Abstract
The spatial organization of cell–cell adherens junctions is distinct in cultured cells from two different tissue types, specifically, epitheliocytes and fibroblasts. In epitheliocytes, contacts are localized tangentially, along contacting cell edges and in association with circumferential actin bundles. Contacts between fibroblasts are radially oriented; that is, they are perpendicular to the overlapping edges of the cells and are associated with straight bundles of actin filaments. In the present study, we establish that the spatial organization of cell–cell contacts in the epithelial cell line IAR-2 can be converted from the typical tangential pattern to the radial pattern observed in fibroblasts. This transition can be induced by treatment with two agents, phorbol 12-myristate 13-acetate and nocodazole, which have different modes of action. Inhibition of myosin contractility reverses tangential-to-radial conversion of cell–cell contacts. These data suggest that formation of radially aligned contacts depends on modulation of contractility within the actin cytoskeleton through the myosin motor protein. The results open the possibility that modulation of the spatial organization of cell–cell contacts may play important roles in regulating organization and physiological functions of epithelial tissues.
Establishment and maintenance of cell–cell and cell–substrate contacts depends on multicomponent interactions between the actin cytoskeleton and specific classes of adhesion proteins (1–5). In previous studies, we described two distinct cell-type specific patterns for the spatial organization of adherens junctions formed by epitheliocytes and fibroblasts (6, 7). In cultures of epithelial cells, adherens junctions are aligned tangentially along the cell–cell boundaries, they expand laterally after cells establish initial contact, and expanding contacts are associated with arc-like bundles of actin filaments (6). By contrast, in cultures of fibroblasts adherens, junctions are aligned perpendicularly to contacting cell edges, and the junctions are colocalized with the ends of straight actin bundles (7).
The aim of the studies presented in this paper was to determine whether epithelial cells are capable of modulating the spatial pattern of cell–cell contacts between tangential and radial alignment. To access pathways that might induce switching between contact orientations, we treated epithelial cells with two reagents, phorbol 12-myristate 13-acetate (PMA) and nocodazole, that have different mechanisms of action. PMA is known to be an activator of protein kinase C that induces epithelial cells to acquire fibroblast-like properties, including polarized lamellipodial activity, elongate cell shape, and increased migratory activity (8). The observed effects of PMA are reminiscent of epithelial-mesenchymal transformation during normal development in vivo and in cultures of epithelial cells treated with certain growth factors (9). Nocodazole is a highly specific inhibitor of microtubule polymerization. Microtubule depolymerization results in increased contractility of the actin cytoskeleton via activation of the small G-protein, Rho (10). We found that PMA and nocodazole induced reorganization of cell–cell contacts, in particular, formation of radial contact strands along with an accompanying change in the structure of the actin cytoskeleton.
To assess the potential mechanism responsible for modulating the orientation of cell–cell contacts, epithelial cells were treated with two pharmacological agents known to inhibit acto-myosin contractility, 2,3-butanedione 2-monoxime (BDM) and HA-1077. BDM suppresses myosin ATPase activity (11) whereas HA-1077, an inhibitor of Rho-kinase, decreases myosin contractility by effecting phosphorylation of myosin phosphatase (12, 13). Treatment with both types of agents resulted in reversal of PMA- and nocodazole-induced formation of radial contact strands. These data suggest that alteration of acto-myosin contractility may provide a common denominator among multiple effects elicited by PMA and nocodazole that result in spatial reorganization of epithelial cell–cell contacts. The reorganization of contacts observed in vitro may possibly be prototypic of the switches in spatial patterns of intercellular contacts in vivo, which may play important roles in the remodeling of epithelial structures during normal development.
MATERIALS AND METHODS
Cell Culture and Drug Treatments.
Rat liver epithelial cell line IAR-2 (14) was cultured and grown as monolayers on glass coverslips (15). In some experiments, cell monolayers were scraped with a hypodermic needle to create a narrow wound and were incubated for 3–4 hr before observation.
To examine the effects of protein kinase C activation or microtubule depolymerization on formation of cell–cell contacts, cells were incubated for 2–3 hr with 0.15 μM PMA (Sigma) or for 50 min with 30–60 μM nocodazole (Sigma). To inhibit myosin contractility, IAR-2 cells incubated in medium containing PMA were treated for 40–50 min with Rho-kinase inhibitor HA-1077 at a concentration of 20 μM or with the myosin inhibitor BDM (Sigma) at a concentration of 40 mM. HA-1077 was a generous gift of V. Small (Institute of Molecular Biology, Salzburg, Austria). In some experiments, pretreatment of cells with 20 μM HA-1077 for 20 min was followed by incubation with HA-1077 and nocodazole. High resolution video differential interference contrast microscopy was performed on a Zeiss Axiophot microscope as described (6).
Immunofluorescence Staining and Confocal Microscopy.
IAR-2 cells were fixed for 10 min at −20°C by using a 1:1 mixture of acetone/methanol and were stained with anti-E-cadherin monoclonal antibodies (Transduction Laboratories, Lexington, KY) at a 1:100 dilution. For simultaneous localization of actin and β-catenin, cells were fixed in PBS containing 3.7% formaldehyde, were permeabilized for 3 min with 1% Triton X-100 in PBS, and were stained with a 1:50 dilution of rhodamine-phalloidin (Molecular Probes) and a 1:50 dilution of anti-β-catenin monoclonal antibodies (Transduction Laboratories). For actin and myosin-II labeling, cells were permeabilized and fixed as described (7) and subsequently were stained with anti-nonmuscle myosin-II polyclonal antibodies (BioMedical Technologies, Stoughton, MA) at a 1:50 dilution and rhodamine-phalloidin. All primary antibodies were visualized by using Oregon-Green labeled secondary antibodies (Molecular Probes). Fluorescence images were collected by using the Bio-Rad MRC 1024 laser scanning confocal microscope system of the Rutgers-Newark Advanced Imaging Facility.
RESULTS
Control IAR-2 Cultures.
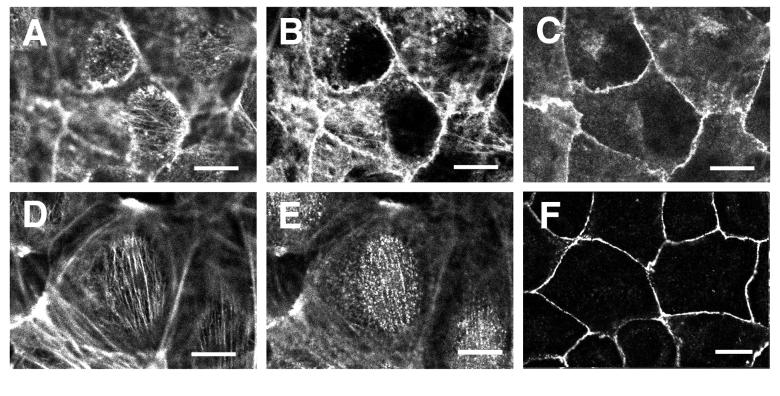
IAR-2 cells formed adherent monolayers of circular or polygonal shaped cells that possessed a defined vertical organization to the actin cytoskeleton. Optical sectioning of fluorescently labeled cells by laser scanning confocal microscopy identified three distinct populations of actin filaments. The uppermost apical region of the cell was traversed by thin parallel bundles of actin filaments that projected downward toward the circumferential bundle of actin, where the ends appeared to terminate (Fig. 1 A and D). Similar apical actin bundles were previously observed in retinal pigment epithelial cells (16). There was no detectable colocalization of adherens junction components with the apical bundles of actin filaments. Circumferential actin bundles outlining the perimeter of the cell were colocalized with smooth, thin adhesion belts containing E-cadherin and β-catenin (Fig. 1 B, C, and F). Lastly, the basal portion of cells contained numerous crisscrossed stress fibers that exhibited no detectable association with E-cadherin or β-catenin (data not shown). Apical actin bundles, circumferential actin bundles, and basal stress fibers exhibited punctate myosin-II staining (Fig. 1 D and E; M.K. and E.M.B., unpublished observations).
Figure 1.
Organization of the actin cytoskeleton and cell–cell contacts in control IAR-2 cells. Monolayers of IAR-2 cells were fixed and either double-labeled for F-actin (A and B) and β-catenin (C), F-actin (D), and myosin-II (E) or single-labeled for E-cadherin (F). Confocal images in A–C are optical sections of the same cells at the level of the apical cell surface (A) or 0.6 μm below the cell surface (B and C). Confocal images of the apical cell surface (A and D) revealed the presence of thin parallel bundles of actin filaments that exhibited punctate localization of myosin-II along the bundles (E). Apical actin bundles appeared to be connected to circumferential actin bundles (B), which colocalized with β-catenin (C). Adhesion belts in IAR-2 cells contained both both β-catenin (C) and E-cadherin (F). (Bars = 10 μm.)
Effects of PMA Treatment on Formation of Cell–Cell Contacts and Organization of the Actin Cytoskeleton.
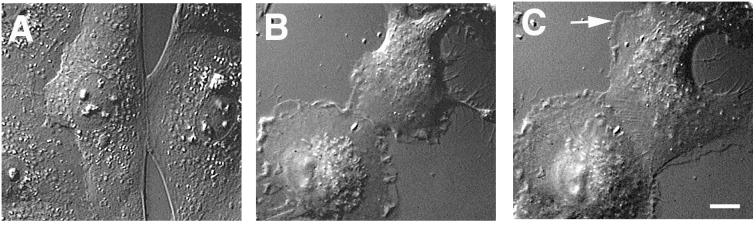
Individual IAR-2 cells treated with PMA lost their circular or polygonal shape and acquired a polarized morphology characterized by having a broad leading lamella with numerous dynamic lamellipodia and stable lateral edges (Fig. 2B). On cell–cell contact, PMA-treated cells continued to exhibit dynamic edge activity forming new lamellipodia along the edge even after establishment of an expanding cell–cell contact (Fig. 2 B and C). The continuation of lamellipodial activity after establishment of cell–cell contact was in direct contrast to the behavior of nontreated IAR-2 cells in which lamellipodial activity was inhibited at the free cell edge adjacent to the site of contact (Fig. 2A; see also ref. 6). Another fibroblast-like feature of PMA-treated cells was the formation of a new leading lamella adjacent to the site of cell–cell contact, which reoriented the direction of cell movement away from the contact (Fig. 2C).
Figure 2.
Effect of PMA on contact formation during wound healing. Contact formation by control (A) or PMA-treated cells (B and C) was documented by using video–differential interference contrast microscopy. B and C represent images of the same cells taken with a 16-min interval. During contact formation in control IAR-2 cells (A), free cell edges adjacent to the contact zone appeared stretched and contained few lamellipodia. Edges of PMA-treated cells contained numerous lamellipodia, which continued to form even after the establishment of the initial cell–cell contact (B). A new leading lamella (arrow) formed at the free edge of a PMA-treated cell resulting in cell reorientation even after contact expansion and increased cell–cell overlapping (C). (Bar = 10 μm.)
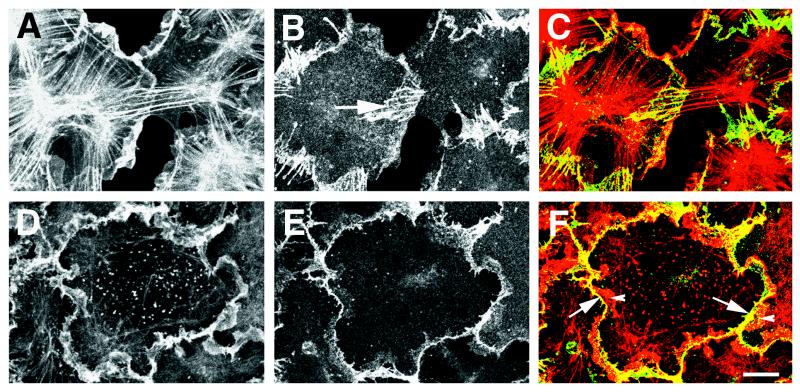
The complex vertical architecture of the actin cytoskeleton was lost after treatment with PMA. PMA-treated cells lost the circumferential bundles of actin filaments that are a major characteristic of cell–cell adherens junctions in epithelial cells (Fig. 3A). Loss of circumferential bundles of actin filaments was accompanied by development of numerous thick stress fibers, which radiated outward from the center of the cell forming star-like arrays. These stress fibers protruded into large actin-rich lamellipodia that had extended and overlapped with adjacent cells (Fig. 3A). Indirect immunofluorescence using anti-E-cadherin and anti-β-catenin antibodies established that PMA-treated cells no longer contained continuous adhesion belts; instead, the cell adhesion proteins were localized to multiple, radially oriented parallel contact strands (Figs. 3B and 4). The radial strands were typically observed in regions in which active lamellae of one cell overlapped the surface of an adjacent cell. Many of the radial contact strands colocalized with the ends of the actin filament bundles emanating from the center of the cell (Fig. 3).
Figure 3.
Organization of the actin cytoskeleton and cell–cell contacts in PMA-treated cells. IAR-2 cells were allowed to form new cell–cell contacts in the presence of PMA alone (A–C) or PMA and p160 Rho-dependent kinase inhibitor HA-1077 (D–F). Cells were fixed and double-stained for actin filaments (A and D) and β-catenin (B and E). C and F are superimposed images of actin and β-catenin staining in which actin is shown in red and β-catenin in green. Yellow color indicates areas in which actin and β-catenin are colocalized. (A–C) PMA-treated cells contained numerous lamellipodia and thick bundles of actin filaments radiating outward from the cell center (A). Cell–cell contacts formed in the presence of PMA consisted of parallel radial strands located in the lamellae that overlapped the surface of the adjacent cell (B, arrow). Radial strands of β-catenin often colocalized with the ends of actin filament bundles (C). (D–F) Inhibition of myosin contractility with HA-1077 resulted in the loss of actin filament bundles (D) and loss of radial cell–cell contacts (E). Thin, scallop-shaped linear cell–cell contacts in cells treated with HA-1077 often formed at the base (F, arrow) of lamellae (F, arrowhead) that overlapped with the surface of an adjacent cell. (Bar = 10 μm.)
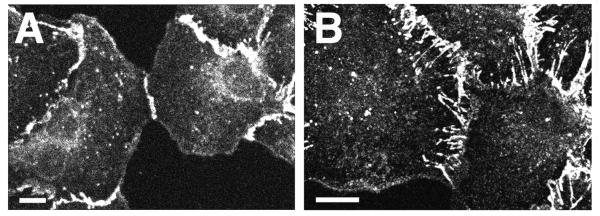
Figure 4.
Localization of E-cadherin to cell–cell contacts in control and PMA-treated epithelial cells. IAR-2 cells forming new cell–cell contacts either in the absence (A) or presence (B) of PMA were fixed and stained with anti-E-cadherin antibodies. In control cells, E-cadherin accumulated as a thin tangential cell–cell contact (A) whereas in PMA-treated cells, E-cadherin was localized to numerous radial strands. (Bars = 10 μm.)
Next, we sought to determine whether there existed a relationship between myosin contractility and formation of radial contact strands in PMA-treated cells. IAR-2 cells were exposed to PMA for 2 hr followed by incubation in PMA and either the myosin inhibitor BDM or the p160 Rho kinase inhibitor, HA-1077, for an additional 40–50 min. Incubation of PMA-treated cells with either BDM (data not shown) or HA-1077 (Fig. 3D) resulted in the loss of stress fibers and retention of actin filament meshworks within lamellipodia that were induced by PMA. Further, myosin inhibition also resulted in the loss of PMA-induced radial contact strands identified by indirect immunofluorescence with either anti-E-cadherin or anti-β-catenin antibodies. β-catenin (Fig. 3E) and E-cadherin (data not shown) were relocalized to the periphery of the cell along lines of cell–cell contact; however, the contacts assumed a “scalloped” shape rather than the linear, smooth adhesion belts seen in control cells. The line of scallop-shaped cell–cell contacts appeared to form at the base of lamellipodia, which continued to extend when cells were treated with PMA and inhibitors of myosin activity (Fig. 3 D–F). Myosin inhibition had no effect on contact structure in monolayers of control cells.
Effects of Microtubule Depolymerization on the Organization of the Actin Cytoskeleton and Cell–Cell Contacts.
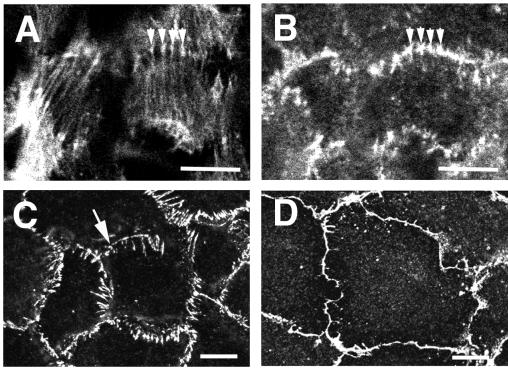
Short-term (50–60 min) treatment of IAR-2 cells with nocodazole (30–60 μM) resulted in almost complete depolymerization of microtubules, although some cells still contained a few short microtubules radiating from the cell center. In contrast to PMA treatment, incubation of IAR-2 cells with nocodazole did not result in a fibroblast-like cell shape, and the overall organization of the actin cytoskeleton was not as dramatically altered as after PMA treatment. Apical and circumferential actin bundles were preserved in nocodazole-treated cells; however, we noted that the ends of apical actin bundles were thickened, and optical sectioning revealed that the dome-like shape of the apical bundles was somewhat flattened relative to control cells. Circumferential bundles of actin filaments and the associated adhesion belt, although still appropriately positioned, often appeared fragmented along their length. Additionally, there were radial contact strands that emanated upward and away from the area containing the adhesion belt and circumferential bundle of actin (Fig. 5 B and C). Examination of cells double-labeled for actin filaments and β-catenin showed that the contact strands were often colocalized with the thickened ends of the apical bundles of actin filaments (Fig. 5 A and B).
Figure 5.
Organization of cell–cell contacts and the actin cytoskeleton after treatment with nocodazole. Monolayers of IAR-2 cells were treated with nocodazole for 1 hr either in the absence (A–C) or presence (D) of HA-1077. Cells were fixed and either were double-stained for actin filaments (A) and β-catenin (B) or were single-labeled with anti-E-cadherin antibodies (C and D). In cells treated with nocodazole, the ends of the apical bundles of actin filaments were thickened, and they appeared to colocalize with β-catenin (compare arrowheads in A and B). Additionally, there were numerous contact strands extending radially away from cell–cell contacts (C) and the tangential cell–cell contacts appeared somewhat fragmented (C, arrow). Inhibition of p160 Rho-dependent kinase using HA-1077 resulted in disappearance of radial strands and restoration of tangential contacts (D). (Bars = 10 μm.)
In fibroblasts, depolymerization of microtubules with nocodazole leads to enhanced contractile activity through a Rho-dependent pathway that stimulates stress fiber formation (10). Likewise, the observed effects of nocodazole on IAR-2 cells suggested activation of myosin contractility on depolymerization of microtubules. To inhibit nocodazole-induced contractility, cells were initially treated with HA-1077, to prevent myosin activation, followed by exposure to nocodazole. Cells treated with HA-1077 retained the tangential organization of adhesion belts typical of control epithelial cells and they did not form the numerous radially aligned contact strands seen in microtubule-disrupted cells. Adherens junctions of HA-1077/nocodazole-treated cells exhibited some scalloping (Fig. 5D).
DISCUSSION
Results of our experiments show that the spatial pattern of adherens contacts in epithelial cultures can be switched from tangential to radial. Radial orientation of contacts was previously shown to be typical of fibroblastic cultures (7), which do not express E-cadherin. However, in our present experiments with cultures of epithelial cells, both unchanged tangential contacts and radial contact strands contained E-cadherin and β-catenin. This result indicates that spatial organization of contacts can be regulated in the absence of changes in the molecular composition of the proteins involved in formation of adherens junctions.
Reorganization of cell–cell contacts in our experiments could be induced by treating epithelial cells with either PMA or nocodazole. PMA, a protein kinase C activator, is a well characterized inducer of epithelial-mesenchymal transformation (8, 17) whereas nocodazole is a specific inhibitor of microtubule polymerization. What is the common link between the mechanisms of contact reorganization induced by PMA and nocodazole? We suggest that the effects of both agents on the organization of the contacts are mediated by alteration of the actin cytoskeleton. Treatment with PMA or nocodazole resulted in distinct changes to the actin cytoskeleton of IAR-2 cells. PMA induced four types of changes in the organization of the actin cytoskeleton: (i) loss of circumferential bundles of actin filaments, (ii) formation of radial arrays of actin stress fibers, (iii) induction of lamellipodial activity, including formation of lamellipodia by cells in monolayers, and (iv) polarization of lamellipodial activity in individual cells. The observed changes in actin organization are in agreement with earlier observations that brief (15–40 min) exposure of epithelial cells to PMA induces disassembly of preexisting stress fibers (17) whereas longer incubation (2 hr) results in formation of novel radial stress fibers (8). Likewise, nocodazole treatment, in addition to changing contact organization from tangential to radial, also modulated actin organization. It induced thickening of the ends of the apical actin bundles and fragmentation of circumferential actin belts. Thus, both treatments promoted actin reorganization, and, in particular, PMA induced formation of stress fibers and nocodazole induced thickening of the apical actin bundles, events that are likely to be driven by myosin activity. Moreover, similarly to cell–cell contacts between fibroblasts (7), radial contact strands formed in the presence of PMA or nocodazole were colocalized with the ends of actin bundles. These observations led us to suggest that, although the mechanisms of action of PMA and nocodazole are substantially different, the signaling pathways activated by these agents may converge on a common downstream target—namely, myosin-driven contraction within the actin cytoskeleton. For example, it has previously been shown that small G-protein Rho activation is involved both in PMA-induced and nocodazole-induced assembly of actin stress fibers (8, 10, 18). Activated Rho is known to increase phosphorylation of myosin II regulatory light chain, thereby up-regulating myosin contractile activity (13). Therefore, modulation of myosin contractility may serve as a common link in the mechanisms of action of PMA and nocodazole on contact organization.
To test our hypothesis regarding possible involvement of acto-myosin contractility in PMA- and nocodazole-induced contact reorganization, we used two inhibitors of myosin activity, BDM and HA-1077. Each inhibitor acts through a different mechanism. BDM directly inhibits myosin ATPase activity (11) whereas HA-1077 is a relatively specific inhibitor of Rho-associated kinase (12) that controls myosin light chain phosphorylation by regulating myosin phosphatase (13). Although these inhibitors have different mechanisms of action, both induced the loss of acto-myosin structures such as radial actin bundles and reversed the transformation of tangential into radial contacts. Similar to our results, inhibition of acto-myosin contractility by BDM and other agents had been found earlier to restore normal epithelial contacts in Ras-transformed mammary cells (19). Consequently, myosin activity is likely to play an important role in contact reorganization. Previously, Volberg et al. (20) postulated that acto-myosin contractility participated in regulating cell–cell junction integrity. This phenomenon of myosin-driven contact reorganization may be similar to the mechanism of maturation of focal contacts between cells and extracellular matrix. An increase in Rho-dependent myosin contractility was found to induce elongation of focal contacts whereas inhibition of contractility by BDM and other agents reversed focal contact elongation (4, 5).
Interestingly, epithelial cells treated with both PMA and Rho kinase inhibitor were still able to form new cell–cell contacts having tangential orientation. Unlike smooth tangential contacts in control cells, contacts formed in the presence of both PMA and Rho kinase inhibitor appeared scalloped. This scalloping may be attributed to lamellipodial activity, which continues, and may even be enhanced, in cells in which myosin activity is inhibited. It is possible that in the absence of acto-myosin contractility physical proximity of lamellipodia in adjacent cells was sufficient to form tangential adhesions.
The reported observations suggest that modulation of the spatial organization of cell–cell contacts similar to that observed in vitro may play important roles in normal development in vivo. Transformation of continuous tangential contacts in epithelial and endothelial sheets into groups of discrete radial contacts may considerably change the physiological properties of cell monolayers and, in particular, promote cell motility and rearrangement. These changes in cell–cell adhesion may facilitate outgrowth of epithelial tubules during organogenesis, elongation of endothelial tubules during angiogenesis, and other types of physiological remodeling of compact epithelial and endothelial structures.
Acknowledgments
We thank Dr. V. Small (Institute of Molecular Biology, Salzburg, Austria) for the gift of reagents. This work was supported by the Gabriella and Paul Rosenbaum Foundation and Mrs. M. Goldman (to I.M.G.), a Russian Foundation of Basic Investigations grant (to J.M.V.), an American Heart Association Predoctoral fellowship (to M.K.), a gracious gift from Mrs. Harold Kaplan to the Research Exchange Program at Rutgers-Newark, and the Charles and Johanna Busch Memorial Fund (to E.M.B.).
ABBREVIATIONS
- PMA
phorbol 12-myristate 13-acetate
- BDM
2,3-butanedione 2-monoxime
References
- 1.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 2.Adams C L, Nelson W J. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- 3.Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- 4.Chrzanowska-Wodnicka M, Burridge K. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pletjushkina O J, Belkin A M, Ivanova O J, Oliver T, Vasiliev J M, Jacobson K. Cell Adhes Commun. 1998;5:121–135. doi: 10.3109/15419069809040286. [DOI] [PubMed] [Google Scholar]
- 6.Gloushankova N A, Alieva N A, Krendel M F, Bonder E M, Feder H H, Vasiliev J M, Gelfand I M. Proc Natl Acad Sci USA. 1997;94:879–883. doi: 10.1073/pnas.94.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gloushankova N A, Krendel M F, Alieva N O, Bonder E M, Feder H H, Vasiliev J M, Gelfand I M. Proc Natl Acad Sci USA. 1998;95:4362–4367. doi: 10.1073/pnas.95.8.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imamura H, Takaishi K, Nakano K, Kodama A, Oishi H, Shiozaki H, Monden M, Sasaki T, Takai Y. Mol Biol Cell. 1998;9:2561–2575. doi: 10.1091/mbc.9.9.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer B, Valles A M, Thiery J P. Acta Anat. 1996;156:227–239. doi: 10.1159/000147849. [DOI] [PubMed] [Google Scholar]
- 10.Liu B P, Chrzanowska-Wodnicka M, Burridge K. Cell Adhes Commun. 1998;5:249–255. doi: 10.3109/15419069809040295. [DOI] [PubMed] [Google Scholar]
- 11.Cramer L P, Mitchison T J. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Nature (London) 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 13.Mackay D J, Hall A. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 14.Montesano R, Saint Vincent L, Drevon C, Tomatis L. Int J Cancer. 1975;16:550–558. doi: 10.1002/ijc.2910160405. [DOI] [PubMed] [Google Scholar]
- 15.Gloushankova N A, Krendel M F, Sirotkin V A, Bonder E M, Feder H H, Vasiliev J M, Gelfand I M. Proc Natl Acad Sci USA. 1995;92:5322–5325. doi: 10.1073/pnas.92.12.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai H, Kalnins V I. Exp Cell Res. 1996;223:63–71. doi: 10.1006/excr.1996.0058. [DOI] [PubMed] [Google Scholar]
- 17.Schliwa M, Nakamura T, Porter K R, Euteneuer U. J Cell Biol. 1984;99:1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danowski B A. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- 19.Zhong C, Kinch M S, Burridge K. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volberg T, Geiger B, Citi S, Bershadsky A D. Cell Motil Cytoskeleton. 1994;29:321–338. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]