Abstract
Pfs25 is a promising target antigen for the development of a malaria transmission-blocking vaccine and prior research has demonstrated induction of high and functionally effective antibodies in mice with IM injection of Pfs25 encoding DNA plasmid. Likewise, Pfs25 DNA vaccine was immunogenic in rhesus macaques but required a protein boost to elicit significant transmission blocking antibodies. The translation of these encouraging findings to human clinical trials has been impeded largely by the relatively poor immunogenicity of DNA plasmids in larger animals. In vivo electroporation (EP) has revealed significant enhancement of the potency of DNA plasmids. The results reported here compared the immunogenicity and functional transmission-blocking effects of immunization with DNA plasmid (25μg) by the traditional IM route compared to coupling the IM injection (0.25μg, 2.5μg and 25μg doses) with in vivo EP. Significantly, a 0.25μg dose of DNA plasmid, when administered with EP, induced antibody titers (1:160,000) and functional transmission blocking effects that were equivalent to those achieved by a one hundred fold higher (25 μg) dose of DNA plasmid given without EP. At a 25.0μg DNA dose with or without EP there was sufficient antigenic stimulation to result in effective antibody titers; however EP method yielded antibody titer of 1:1,280,000 as compared to only 1:160,000 titer without EP. This observed two log reduction in the amount of DNA plasmid required to induce significant transmission-blocking effects makes a compelling argument in favor of further evaluation of DNA vaccines by in vivo EP method in larger animals. Further experiments in non-human primates and eventually in phase I human trials will determine if the use of EP will induce effective and sustained malaria transmission blocking effects at acceptable doses of plasmid DNA.
1. Introduction
Plasmodium falciparum infection continues to exact a tremendous toll of morbidity and mortality in, primarily, sub-Saharan African and Asian countries, with estimates of 1–1.5 million attributable deaths and more than 350 million incidents of clinical illness per year [1]. Historical and current malaria control efforts have relied upon vector control, prompt diagnosis and treatment of clinical disease and selective chemoprophylaxis. Malaria vaccines could augment such control efforts however successful deployment of effective malaria vaccines would depend upon overcoming numerous obstacles, a key one being the optimization of the potency of such vaccine constructs in humans.
The sexual stage of the malaria parasite is essential to the transmission of the disease between humans via the bite of the female anopheline mosquito, and is the stage at which malaria transmission blocking vaccines (TBVs) exert their effects [2–4]. A small percentage of malaria parasites in infected red blood cells differentiate from asexual forms into sexual forms termed gametocytes [5]. Several P. falciparum proteins, such as Pfs48/45 and Pfs230, expressed in the gametocytes and exposed on the surface of gametes, have shown promise as transmission blocking antigens in small animal models [2]. Proteins expressed within the gametocytes while in the human host, prior to gametogenesis which occurs in the mosquito midgut, are termed “pre-fertilization” antigens. These proteins are targets of natural immune responses and are likely subject to immune selective pressure. After the mosquito blood meal, gametocytes rapidly transform into micro (male) and macro (female) gametes, fertilization occurs followed by successive transformation of zygotes to motile ookinetes and then formation of oocysts. Proteins (Pfs25 and Pfs28) expressed after fertilization of the gamete in the mosquito midgut are termed “post-fertilization” antigens and are not exposed to the human immune system during natural infection [2].
Pfs25 is a 25-kDa surface protein of the sexual stage of the malaria parasite. Although transcripts of this gene are detectable in the human host, actual protein expression only occurs from the onset of gametogenesis in the mosquito midgut through the zygote-ookinete transformation stages [6]. Prior research has clearly demonstrated that antibodies to Pfs25 can be induced by either DNA or recombinant protein immunization and that such antibodies recognize reduction-sensitive conformational epitopes and mediate transmission blocking in a complement-dependent manner [2, 7–9].
TBVs could be a valuable tool in ameliorating the impact of malaria in endemic areas. Several major obstacles have impeded the development of TBVs in spite of solid transmission blocking effect exhibited by monoclonal and polyclonal antibodies against pre-fertilization and post-fertilization antigens. Pfs25, as recombinant protein adjuvant formulations, has undergone preclinical evaluation in small animal and non-human primates and such studies have revealed strong transmission blocking efficacy[7]. DNA vaccines based on Pfs25 have shown highly effective immunogenicity in mice, however a major obstacle has been the relatively poor immunogenicity of Pfs25 DNA-based vaccines in non-human primates [8, 9]. Although Pfs25 has been successfully expressed in several recombinant expression systems [10], it has proven extremely difficult to develop an appropriate formulation for optimal immunogenicity in humans. Numerous attempts and approaches have been employed to improve suboptimal immunogenicity of DNA vaccines in larger animals. These include various routes of administration of DNA plasmids with or without immunomodulatory cytokines and chemokines [11,12], particle mediated or needle free delivery of DNA vaccines [13,14], vaccine formulations using cationic lipids [15,16] and use of various polymers for sustained release of DNA plasmids [17,18].
In vivo electroporation [EP] mediated DNA vaccine delivery utilizes the brief application of electrical fields to a target region of tissue (typically muscle or skin) following the injection of a DNA vaccine in order to enhance its potency. Transient changes in plasma membrane permeability contribute to increased intracellular uptake and expression of injected DNA. Pro-inflammatory cytokine induction and mobilization of monocyte/macrophages to the site of EP are additional factors that result in improved antigen presentation [19–28].
The study described here was a dose-response evaluation (0.25μg, 2.5μg and 25μg doses) of the effect of EP on Pfs25 DNA vaccine immunogenicity (antibody responses and functional transmission blocking activity in BALB/c mice) and a direct comparison with a fixed dose (25μg) of DNA plasmid without EP. Results indicate that administration of Pfs 25 DNA vaccines along with EP lowers the DNA dose by as much as two logs. A further extrapolation of this outcome is that the EP approach may allow delivery of 10—100 times more effective dose in larger animals which in turn may significantly improve efficacy of DNA vaccines in non-human primates and humans.
2. Materials and Methods
2.1. DNA plasmids
DNA vaccine vector VR1020 (Vical, Inc., San Diego, CA) with Pfs25 coding sequence optimized for expression in mammalian cells (minus the N-terminal signal and C-terminal anchor sequence) was prepared using an Endo Free® Plasmid Purification Giga Kit (Qiagen, Valencia, CA). Plasmid DNA sequence was verified by PCR amplification of inserts and DNA sequence analysis. Endotoxin level of the purified DNA plasmid was assessed by the Limulus amoebocyte lysate assay (BioWhittaker, Inc., Walkersville, MD) and found to be lower than 0.1 ETU/μg DNA.
2.2. Immunization scheme
Four groups of six week old female BALB/c mice (six per group) were used in a dosing schedule of one priming immunization and two booster immunizations at four week intervals. Mice were bled prior to each dose and at four weeks after each boost. Group one mice received a dose of 0.25μg Pfs25 plasmid DNA per injection; group two mice received 2.5μg per injection and group three mice received 25 μg per injection. In these three groups, the DNA plasmid was administered IM with in vivo EP. Group four mice received 25 μg Pfs25 plasmid DNA by standard IM injection without EP. All injections were done in the anterior tibialis muscle in a volume of 25μl of endotoxin-free PBS. Electroporation was accomplished using an Ichor pulse generator and a TriGrid Electrode Array as previously described [19]. The intraelectrode spacing of the TriGrid electrode array used for these studies was 2.5 mm. Injection was performed using a 0.3cc U-100 Insulin syringe (Becton-Dickinson, Franklin Lakes, NJ) with a 29 gauge needle. Injection of DNA was followed four seconds later by electrical stimulation at amplitude of 250 volts/centimeter of electrode spacing. The total duration of electrical stimulation was 40 mS, applied over a 400mS interval (a 10% duty cycle).
2.3. Antibody analysis
The sera obtained were analyzed for end point titers, immunoglobulin isotype specificity and avidity using ELISA. 96 well, Immulon-2 microtiter plates were coated with rPfs25 at a concentration 1.0μg/ml in PBS buffer, 100μl per well, and incubated overnight at 4 C. Plates were then blocked with 5% nonfat milk in PBS plus 0.05% Tween 20 (PBS-T). The endpoint titer determination was accomplished by serial dilution of either individual mouse sera or pooled group sera. Plates were incubated with various dilutions of sera (100μl/well in the blocking buffer) for two hours at room temperature, and then washed six times in PBS-T. Secondary antibody, goat anti-mouse IgG, heavy plus light chains, conjugated with horseradish peroxidase (Gibco-BRL) was then added at a concentration of 0.1 μg/ml, 100 μl per well and incubated for 1 hour at room temperature and then washed six times in PBS-T. Plates were then developed with ABTS single-reagent substrate 2,2′ –azinobis (3-ethylbenzthiazolinesulfonic acid) (KPL, Gaithersburg, MD) and absorbance was read at 405nm. Pre-immunization murine sera served as negative control. For antibody isotype analysis the secondary antibodies used were goat, anti-mouse IgG1, IgG2a, IgG2b and IgG3, conjugated with PO (Gibco-BRL). To determine avidity of antibody various concentrations (0, 1, 2, 4 and 6M) of sodium thiocyanate (NaSCN) washes were employed in the above ELISA protocol, using a modification of the method of Pullen et.al. [29] to disrupt antigen-antibody binding. The total binding of antigen to antibody was represented by the absorbance without NaSCN wash.
2.4. Standard Membrane Feeding Assay (MFA)
The MFA evaluates the functional ability of sera to affect the development of oocysts in the mosquito mid-gut [2]. Mature gametocytes (14–18 days old) of P. falciparum strain NF54 were produced in-vitro as described before [30]. Gametocyte cultures at 0.3 to 0.4% final gametocytemia were mixed with various dilutions of experimental sera, human sera and washed human RBCs to a final 50% hematocrit. The mixture was fed to starved (6 h) female Anopheles gambiae mosquitoes for 15 minutes through a Parafilm membrane warmed to 39 C with a glass water-jacket. After the feed the blood-fed mosquitoes were maintained at 26 C and 70–80% relative humidity for 7–9 days and then dissected. Midguts were stained using 0.1% mercurochrome and examined for the presence of oocysts. Each diluted serum was fed to a group of mosquitoes in duplicate cages and negative controls were either pre-immunization murine sera or normal human sera. Transmission blocking activity was assessed by reduction in the percent of infected mosquitoes and reduction in the number of oocysts per midgut. Percent reduction in the number of oocysts was calculated using the following formula: percentage oocyst reduction = 100 − [(geometric mean number of oocysts with test sera/geometric mean number of oocsysts with pre-immune sera) X100].
2.5. Statistical analysis
Differences in the antibody responses were analyzed by ANOVA, and Levene’s test for equality of variance, and mosquito infectivity differences were analyzed by Mann Whitney t-test, all included in the SPSS software package.
3. Results
Pfs25 antigen induces antibodies in mice that interfere with the development of oocysts in the mosquito midgut when tested by MFA. We have earlier shown that a DNA plasmid dose of 25–50μg, administered as prime and two subsequent boosts, is required to elicit effective transmission-blocking antibodies in mice [8], however immunogenicity in rhesus macaques (Macaca mulatta) was found to be rather low [9] thus stressing the need for evaluation of various approaches to increase immunogenicity of DNA vaccines in larger animals.
3.1. DNA dose-dependent total antibody titers
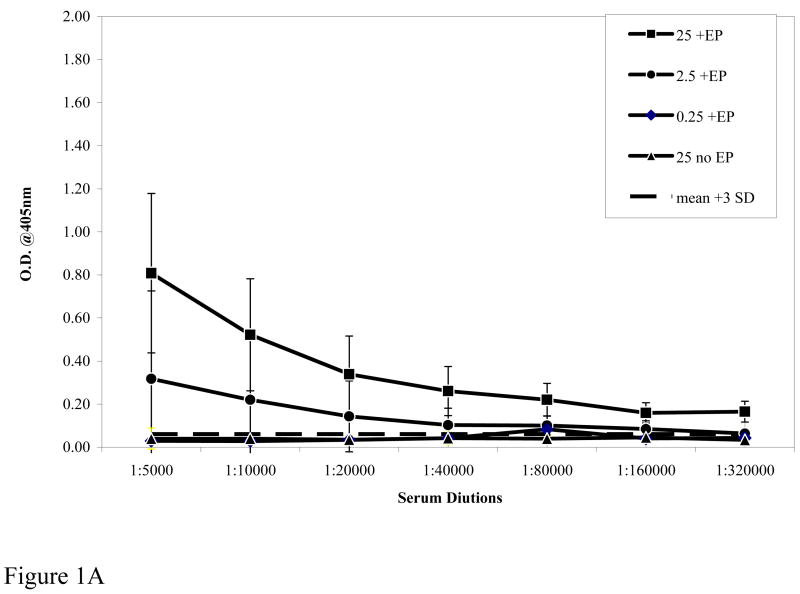
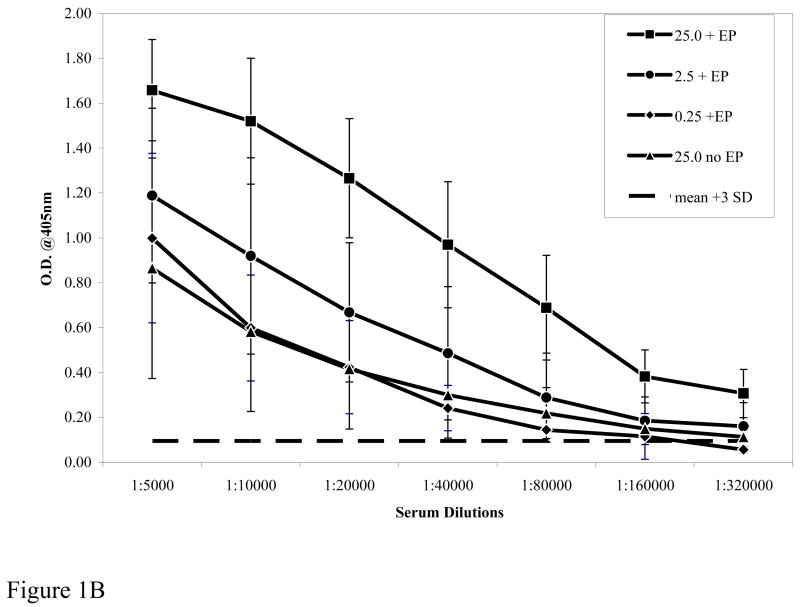
We hypothesized that DNA delivery by in vivo EP would boost the potency of the Pfs25 DNA plasmid compared to conventional IM injection by increasing the magnitude of elicited antibody response and/or reducing the DNA dose required to consistently achieve target levels of response. Figure 1A shows the elicited antibody titers after a prime and one boost at various doses of plasmid DNA with EP (+ EP) and at the 25μg DNA dose without EP (No EP). EP clearly enhanced the immunogenicity of the injected DNA with a 0.25μg dose eliciting titers similar to those elicited by a 25μg dose without EP. Similarly, as shown in Figure IB (prime and two boosts) antibody titer with a 25 μg dose of DNA without EP (No EP) was 1:160,000 and similar to that elicited by a 100 times lower DNA dose (0.25μg) with EP. Figure 1B also demonstrates the total antibody responses after three immunizations to various doses of Pfs25 plasmid DNA given with EP. There is clearly a correlation between the dose of Pfs25 DNA administered by EP and the induced antibody titers. Equally important, the antibody titers (1:160,000 to 1:320,000) so induced with each of the three doses is within the range that has been shown to be sufficient to mediate transmission-blocking effects in MFA. Further, titration of sera and analysis revealed that the endpoint titer of antibody elicited by 25 μg DNA with EP was in excess of 1:1,280,000. These results clearly demonstrate that coupling in vivo EP with DNA vaccine administration can significantly lower the amount of DNA required without EP; in the present case as much as a two log reduction.
Figure 1A and 1B.
Antibody titers measured by ELISA to rPfs25. Figure 1A shows the serum dilution of antibody responses from pooled sera of immunized mice after prime and one boost. Dotted line represents pre-bleed mean plus 3 standard deviations used as a cut off. Figure 1B shows antibody response after prime and two boosts. The differences between 25μg plasmid dose with or without EP were significant at p<0.0.1 by ANOVA. Symbols: 25 μg +EP [filled square], 2.5μg +EP [filled circle], 0.25μg +EP [filled diamond], 25 μg No EP [filled triangle].
3.2. Immunogenicity differences with or without EP
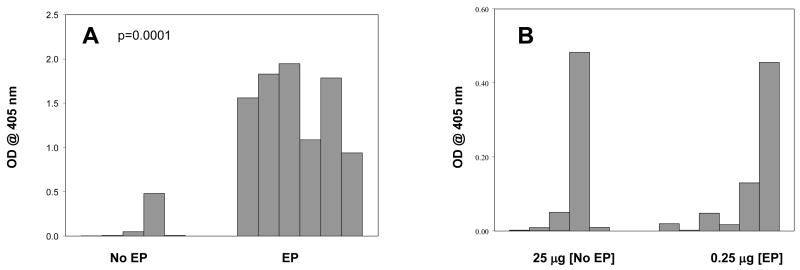
To allow further comparison of the immunogenicity differences with or without EP we analyzed the antibody responses after a prime and a single boost at a fixed DNA dose (25 μg). Figure 2A shows the antibody reactivity at a 1:4,000 serum dilution. Mice given plasmid without EP demonstrated low and inconsistent antibody levels with only one of the six mice exhibiting a meaningful response following a prime and one boost immunization. In contrast, mice receiving the same 25 μg dose with EP exhibited much higher antibody reactivity with 100% of the mice responding in a robust fashion. Data in Figure 2B shows the comparison of antibody responses after a prime and one boost when 25 μg of plasmid DNA was administered without EP and 0.25μg was administered with EP. The magnitude of antibody reactivity and number of responder mice were very similar even though only one-hundredth dose of DNA was given with EP. These results also demonstrate animal to animal variation especially when immunologically lower effective doses are being evaluated. Comparing results in Figure 1 and Figure 2, it is clear that when administered with in vivo EP, the Pfs25 DNA vaccine exhibits robust immunogenicity after just two doses. In contrast, immunological responses following two immunizations by conventional injection were low and inconsistent, even when applied at the relatively high 25μg dose level.
Figure 2.
[A] Comparison of the immunogenicity of 25 μg DNA administered with EP [EP] or without EP [No EP]. Optical density at 405nm of individual mouse sera at 1:4,000 dilution after prime and one boost are shown. [B] shows Comparison of the immunogenicity of 25 μg DNA administered without EP [25μg, No EP] and 0.25μg with EP [0.25μg, EP]. Optical density at 405nm of individual mouse sera at 1:4,000 dilution after prime and one boost are shown. The antibody responses in the two groups, i.e., 25 μg without EP and 0.25 μg without EP were similar with a significance of 91.6% as assessed by Levenne’s test for equality of variance.
3.3. IgG isotype comparison
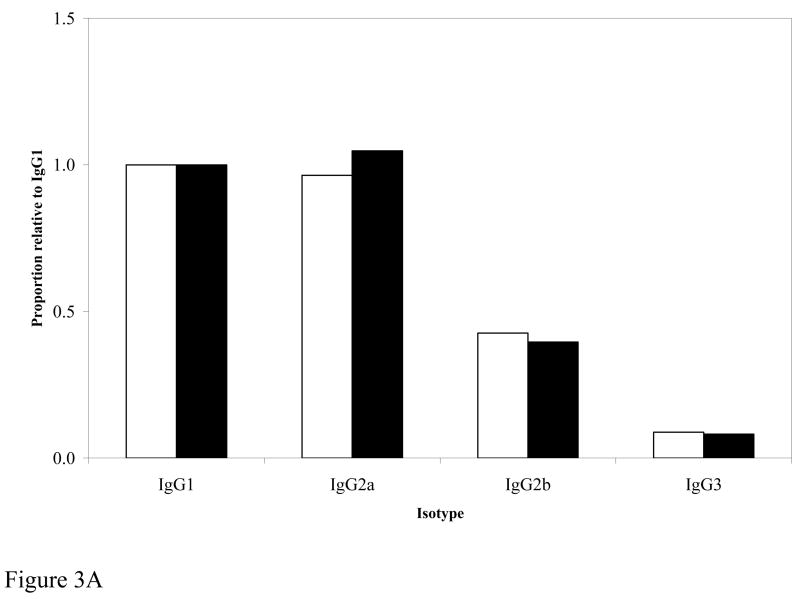
Since comparison with or without EP revealed enhanced immunogenicity with EP, we wanted to determine if such increased immunogenicity was skewed to a particular immunoglobulin isotype. Figure 3A demonstrates that the distribution of isotypes was identical between the two groups of mice immunized with 25μg of DNA with or without EP.
Figure 3.
Figure 3A. Comparison of IgG isotypes. Pooled sera from mice immunized with 25 μg DNA with EP (white bars) or 25 μg NO EP (filled bars) were analyzed at 1:10,000 dilution. For comparison between the two groups, IgG isotypes were expressed as a proportion relative to IgG1.
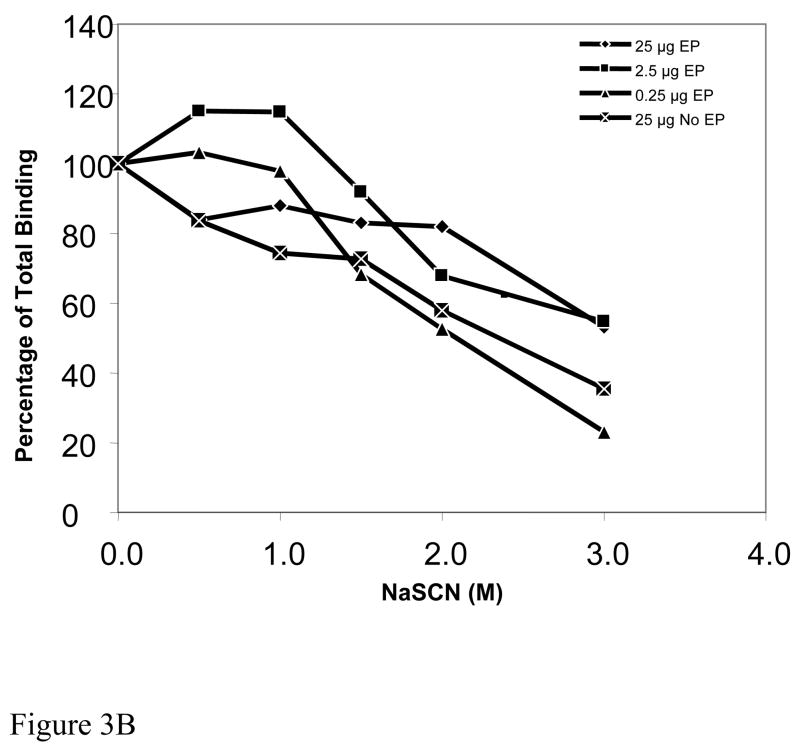
Figure 3B. Antibody avidity of sera. The plot shows the molar concentration of NaSCN required to reduce antigen-antibody binding. Zero molar NaSCN is the baseline avidity and is represented on the plot as 100 percent total binding. Pooled mice sera from the four groups are identified by the following symbols: 25μg EP [filled diamond], 2.5μg EP [filled square], 0.25μg EP [filled triangle], 25μg No EP [X in square].
3.4. Antibody avidity
The strength with which antibody binds to antigen may greatly affect the function of such antibody and therefore we wanted to determine whether EP or dose of plasmid had any demonstrable effect on the avidity of the induced antibodies. Figure 3B shows that at doses of 25 μg and 2.5 μg plasmid, with EP, a 3M concentration of NaSCN was required for a 50% reduction in antibody-antigen binding, whereas antibodies induced with 0.25μg DNA with EP and with 25 μg DNA without EP had modestly reduced avidity of interaction revealed by 50% reduction in binding with only 2M NaSCN. These studies indicate that both dose of plasmid and presence or absence of EP can affect antibody avidity, however the biological significance of such differences, if any, remains to be determined.
3.5. Evaluation of sera transmission blocking activity
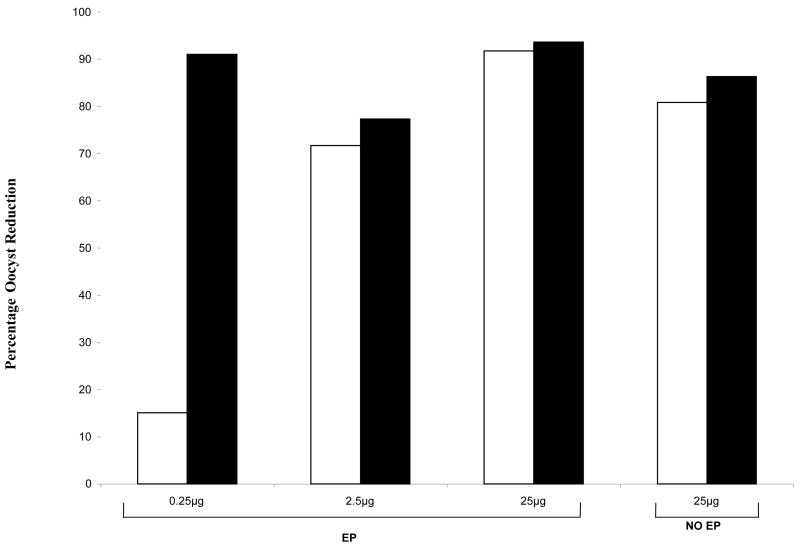
To assess the functional transmission blocking effect of the immunization-induced antibodies, sera were tested in MFA at various sera dilutions (1:2 to 1:16). Both pre-immunization murine serum and normal human serum were used as negative controls. Initially we tested sera from mice immunized with various DNA doses at a serum dilution of 1:2 in MFA. As shown in Figure 4 there was >85% reduction in the number of oocyst in mosquitoes fed on sera from mice immunized with 25μg DNA with or without EP. This finding is consistent with earlier studies that evaluated Pfs-25 DNA vaccine administered in the traditional intramuscular manner [8]. More importantly sera from mice immunized with a two log lower dose (0.25 μg) Pfs25 DNA vaccine given with EP also yielded a comparable ~85% reduction in the number of oocysts. This demonstrated that EP method not only enhances immune responsiveness but also the functional activity of antibodies elicited even at the lowest dose of vaccine plasmid. For further functional assessment of antibodies we looked at comparative effectiveness of antibodies at a fixed dose (25μg). In these studies various anti-sera were tested at 1:2 to 1:16 dilutions in MFA. As shown in Table I sera from mice immunized with EP effectively reduced P. falciparum infectivity in A. gambiae at 1:2 and 1:4 dilutions. Even at 1:8 dilutions the sera from these mice showed 76% oocyst infectivity reduction. In contrast, sera from mice immunized with the same 25μg DNA dose but without EP blocked infectivity only at 1:2 dilutions, thus demonstrating further superiority of DNA immunization with EP.
Figure 4.
Membrane Feeding Assay comparing percentage oocyst reduction after a prime and one boost [white bars] or a prime and two boosts [filled bars]. Pooled sera were tested at 1:2 dilution and the percentage oocyst reduction was calculated as discussed in materials and methods section. Three of the four groups of mice received EP [EP] and one group did not receive EP [No EP]. The doses of DNA administered to each group are indicated on the horizontal axis.
4. Discussion
Pfs25 is a well established target for the development of a malaria transmission blocking vaccine. Elicited antibodies must recognize reduction sensitive conformational epitopes in the parasite and be present in relatively high titer in order to be effective [7, 31]. Attempts to induce such response using adjuvant formulated recombinant Pvs25, a P. vivax homologue of Pfs25, have been only partially successful, especially in phase I clinical trials [32,33]. Poor immunogenicty of such vaccines has been attributed largely to lack of appropriate conformation as well as lack of a potent adjuvant. DNA vaccine based approach for Pfs25 was employed to overcome some of these potential difficulties with protein based vaccines. Indeed, DNA vaccines encoding Pfs25and Pvs25 do induce the formation of functionally effective antibodies in mice and in non-human primates and these antibodies can recognize such conformation-dependent epitopes in respective antigens. In mice, while DNA immunization by conventional injection alone was sufficient to elicit transmission blocking antibodies [8, 34], our studies have revealed that in non human primates, a heterologous protein boost was needed to enhance the immunogenicity and to elicit functional antibodies of high titer [9].
The many potential advantages of DNA-based vaccines have been extensively reviewed [35, 36] and these advantages argue in favor of further optimization of a DNA based TBVs for malaria, especially since protein based TBVs for malaria (full length or sub-unit) have proven to be difficult to produce in a manner that mimics native protein conformation. Moreover, when such recombinant protein has been attainable, there is a strict requirement for specific and effective adjuvant to augment the immunogenicity of such protein constructs. The relative ease of production, low potential production costs compared to protein-based vaccines, relative ease of modifying the vaccine construct to accommodate multiple epitopes of vaccine antigens, and more importantly the stability without requirement of maintaining a “cold chain” are some of the significant possible advantages of DNA vaccines. Previously it has been shown that vaccine induced transmission blocking activity is dependent upon antibody titers [31]. It thus appears that the dose of DNA plasmid employed might be one of the critical factors in inducing sufficient antibody titers to mediate transmission blocking effects, especially for larger animals. As discussed earlier, DNA vaccines in general have shown rather poor immunogenicity in larger animals and humans and attempts to enhance the potency of DNA plasmids include various approaches such as codon optimization of antigen coding gene, use of genetic and chemical immunomodulatory adjuvants, administration of plasmids as cationic lipid formulations and alternate methods of delivery, including in vivo EP [11–28].
Results from animal studies indicate that a vaccine delivery method employing EP can induce prolonged antigen production in myocytes which have a slow cellular turnover rate [21]. Consequently, EP shows significant promise as an adjunct to standard IM immunization and has demonstrated the ability to consistently enhance immunogenicity of DNA vaccine constructs across animal species [19, 20–22]. The results from our study clearly establish the value of EP for enhancing the potency of Pfs25 DNA plasmid malaria TBV. In our previous studies of conventional intramuscular DNA immunization in mice [8] (body weight approximately 25g) and rhesus monkeys [9] (average body weight 4,000g), the latter received a log lower dose of DNA based on body weight. Although the Pfs25 DNA vaccine (0.5 and 1 mg doses) were immunogenic in nonhuman primates, the inability to elicit antibody levels sufficient to effect transmission blocking in MFA suggests that the immunization procedure was of suboptimal potency. The present study demonstrates that, even with a two-log reduction of DNA dose, Pfs25 DNA immunization with EP elicited a robust antibody response and functional transmission blocking activity. These data indicate that the EP approach can lower the actual effective DNA dose needed for potent immunogenicity of a malaria vaccine and may provide the means to extrapolate the promising immune responses observed in mice to larger animals. These findings thus warrant further evaluation of the EP method for Pfs25 DNA vaccine in non-human primates to validate the conclusions of the present study. With a number of Phase I clinical trials for EP mediated intramuscular DNA vaccine delivery now underway [37–39] these findings may have significant implications for the application of this methodology to malaria vaccine trials in non human primates and, eventually, in phase I human clinical trials.
Table 1.
Membrane Feeding Assay, immunization with 25μg with or without EP
| Group | Dilution | Uninfected/Total | G.M. Oocyst | Percent Oocyst Reduction | % Infected |
|---|---|---|---|---|---|
| EP | 1:2 | 21/23 | 1.0a,b | 97.5 | 8.5 |
| EP | 1:4 | 8/28 | 3.4a,b | 91.0 | 71.4 |
| EP | 1:8 | 1/23 | 9.5 | 76.0 | 95.6 |
| EP | 1:16 | 0/30 | 51.9 | 0 | 100 |
| No EP | 1:2 | 10/26 | 3.6a | 91.0 | 61.5 |
| No EP | 1:4 | 3/25 | 38.8 | 1.0 | 88 |
| No EP | 1:8 | 1/26 | 55.3 | 0 | 96 |
| No EP | 1:16 | 7/22 | 89.9 | 0 | 68 |
| Pre- immune | 1:2 | 4/22 | 28.9 | 81 | |
| Pre- immune | 1:4 | 3/26 | 49.7 | 85 |
Column “Uninfected/Total” shows the number of uninfected mosquitoes over total number mosquitoes in that group, and column “% Infected” shows the percentage total infected mosquitoes per group at a given dilution. Column “GM Oocyst” indicates the geometric mean [GM] oocyst burden per mosquitoe for that group and column “Percent Oocyst Reduction” shows the percentage oocyst reduction for that group compared to the average GM oocyst burden of the two pre-immunization control feeds. Statistical significance was assessed by Mann-Whitney t-test. Statistical significance for oocyst count differences when compared between immune and pre-immune sera are represented by ‘a’ (p<0.05) and those between sera from mice immunized with or without EP are represented by ‘b’ (p<0.0.025).
Acknowledgments
These studies were supported by NIH research grant 2RO1AI47089-05A2. RL was supported by a pre-doctoral fellowship from the Johns Hopkins Malaria Research Institute. Supply of human RBCs for parasite culture was supported by RR00052. We gratefully acknowledge the gift of recombinant Pfs25 protein from the malaria vaccine development branch of the NIAID, NIH. Authors also thank Sanaria Inc. for NF54 parasites maintained at the JHMRI core facility and JHMRI mosquito core for supplying mosquitoes used for these studies.
Abbreviations used
- TBVs
Transmission blocking vaccines
- EP
in vivo electroporation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–17. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar N. A vaccine to prevent transmission of human malaria: a long way to travel on a dusty and often bumpy road. Current Science. 2007;92:1535–44. [Google Scholar]
- 3.Carter R. Transmission blocking malaria vaccines. Vaccine. 2001;19:2309–14. doi: 10.1016/s0264-410x(00)00521-1. [DOI] [PubMed] [Google Scholar]
- 4.Stowers A, Carter R. Current developments in malaria transmission-blocking vaccines. Expert Opin Biol Ther. 2001;1:619–28. doi: 10.1517/14712598.1.4.619. [DOI] [PubMed] [Google Scholar]
- 5.Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100:191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 6.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–6. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaslow DC, Bathurst IC, Lensen T, Ponnudurai T, Barr PJ, Keister DB. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994;62:5576–80. doi: 10.1128/iai.62.12.5576-5580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobo CA, Dhar R, Kumar N. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect Immun. 1999;67:1688–93. doi: 10.1128/iai.67.4.1688-1693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coban C, Philipp MT, Purcell JE, Keister DB, Okulate M, Martin DS, et al. Induction of Plasmodium falciparum transmission-blocking antibodies in nonhuman primates by a combination of DNA and protein immunizations. Infect Immun. 2004;72:253–9. doi: 10.1128/IAI.72.1.253-259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou L, Miles AP, Wang J, Stowers AW. Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine. 2003;21:1650–57. doi: 10.1016/s0264-410x(02)00701-6. [DOI] [PubMed] [Google Scholar]
- 11.Sheerlink J-PY. Genetic adjuvants for DNA vaccines. Vaccine. 2001;19:2647–56. doi: 10.1016/s0264-410x(00)00495-3. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki S, Takeshita F, Xin K, Ishii N, Okuda K. Adjuvant formulations and delivery systems for DNA vaccines. Methods. 2003;31:243–54. doi: 10.1016/s1046-2023(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 13.Kendall M. Engineering of needle-free physical methods to target epidermal cells for DNA vaccination. Vaccine. 2006;24:4651–6. doi: 10.1016/j.vaccine.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 14.Drape R, Macklin M, Barr L, Jones S, Haynes J, Dean H. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006;24:4475–81. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Hermanson G, Whitlow V, Parker S, Tonsky K, Rusalov D, Ferrari M, et al. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. PNAS. 2004;101:13601–06. doi: 10.1073/pnas.0405557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedegah M, Rogers WO, Belmonte A, Belmonte M, Banania G, Patterson N, et al. Vaxfectin enhances immunogenicity and protective efficacy of P. yoelii circumsporozoite DNA vaccines. Vaccine. 2006;24:1921–7. doi: 10.1016/j.vaccine.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 17.O’Hagan D, Singh M, Ulmer J. Microparticles for the delivery of DNA vaccines. Immunological Reviews. 2004;199:191–200. doi: 10.1111/j.0105-2896.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 18.Mollenkopf H, Dietrich G, Fensterle J, Grode L, Diehl K, Knapp B, et al. Enhanced protective efficacy of a tuberculosis DNA vaccine by adsorption onto cationic PLG microparticles. Vaccine. 2004;22:2690–5. doi: 10.1016/j.vaccine.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Luxembourg A, Hannaman D, Ellefsen B, Nakamura G, Bernard R. Enhancement of immune responses to an HBV DNA vaccine by electroporation. Vaccine. 2006;24:4490–93. doi: 10.1016/j.vaccine.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand VD, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007;81:5257–69. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang C, Babiuk S, Van Drunen Littel-van den Hurk S, Babiuk LA, Griebel P. A single DNA immunization in combination with electroporation prolongs the primary immune response and maintains immune memory for six months. Vaccine. 2007;30:5485–94. doi: 10.1016/j.vaccine.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22:2493–98. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 23.Dobano C, Widera G, Rabussay D, Doolan D. Enhancement of antibody and cellular immune responses to malaria DNA vaccines by in vivo electroporation. Vaccine. 2007;25:6635–45. doi: 10.1016/j.vaccine.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Peng B, Zhao Y, Xu L, Xu Y. Electric pulses applied prior to intramuscular DNA vaccination greatly improve the vaccine immunogenicity. Vaccine. 2000;25:2064–73. doi: 10.1016/j.vaccine.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Cheung YK, Cheng SC, Sin FW, Xie Y. Plasmid encoding papillomavirus Type 16 (HPV 16) DNA constructed with codon optimization improved the immunogenicity against HPB infection. Vaccine. 2004;23:629–38. doi: 10.1016/j.vaccine.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Zhang H, Fan X, Zhang Y, Huang J, Liu Q, Tjelle T, et al. DNA electroporation prime and protein boost strategy enhances humoral immunity of tuberculosis DNA vaccine in mice and non-human primates. Vaccine. 2006;24:4565–8. doi: 10.1016/j.vaccine.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Hebel H, Attra H, Khan A, Draghia-Akli R. Successful parallel development and integration of a plasmid-based biologic, container-closure system and electrokinetic delivery device. Vaccine. 2006;24:4607–14. doi: 10.1016/j.vaccine.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 28.Tjelle T, Salte R, Mathiesen I, Kjeken R. A novel electroporation device for gene delivery in large animals and humans. Vaccine. 2006;24:4667–70. doi: 10.1016/j.vaccine.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 29.Pullen G, Fitzgerald G, Hosking C. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–7. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 30.Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–6. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 31.Miura K, Keister DB, Muratova OV, Sattabongkot J, Long CA, Saul A. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs 25 is a direct and predictable function of antibody titer. Malar J. 2007;6:107–20. doi: 10.1186/1475-2875-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles AP, Zhang Y, Saul A, Stowers AW. Large scale purification and characterization of malaria vaccine candidate antigen Pvs25H for use in clinical trials. Protein Expr Purif. 2002;25:87–96. doi: 10.1006/prep.2001.1613. [DOI] [PubMed] [Google Scholar]
- 33.Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, et al. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine. 2005;23:3131–8. doi: 10.1016/j.vaccine.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kongkasuriyachai D, Bartels-Andrews L, Stowers A, Collins WE, Sullivan J, Sattabongkot J, et al. Potent immunogenicity of DNA vaccines encoding Plasmodium vivax transmission-blocking vaccine candidates Pvs25 and Pvs28-evaluation of homologous and heterologous antigen-delivery prime-boost strategy. Vaccine. 2004;22:3205–13. doi: 10.1016/j.vaccine.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005;175:633–39. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 36.Liu MA, Ulmer JB. Human clinical trials of plasmid DNA vaccines. Adv Genet. 2005;55:25–40. doi: 10.1016/S0065-2660(05)55002-8. [DOI] [PubMed] [Google Scholar]
- 37.http://www.abedia.com/wiley/record_detail.php?ID=395Gene Therapy Clinical Trials Worldwide- Trial ID: UK-112 (2007)
- 38.http://www.ClinicalTrials.gov NIH Clinical Trials website-Identifier:NCT00250419 (2007)
- 39.http://www.ClinicalTrials.gov NIH Clinical Trials website- Identifier: NCT00471133 (2007)