Abstract
In the hermaphrodite ascidian Ciona intestinalis, the egg vitelline coat (VC) controls gamete self–nonself discrimination. Oocytes, after germinal vesicle breakdown, can be fertilized by both self and nonself sperm. However, a barrier to fertilization by self sperm progressively develops in the VC in the 3 hours after germinal vesicle breakdown. During this period, follicle cells attached to the outer surface of the VC release self-sterility factors that bind to the VC. Within the follicle cells, these factors (possibly peptides) are thought to be shuttled to the cell membrane by an hsp70 homolog (Cihsp70). In fact, antibodies to hsp70 block the development of self-sterility. Proteasomes are central to the production of antigen peptides. Specific inhibition of proteasome activity with clasto-lactacystin β-lactone (CLβL) prevented the onset of self-sterility, but had no effect once this process had started. CLβL did not block fertilization by nonself sperm. The self-sterility factors were removed from mature oocytes by exposure to acidified media, and their biological activity was transferred to immature oocytes treated with CLβL. The obvious high multiplicity of self–nonself recognition alleles involved in fertilization, and the involvement of an hsp70 and a proteasome in processing self-sterility factors, suggests that this system may be evolutionarily related to the vertebrate immune system.
In the hermaphrodite ascidian Ciona intestinalis, gamete self-incompatibility is a mechanism that prevents self-fertilization and is based on the ability of the oocyte vitelline coat (VC) to distinguish and accept only heterologous spermatozoa (1). The self-incompatibility barrier is established during oogenesis and is controlled by the overlying follicle cells. Indeed, ablation of follicle cells prevents the onset of self-sterility (2, 3). Follicle cells exert their effect even when detached from the VC and cocultured with maturing oocytes (2). This process is strictly individual-specific because it occurs only with oocytes and follicle cells from the same animal (3). Hence, the self-discrimination determinants on the VC result from a highly specific match between the molecules produced by follicle cells and those present on the oocyte VC of the same animal (3). Given the invariable success of fertilization between heterologous gametes, we conclude that self–nonself discrimination is regulated by a highly polymorphic system. This suggests that the factor released by the follicle cells, which modifies the VC (i.e., the gateway to nonself spermatozoa), is of a protein nature.
Using a specific antibody, we recently demonstrated that an hsp70 protein (Cihsp70), constitutively expressed at the plasma membrane of the follicle cells in the vitellogenic oocytes at the onset of self-sterility, is required for the switch from self-fertility to self-sterility (4). We suggested that Cihsp70 shuttles peptides from internal compartments of follicle cells to the plasma membrane facing the VC (4). A similar mechanism and function have been attributed to an hsp72 molecule expressed on the cell surface of human tumor cells but not normal cells (5, 6). Furthermore, hsps isolated from cancer cells or virus-infected cells elicit an immune response to the cognate tumor or viral antigens (7).
Proteasomes, which regulate many cellular processes, are central also to the production of antigenic peptides that bind to MHC class I glycoproteins (8). Proteolysis is catalyzed by β-subunits X, Y, Z and their homologues LMP2, LMP7, MECL-1 (9, 10). The processing of polypeptides by proteasomes is conserved in the evolution between vertebrates and invertebrates (11). Accordingly, Niedermann et al. (11) proposed that the immune system of vertebrates has recruited the proteasomes, which are phylogenetically ancient multicatalytic high molecular weight endoproteases (11).
We used clasto-lactacystin β-lactone (CLβL), a specific and irreversible inhibitor of all proteasome catalytic β-subunits (12), to investigate the function of 20S proteasome catalytic β-subunits in the follicle cells of maturing oocytes of C. intestinalis in the building of the gamete self-incompatibility barrier. Self-sterility in C. intestinalis eggs is abolished by acidic sea-water treatment (13). Here we demonstrate that a VC acid extract is able to supply self-sterility factors and so to induce self-sterility in both immature follicle cell-free oocytes and in CLβL-treated oocytes.
MATERIALS AND METHODS
Effect of CLβL on Germinal Vesicle Breakdown (GVBD) Oocytes.
Self-fertile GVBD oocytes isolated from the ovary of a single animal (2) were split into two groups: One group was cultured with 50 μM CLβL, and the other was the control. After a 3-hour incubation, the oocytes were washed and fertilized with autologous spermatozoa. Cross-fertilization controls were run in parallel to verify the viability of the oocytes throughout the experiment. To verify whether CLβL affects the timing of the onset of self-sterility, GVBD oocytes cultured with the inhibitor were scored for self-fertilization every 30 minutes for 3 hours.
Effect of CLβL on GV Oocytes.
Vitellogenic oocytes at germinal vesicle (GV) stage were incubated with and without 50 μM CLβL until GVBD (60–90 minutes). After GVBD, oocytes were washed and divided into two aliquots: One was allowed to complete maturation in fresh seawater for 3 hours, the other was inseminated with autologous sperm and checked for self-fertilization. Untreated oocytes samples also were checked for self-fertilization. On maturation, oocytes were fertilized with autologous spermatozoa and were scored for the first cleavage. Also in this case, nonself fertilization was tested.
Preparation of the VC Acid Extract.
Eggs from the gonoduct of a single self-sterile animal were deprived of follicle cells by shaking. The supernatant was removed, and 250 μl of packed eggs (corresponding to 32,000 eggs) were incubated in 1 ml of Millipore-filtered seawater (pH 2.6) for 5 minutes. This suspension was neutralized by 0.5 ml of artificial seawater buffered with 20 mM Tris⋅HCl (pH 8.2). Eggs were packed by hand-centrifuge, and the supernatant, which represents the VC acid extract, was recovered.
Rescue of the Onset of Self-Sterility in CLβL-Treated GV Oocytes by Follicle Cells and Acid Extract.
In a typical experiment, 50 μl of acid extract were added to 50 μl of Millipore-filtered seawater containing either self or nonself GVBD oocytes free of follicle cells. Three hours later, oocytes were washed and fertilized with autologous sperm. To verify the rescue of self-sterility by acid extract and follicle cells, GV oocytes, incubated with the inhibitor as described earlier, after GVBD, were washed and cultured with either follicle cells detached from untreated autologous GVBD oocytes or autologous acid extract. Three hours later, the oocytes were inseminated with autologous spermatozoa, and fertilization was checked.
Western Blot Analysis.
Self-fertile ovarian GVBD oocytes were allowed to mature with and without 50 μM CLβL in seawater. Fifty oocytes in 40 μl of seawater were withdrawn at time 0 and after 90 minutes of incubation. They were solubilized by adding 40 μl of Laemmli (14) sample buffer 2× containing 10% 2β-mercaptoethanol. After gentle pipetting, samples were incubated for 4 minutes in boiling water and then were centrifuged for 10 minutes at 10,000 × g. Samples were separated by 11% SDS/PAGE. A control sample of proteins extracted from C. intestinalis ovary and prepared as described (4) was loaded on the same gel.
Proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell) and were treated with a monoclonal anti-hsp70 antibody (clone BRM-22, Sigma) at a dilution of 1:500. A 20 mM Tris⋅HCl buffer (pH 7.6) containing 137 mM NaCl, 0.1% Tween-20, and 5% nonfat dry milk (Bio-Rad) was used throughout the immunostaining procedure, and enhanced chemiluminescence (Amersham) was used for detection. The increase of the signal due to hsp70 was quantitated by densitometric analysis of the autoradiograph.
RESULTS
Effect of CLβL on the Onset of Self-Sterility in Ovarian Oocytes.
Preliminary observations showed that a proteasome Z subunit (European Molecular Biology Laboratory accession no. AJ002142) is highly expressed in the accompanying cells of young previtellogenic oocytes and throughout oocyte maturation in follicle cells (data not shown). To establish whether the self-sterility factors produced by the follicle cells arise from a proteolytic process involving proteasome activity, we tested the effect of CLβL on maturation of ovarian oocytes. First, we exposed ovarian oocytes, which had undergone GVBD, to CLβL for 3 hours and found that self-sterility developed on schedule. This indicates that either no proteasome activity is involved or the processing of self-sterility molecules occurs at earlier stages of maturation.
Next we exposed to CLβL GV oocytes, namely, oocytes that had not undergone GVBD, and that did process fully formed VC. After 60–90 minutes of exposure, (time necessary for GVDB to occur) the CLβL was washed out, and the oocytes were cultured for 3 hours and then were test-fertilized (Table 1). These oocytes remained self-fertile.
Table 1.
Inhibition of the onset of self-sterility in GV oocytes by CLβL
| Percent self-fertilization
| |||||
|---|---|---|---|---|---|
| Sample | T0 | T0L | T3 | T3L | |
| GV oocytes | Experiment 1 | 90 | 90 | 0 | 90 |
| Experiment 2 | 100 | 100 | 0 | 100 | |
| Experiment 3 | 95 | 95 | 0 | 95 | |
| Experiment 4 | 88 | 88 | 0 | 88 | |
| Experiment 5 | 92 | 92 | 0 | 92 | |
| Experiment 6 | 100 | 100 | 0 | 100 | |
The germinal vesicle oocytes (GV oocytes) were incubated with the inhibitor up to GVBD. T0 indicates the time corresponding to GVBD and when oocytes were checked for self-fertilization. L, CLβL-treated oocytes; T3, oocytes inseminated after 3 hours of maturation.
To establish whether the target of CLβL-induced inhibition was indeed follicle cell proteasome, GV oocytes were exposed to CLβL for 60–90 minutes, at which time GVBD had occurred. They then were washed, and their follicle cells were removed. The washed oocytes were divided into two groups: One group was cultured with autologous follicle cells from untreated oocytes, and the other group was used as a control (Table 2). After 3 hours, the control oocytes remained self-fertile, and the oocytes exposed to untreated self-follicle cells were self-sterile. These biological assays show that only proteasome activity of the follicle cells, and not that of the oocyte, is required to provide self-sterility factors.
Table 2.
Effect of autologous follicle cells from untreated oocytes on CLβL-treated GV oocytes
| Percent self-fertilization
| |||
|---|---|---|---|
| Experiment | T0 | T3 | T3 + AFC |
| 1 | 90 | 90 | 0 |
| 2 | 100 | 100 | 0 |
| 3 | 95 | 95 | 0 |
All oocytes were treated with CLβL until GVBD. T0 indicates the time corresponding to GVBD and when oocytes were checked for self-fertilization. T3, oocytes inseminated after 3 hours of maturation; AFC, autologous follicle cells detached from GVBD untreated oocytes.
Effect of CLβL on the Onset of Self-Sterility by GVBD Oocytes.
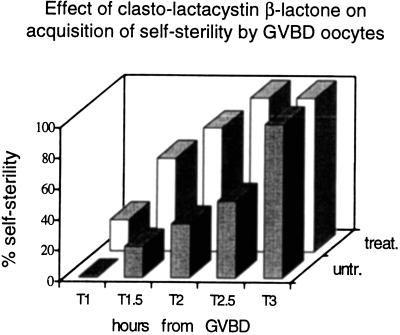
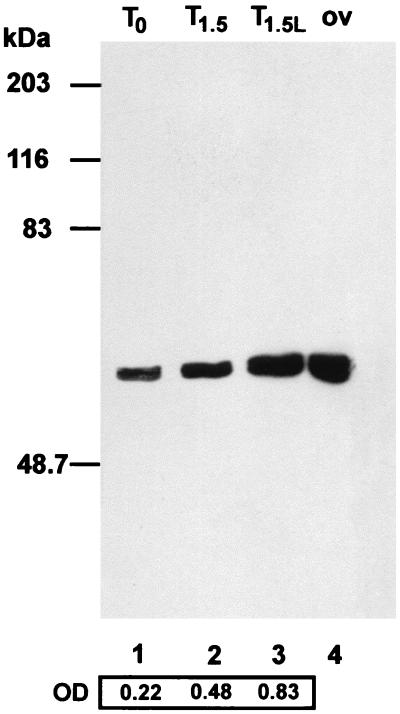
A more accurate analysis of the onset of self-sterility in the GVBD oocytes incubated with CLβL revealed that, under these conditions, oocytes became self-sterile before the control (Fig. 1). Data in the literature indicate that proteasome inhibitors stimulate the expression of cytosolic hsps (15, 16). This seems to be the case also in our system, as shown by using an anti-hsp70 antibody in Western blot analysis of proteins from untreated and treated oocytes from single individuals at different times. Although the time-dependent increase in hsp70 synthesis was higher in the CLβL-treated oocytes than in the untreated oocytes (Fig. 2), no conclusion can be reached as to a direct effect of the increase of the hsps on the early acquisition of self-sterility. However, the results of the biological assays point to an effect of the inhibitor on any of the molecules, including hsp70s, involved in the release of the self-discrimination factors.
Figure 1.
Time course of self-sterility acquisition by GVBD oocytes treated with CLβL. Ovarian oocytes immediately after GVBD were incubated with (□) or without (░⃞) 50 μM CLβL. Aliquots of oocytes were scored for self-fertilization at different times. Each column represents the percentage of self-sterile oocytes.
Figure 2.
Western blot analysis of proteins extracted from untreated oocytes at T0, time corresponding to GVBD (lane 1), and from untreated (T1.5) and CLβL-treated oocytes (T1.5L) 90 minutes after GVBD (lanes 2 and 3). An anti-hsp70 antibody was used. As a control, a sample of protein extracted from ovary (ov) is shown in lane 4. Hsp70 increase was quantitated by densitometric analysis of the autoradiograph. Molecular size standards are indicated on the left (in kilodaltons).
Effect of the VC Acid Extract on the Induction of Self-Sterility in Oocytes Treated with CLβL.
Because the characterization of self-sterility factors requires large amounts of material from single animals, we tried to recover them not only from the culture medium of follicle cells but also from mature oocytes. This idea was prompted by T. H. Morgan’s observations (13) that, in C. intestinalis, the egg block to self-fertilization can be removed by treating eggs with acidic sea water without affecting their viability.
We applied acidic sea water to mature self-sterile eggs deprived of follicle cells. Then we added the acid extract to either autologous or heterologous follicle cell-free self-fertile ovarian oocytes. After 3 hours of incubation, the oocytes exposed to the self acid extract were self-sterile, and the oocytes exposed to nonself acid extract were self-fertile (data not shown). This experiment shows that, like follicle cells (3) the VC acid extract induces self-sterility in an individual-specific manner.
To check whether the VC acid extract contains the follicle cell individual-specific factors, whose production is prevented by CLβL, GV oocytes were exposed to CLβL until GVBD, and, after washing, they were incubated with homologous acid extract. This procedure resulted in self-sterility. These experiments (results not shown because they coincide with those in Table 2) demonstrate that the VC acid extract contains the individual-specific factors produced by follicle cell proteasome and that the VC is their final target.
DISCUSSION
CLβL Inhibits the Onset of Self-Sterility.
Our hypothesis is that the ultimate step in the acquisition of self-sterility is the apposition to the VC of the individual-specific factors that “dock” into a preexisting receptor molecule of the VC, thus making it functional in discrimination between self and nonself sperm (4). Because of the lack of heterosterility and the small size of factors released by follicle cells, we propose that the self-sterility factors are of a protein nature. Accordingly, the generation of the peptides should result from proteasome activity.
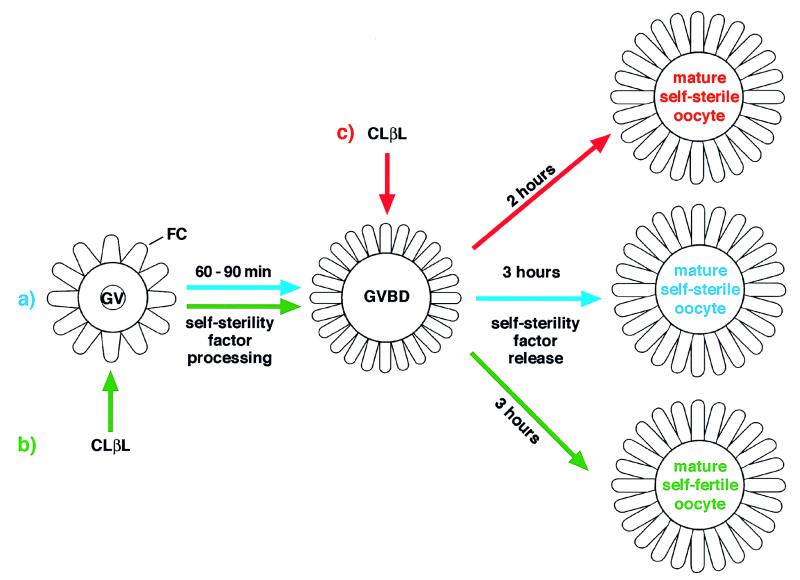
Here we demonstrate that proteasome activity is required for the onset of gamete self-sterility in C. intestinalis. We show that CLβL, an irreversible inhibitor of all proteasome catalytic β-subunits (12), prevents the onset of self-sterility only when applied to GV stage oocytes. This is in line with results that protein synthesis inhibitors applied at GVBD stage do not prevent acquisition of self-sterility (2). Hence, we conclude that CLβL is effective only on GV oocytes because at this stage, and not later, is the substrate for proteasome available (Fig. 3). This finding provides a system for in-depth studies on the timing of self-discrimination factor processing.
Figure 3.
Schematic representation of the effect of CLβL on the onset of self-sterility. (a) Self-sterility maturation process. GV oocytes mature to viable self-fertile oocytes in 60–90 minutes, the time required for GVBD to occur. During this time, self-sterility factors are processed in the follicle cells. After GVBD, the self-sterility factor is released and oocytes become fully mature self-sterile oocytes within 3 hours. (b) When CLβL is added to GV oocytes and maintained in the medium until they undergo GVBD (≈90 minutes), self-sterility factor processing does not occur, and, consequently, oocytes remain self-fertile. (c) If GVBD oocytes, which have already processed the self-sterility factors, are allowed to complete maturation in the presence of CLβL, they become self-sterile in a time considerably shorter than do controls. FC, follicle cells.
The inhibition of the onset of self-sterility caused by CLβL is caused specifically by inhibition of follicle cell function because self-sterility was induced in CLβL-treated oocytes by supplementing the medium with follicle cells of untreated autologous oocytes. This experiment also suggests that the proteasome inhibitor does not interfere with the production and assemblage of the VC individual-specific molecules that bind factors released from follicle cells.
CLβL Accelerates the Onset of Self-Sterility in GVBD Oocytes.
The observation that the application of proteasome inhibitor after GVBD, instead of preventing the acquisition of self-sterility, accelerates this process (Fig. 1) suggests that the inhibitor acts positively on the molecular pathway that enforces self-sterility. Even though we cannot draw any conclusion about the factors involved in this process or the molecules that are specifically affected by CLβL, it is interesting that hsp70s are induced by CLβL (15, 16). Previous data from our laboratory suggest that an hsp70 is involved in the final step of the acquisition of self-sterility in physiological conditions (4). Hence, one may speculate that the faster acquisition of self-sterility induced by CLβL may be due either to an increase of hsp70s or to an indirect effect of these proteins on the molecules located downstream from the processing of the self-sterility factors whose apposition to the VC leads to the onset of self-sterility (Fig. 3).
VC Acid Extract Induces Self-Sterility in Oocytes Treated with CLβL.
Kawamura et al. (17) confirmed Morgan’s finding (13) that acidic sea water abolishes the self-incompatibility barrier in mature oocytes and partially characterized the supernatant of acid-treated eggs. Kawamura showed that the acid extract is constituted mainly by neutral sugars that act in a biological assay as nonspecific inhibitors of binding of heterologous sperm to the VC of mature oocytes and small peptides. However, the function, if any, of the acid extract in self–nonself recognition has not been demonstrated.
Our result that both follicle cell-free ovarian oocytes and CLβL-treated ovarian oocytes become self-sterile when incubated with autologous VC acid extract suggests that VC acid extract contains, in a biologically active form, the self-sterility molecules released from follicle cells during oogenesis. This work demonstrates that the VC acid extract is biologically active in the induction of oocyte self-sterility—a process that in nature is controlled by autologous follicle cells.
In an earlier study, it was suggested that fertilization between heterologous gametes could result from a cytotoxic reaction triggered by the absence of self molecular markers (4) and that sperm would behave as natural killer cells. In fact, its individual-specific receptor, like the inhibitory natural killer cell receptor (KIR) (18), once engaged by the determinants on the VC, prevents binding to the egg and, therefore, fertilization. In this context, the involvement of proteasome in the production of self-specific factors transferred to the VC and able to prevent the binding of self sperm would be in agreement with Niedermann’s finding that the ability of proteasomes to generate potentially immunocompetent peptides is conserved in evolution among vertebrates and invertebrates (11).
Acknowledgments
Thanks go to Jean Gilder for editing the text.
ABBREVIATIONS
- GVBD
germinal vesicle breakdown
- GV
germinal vesicle
- CLβL
clasto-lactacystin β-lactone
- VC
vitelline coat
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Rosati F, De Santis R. Exp Cell Res. 1978;121:111–119. doi: 10.1016/0014-4827(78)90531-1. [DOI] [PubMed] [Google Scholar]
- 2.De Santis R, Pinto M R. Mol Reprod Dev. 1991;29:47–50. doi: 10.1002/mrd.1080290108. [DOI] [PubMed] [Google Scholar]
- 3.Pinto M R, De Santis R, Marino R, Usui N. Dev Growth Differ. 1995;37:287–291. doi: 10.1046/j.1440-169X.1995.t01-2-00006.x. [DOI] [PubMed] [Google Scholar]
- 4.Marino R, Pinto M R, Cotelli F, Lora Lamia C, De Santis R. Development (Cambridge, UK) 1998;125:899–907. doi: 10.1242/dev.125.5.899. [DOI] [PubMed] [Google Scholar]
- 5.Multhoff G, Botzler C, Meier T, Wiesnet M, Issels L D. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 6.Multhoff G, Botzler C. Ann NY Acad Sci. 1998;851:86–93. doi: 10.1111/j.1749-6632.1998.tb08980.x. [DOI] [PubMed] [Google Scholar]
- 7.Suto R, Srivastava P K. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Kasahara M. Immunol Rev. 1998;163:161–176. doi: 10.1111/j.1600-065x.1998.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 9.Grziwa A, Baumeister W, Dahlmann B, Kopp F. FEBS Lett. 1991;290:186–192. doi: 10.1016/0014-5793(91)81256-8. [DOI] [PubMed] [Google Scholar]
- 10.Lowe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber A. Science. 1995;268:533–539. doi: 10.1126/science.7725097. [DOI] [PubMed] [Google Scholar]
- 11.Niedermann G, Grimm R, Geier E, Maurer M, Realini C, Gartmann C, Soll J, Omura S, Rechsteiner M C, Baumeister W, et al. J Exp Med. 1997;21:209–220. doi: 10.1084/jem.186.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenteany G, Standaert R F, Lane S W, Soongyu C, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 13.Morgan T H. J Exp Zool. 1939;80:19–54. [Google Scholar]
- 14.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Bush K T, Goldberg A L, Nigam S K. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- 16.Kawazoe Y, Nakai A, Nagata K. Eur J Biochem. 1998;255:356–362. doi: 10.1046/j.1432-1327.1998.2550356.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura K, Nomura M, Kameda T, Shimamoto H, Nakauchi M. Dev Growth Differ. 1991;33:139–148. doi: 10.1111/j.1440-169X.1991.00139.x. [DOI] [PubMed] [Google Scholar]
- 18.Lanier L L, Philips J H. Immunol Today. 1996;17:86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]