Full text
PDF

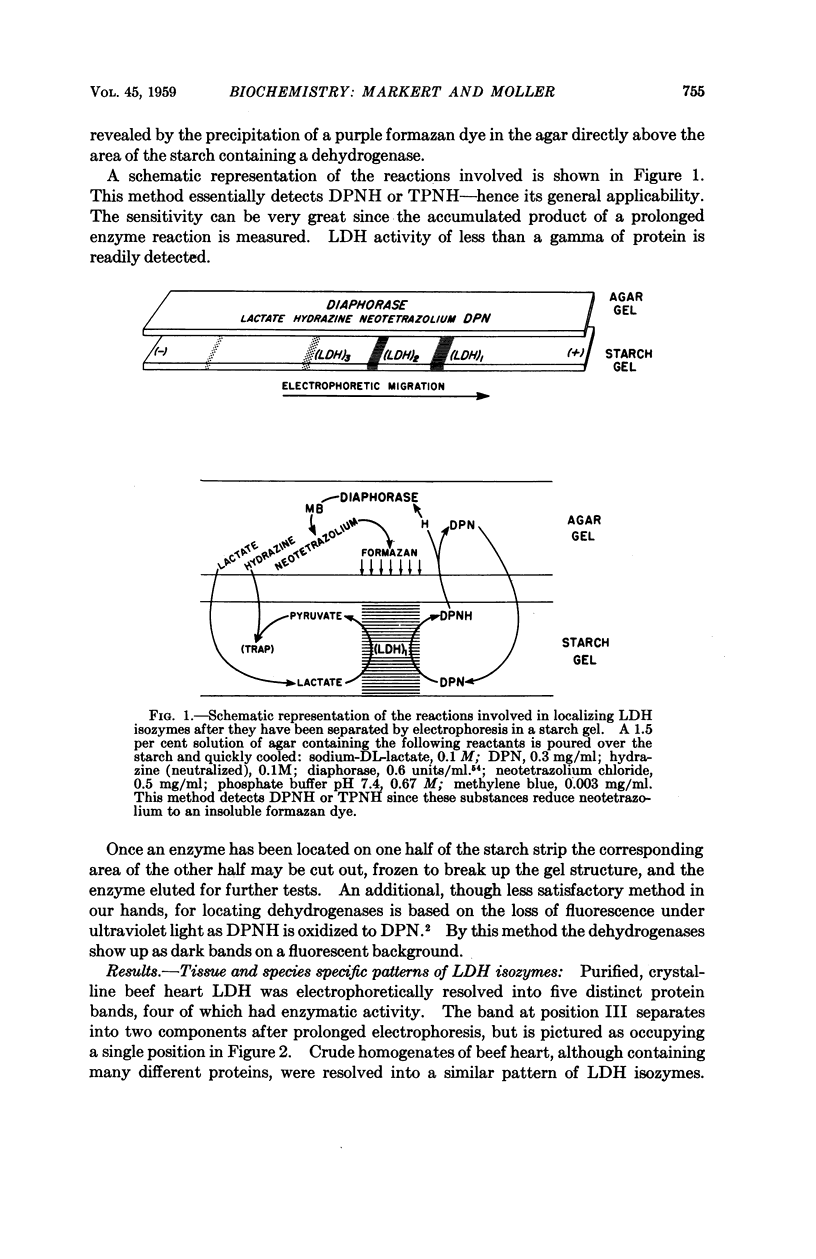

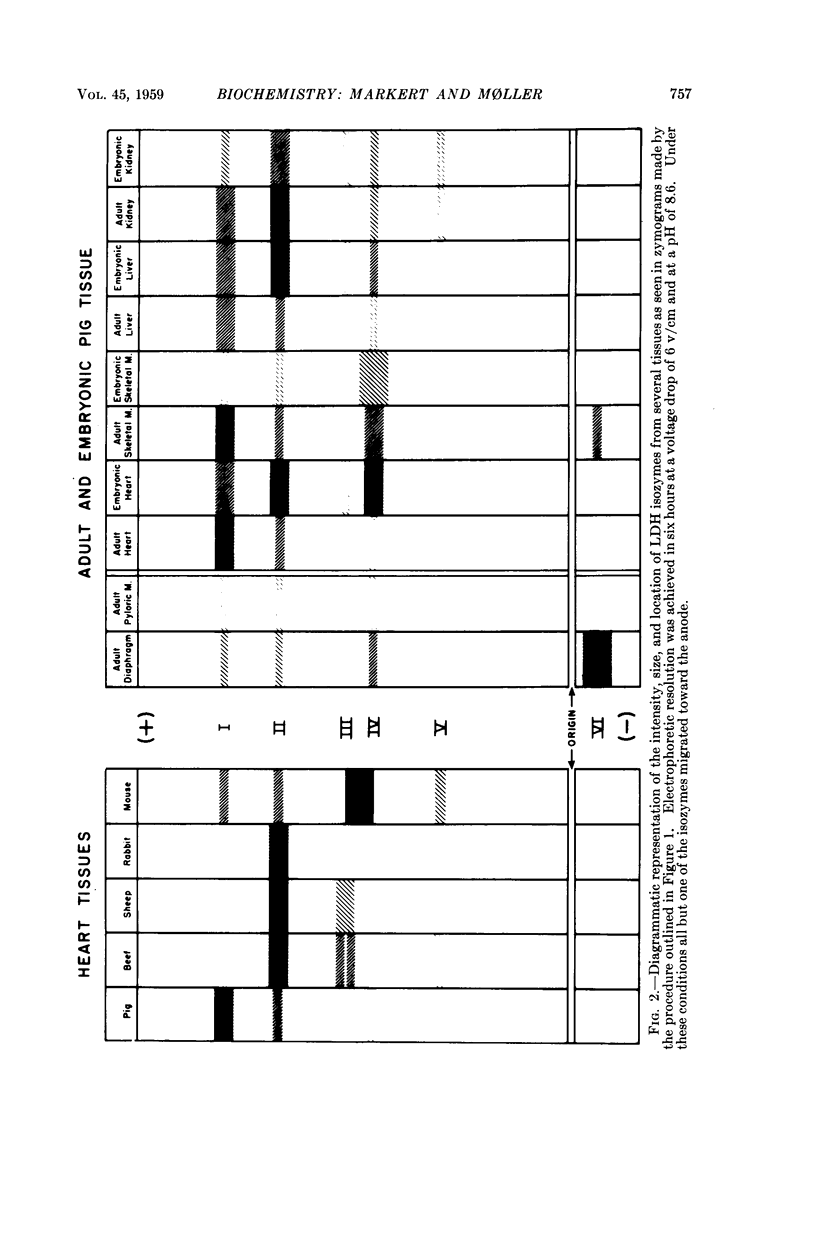
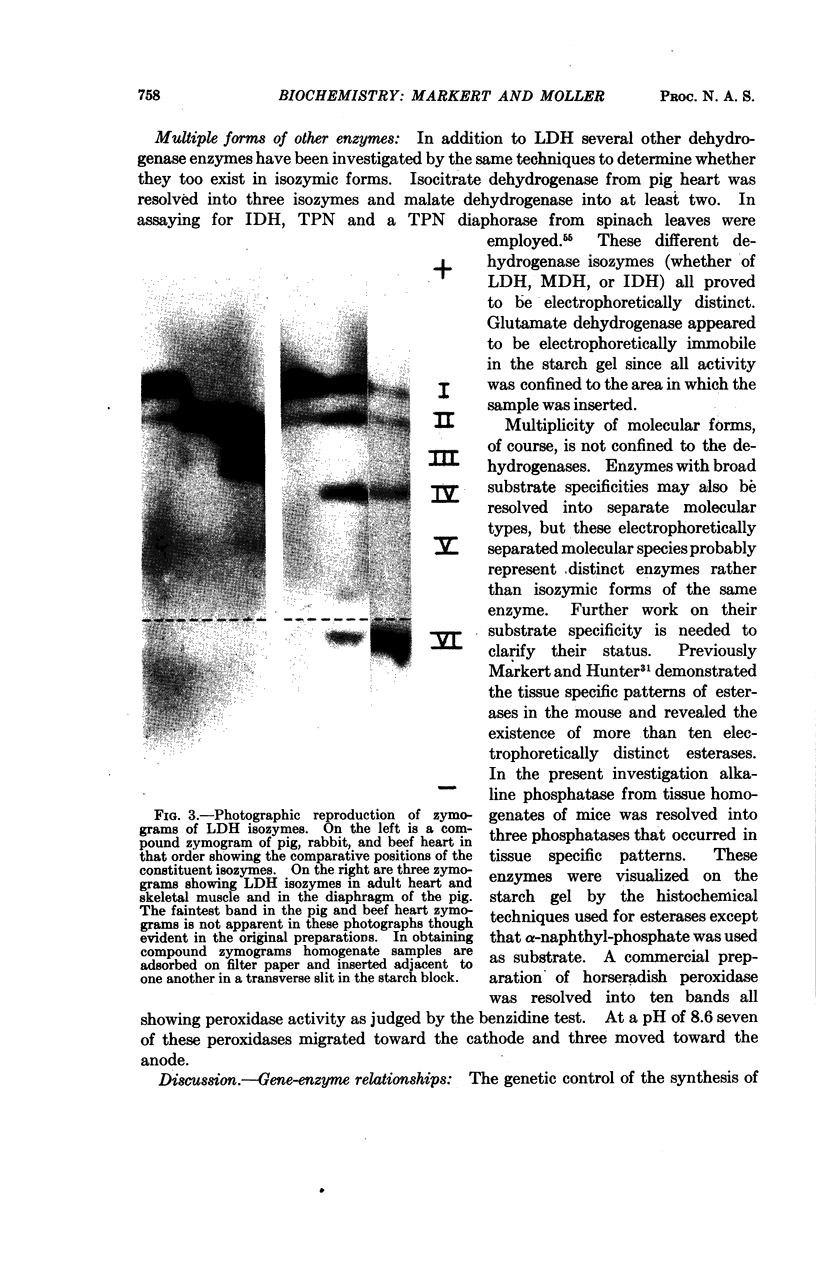





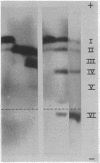
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. A TPNH diaphorase from chloroplasts. Arch Biochem Biophys. 1956 Dec;65(2):475–490. doi: 10.1016/0003-9861(56)90207-7. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., BLUMBERG G. S. Distribution of electrophoretically different haemoglobins among some cattle breeds of Europe and Africa. Nature. 1958 May 31;181(4622):1551–1552. doi: 10.1038/1811551a0. [DOI] [PubMed] [Google Scholar]
- BEARN A. G., VESELL E. S. Localization of lactic acid dehydrogenase activity in serum fractions. Proc Soc Exp Biol Med. 1957 Jan;94(1):96–99. doi: 10.3181/00379727-94-22866. [DOI] [PubMed] [Google Scholar]
- BLUMBERG B. S., TOMBS M. P. Possible polymorphism of bovine alpha-lactalbumin. Nature. 1958 Mar 8;181(4610):683–684. doi: 10.1038/181683a0. [DOI] [PubMed] [Google Scholar]
- BROWN H., SANGER F., KITAI R. The structure of pig and sheep insulins. Biochem J. 1955 Aug;60(4):556–565. doi: 10.1042/bj0600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CINADER B. Antibodies against enzymes. Annu Rev Microbiol. 1957;11:371–390. doi: 10.1146/annurev.mi.11.100157.002103. [DOI] [PubMed] [Google Scholar]
- CIOTTI M. M., KAPLAN N. O., STOLZENBACH F. E. Reaction of pyridine nucleotide analogues with dehydrogenases. J Biol Chem. 1956 Aug;221(2):833–844. [PubMed] [Google Scholar]
- DIXON G. H., SMITHIES O. Zone electrophoresis of cabbage enzymes in starch gels. Biochim Biophys Acta. 1957 Jan;23(1):198–199. doi: 10.1016/0006-3002(57)90305-0. [DOI] [PubMed] [Google Scholar]
- GREGORY K. F., WROBLEWSKI F. Preparation and properties of purified antilactic dehydrogenase. J Immunol. 1958 Nov;81(5):359–367. [PubMed] [Google Scholar]
- HARRIS J. I., NAUGHTON M. A., SANGER F. Species differences in insulin. Arch Biochem Biophys. 1956 Nov;65(1):427–438. doi: 10.1016/0003-9861(56)90203-x. [DOI] [PubMed] [Google Scholar]
- HENION W. F., MANSOUR T. E., BUEDING E. The immunological specificity of lactic dehydrogenase of Schistosoma mansoni. Exp Parasitol. 1955 Jan;4(1):40–44. doi: 10.1016/0014-4894(55)90021-7. [DOI] [PubMed] [Google Scholar]
- HENION W. F., SUTHERLAND E. W. Immunological differences of phosphorylases. J Biol Chem. 1957 Jan;224(1):477–488. [PubMed] [Google Scholar]
- HESS B. DPN-dependent enzymes in serum. Ann N Y Acad Sci. 1958 Oct 13;75(1):292–303. doi: 10.1111/j.1749-6632.1958.tb36877.x. [DOI] [PubMed] [Google Scholar]
- HILL B. R. Further studies of the fractionation of lactic dehydrogenase of blood. Ann N Y Acad Sci. 1958 Oct 13;75(1):304–310. doi: 10.1111/j.1749-6632.1958.tb36878.x. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. W., MOORE S., STEIN W. H. A chromatographic investigation of pancreatic ribonuclease. J Biol Chem. 1953 Feb;200(2):493–506. [PubMed] [Google Scholar]
- HUNTER R. L., MARKERT C. L. Histochemical demonstration of enzymes separated by zone electrophoresis in starch gels. Science. 1957 Jun 28;125(3261):1294–1295. doi: 10.1126/science.125.3261.1294-a. [DOI] [PubMed] [Google Scholar]
- HUNT J. A., INGRAM V. M. Allelomorphism and the chemical differences of the human haemoglobins A, S and C. Nature. 1958 Apr 12;181(4615):1062–1063. doi: 10.1038/1811062a0. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957 Aug 17;180(4581):326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- ISHIHARA Y., SAITO T., ITO Y., FUJINO M. Structure of sperm- and sei-whale insulins and their breakdown by whale pepsin. Nature. 1958 May 24;181(4621):1468–1469. doi: 10.1038/1811468b0. [DOI] [PubMed] [Google Scholar]
- KREBS E. G. Yeast Glyceraldehyde-3-phosphate dehydrogenase. I. Electrophoresis of fractions precipitated by nucleic acid. J Biol Chem. 1953 Feb;200(2):471–478. [PubMed] [Google Scholar]
- MALMSTROM B. G. The purification of yeast enolase by zone electrophoresis and ion-exchange chromatography, and the existence of several active forms of the enzyme. Arch Biochem Biophys. 1957 Jul;70(1):58–69. doi: 10.1016/0003-9861(57)90080-2. [DOI] [PubMed] [Google Scholar]
- MANSOUR T. E., BUEDING E., STAVITSKY A. B. The effect of a specific antiserum on the activities of lactic dehydrogenase of mammalian muscle and of Schistosoma mansoni. Br J Pharmacol Chemother. 1954 Jun;9(2):182–186. doi: 10.1111/j.1476-5381.1954.tb00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKERT C. L., HUNTER R. L. The distribution of esterases in mouse tissues. J Histochem Cytochem. 1959 Jan;7(1):42–49. doi: 10.1177/7.1.42. [DOI] [PubMed] [Google Scholar]
- MARTIN A. J. P., PORTER R. R. The chromatographic fractionation of ribonuclease. Biochem J. 1951 Jul;49(2):215–218. doi: 10.1042/bj0490215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A. Reduction of alpha gamma-diketo and alpha-keto acids catalyzed by muscle preparations and by crystalline lactic dehydrogenase. J Biol Chem. 1950 May;184(1):117–129. [PubMed] [Google Scholar]
- MITIDIERI E., RIBEIRO L. P., AFFONSO O. R., VILLELA G. G. Localization of xanthine dehydrogenase in rat serum by paper electrophoresis. Biochim Biophys Acta. 1955 Aug;17(4):587–587. doi: 10.1016/0006-3002(55)90426-1. [DOI] [PubMed] [Google Scholar]
- Markert C L, Owen R D. Immunogenetic Studies of Tyrosinase Specificity. Genetics. 1954 Nov;39(6):818–835. doi: 10.1093/genetics/39.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAJJAR V. A., FISHER J. Mechanism of antibody-antigen reaction. Science. 1955 Dec 30;122(3183):1272–1273. doi: 10.1126/science.122.3183.1272-a. [DOI] [PubMed] [Google Scholar]
- NEILANDS J. B. Studies on lactic dehydrogenase of heart. I. Purity, kinetics, and equilibria. J Biol Chem. 1952 Nov;199(1):373–381. [PubMed] [Google Scholar]
- PFLEIDERER G., JECKEL D. Individuelle Milchsäuredehydrogenasen bei verschiedenen Saugetieren. Biochem Z. 1957;329(5):370–380. [PubMed] [Google Scholar]
- PFLEIDERER G., JECKEL D., WIELAND T. Uber den Wirkungsmechanismus der Milchsäure-dehydrogenasen. II. Zur Frage des Zinkgehalts und der SH-Gruppen. Biochem Z. 1958;330(4):296–302. [PubMed] [Google Scholar]
- SAYRE F. W., HILL B. R. Fractionation of serum lactic dehydrogenase by salt concentration gradient elution and paper electrophoresis. Proc Soc Exp Biol Med. 1957 Dec;96(3):695–697. doi: 10.3181/00379727-96-23580. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLAN H. H., STEIN W. H. Chromatographic studies on lysozyme. J Biol Chem. 1953 Feb;200(2):507–514. [PubMed] [Google Scholar]
- TIMASHEFF S. N., STURTEVANT J. M., BIER M. On the electrophoretic heterogeneity of trypsin. Arch Biochem Biophys. 1956 Jul;63(1):243–246. doi: 10.1016/0003-9861(56)90027-3. [DOI] [PubMed] [Google Scholar]
- VAN DER HELM H. J., VAN VLIET G., HUISMAN T. H. Investigations on two different hemoglobins of the sheep. Arch Biochem Biophys. 1957 Dec;72(2):331–339. doi: 10.1016/0003-9861(57)90209-6. [DOI] [PubMed] [Google Scholar]
- VESELL E. S., BEARN A. G. Observations on the heterogeneity of malic and lactic dehydrogenase in human serum and red blood cells. J Clin Invest. 1958 May;37(5):672–677. doi: 10.1172/JCI103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VESELL E. S., BEARN A. G. The heterogeneity of lactic and malic dehydrogenase. Ann N Y Acad Sci. 1958 Oct 13;75(1):286–291. doi: 10.1111/j.1749-6632.1958.tb36876.x. [DOI] [PubMed] [Google Scholar]
- WIELAND T., PFLEIDERER G. Nachweis der Heterogenität von Milchsäure-dehydrogenasen verschiedenen Ursprungs durch Trägerelektrophorese. Biochem Z. 1957;329(2):112–116. [PubMed] [Google Scholar]