Abstract
Wiskott-Aldrich syndrome protein (WASp) is a hematopoietic-specific, multidomain protein whose mutation is responsible for the immunodeficiency disorder Wiskott-Aldrich syndrome. WASp contains a binding motif for the Rho GTPase CDC42Hs as well as verprolin/cofilin-like actin-regulatory domains, but no specific actin structure regulated by CDC42Hs-WASp has been identified. We found that WASp colocalizes with CDC42Hs and actin in the core of podosomes, a highly dynamic adhesion structure of human blood-derived macrophages. Microinjection of constitutively active V12CDC42Hs or a constitutively active WASp fragment consisting of the verprolin/cofilin-like domains led to the disassemly of podosomes. Conversely, macrophages from patients expressing truncated forms of WASp completely lacked podosomes. These findings indicate that WASp controls podosome assembly and, in cooperation with CDC42Hs, podosome disassembly in primary human macrophages.
Wiskott-Aldrich syndrome (WAS) is a X chromosome-linked recessive disorder originally characterized by the clinical triad eczema, thrombocytopenia, and severe immunodeficiency (1). Cellular defects in WAS range from loss of microvilli (2) and CD43 expression on lymphocytes (3) and reduced size and quantity of platelets (reviewed in ref. 4) to compromised motility of dendritic cells (5) and defective chemotaxis of macrophages (6, 7). The gene responsible for WAS has been identified, and a plethora of mutations spread over the WAS gene has been reported (4, 8, 9).
A clue as to how mutations in one gene could produce such a variety of symptoms came from characterization of the WAS protein (WASp). WASp is a hematopoietic cell-specific protein that consists of several functional domains, including a WASp homology 1 (WH1) domain, which overlaps with a pleckstrin homology domain, a CDC42/Rac-interactive binding domain, also called GTPase-binding domain, a polyproline domain, a verprolin homology domain, a cofilin homology domain, and a short stretch of acidic amino acids at the C terminus (10–12). Some of these domains were also found in the WASp homologues N-WASp (13) and WAVE/Scar (14).
The CDC42/Rac-interactive binding domain of WASp was shown to bind relatively specific to the active, GTP-bound form of the Rho-GTPase CDC42Hs, which qualifies WASp as a CDC42Hs effector protein (10–12). CDC42Hs, like Rho and Rac, belongs to the Rho family of GTPase-binding proteins that plays a pivotal role in the dynamic organization of cellular actin structures (reviewed in ref. 15). That CDC42Hs might also regulate actin through WASp was first indicated by the finding that actin clustering induced by overexpression of WASp in aortic endothelial cells could be blocked by coexpression of dominant-negative N17CDC42Hs (12).
The recent findings that different actin regulatory molecules and actin-related proteins can bind to WASp (16–18), the implication of N-WASp and a further WASp-related protein, WAVE, in the generation of filopodia and lamellipodia, respectively (14, 19, 20), as well as the long-known abnormalities in the cytoarchitecture of WAS lymphocytes and platelets, strongly suggest an important role for WASp in actin regulation. Until now, however, direct evidence for abnormal actin regulation in hematopoietic cells of WAS patients has been scarce (5, 21), and no physiologically relevant actin structures controlled by CDC42Hs-WASp have been identified.
In the present study, we provide evidence that WASp, in cooperation with CDC42Hs, controls podosomes, dynamic actin-containing adhesion structures of primary human macrophages. Podosomes seemingly play an important role in migratory processes, and their impaired regulation may provide a molecular basis for the cellular immunodeficiency in WAS.
MATERIALS AND METHODS
Cell Isolation and Cell Culture.
Human peripheral blood monocytes were isolated by centrifugation of heparinized blood in Ficoll (Seromed, Munich). Monocytic cells were isolated with magnetic anti-CD14 antibody beads and an MS+ Separation Column (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions and seeded onto Cellocate coverslips (Eppendorf) at a density of 5 × 104 cells. Cells were cultured in RPMI containing 20% autologous serum at 37°C, 5% CO2, and 90% humidity. Medium was changed every 3–4 days.
Cell Polarization.
Slices of 200 μl of 2% agarose containing 50 μg/ml formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma) were added to macrophages cultured coverslips in 2 ml of medium for the indicated times. Agar slices and coverslips were placed at opposite ends of wells in eight-well plates (Nunc). After a 6-h incubation, coverslips were removed and fixed in 3.7% formaldehyde.
Generation of WASp Constructs.
WASp-domain constructs were created by cloning PCR-generated inserts into the EcoRI and BamHI sites (in the case of constructs GP and C-V) or into the BamHI site (in the case of constructs GPC, C, V, and C-Co) of vector pGEX-2T (Amersham Pharmacia). Inserts of constructs were checked for correct orientation and fully sequenced.
Protein Expression.
Proteins were expressed in Escherichia coli as glutathione S-transferase (GST) fusions as described (23). Proteins were dialyzed against microinjection buffer (50 mM Tris⋅HCl/150 mM NaCl/5 mM MgCl2), concentrated in Centricon or Microcon (Amicon), shock-frozen, and stored at −80°C. Purity was tested by SDS/PAGE and Coomassie staining.
Actin Binding Assay.
Cells (4 × 107) were lysed for 30 min at 4°C in 1 ml of RIPA buffer (10 mM Tris⋅HCl, pH 8.0/1% Triton X-100/140 mM NaCl) with protease inhibitors. After centrifugation (15000 rpm, 15 min, 4°C), the supernatant was added to 200-μl aliquots of a 50% slurry of glutathione Sepharose beads. The beads had been previously incubated for 1 h with 50 μg of GST-fusion proteins. Beads were incubated with lysate for 3 h at 4°C with mixing, pelleted, washed four times with 1 ml of washing buffer (10 mM Tris⋅HCl, pH 7.5/0.1% Triton X-100/10% glycerol), and incubated with 1 ml of washing buffer for 15 min at 4°C with mixing. Beads were pelleted, 50 μl of SDS sample buffer was added, and an aliquot was run on a 12.5% SDS gel. Actin binding was tested by Western blot using actin-specific antibody MAB1501 (Chemicon). No actin binding was detected when GST alone was bound to beads.
Immunoblotting.
Western blots were prepared as described (24). Actin was detected with mAb MAB 1501 (Chemicon), CDC42Hs by using a polyclonal antibody raised against amino acid 167–183 of CDC42Hs (22). For WASp, mAb 3D8.H5 (25), recognizing an epitope between amino acids 102 and 201 of WASp, was used. Secondary antibodies were horseradish peroxidase-coupled sheep anti-mouse or donkey anti-rabbit IgGs (Amersham Pharmacia). Protein bands were visualized by using Super Signal kit (Pierce) and X-Omat AR film (Kodak).
Microinjection of Proteins.
Cells for microinjection experiments were cultured for 6–10 days. Microinjection was performed by using transjector 5246 (Eppendorf) and a compic inject micromanipulator (Cell Biology Trading, Hamburg, Germany). Proteins were injected into the cytoplasm at 300 μg/ml in the case of CDC42 and 0.5–2 mg/ml in the case of WASp domains, unless indicated otherwise. Injected cells were identified by labeling coinjected rat IgG (5 mg/ml; Dianova, Hamburg, Germany) with FITC-labeled goat anti-rat IgG (Dianova). Control injections were performed with GST.
Immunofluorescence Microscopy.
Cells were fixed for 10 min in 3.7% formaldehyde solution and permeabilized for 10 min in ice-cold acetone. Actin was stained with Alexa 568-labeled phalloidin (Molecular Probes) or mAb MAB 150, CDC42 with the above-mentioned antibody, WASp with antibody 3D8.H5, and vinculin with antibody VIN-11–5 (Sigma). Secondary antibodies were Alexa 488- and 568-labeled goat anti-mouse or goat anti-rabbit (Molecular Probes). Coverslips were mounted in Moviol (Calbiochem) containing p-phenylendiamine (Sigma) as anti-fading reagent and sealed with nail polish.
RESULTS
WASp Colocalizes with CDC42Hs and Actin in Podosomes of Primary Human Macrophages.
Previously, we reported that human peripheral blood monocytes show a large increase of membrane-bound CDC42Hs when differentiating into adherent macrophages (22, 24). We therefore speculated that CDC42Hs might have a specific function in macrophage adhesion. To obtain better evidence for this hypothesis, we first performed a series of immunofluorescence experiments.
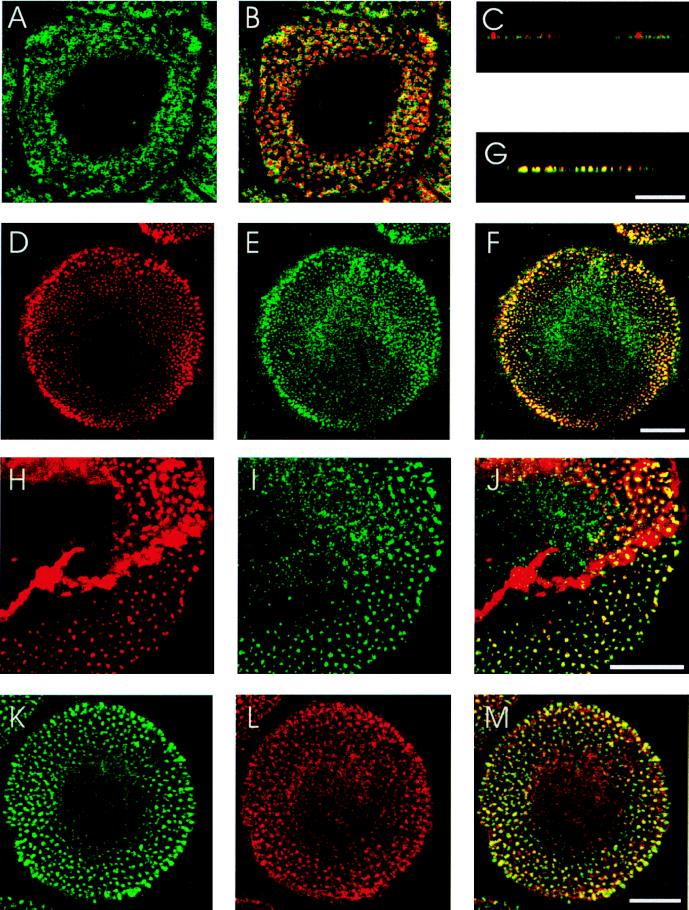
Unstimulated human macrophages show a rounded, epitheloid cell shape after 4–7 days in culture (Fig. 1). Staining with rhodamine phalloidin revealed filamentous actin in the cytoplasm, in a peripheral ring and in numerous dot-like structures, called podosomes (Fig. 1 B, D, and H). Podosomes have been described as zones of close contact to the substratum, consisting of an actin-rich core together with associated actin-regulatory proteins and surrounded by a ring of vinculin (Fig. 1 A–C; ref. 26). After prolonged macrophage culture (>7 days), podosomes are mostly organized in a peripheral band surrounding a central podosome-free area containing the nucleus (Fig. 1B).
Figure 1.
Localization of actin, vinculin, CDC42Hs, and WASp in podosomes of primary human macrophages. Confocal laser scanning micrographs showing horizontal sections of ventral parts of cells (A, B, D–F, and H–M), vertical sections of peripheral parts of cells (C and G), vinculin staining(A), overlays of vinculin (green) and actin staining (red) (B and C), actin staining (D), CDC42Hs staining (E), overlay of D and E (F), overlay of actin (red) and CDC42HS staining (G), actin staining (H), WASp staining (I) overlay of H and I (J), WASp staining (K), CDC42Hs staining (L), and overlay of K and L (M). Yellow color indicates colocalization of red and green. [Bar = 10 μm for each row.]
Double staining with rhodamine phalloidin and a specific anti-CDC42Hs antibody (22) revealed colocalization of actin and CDC42Hs to the core of podosomes (Fig. 1 D–G). Besides in podosomes, CDC42Hs staining was also detected in a granular distribution in the cytoplasm surrounding the nucleus (Fig. 1E). Between 50 and 90% of podosomes from whole cells stained positive for both actin and CDC42Hs.
Given that WASp is a hematopoietic-specific effector of CDC42Hs and that WASp can localize to clusters of actin polymerization (12), we tested whether it is also present in podosomes. Double-staining experiments demonstrated colocalisation of actin and WASp in the core of podosomes (Fig. 1 H–J). WASp staining could also be detected in the cytoplasm as described earlier for other cell types (Fig. 1I; refs. 25 and 27). Between 50 and 90% of podosomes stained positive for both actin and WASp. Not surprisingly, double staining for WASp and CDC42Hs also revealed a high degree of colocalization in podosomes (Fig. 1 K–M).
Microinjection of V12CDC42Hs or WASp Verprolin/Cofilin-Like Domain Leads to Podosome Disruption.
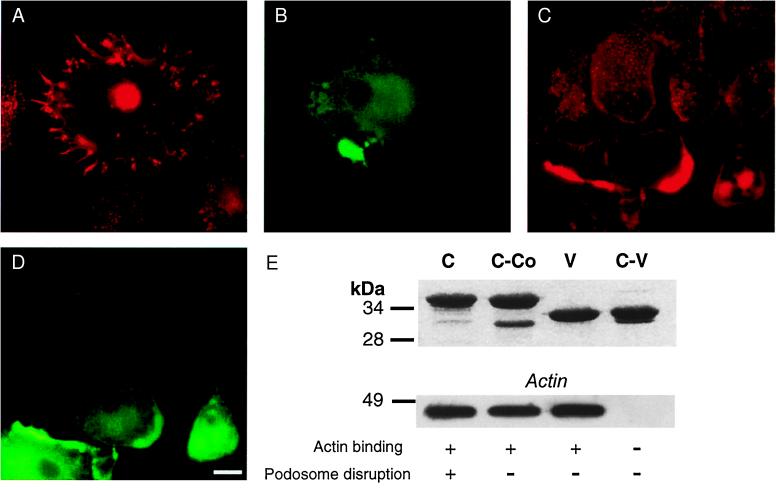
We next performed microinjection experiments to gather evidence for the potential involvement of CDC42Hs and WASp in the regulation of podosomes. When injected with constitutively active V12CDC42Hs (28), macrophages responded by (i) formation of microspikes/filopodia, (ii) cell spreading, or (iii) disassembly of podosomes, or different combinations and degrees thereof. The most prevalent (50–70% of injected cells) response was filopodia formation combined with podosome disappearance (Fig. 2 A and B).
Figure 2.
CDC42Hs and WASp control podosomes. Immunofluorescence micrographs of primary human macrophages. (A) Cell injected with V12CDC42Hs, actin staining; (B) injection control of A, staining against coinjected rat IgG. (C) Macrophages injected with C-terminal WASp polypeptide C, actin staining; (D) injection control of C, staining against coinjected rat IgG. [Bar = 10 μm.] (E) (Upper) GST-fusions of C-terminal WASp polypeptides. SDS/polyacrylamide gel stained with Coomassie blue. Denominations of polypeptides are given above each lane. Running behavior of polypeptides differs in some cases from theoretical molecular mass, which is indicated on the left in kDa. (Lower) Actin binding of C-terminal WASp polypeptides. Western blot probed with actin-specific antibody. Molecular mass is indicated on the left in kDa. Abilities of polypeptides to bind actin and disrupt podosomes are indicated below each lane by + or −.
For evaluating the role of WASp in podosome regulation, we designed and bacterially expressed GST-polypeptides representing various WASp domains. Microinjection into macrophages of polypeptide GPC, corresponding to amino acids 187–489 (containing the GTPase binding, the polyproline and the C-terminal verprolin- and cofilin-like domains of WASp) and of polypeptide C, corresponding to amino acids 422–489 (containing only the C-terminal domain, but not the final acidic stretch) led to the disruption of podosomes (Fig. 2 C and D). It also caused clumping of actin in the cell periphery (Fig. 2C), an effect described earlier in porcine aortic endothelial cells overexpressing WASp (12). Injection of polypeptide GP, corresponding to amino acids 238–404 (containing the GTP-binding and the polyproline domain) did not have any discernible effect on the actin cytoskeleton of macrophages.
The active polypeptide C contains both verprolin- and cofilin-like subdomains, thereby displaying homology to regions of known regulators of the actin cytoskeleton. Indeed, corresponding domains in the WASp-related protein N-WASp have already been described as being absolutely necessary for actin binding and severing as well as microspike formation by N-WASp (13, 19, 20). To further elucidate the function of the C-terminal verprolin- and cofilin-like domains, we created constructs corresponding to (i) the verprolin-like domain of WASp (amino acids 430–449; construct V), (ii) the C-terminal domain without the cofilin-like domain (amino acids 430–468, construct C-Co), and (iii) the C-terminal domain without the verprolin-like domain (amino acids 450–489; construct C-V). On injection into macrophages, none of these domains disrupted podosomes or produced any other discernible effect on the actin cytoskeleton. Yet, in GST pull-down assays, polypeptides C, C-Co, and V, but not polypeptide C-V, bound to actin, either from macrophage lysate supernatants (Fig. 2E) or added as a purified protein from rabbit skeletal muscle (data not shown). These results indicate that the verprolin-like domain of WASp is necessary and sufficient for direct binding of actin but does need the cofilin-like domain to disrupt podosomes and induce actin clumping.
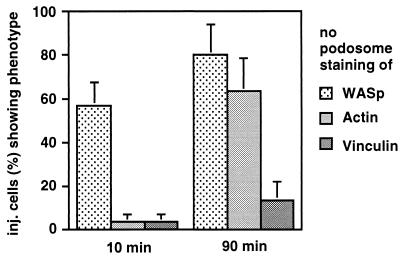
To gain insight into the potential mode of action of the C-terminal WASp domain, we injected cells with diluted preparations of polypeptide C (200 ng/μl) and after different time points stained them for actin, vinculin, and WASp. Staining for WASp was done by using antibody 3D8.H5, which does not recognize polypeptide C. Ten minutes after injection, 57% of injected cells had completely lost WASp from their podosomes, whereas only 3% had lost actin and 3% had lost vinculin. Ninety minutes after injection, 80% of cells were without podosomal WASp, 63% were without podosomal actin, and 13% were without podosomal vinculin (Fig. 3). After prolonged incubation (6 h) or injection of more concentrated protein solutions, vinculin also disappeared almost completely from podosomes. This result shows that podosome disruption through the C-terminal domain of WASp is a specific and sequential process, with WASp being the first protein dislocated from its podosomal localization, followed by actin and finally by vinculin.
Figure 3.
Time course of podosome disruption after injection of C-terminal WASp domain. Cells were fixed at the indicated time points after injection. Percentage of cells without podosomal staining of the respective protein is represented by bars. For each value, three separate injections of 30 cells were performed. Standard errors are indicated by error bars. Uninjected cells or cells injected with GST show values below 3% for loss of podosomal staining for each protein (data not shown).
Podosomes Are Not Formed in Macrophages from WAS Patients.
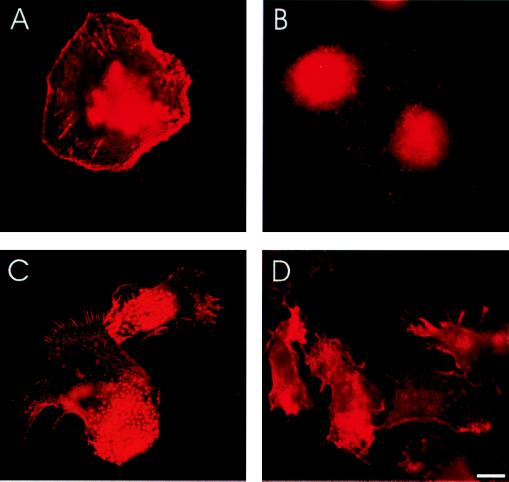
To get direct proof that WASp regulates podosomes, we investigated macrophages from two molecular genetically well characterized WAS patients. One patient is at best able to express an extremely truncated form of WASp, because of the insertion of a guanine nucleotide at position 69 resulting in a frameshift mutation and termination of expression after amino acid 45 (patient 1). The WASp gene of the other patient shows the mutation C995T, leading to a premature stop codon and expression of a C-terminally truncated form of WASp (patient 3,603; ref. 9). The actin distribution and the overall shape did not differ markedly between macrophages of the two patients. However, macrophages from patient 3,603 seemed to adhere better to glass coverslips than those from patient 1. In none (0%) of the observed macrophages from the two WAS patients we could detect podosomes, as defined by a lack of the typical localization of actin (Fig. 4A), vinculin (Fig. 4B), WASp, and CDC42 (data not shown). Thus, macrophages from WAS patients expressing only truncated forms of WASp are not able to form podosomes.
Figure 4.
WAS macrophages have defects in podosome formation and cell polarization. Immunofluorescence micrographs of primary human macrophages. (A) Macrophage of WAS patient 3603, actin staining. (B) Macrophages of WAS patient 3,603, vinculin staining. (C and D) Macrophages stimulated with fMLP, actin staining. (C) Macrophages from healthy donor. (D) Macrophages from WAS patient 3,603. [Bar = 10 μm.]
WAS Macrophages Have a Defect in Polarization.
We finally asked what further consequences the inability to form podosomes could have for WAS macrophage function. For this purpose, we induced a polarized phenotype in macrophages by applying a gradient of the chemoattractant fMLP. On polarization of normal macrophages, podosomes reoriented to the trailing edge of the cell, whereas filopodia were confined to the leading edge (Fig. 4C). When stimulated with an fMLP gradient, WAS macrophages were also able to acquire a polarized cell shape. This was evaluated by counting cells (n = 100) with elongated shape (ratio of length to breadth ≥2; 74% with fMLP compared with 27% without fMLP) and cells displaying filopodia (70% with fMLP compared with 19% without fMLP). However, in addition to the absence of podosomes, the cell body of stimulated WAS macrophages seemed to be more irregular, fewer filopodia were generated per cell and, more interestingly, filopodia were distributed over a larger part of the cell circumference and were not confined to one edge of the cell (Fig. 4D). Hence, podosomes and filopodia seem to display a mutually exclusive polarized distribution in a migratory phenotype of macrophage, and this distribution is severely compromised in WAS macrophages.
DISCUSSION
The results of our study suggest that WASp, in cooperation with the Rho-GTPase CDC42Hs, regulates podosomal adhesion structures in primary human macrophages. This conclusion is based on the following findings: (i) WASp, CDC42Hs, and actin colocalize in the core of podosomes, (ii) WASp coprecipitates with active CDC42Hs in macrophage lysates (data not shown), (iii) macrophages from WAS patients cannot assemble podosomes, (iv) microinjection of active V12CDC42Hs induces filopodia and disassembles podosomes in parallel, and (v) microinjection of a C-terminal WASp fragment led to the sequential displacement of WASp, actin, and vinculin from podosomes and thereby induced their disappearance.
Interestingly, long-term overexpression (48 h) of V12CDC42Hs in HeLa-derived HtTA-1 cells was reported to result in the formation of actin-rich structures resembling podosomes (29). These artificial actin dots were shown to contain vinculin and to contact the substratum. However, in these structures, no vinculin ring was found, phosphotyrosine is stained only faintly, whereas we and others detect high amounts of phosphotyrosine in macrophage podosomes, and V12CDC42Hs is present and proformatory, whereas macrophage podosomes are disrupted by V12CDC42Hs. Hence, these actin-rich dots clearly differ from podosomes in many structural and functional regards.
Podosomes are highly dynamic adhesion structures with a half-life of 2–12 min (30, 31). They have so far been described in monocyte-derived cells such as macrophages and osteoclasts (32, 33), as well as in transformed fibroblasts (32, 34), and their presence has been linked to the invasiveness and ability of these cells to move across anatomical boundaries (32). Consistent with this, it could be shown that the subcellular position of podosomes corresponds to sites of localized proteolytic activity (35).
We identified the hematopoietic-specific WASp as an important regulator of podosomes in macrophages. One can speculate that there is a corresponding regulator in transformed fibroblasts that might be a new member of the steadily growing WASp family of proteins (14, 20).
Microinjection of a WASp fragment encompassing the C-terminal verprolin- and cofilin-like domains released podosomal components time-dependently with the sequence WASp–actin–vinculin. This indicates that podosome disassembly by injection of the C-terminal WASp domain could be due to dislocation of endogenous WASp. Interestingly, the C-terminal WASp domain also led to the formation of actin clusters at the cell periphery. Actin clustering was reported previously for Jurkat T cells or endothelial cells overexpressing WASp (12). Hence, disassembly of podosomes might be a combination of binding competition and actin depolymerization/polymerization through a constitutively active C-terminal WASp fragment. Consistent with this idea, it was proposed that the C terminus in N-WASp is masked in the inactive molecule and on binding of GTP-CDC42Hs to the CDC42/Rac-interactive binding domain gets unmasked and thereby active (20). This may also be true for WASp, considering that constitutively active V12CDC42Hs disassembled podosomes just like the isolated C-terminal WASp domain. The form of CDC42Hs that we could detect in fully assembled podosomes might conceivably be in an inactive state, not directly bound to WASp, but part of a preformed machinery that, on activation, disassembles podosomes. However, our finding that WAS macrophages do not contain podosomes clearly demonstrates the requirement of WASp for assembly of podosomes also. The recent report that WASp is able to bind the Arp2/3 (Actin-related protein) complex, a nucleator of actin filaments (17), may provide an explanation for this. In a possible scenario, WASp may receive a signal from receptor tyrosine kinases and subsequently initiate the formation of podosomes by concentrating the Arp2/3 complex to sites of actin assembly.
Cell polarization of leukocytes, mostly associated with generation of filopodia, can be induced by external cues like fMLP (6) and is thought to be the first step in cell migration (reviewed in ref. 36). Because we observed that CDC42Hs in the same macrophage can regulate both, filopodia, presumably via N-WASp, and podosomes, through WASp, we asked what the effect of WAS deficiency on macrophage polarization would be. Normal macrophages stimulated with a gradient of the bacterial chemoattractant fMLP developed a polarized phenotype displaying filopodia at the leading edge and podosomes at the trailing edge. In comparison, macrophages from WAS patients not only lacked podosomes at the trailing edge but also failed to generate the densely arranged filopodia at the leading edge. Instead, filopodia were less numerous and were also distributed over a larger part of the cell. Such a behavior could possibly be explained by compartmentalized and mutually exclusive regulation of podosomes via CDC42Hs-WASp at one edge of the cell and filopodia via CDC42Hs–N-WASp at the other edge of the cell. A consequence of this model would be that if the CDC42Hs–WASp part is missing, the CDC42Hs–N-WASp part will be disorganized also. Supporting this hypothesis, coexpression of WASp and CDC42Hs was found to suppress microspike formation in COS-7 cells (20). Moreover, a defect in chemotaxis, which requires directed polarization, but not chemokinesis, which does not require directed polarzation, was shown recently in WAS macrophages (7). Furthermore, in nonadherent WAS monocytes, a loss of pseudopodial polarization in response to fMLP was reported (6). All of these data support an essential role of WASp in macrophage polarization/migration.
Unsuccessful chemotactic migration of WAS macrophages could result in impaired recruitment to inflamed tissues (6) and the inability of antigen-presenting cells to travel to secondary lymphoid organs (37), and this could be partly accountable for the observed immune defects in WAS patients. Given the occurrence of podosomes in macrophages as well as in tumor cells (38), WASp and/or other WASp-family proteins may also be a contributing factor to pathogenic processes involving cellular emigration and invasion like atherosclerosis or tumor metastasis.
Acknowledgments
We thank the WAS patients for participating in this study. We also thank Peter C. Weber, Jürgen Heesemann, and Bernd H. Belohradsky for continuous support, Barbara Böhlig for expert technical assistance, Alan Hall for providing the V12CDC42Hs construct, Daniela Rieger for rabbit skeletal muscle actin, Markus Essler for help and discussions, Markus Bauer for help with computer software, Rudolf Kern (Cellbiology Trading) for maintaining the microinjection device, and Michael Schleicher and Wolfgang Siess for helpful advice and critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 413, Ae 11), by Wilhelm Sander Stiftung, and by August Lenz Stiftung.
ABBREVIATIONS
- fMLP
formyl-methionyl-leucyl-phenylalanine
- GST
glutathione S-transferase
- WAS
Wiskott-Aldrich syndrome
- WASp
Wiskott-Aldrich syndrome protein
References
- 1.Kirchhausen T. Mol Med Today. 1998;9:300–304. doi: 10.1016/s1357-4310(98)01268-4. [DOI] [PubMed] [Google Scholar]
- 2.Kenney D M, Cairns L, Remold-O′Donnel E, Peterson J, Rosen F S, Parman R. Blood. 1986;68:1329–1332. [PubMed] [Google Scholar]
- 3.Remold-O’Donnell E, Rosen F S, Kenney D M. Blood. 1996;87:2621–2631. [PubMed] [Google Scholar]
- 4.Ochs H D. Springer Semin Immunopathol. 1998;19:459–478. doi: 10.1007/BF00792601. [DOI] [PubMed] [Google Scholar]
- 5.Binks M, Jones G E, Brickell P M, Kinnon C, Katz D R, Thrasher A J. Eur J Immunol. 1998;28:3259–3267. doi: 10.1002/(SICI)1521-4141(199810)28:10<3259::AID-IMMU3259>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Badolato R, Sozzani S, Malacarne F, Bresciani S, Fiorini M, Borsatti A, Albertini A, Mantovani A, Ugazio A, Notarangelo L D. J Immunol. 1998;161:1026–1033. [PubMed] [Google Scholar]
- 7.Zicha D, Allen W E, Brickell P M, Kinnon C, Dunn G A, Jones G E, Thrasher A J. Brit J Haematol. 1998;101:659–665. doi: 10.1046/j.1365-2141.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 8.Derry J M J, Ochs H D, Francke U. Cell. 1994;78:635–644. [PubMed] [Google Scholar]
- 9.Schindelhauer D, Weiss M, Hellebrand H, Golla A, Hergersberg M, Seger R, Belohradsky B H, Meindl A. Hum Genet. 1996;98:68–76. doi: 10.1007/s004390050162. [DOI] [PubMed] [Google Scholar]
- 10.Aspenström P, Lindberg U, Hall A. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 11.Kolluri R, Fuchs Tolias K, Carpenter C L, Rosen F S, Kirchhausen T. Proc Natl Acad Sci USA. 1996;93:5615–5618. doi: 10.1073/pnas.93.11.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Symons M, Derry J M J, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 13.Miki H, Miura K, Takenawa T. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 14.Miki H, Suetsugu S, Takenawa T. EMBO J. 1998b;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall A. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 16.Ramesh N, Antón I M, Hartwig J H, Geha R S. Proc Natl Acad Sci USA. 1997;94:14671–14676. doi: 10.1073/pnas.94.26.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machesky L M, Insall R H. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Spencer S D, Lasky L A. J Biol Chem. 1998;273:5765–5770. doi: 10.1074/jbc.273.10.5765. [DOI] [PubMed] [Google Scholar]
- 19.Miki H, Takenawa T. Biochem Biophys Res Commun. 1998;243:73–78. doi: 10.1006/bbrc.1997.8064. [DOI] [PubMed] [Google Scholar]
- 20.Miki H, Sasaki T, Takai Y, Takenawa T. Nature (London) 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 21.Gallego M D, Santamaria M, Pena J, Molina I J. Blood. 1997;90:3089–3097. [PubMed] [Google Scholar]
- 22.Aepfelbacher M, Vauti F, Weber P C, Glomset J A. Proc Natl Acad Sci USA. 1994;91:4263–4267. doi: 10.1073/pnas.91.10.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridley A, Hall A. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 24.Aepfelbacher M, Essler M, Huber E, Czech A, Weber P C. J Immunol. 1996;157:5070–5075. [PubMed] [Google Scholar]
- 25.Stewart D M, Treiber-Held S, Kurmann C C, Facchetti F, Notarangelo L D, Nelson D L. J Clin Invest. 1996;97:2627–2634. doi: 10.1172/JCI118712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarone G, Cirillo D, Giancotti F G, Comoglio P M, Marchisio P C. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 27.Rivero-Lezcano O M, Marcilla A, Sameshima J H, Robbins K C. Mol Cell Biol. 1995;15:5725–5731. doi: 10.1128/mcb.15.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart M J, Shinjo K, Hall A, Evans T, Cerione R A. J Biol Chem. 1991;266:20840–20848. [PubMed] [Google Scholar]
- 29.Dutartre H, Davoust J, Gorvel J-P, Chavrier P. J Cell Sci. 1996;109:367–377. doi: 10.1242/jcs.109.2.367. [DOI] [PubMed] [Google Scholar]
- 30.Stickel S K, Wang Y L. J Cell Biol. 1987;104:1521–1526. doi: 10.1083/jcb.104.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa J, Yamanaka T, Doi S, Turksen K, Heersche J M N, Aubin J E, Takeuchi H. Bone. 1990;6:287–293. doi: 10.1016/8756-3282(90)90082-a. [DOI] [PubMed] [Google Scholar]
- 32.Marchisio P C, Cirillo D, Teti A, Zambonin Zallone A, Tarone G. Exp Cell Res. 1987;169:202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- 33.Hiura K, Lim S S, Little S P, Lin S, Sato M. Cell Motil Cytoskeleton. 1995;30:272–284. doi: 10.1002/cm.970300405. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka J, Watanabe T, Nakamura N, Sobue K. J Cell Sci. 1993;104:595–606. doi: 10.1242/jcs.104.2.595. [DOI] [PubMed] [Google Scholar]
- 35.Chen W-T, Olden K, Bernard B A, Chu F-F. J Cell Biol. 1984;98:1546–1555. doi: 10.1083/jcb.98.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-Madrid F, del Pozo M A. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thrasher A J, Jones G E, Kinnon C, Brickell P M, Katz D R. Trends Immunol Today. 1998;19:537–539. doi: 10.1016/s0167-5699(98)01350-4. [DOI] [PubMed] [Google Scholar]
- 38.Schuuring E, Verhoeven E, Litvinov S, Michalides R J. Mol Cell Biol. 1993;13:2981–2898. doi: 10.1128/mcb.13.5.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]