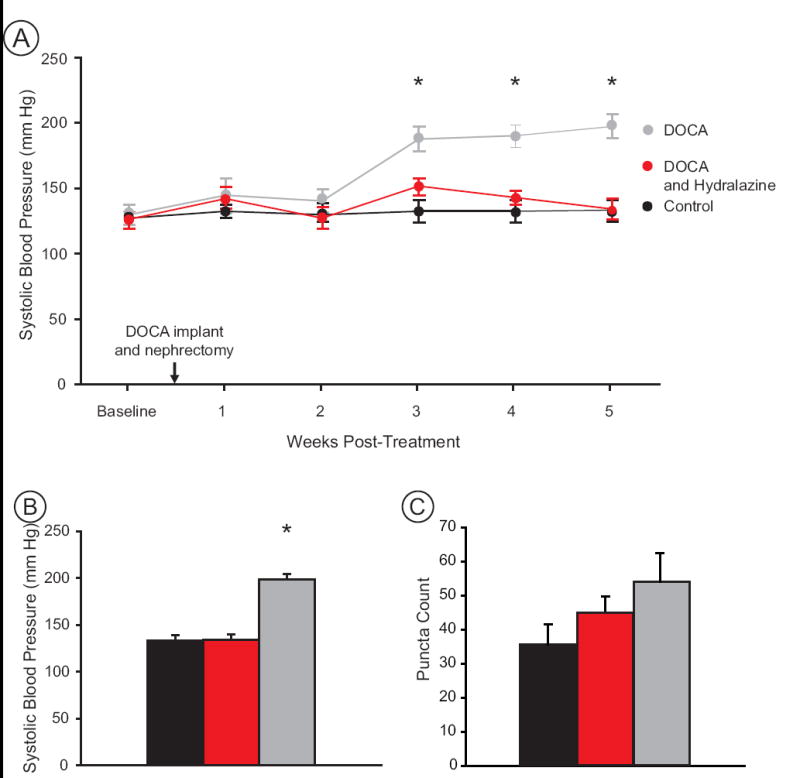
Blood pressure and puncta counts in DOCA (grey), DOCA-hydralazine (red) and Control (black) groups. A) The timecourse of blood pressure changes shows that DOCA rats became hypertensive beginning at 3 weeks post-implant while hydralazine treatment maintained the blood pressure of DOCA rats at normotensive levels. B) At 5 weeks post-implant DOCA rats (n=7) were hypertensive while controls (n=7) and DOCA-hydralazine rats (n=7) were normotensive. C) The density of GluR1 puncta in NTS was not significantly different between groups (see text). Asterisks indicate significant differences between groups (see text) (n=7 animals per treatment group).
GluR1-ir puncta were counted in a fixed area of medial NTS (Fig. 3A) from light micrographs for all conditions. GluR1-ir puncta counts were greatest in DOCA rats (54.0 ± 8.4) compared to controls (35.6 ± 5.8) and DOCA-hydralazine rats (44.9 ± 4.9) (Fig. 6C). Although we saw the pattern we expected, namely that chronically hypertensive animals had more GluR1-ir puncta than animals with normotensive blood pressure, these differences were not statistically significant (one-way ANOVA, p = 0.164; paired t-test between DOCA and DOCA-hydralazine rats, t = 0.94, p = 0.36), suggesting that additional factors in the DOCA model (such as salt water consumption) may influence GluR1-ir within NTS.
2.6 Hydralazine treatment reduces GluR1-ir of SHRs within NTS
Due to potential gustatory complexities of the DOCA-salt model, we also examined the effects of hydralazine treatment on GluR1-ir in the SHR model of hypertension by comparing SHRs, SHR-hydralazine, and WKY controls. Prior to beginning the hydralazine protocol, all SHRs had elevated systolic pressure compared to the control group (Fig. 7A). However, over the 4-week course of treatment, SHR-hydralazine rats showed a reduction in systolic pressure that reached normotensive levels after two weeks of treatment (Fig. 7A) and remained normotensive for the duration of the experiment. After 4 weeks of treatment, systolic pressure of WKY control rats (137.5 ± 3.0 mm Hg) was not different from SHR-hydralazine rats (142.0 ± 4.8 mm Hg), while untreated SHRs remained hypertensive (197.7 ± 3.5 mm Hg) (one-way ANOVA, p<0.001; Tukey test pair-wise comparisons, SHR versus WKY and SHR versus SHR-hydralazine, p<0.001; Fig. 7B).
Figure 7.
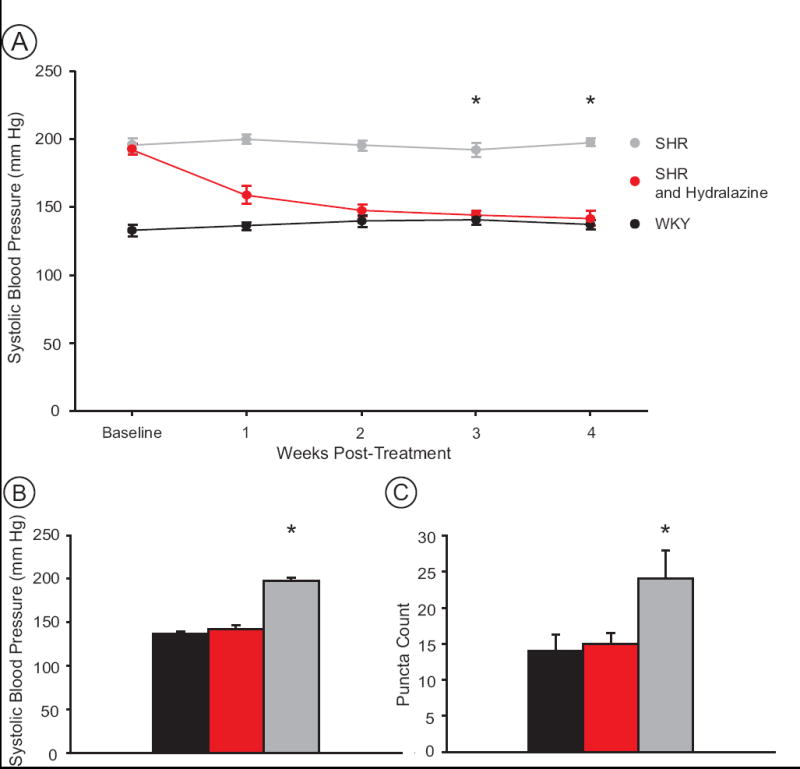
Blood pressure and puncta counts in SHR (grey), SHR-hydralazine (red) and WKY (black) groups. A) The timecourse of blood pressure changes shows that by 3 weeks post-treatment, hydralazine treatment reduced the blood pressure of SHRs to normotensive levels of age-matched WKY rats. Hydralazine treatment began the day after the final baseline blood pressure measurement. B) After 4 weeks, SHRs (n=7) were hypertensive, while WKY (n=7), and SHR-hydralazine rats (n=7) were normotensive. C) Hypertensive SHRs show a greater density of GluR1 puncta in NTS than both WKY, and SHR-hydralazine rats. Asterisks indicate significant differences between groups (see text) (n=7 animals per treatment group).
GluR1-ir puncta counts revealed a significant increase (one-way ANOVA, P = 0.033) in the number of GluR1 puncta within the NTS of SHRs (24.1 ± 3.9) compared to WKY controls (14.0 ± 2.3) and SHR-hydralazine rats (15.0 ± 1.5). A paired t-test showed a significant difference between SHRs and SHR-hydralazine rats (t = 2.186, p = 0.049) (Fig. 7C). These results show that the increase in GluR1-ir induced by hypertension can be reversed by sustained normotensive blood pressure in SHRs.
2.7 Technical considerations
These studies emphasize the importance of making direct numerical comparisons only within an experiment, where the experimental and control tissues are processed simultaneously. GluR1-ir puncta counts were consistent within studies but exhibited considerable variance between studies. In addition to slight differences that may occur during immunocytochemical processing, several iterations of the polyclonal GluR1 antibody were used that resulted in slightly different staining patterns over the years. However, the underlying trend has been consistent. All studies, including those at the light microscopic level and those based on ultrastructural analyses resulted in an increase in GluR1-ir in hypertensive animals compared to normotensive animals, whether the normotensive state was due to control conditions (DOCA control animals), genetic strain (WKY rats) or due to drug treatment (hydralazine treatment in SHRs).
3. Discussion
The hypertensive state represents a condition of altered homeostatic control mechanisms that includes poorly understood adjustments with the central nervous system, including NTS. Our results indicate that fundamental structural changes that take place in NTS are likely contributors to altered pathways regulating autonomic function during the development of hypertension. We found a strong correlation between central synaptic plasticity and systolic pressure, an index of sympathetic nervous system activity. Elevating blood pressure increased both the density of the GluR1 subunit of the AMPA receptor within dendritic spines as well as the number of such spines on NTS neurons. Furthermore, ultrastructural analyses demonstrated that the overall increase in the density of GluR1-ir dendritic spines in NTS is not simply due to changes in trafficking of the receptor. Chronic hydralazine treatment lowered arterial pressure in two distinct models of hypertension and decreased these GluR1 effects, a result which suggests a universal adjustment related to pressure but not specific to the origin of the elevated blood pressure. Together with previous studies in SHR, the present results suggest that hypertension induces both glutamate receptor plasticity and synaptic plasticity in NTS neurons. Our demonstration that GluR1-ir is present in second-order NTS neurons suggests these neurons may contribute to the structural changes seen during hypertension.
3.1 GluR1 Localization in NTS
AMPA receptors mediate afferent transmission at the first synapse of the baroreflex (Andresen and Yang, 1990; Botsford et al., 1999), but the subunit composition of these receptors on second-order NTS neurons remain undefined. In the present study, we found the GluR1 subunit in dendritic targets of vagal afferent terminals and in dendritic spines of second-order neurons. Medial NTS is predominantly involved with cardiovascular and barosensory function (Andresen and Mendelowitz, 1996; Lawrence and Jarrott, 1996), and presumably, many of the neurons that display GluR1-ir are involved in these functions. The presence of other AMPA receptor subunits within the NTS (Aicher et al., 2002; Ambalavanar et al., 1998; Kessler and Baude, 1999) makes it likely that the receptors on second-order neurons in NTS are heteromeric. However, ultrastructural analyses have demonstrated that the GluR2 subunit is often excluded from GluR1-containing AMPA receptors on dendritic spines (Aicher et al., 2002). This suggests that GluR1-containing AMPA receptors on dendritic spines may be permeable to calcium (Engelman et al., 1999).
The number of dendritic spines and the proportion of those spines that contain GluR1 in the NTS increase in hypertensive SHRs compared to normotensive controls (Aicher et al., 2003). These increases were specific to medial NTS in hypertensive SHRs and were not seen in either rostral NTS or in young non-hypertensive SHRs. The current study showed that DOCA-salt hypertension also increased the number of GluR1-ir spines in NTS compared to normotensive controls with a similar genetic background. Thus, the differences observed in SHRs are unlikely to reflect genetic differences between strains, and together, suggest that hypertension induces structural changes in GluR1-ir in NTS neurons.
3.2 GluR1 plasticity and blood pressure
To assess the contribution of blood pressure to GluR1-ir we tested whether antihypertensive therapy prevented these central changes (Vial et al., 1989). In both DOCA-salt and SHR models, hydralazine tended to reduce the density of GluR1-ir puncta in NTS compared to untreated hypertensive rats. The higher variance in the DOCA-hydralazine model prevented detection of statistically significant differences in GluR1-ir puncta. For example, drinking salt solution may stimulate changes in glutamate receptors in NTS (Nakamura and Norgren, 1993) and obfuscate effects due to blood pressure. Hydralazine treatment robustly reversed the GluR1-ir increase that normally develops in SHRs.
The heterogeneous nature of NTS confounds detection of localized receptor changes and may result in the varied reports associated with hypertension. Our results suggest that alterations to spines within medial NTS may be occurring only at specific sites on a subset of NTS neurons and would probably not be detectable utilizing global assessment methods. Prior studies examining subregions of the NTS with in vitro autoradiography revealed no difference between AMPA receptor binding sites between hypertensive and control rats (Ashworth-Preece et al., 1999). However, semi-quantitative PCR on punches of tissue from NTS indicated significantly higher GluR3 mRNA and total AMPA receptor mRNA in hypertensive rats than controls (Saha et al., 2004).
Increases in the GluR1 and the GluR3 subunits are not mutually exclusive, and both may contribute to the increase in the total AMPA receptor mRNA seen with semi-quantitative PCR (Saha et al., 2004). Activity-dependent plasticity is predicated upon Ca2+ influx (Liu and Cull-Candy, 2000; Yang et al., 1999) and would presumably be strengthened by either removing GluR2-containing calcium-impermeable receptors, or incorporating additional calcium-permeable, GluR2-deficient, AMPA receptors. Heteromeric receptors consisting of GluR1 and GluR3, or homomeric receptors consisting of either in isolation would suffice to yield an increase in calcium-permeable AMPA receptors.
3.3 Functional implications of increased GluR1-ir spines in NTS
The observed increase in AMPA receptors containing GluR1 might be expected to enhance glutamatergic transmission in NTS, thereby enhancing baroreflex function during chronic hypertension. However, decreases in baroreflex function are well documented following increased glutamatergic transmission. For example, vasodepressor responses to direct injections of glutamate into NTS are blunted in SHRs (Talman and Lewis, 1991).
There are at least three potential mechanisms that might reconcile the present results with baroreflex depression during hypertension. Two proposed mechanisms are predicated upon decreased excitability of second-order NTS neurons, while the third proposes a rationale for increased inhibitory signaling within NTS during chronic hypertension. The increased prevalence of spines during chronic hypertension may lead to spatial isolation of afferent inputs by concentrating AMPA receptors within the spiny domain. This physical isolation of inputs may reduce afferent summation at the soma near the spike initiation zone and reduce the efficacy of activation of post-synaptic cells (Fig. 8). Alternatively, a postulated increase in calcium influx via GluR2-deficient AMPA receptors may lead to activation of a calcium-dependent inhibitory conductance (chloride or potassium) that may also reduce the excitability of second-order baroreceptive NTS neurons. A third potential mechanism that may be responsible for the decrease in baroreflex function during hypertension may involve enhanced GABA receptor responses in NTS (Durgam et al., 1999; Tsukamoto and Sved, 1993). Most GABAergic neurons within NTS are directly activated by solitary tract afferents [Bailey, T.W.; Appleyard, S.M., Andresen, M.C. Cranial visceral afferent transmission to GABAergic neurons in nucleus tractus solitarius (NTS) from GAD-EGFP mice 35th Annual Meeting of Society for Neuroscience, 2005], suggesting that enhanced glutamatergic transmission to those GABAergic interneurons may enhance GABAergic transmission to depress overall NTS functional throughput. These potential mechanisms are not mutually exclusive, and the heterogeneity of NTS may allow for them all to play a role in regulating autonomic transmission in NTS. However, as yet, none have been directly assessed.
Figure 8.
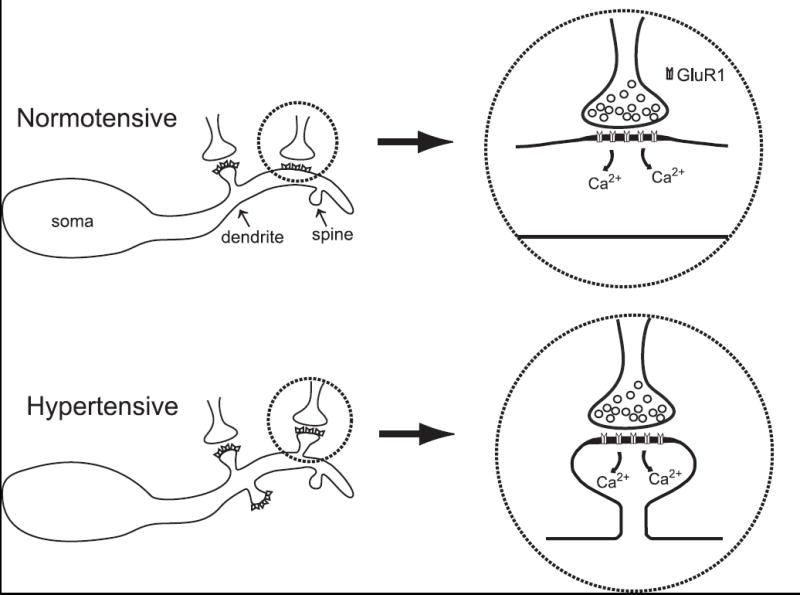
Potential model of cellular changes in NTS after the development of hypertension. An increase in afferent input to barosensitive NTS neurons may lead to an increase in local calcium influx. Increased activity, in addition to increased local calcium may contribute to the formation of dendritic spines. GluR1 homomeric AMPA receptors or GluR2-deficient receptors would be calcium permeable. The resulting increase in dendritic spines may reduce the synaptic efficacy of afferent activity by spatially isolating inputs; or through the activation of a calcium-sensitive chloride conductance.
We do not know if the afferents to the newly formed GluR1 spines are baroreceptive primary afferents. We also do not know if the neurons containing newly formed GluR1 spines are excitatory or inhibitory interneurons (Durgam et al., 1999) or output neurons. Our recent findings of distinct populations of NTS neurons projecting to the caudal ventrolateral medulla and paraventricular nucleus of the hypothalamus (Hermes et al., 2006) support the notion that the functional consequences of these structural changes will greatly depend on the specific population(s) of neurons effected. Future studies will address the intriguing possibility that only select populations of NTS neurons undergo morphological changes in response to elevations in arterial pressure.
The effects of changes in dendritic spines in NTS may depend on which cells possess the spines. A recent study (Ito et al., 2005) found that microinjection of Rho-kinase inhibitor, which promotes spine formation in the hippocampus, enhanced glutamate sensitivity within NTS, making glutamate more effective at decreasing blood pressure in SHRs than WKYs. Although this study only involved acute injections, it raises the possibility that the effects of changes in dendritic spines in NTS may not generalize to any manipulation. The mechanisms underlying spine formation, the stability of the dendritic spines, the type of afferent input to the neuron, and the degree of calcium permeability of the AMPA receptors in these spines may all influence the functional consequences of changes in dendritic spine density in NTS. Future studies must address what types of NTS neurons undergo synaptic plasticity following sustained hypertension, but the present results offer the intriguing possibility that activity-dependent synaptic plasticity may occur in the NTS in response to a physiological stimulus.
4. Experimental Procedure, Acknowledgements, References
Male Sprague-Dawley rats (200-300 g, Taconic Laboratories, Germantown, NY) were utilized for tract tracing, electrophysiology, and DOCA-salt experiments (n=52). Age-matched pairs of male SHR (n=14) and Wistar-Kyoto rats (WKY) (n=7; Taconic Laboratories) were used for hydralazine studies (11-13 weeks of age at beginning of protocol). All methods were approved by the Institutional Animal Care & Use Committee at Oregon Health & Science University. All surgical procedures were conducted under 4% isoflurane anesthesia administered through a face mask.
4.1 Labeling of vagal afferents
Vagal afferents were identified using anterograde tracing following pressure injection of biotinylated dextran amine (BDA) (5 – 7 μl, 10% in 0.1 M phosphate buffer, PB, Molecular Probes, Eugene, OR) into the left nodose ganglion as previously described (Aicher et al., 2000). Following injections (n=6), surgical wounds were closed and rats were monitored during recovery before being returned to the colony.
Seven days after injections, each rat was perfused transcardially as described below (Perfusion and immunocytochemistry). Coronal vibratome sections (40 μm) through the NTS were processed for detection of BDA and GluR1 labeling utilizing a previously published combined peroxidase and immunogold localization method for two antigens (Aicher et al., 2000). BDA was visualized using the avidin-biotin detection method and GluR1 was detected with a rabbit polyclonal antibody directed against the GluR1 subunit of the AMPA receptor (1:25; Chemicon, Temecula, CA), and visualized with a goat anti-rabbit IgG conjugated to colloidal gold (1 nm; 1:50; Electron Microscopy Sciences, Fort Washington, PA). Silver intensification was performed to enhance the particle size and vibratome sections were embedded in plastic and prepared for electron microscopy (Aicher et al., 2000). Ultrathin sections (75 nm) were obtained from the surface of vibratome sections in cases with successful anterograde and immunogold labeling (n=4). The region of medial NTS examined was similar to the area used in our previous ultrastructural analyses and areas of tissue containing both labels in close proximity (less than 1.0 μm) were photographed (see details below for Electron microscopy). Cases exhibiting retrograde labeling, suboptimal morphology, or lacking immunogold labeling were excluded from the analysis.
4.2 Labeling second-order neurons
Neurons within the medial portions of NTS in horizontal slices were visualized under DIC optics and whole-cell patch recordings were used to characterize the neurons, as previously described (Bailey et al., 2006; Doyle and Andresen, 2001). Electrophysiological synaptic criteria based on responses to stimulation of the solitary tract including low jitter, and relatively few synaptic failures were used to differentiate second- from higher-order neurons (Doyle and Andresen, 2001). Second-order neurons (n=6) were filled with biocytin during the recording and then successfully processed for immunocytochemical detection of GluR1. Slices in which non-second order neurons were patched were discarded and only one neuron per slice was filled.
After recording was completed, the slice was placed into 4% paraformaldehyde/1% gluteraldehyde and microwaved (800 watt) at 50% power for 5 seconds. Tissue was rinsed in cold 0.1 M PB, followed by 0.1 M Tris-saline and incubated with streptavidin conjugated to Alexa 594 (6.25 μg/ml, Molecular Probes) for 4 hrs to allow for visualization of the biocytin filled neurons. Tissue was rinsed and incubated with a rabbit anti-GluR1 antibody (1:25, Chemicon) for 40 hours at 4°C, and the bound antibody was detected with a donkey anti-rabbit IgG conjugated to Alexa 488 (1:800, Molecular Probes). Two cases containing second-order neurons were successfully filled, immunolabeled for GluR1, and resulted in a single cell fill in each slice.
4.3 Assessment of blood pressure
Systolic blood pressure was assessed in restrained rats by use of an indirect tail-cuff method (Kent Scientific Corp, Torrington, CT) two days per week as previously described (Aicher et al., 2003). Baseline blood pressure measurements were obtained for one week for all experiments before rats were randomly assigned to treatment or control groups.
4.4 Perfusion and immunocytochemistry
At the conclusion of all blood pressure experiments (5 weeks for experiments involving DOCA-salt rats or 4 weeks for experiments involving SHRs), rats were overdosed with sodium pentobarbital (150 mg/kg) and perfused transcardially with a mixture of 3.8% acrolein in 2% paraformaldehyde; followed by 2% paraformaldehyde (in 0.1 M PB) as previously described (Aicher et al., 2003). The brain stem was sectioned (40 μm) on a vibrating microtome and collected into 0.1 M PB. Coronal sections through the NTS were processed for immunoperoxidase localization of GluR1 (Aicher et al., 2003). Vibratome sections through NTS were incubated in a rabbit anti-GluR1 antibody (1:25, Chemicon) for 40 hours at 4°C, and the bound antibody was detected with a biotinylated goat anti-rabbit IgG (1:400, Vector Laboratories, Burlingame, CA).
4.5 DOCA-salt rats
DOCA-salt rats (DOCA; n=7) underwent a unilateral nephrectomy and a silastic implant with deoxycoricosterone acetate (200 mg/kg, Sigma-Aldrich, Milwaukee, WI) was placed subcutaneously. DOCA-salt rats were given 1% NaCl and 0.2% KCl in their drinking water for the duration of the experiment (Watts and Fink, 1999). Control animals (n=7) were given a unilateral nephrectomy and regular tap water to drink.
4.5.1 DOCA-salt rats treated with hydralazine
Control (n=7) and DOCA rats (n=7) were treated as described above. A third group (DOCA-hydralazine; n=7) received the same DOCA implant procedure as well as hydralazine hydrochloride (1-3 mg/10 mL hydralazine, Sigma-Aldrich) in their salted drinking water. The hydralazine concentration was titrated to a dosage that prevented the development of hypertension, based on bi-weekly monitoring of blood pressure for each animal, as well as monitoring consumption of the treated water.
4.6 SHR, SHR with hydralazine, and WKY
One group of SHRs (n=7) was given tap water to drink. The second group of SHRs (n=7) was given hydralazine (SHR-hydralazine; 1-3 mg/10 mL) in their drinking water, at a dose that reversed hypertension. A third group of WKY rats (n=7) served as controls.
4.7 Light Microscopic Analysis
For GluR1 immunoperoxidase labeling in the NTS, pairs (DOCA study) or trios (hydralazine studies) of animals were processed through identical immunocytochemical conditions to ensure comparable labeling conditions. Tissue punctures through the lateral edge of the vibratome sections were used to distinguish cases, and tissue sections from matched pairs or trios were combined during all antibody incubations. For the DOCA only experiment, alternate sections were processed for light microscopic and electron microscopic analyses. An observer blinded to the experimental conditions selected the area within medial NTS to be examined for each animal. GluR1-ir puncta were counted in fixed regions for the DOCA (0.02 mm2), DOCA-hydralazine (0.02 mm2), and SHR-hydralazine (0.01 mm2) experiments. The selected regions were lateral to the midline, medial to the solitary tract, and dorsal to the motor nucleus of the vagus. The selected region was identified on a vibratome section from the subpostremal level of NTS (13.8 to 14.0 mm caudal to Bregma (Paxinos and Watson, 1998)).
4.8 Electron microscopy
Ultrathin tissue sections were prepared as previously described (Aicher et al., 2003) and examined with an FEI Tecnai 12 electron microscope. Images were captured using a digital camera (Advanced Microscopy Techniques (AMT) Corporation, Danvers, MA). All cases were prescreened for successful labeling and optimal morphological preservation before inclusion in the analyses. For the analysis of GluR1-ir in the DOCA-only experiment, a 1927-μm2 area of medial NTS (DOCA, n= 3; control, n =3) was examined. The subpostremal region of NTS described above for light microscopic analyses was also examined in the EM analysis. For each case, an identical number of non-overlapping micrographs at the same magnification were collected. All GluR1-ir labeled and unlabeled dendrites and spines were counted, measured, and categorized by 2 observers who were blinded to the experimental conditions. Based on cross-sectional diameter and synaptic input, dendrites were classified as either large dendrites (>1.0 μm) or spines (<1.0 μm).
Acknowledgments
We thank Dr. Stephanie Watts for her assistance with the DOCA-salt protocol and Dr. John Welsh for assistance with confocal microscopy. We are also grateful to Lisa Brown-Istvan for technical assistance. This work was supported by a grant (HL56301) from NIH (SAA). The confocal (NIH RR016858, John P. Welsh, PI) and electron microscopy (M.J. Murdock Charitable Trust) were made possible by shared instrumentation grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher SA, Goldberg A, Sharma S, Pickel VM. μ-Opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol. 2000;422:181–190. doi: 10.1002/(sici)1096-9861(20000626)422:2<181::aid-cne3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Sharma S, Mitchell JL. Co-localization of AMPA receptor subunits in the nucleus of the solitary tract in the rat. Brain Res. 2002;958:454–458. doi: 10.1016/s0006-8993(02)03693-4. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Sharma S, Mitchell JL. Structural changes in AMPA-receptive neurons in the nucleus of the solitary tract of spontaneously hypertensive rats. Hypertension. 2003;41:1246–1252. doi: 10.1161/01.HYP.0000069007.98987.E0. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Sharma S, Pickel VM. N-methyl-D-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neuroscience. 1999;91:119–132. doi: 10.1016/s0306-4522(98)00530-2. [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Ludlow CL, Wenthold RJ, Tanaka Y, Damirjian M, Petralia RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp Neurol. 1998;402:75–92. [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius--Gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius - Common denominators. Chem Senses. 1996;21:387–395. doi: 10.1093/chemse/21.3.387. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol. 1990;90:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Ashworth-Preece MA, Chen F, Jarrott B, Lawrence AJ. Visualisation of AMPA binding sites in the brain stem of normotensive and hypertensive rats. Brain Research. 1999;834:186–189. doi: 10.1016/s0006-8993(99)01560-7. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford SA, Dean C, Hopp FA, Seagard JL. Presence of glutamate receptor subtypes on barosensitive neurons in the nucleus tractus solitarius of the dog. Neurosci Lett. 1999;261:113–117. doi: 10.1016/s0304-3940(98)00987-2. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Durgam VR, Vitela M, Mifflin SW. Enhanced gamma-aminobutyric acid-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension. 1999;33:530–536. doi: 10.1161/01.hyp.33.1.530. [DOI] [PubMed] [Google Scholar]
- Engelman HS, Allen TB, MacDermott AB. The distribution of neurons expressing calcium-permeable AMPA receptors in the superficial laminae of the spinal cord dorsal horn. J Neurosci. 1999;19:2081–2089. doi: 10.1523/JNEUROSCI.19-06-02081.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon FJ, Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Res. 1991;568:319–322. doi: 10.1016/0006-8993(91)91418-z. [DOI] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirooka Y, Hori N, Kimura Y, Sagara Y, Shimokawa H, Takeshita A, Sunagawa K. Inhibition of rho-kinase in the nucleus tractus solitarius enhances glutamate sensitivity in rats. Hypertension. 2005;46:360–365. doi: 10.1161/01.HYP.00001747119.23178.05. [DOI] [PubMed] [Google Scholar]
- Jones JV, Thoren PN. Characteristics of aortic baroreceptors with non-medullated afferents arising from the aortic arch of rabbits with chronic renovascular hypertension. Acta Physiol Scand. 1977;101:286–293. doi: 10.1111/j.1748-1716.1977.tb06010.x. [DOI] [PubMed] [Google Scholar]
- Kessler JP, Baude A. Distribution of AMPA receptor subunits GluR1-4 in the dorsal vagal complex of the rat: A light and electron microscope immunocytochemical study. Synapse. 1999;34:55–67. doi: 10.1002/(SICI)1098-2396(199910)34:1<55::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Liu S-QJ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. Taste responses of neurons in the nucleus of the solitary tract of awake rats: An extended stimulus array. J Neurophysiol. 1993;70:879–891. doi: 10.1152/jn.1993.70.3.879. [DOI] [PubMed] [Google Scholar]
- Pang CC, Sutter MC. Hydralazine prevents changes in the contractile response of aortic but not portal vein strips in hypertensive rats. Blood Vessels. 1980;17:293–301. doi: 10.1159/000158260. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Saha S, Spary EJ, Maqbool A, Asipu A, Corbett EK, Batten TF. Increased expression of AMPA receptor subunits in the nucleus of the solitary tract in the spontaneously hypertensive rat. Brain Res Mol Brain Res. 2004;121:37–49. doi: 10.1016/j.molbrainres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Talman WT, Lewis SJ. Altered cardiovascular responses to glutamate and acetylcholine microinjected into the nucleus tractus solitarii of the SHR. Clin Exp Hypertens [A] 1991;13A:661–668. doi: 10.3109/10641969109042069. [DOI] [PubMed] [Google Scholar]
- Tomita S, Nicoll RA, Bredt DS. PDZ protein interactions regulating glutamate receptor function and plasticity. J Cell Biol. 2001;153:F19–F24. doi: 10.1083/jcb.153.5.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension. 1993;22:819–825. doi: 10.1161/01.hyp.22.6.819. [DOI] [PubMed] [Google Scholar]
- Vial JH, Yong AC, Boyd GW. Structural change in the rat hindlimb during deoxycorticosterone acetate hypertension; its reversibility and prevention. J Hypertens. 1989;7:143–150. [PubMed] [Google Scholar]
- Watts SW, Fink GD. 5-HT2B-receptor antagonist LY-272015 is antihypertensive in DOCA-salt-hypertensive rats. Am J Physiol Heart Circ Physiol. 1999;276:H944–H952. doi: 10.1152/ajpheart.1999.276.3.H944. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yen JC, Chan JYH, Chan SHH. Differential roles of NMDA and non-NMDA receptors in synaptic responses of neurons in nucleus tractus solitarii of the rat. J Neurophysiol. 1999;81:3034–3043. doi: 10.1152/jn.1999.81.6.3034. [DOI] [PubMed] [Google Scholar]


