Abstract
c-fos-induced growth factor/vascular endothelial growth factor D (Figf/Vegf-D) is a secreted factor of the VEGF family that binds to the vessel and lymphatic receptors VEGFR-2 and VEGFR-3. Here we report that Figf/Vegf-D is a potent angiogenic factor in rabbit cornea in vivo in a dose-dependent manner. In vitro Figf/Vegf-D induces tyrosine phosphorylation of VEGFR-2 and VEGFR-3 in primary human umbilical cord vein endothelial cells (HUVECs) and in an immortal cell line derived from Kaposi’s sarcoma lesion (KS-IMM). The treatment of HUVECs with Figf/Vegf-D induces dose-dependent cell growth. Figf/VEGF-D also induces HUVEC elongation and branching to form an extensive network of capillary-like cords in three-dimensional matrix. In KS-IMM cells Figf/Vegf-D treatment results in dose-dependent mitogenic and motogenic activities. Taken together with the previous observations that Figf/Vegf-D expression is under the control of the nuclear oncogene c-fos, our data uncover a link between a nuclear oncogene and angiogenesis, suggesting that Figf/Vegf-D may play a critical role in tumor cell growth and invasion.
During development and in the vascularization of tumors inductive signaling leads to the formation of capillaries throughout the new-forming tissues (1, 2). Inhibitors who regulate proliferation, migration, differentiation of endothelial cells, degradation of the extracellular matrix, and tube formation finely tune this complex process, known as angiogenesis (3–5). The prototype of angiogenic factors is represented by vascular endothelial growth factor (VEGF) A, also known as vascular permeability factor. VEGF-A induces endothelial cell differentiation and is essential for embryonic vessel development (4, 6, 7). It belongs to a multigene family of angiogenic factors in which several new members were discovered recently. It generally is thought that each member of this family plays a specific role in the angiogenic process (2). In addition to VEGF-A, this family includes the placental growth factor (PlGF), VEGF-B/VRF, VEGF-C/VRP, c-fos-induced growth factor (Figf)/VEGF-D, and VEGF-E (8–18). All of these factors show a conserved cysteine-rich domain characteristic of the family. Differences in the patterns of expression suggest a specific role for at least some of the factors in the vascularization of different tissues. Plgf is expressed mostly in the placenta, whereas VEGF-B is prevalent in skeletal and cardiac muscle tissues (12, 19). These two factors can form heterodimers with VEGF-A adding an additional level of specificity (12, 20). Interestingly, VEGF-C is involved in both blood and lymphatic vessel growth (21, 22).
Figf/Vegf-D initially was identified by using a differential screening strategy aimed at the identification of new c-fos-responsive genes in mouse fibroblasts and therefore named c-fos-induced growth factor (15). Its human orthologue shares 84% identity and was named VEGF-D because it encodes for a secreted protein whose primary sequence is most similar to VEGF-C (16, 23–25). Both VEGF-C and VEGF-D are recognized by VEGF receptors (VEGFR)-2 and -3, which are present on endothelial cells (14, 23). In mouse embryos Figf/Vegf-D is expressed in several organs, including limb buds, teeth, heart, and pituitary as well as lung and kidney mesenchyme, liver, derma, and periosteum of the vertebral column that partially overlaps Vegf-C expression (26, 27). In cultured fibroblasts Figf/Vegf-D regulation differs from VEGF-C. Whereas the expression of Figf/Vegf-D depends on c-fos (15), VEGF-C is induced by serum, tumor promoter phorbol myristate 13-acetate, IL-1β, and tumor necrosis factor α, and its expression is independent from c-fos (15, 28, 29).
We produced a recombinant form of mature mouse Figf/Vegf-D and analyzed its biological activity both in vivo and in vitro. Figf/Vegf-D, expressed in Chinese hamster ovary (CHO) cells or purified from yeast, is a potent angiogenic factor in rabbit cornea assays. In vitro it activates tyrosine phosphorylation of VEGFR-2 and VEGFR-3 present on human umbilical cord vein endothelial cells (HUVECs) and on the Kaposi’s sarcoma immortalized cell line (KS-IMM). In KS-IMM cells Figf/Vegf-D induces proliferation and chemotaxis. In HUVECs Figf/Vegf-D induces growth and morphological changes within a three-dimensional matrix.
MATERIALS AND METHODS
Expression of Figf/Vegf-D.
To express the mature factor in CHO cells, the Figf/Vegf-D cDNA with a segment coding for the FLAG octapeptide (IBI/Kodak) at C terminal was amplified by PCR and inserted into the mammalian expression vector pcDNA3 (Invitrogen) under the control of the cytomegalovirus promoter (construct LM357). CHO cells were transfected with LM357 by using calcium phosphate precipitation. Stable clones were selected in DMEM containing 10% FCS and 800 μg/ml G418. To assay the presence of Figf/Vegf-D in CHO supernatants, isolated clones were grown in DMEM containing 2% FCS and 800 μg/ml G418 and analyzed by ELISA using anti-Figf/Vegf-D rabbit polyclonal antiserum (15). Supernatant from positive clones was precipitated with deoxycholate acid and analyzed by Western blot. Different CHO clones expressed different Figf/Vegf-D levels. Specifically, clone 65 expressed less than 0.1 ng/ml of Figf/Vegf-D in the cell supernatant in vitro whereas clone 79 expressed approximately 0.5 ng/ml of Figf/VEGF-D in the same conditions.
To express Figf/Vegf-D in yeast a cDNA fragment encoding the portion of the mouse Figf/Vegf-D polypeptide from residues 91 to 208 with six histidine residues at N terminus was amplified by PCR and inserted into the expression vector Yepsec1 immediately downstream from DNA sequence encoding the Kluyveromyces lactis toxin leader peptide (LM375) (30). The protein was expressed in Saccharomyces cerevisiae yeast strain by adding galactose to the yeast culture medium because Yepsec1 construct contains a galactose upstream activation sequence and the 5′ nontranslated leader of the yeast CYC1 gene, up to position −4 from the ATG translation initiation codon (30). Figf/Vegf-D glycosilation mutant was obtained by PCR with the substitution N160P (LM376). Figf/Vegf-D and Figf/Vegf-D N160P proteins were purified from the yeast supernatant by using a nickel column (HiTrap Chelating columns Pharmacia Biotech) under native conditions.
In Vivo Angiogenic Assay.
The angiogenic activity of Figf/Vegf-D was assayed in vivo by using the rabbit cornea assay previously described (31). Corneal assays were performed in male New Zealand albino rabbits (Charles River, Calco, Lecco, Italy) in accordance with the guideline of the European Economic Community for Animal Care and Welfare (EEC Law No. 86/609). Briefly, after being anaesthetized with sodium pentotal (30 mg/Kg), a micro pocket (1.5 × 3 mm) was surgically produced by using a pliable iris spatula 1.5 mm wide in the lower half of the cornea. The cell suspension (from 2.5 to 4 × 105 cells/5 ml) or slow-release pellets of Elvax-40 (DuPont) containing the purified growth factor were implanted into the micro pocket. Subsequently daily observation of the implants was made with a slit lamp stereomicroscope without anesthesia. An angiogenic response was scored positive when budding of vessels from the limbal plexus occurred after 4 days and capillaries progressed to reach the implanted pellet according to the scheme previously reported (32). The potency of angiogenic activity was evaluated on the basis of the number and growth rate of newly formed capillaries, and an angiogenesis score was calculated as described (32). Corneas were removed at the end of the experiment as well as at defined intervals after surgery and/or treatment and fixed in formalin for histological examination. A minimum of four independent experiments was performed for each condition.
Cell Cultures.
Human endothelial cells were isolated from umbilical cord vein by collagenase treatment as described (33) and used at passage 1–4. KS-IMM cells were derived from a non-AIDS patient and are immortalized without signs of senescence after more than 120 in vitro passages. This cell line shares common markers and similar biological behavior with typical KS “spindle cells” (34). Cells were grown on gelatin-coated plastic, in medium M 199 supplemented with 20% heat-inactivated FCS, penicillin (100 units/ml), streptomycin (50 μg/ml), heparin (50 μg/ml), and bovine brain extract (100 μg/ml) (Life Technologies, Milan, Italy).
In Vitro Angiogenesis.
Because Matrigel can induce spontaneously in vitro angiogenesis, we tested more preparations and used batches devoid of this activity. Fifty microliters of Matrigel (Collaborative Research, lot 901448) (35) was added per well of 96-well tissue culture plates and allowed to gel at 37°C for 10 min. HUVECs were starved for 24 h in M199 with 1% FCS before being harvested in PBS-EDTA. Cells (104) were gently added to each of triplicate wells and allowed to adhere to the gel coating for 30 min at 37°C. Then, medium was replaced with indicated concentrations of Figf/Vegf-D. The plates were monitored after 24 h and photographed with a Canon microscope. Each experiment was repeated at least three times with identical results.
Immunoprecipitation and Western Blotting.
Subconfluent cultures were starved as above and then cells were stimulated with the indicated concentrations of Figf/Vegf-D for 10 min at room temperature. Positive control was done by incubating cells with sodium orthovanadate (0.1 mM H2O2, 1 mM Na3OV4) for 20 min at 37°C. After three washes with cold PBS containing 1 mM sodium orthovanadate, cells were lysed for 20 min on ice in 50 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, 1 mM Ne3VO4, 1 mM PMSF, 0.1 mM ZnCl2, 1% Triton. Lysates (1 mg of total proteins) were incubated at 4°C for 2 h with 100 μl of a 50% solution of protein A-Sepharose (Amersham-Pharmacia Biotech) in 50 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, and anti-VEGFR-2 (Santa Cruz Biotechnology, sc-504) or anti-VEGFR-3 (Santa Cruz Biotechnology, sc-321). Immunoprecipitates were washed four times with lysis buffer and analyzed by 8% SDS/PAGE. Proteins were transferred onto a nylon membrane [poly(vinylidene difluoride), Millipore] and analyzed by immunoblotting with antiphosphotyrosine mAb (Upstate Biotechnology, Lake Placid, NY). Staining was performed by a chemiluminescence assay (ECL, Amersham-Pharmacia Biotech).
Cell Growth Assay.
HUVECs (2.5 × 103) or KS-IMM cells were plated in 96-well plates (Costar) coated with gelatin (Difco; 0.05%, for 1 h at 22°C) in M199 medium containing 20% FCS (Irvine Scientific). After 24 h the medium was removed and replaced with M199 containing 1% FCS with or without Figf/Vegf-D; fresh factor was added every 2 days. Endothelial cell numbers were estimated after staining with crystal violet by a colorimetric assay described by Keung et al. (36).
Chemotaxis Assay.
Chemotaxis assays on HUVECs and KS-IMM were performed as described (33, 37) with the Boyden chamber technique using a 48-well micro chemotaxis chamber. Polyvinylpyrrolidone-free polycarbonate filters (Nucleopore, Corning-Costar) with a pore size of 5 μm were coated with 1% gelatin for 10 min at room temperature and equilibrated in M199 supplemented with 1% FCS. Indicated concentrations of purified Figf/Vegf-D were placed in the lower compartment of a Boyden chamber. Subconfluent cultures were starved as above, harvested in PBS (pH 7.4) with 10 mM EDTA, washed once in PBS, and resuspended in M199 containing 1% FCS, at a final concentration of 2.5 × 106 cells/ml. After placing the filter between the lower and upper chambers, 50 μl of the cell suspension was seeded in the upper compartment. Cells were allowed to migrate for 7 h at 37°C in a humidified atmosphere with 5% CO2. The filter then was removed, and cells on the upper side were scraped with a rubber policeman. Migrated cells were fixed in methanol, stained with Giemsa solution (Diff-Quick, Baxter Diagnostics, Rome) and counted from five random high-power fields (magnification ×100) in each well. Each experimental point was studied in triplicate.
RESULTS
Induction of Angiogenesis in Vivo.
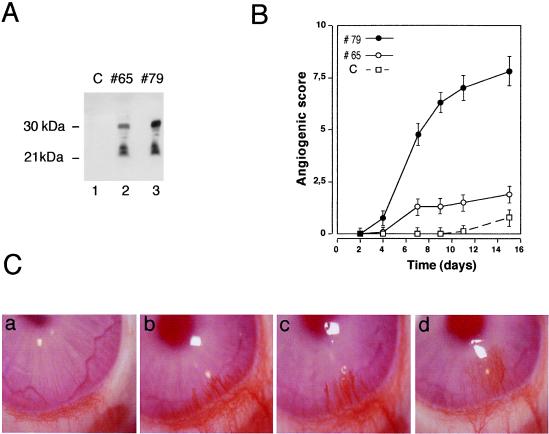
Mature VEGF-C and Figf/VEGF-D factors are generated by proteolytic cleavages of both of the N- and C-terminal domains during secretion (15, 23, 38). To obtain recombinant mature Figf/Vegf-D we generated CHO clones by stable transfection of constructs containing the mouse Figf/Vegf-D cDNA truncated at the C-terminal proteolytic site (38). To assess in vivo the angiogenic activity of increasing concentrations of the recombinant protein administered to avascular tissue two clones expressing different levels of secreted Figf/Vegf-D were selected for implantation into rabbit corneas. These clones secrete in the culture medium Figf/Vegf-D in two main forms of molecular mass of 30 and 21 kDa, respectively (Fig. 1A). Both clone 65 and clone 79 induced corneal vascularization whereas the CHO mock transfectant clone did not show any angiogenic effect (Fig. 1B). Although a direct dose response could not be made in this assay, the efficiency of the angiogenic response correlated with the amount of growth factor released in vitro as clone 79 secreted about 5-fold more Figf/Vegf-D than clone 65 in the same conditions (Fig. 1A). Consistently, neovascular growth induced by clone 79 was more efficient and persisted in 100% of the implants whereas clone 65 did so in only 30% of corneas (Fig. 1B). This angiogenic activity also was suggested by the direct correlation between neovascular growth observed and the number of cells implanted into corneal micro pocket (data not shown). The angiogenic response obtained with clone 79 (Fig. 1C) was comparable to the one elicited with cells expressing VEGF-A121 (39) both in intensity and appearance.
Figure 1.
Implanted Figf/Vegf-D-expressing cells induce neovascularization in rabbit corneas. (A) Figf/Vegf-D expressed in CHO cells. Equal volumes of culture supernatants from clones 65 and 79 were precipitated and analyzed by Western blot using an anti-Figf/Vegf-D rabbit polyclonal antiserum. (B) CHO cells (4 × 104) expressing Figf/Vegf-D were surgically implanted into the corneas. New blood vessel growth was recorded every other day with a slit lamp stereomicroscope. Angiogenic scores were calculated on the basis of the number of vessels and their growth rate and plotted versus time (for experimental details see Materials and Methods). Angiogenic score data are the mean values obtained from the response scored in all animals in this study. C, CHO mock transfectant clone; #65, clone expressing low levels of Figf/Vegf-D (0.1 ng/ml protein in supernatant); #79 clone expressing higher levels of Figf/Vegf-D (approximately 0.5 ng/ml protein in supernatant). (C) Pictures of rabbit corneas from a representative experiment. (a) Corneal implant of CHO mock transfectant. Clone 79 promotes and sustains vascular growth over time at day 6 (b), 9 (c), and 14 (d). Corneas were photographed with a stereomicroscope. Magnification: ×18.
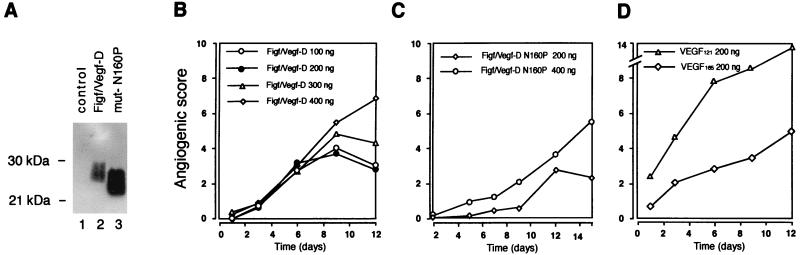
To obtain larger amounts of pure Figf/Vegf-D it also was expressed in yeast S. cerevisiae. To obtain a secreted Figf/Vegf-D form in yeast supernatants the cDNA fragment encoding the portion of the mouse Figf/Vegf-D polypeptide from residues 91 to 208 plus a segment coding for six histidine residues at the N terminus was cloned in a yeast vector containing a secretion signal. This recombinant protein expressed in yeast was secreted into the culture medium (Fig. 2A). By contrast with the other members of the VEGF family, VEGF-C and Figf/Vegf-D contain two putative glycosylation sites in the mature protein. Secreted Figf/Vegf-D is glycosilated at asparagine-160 residue in both mammalian and yeast cells (data not shown). To test the activity of both the glycosylated and unglycosylated forms we also generated a Figf/Vegf-D mutant in which the glycosylation site was mutated by the introduction of a proline residue at position 160, which is present in all other known VEGF family members. Consistent with N-linked glycosylation, the wild-type protein shows about 2-kDa molecular mass increase with respect to the mutant Figf/Vegf-D N160P (Fig. 2A) and it is sensitive to endoglycosidase H (not shown).
Figure 2.
Figf/Vegf-D sustains dose-dependent angiogenesis in vivo. (A) Supernatant of S. cerevisiae yeast strains expressing Figf/Vegf-D and Figf/Vegf-D mutant as indicated. (B) The angiogenic activity of various concentrations of Figf/Vegf-D were tested as slow-release preparations in the rabbit cornea assay. (C) Angiogenic activity of 200 and 400 ng/pellet of Figf/Vegf-D N160P. (D) Angiogenic activity of 200 ng/pellet of VEGF-A121 and VEGF-A165 is shown for comparison. Angiogenic score data are the mean values obtained from the responses scored in all animals in this study. Variations were below 10% of the mean values. Angiogenic scores are calculated as described in Fig. 1 and in Materials and Methods.
Figf/Vegf-D purified to homogeneity was analyzed in the corneal micro pocket assay in vivo. Similar to the results obtained with implanted CHO cells, purified Figf/Vegf-D induced a strong angiogenic response. After the implant of a single dose of protein in the slow-release pellets all Figf/Vegf-D doses of 100–400 ng/pellet induced capillary growth after just 3 days. However, a clear effect of increasing Figf/Vegf-D concentration was evident at later time points (Fig. 2B). The Figf/Vegf-D N160P mutant showed less potent angiogenic activity with respect to the wild-type protein (Fig. 2C), suggesting that Figf/Vegf-D glycosylation is involved in receptor recognition. In this assay, recombinant Figf/Vegf-D showed intermediate activity when compared with VEGF-A121 and VEGF-A165 (Fig. 2D) when used at doses of 300–400 ng. Corneal angiogenesis induced by either Figf/Vegf-D or VEGF-A was noninflammatory (not shown).
Figf/Vegf-D Induces in Vitro Angiogenesis.
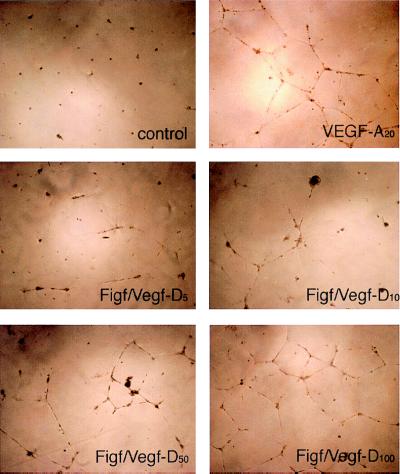
Studying endothelial cell behavior in a three-dimensional culture system, consisting of extra cellular matrix proteins, allows for in vitro conditions that more closely mimic the in vivo environment permissive for cell differentiation into capillary-like structures. This assay system is called in vitro angiogenesis (40). To examine whether Figf/Vegf-D induces in vitro morphological changes resembling of capillary like-structure formation, endothelial cells were plated on a three-dimensional matrix of Matrigel (41) and then stimulated with increasing concentrations of Figf/Vegf-D. Endothelial cells grown under these conditions in the presence of 1% BSA exhibited a small round shape and did not spread. Treatment with Figf/Vegf-D for 24 h resulted in dramatic dose-dependent morphological changes. The cells became elongated, forming thin cords of interconnecting cells (Fig. 3). The effect was investigated in a range of concentrations between 5 and 200 ng/ml and was maximal at 100 ng/ml. Similar effects also were observed with 20 ng/ml VEGF-A. These data demonstrate that Figf/Vegf-D, like VEGF-A, is able to mediate dramatic cell reorganization, which would be necessary in vivo for the sprouting of endothelial cells and tube formation. No morphological alterations could be observed in KS-IMM cells, either by treating the cells with VEGF-A or with Figf/Vegf-D (not shown).
Figure 3.
Figf/Vegf-D-induced endothelial cell morphological changes. VEGF-A or Figf/Vegf-D were added to HUVECs cultured in three-dimensional Matrigel in low serum conditions. Photographs were taken 24 h after Figf/Vegf-D treatment. Protein concentrations (ng/ml) used are indicated.
Figf/Vegf-D Induction of VEGFR-2 and VEGFR-3 Tyrosine Phosphorylation.
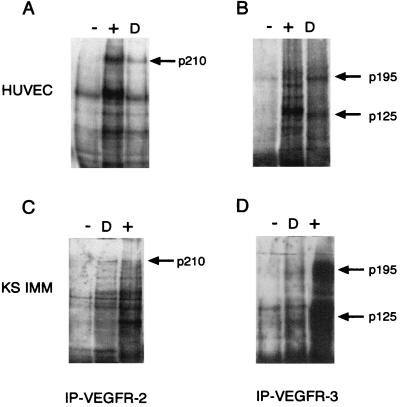
It has been reported recently that VEGF-D and VEGF-C are the ligands of the endothelial tyrosine kinase receptors VEGFR-2 and VEGFR-3 (14, 23). To examine the cellular response of endothelial cells to Figf/Vegf-D in vitro, we first tested whether Figf/Vegf-D could stimulate signal transduction from VEGFR-2 and VEGFR-3 in HUVECs and KS-IMM cells because these cells express both receptors. Tyrosine phosphorylation of these receptors was assayed in serum-starved cells treated with Figf/Vegf-D. VEGFR-2 and VEGFR-3 were immunoprecipitated with specific antibodies and analyzed by Western blotting with antiphosphotyrosine-specific antibodies. Figf/Vegf-D stimulated tyrosine phosphorylation of the 210-kDa VEGFR-2 and both the 125- and 195-kDa processed and unprocessed forms of VEGFR-3 in both HUVECs and KS-IMM cells (Fig. 4). Thus, Figf/Vegf-D, like VEGF-C, binds and activates these receptors on endothelial cells.
Figure 4.
Figf/Vegf-D-induced tyrosine phosphorylation of VEGFR-2 and VEGFR-3. HUVECs and KS-IMM cells were incubated with Figf/Vegf-D. After stimulation receptors were immunoprecipitated with antireceptor antibodies and analyzed by Western blotting with an antiphosphotyrosine mAb. (A and B) Phosphorylation of VEGFR-2 and VEGFR-3 in HUVECs. (C and D) Phosphorylation of VEGFR-2 and VEGFR-3 in KS-IMM cells. Positive control (+) and Figf/Vegf-D stimulation (D) is indicated. Arrows denote the position of the phosphorylated 210-kDa VEGFR-2 protein and the positions of the phosphorylated, proteolytically processed 125-kDa and unprocessed 195-kDa forms of VEGFR-3.
Figf/Vegf-D Induction of Growth and Chemotaxis in HUVECs and KS-IMM Cells.
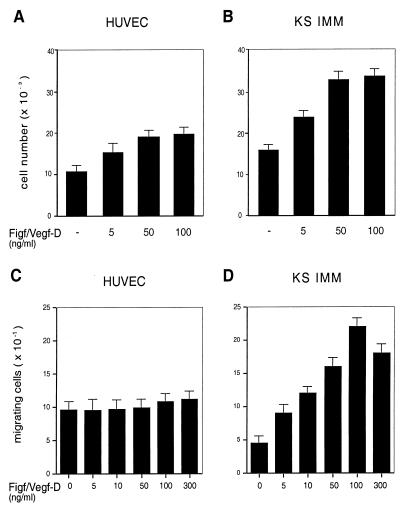
To investigate further the proliferative effect of Figf/VEGF-D on endothelial cells, we incubated both the cultured HUVECs and KS-IMM cells in the presence of increasing concentrations of Figf/Vegf-D. Proliferation of both cell types was stimulated in a dose-dependent manner (Fig. 5 A and B). The effect was investigated in a range of concentrations between 5 and 100 ng/ml and was maximal at 50 ng/ml for both cell types. Interestingly, when suboptimal concentrations of VEGF-A165 and Figf/Vegf-D were coadded to HUVECs the resulting proliferation was higher than the treatment of each alone (not shown).
Figure 5.
Figf/Vegf-D-induced cell proliferation and chemotactic activity. (A and B) Proliferative effects of Figf/Vegf-D were assayed on HUVECs and KS-IMM cells as indicated. Experiments were performed in medium containing 1% FCS. After 72 h cells were enumerated by using a Coulter counter and values represent the mean (±SEM) of triplicate samples. (C and D) Cells were seeded in the upper wells of a 48-well micro chemotaxis Boyden chamber and incubated for 7 h at 37°C in medium containing 1% FCS. The lower wells contained the indicated concentrations of Figf/VEGF-D. Cells migrating through a polycarbonate membrane with a pore size of 5 μm were quantified by staining the cells with Giemsa solution and counting was performed on a light microscope of five high-power fields (×100). The results are expressed as the mean ± 1 SD of three independent experiments performed in triplicate.
The chemotactic effect of Figf/Vegf-D on HUVECs and KS-IMM cells was analyzed in a modified Boyden chamber assay. The migration of the cells through collagen-coated micropore filters toward chemoattractants was scored in the absence of serum. Figf/Vegf-D stimulated the migration of KS-IMM cells in a dose-dependent manner. In HUVECs, under identical conditions Figf/Vegf-D induced little or no migration (Fig. 5 C and D).
DISCUSSION
The results reported in this work show that Figf/Vegf-D is a potent angiogenic factor. In rabbit corneas Figf/Vegf-D, expressed either in CHO or yeast cells, can efficiently induce angiogenesis. The dose dependency and the early response suggest a direct effect of Figf/Vegf-D on endothelial cell recruitment in vivo. This observation has been confirmed by in vitro experiments that show direct Figf/Vegf-D activities on endothelial cells. In addition to Figf/Vegf-D, other factors, including VEGF-A, basic fibroblast growth factor, placental growth factor, VEGF-C, and VEGF-E induce angiogenesis, suggesting a redundancy or a coordination among factors performing the same function (2, 10, 42, 43). The generation of VEGF-A knockout mice demonstrated that this factor is essential for angiogenesis during development (6, 7). Thus, simple redundancy of all members of the family is unlikely. We favor the hypothesis that the complex process of angiogenesis normally requires the cooperation of multiple factors and the experimental overexpression of some key members is able to trigger the process both directly and indirectly, inducing the expression of other factors. Each of these factors shows a peculiar pattern of expression, suggesting that a complex balance of factors in different developing organs may be relevant. Moreover, the biological function of VEGFs may not be limited to angiogenesis. For instance, fibroblast growth factors not only induce angiogenesis, but are also regulators of embryonic development, influencing the formation of several structures including body axis, limbs, heart, and lung differentiation (44–47). Similarly, the expression of Figf/Vegf-D in tissues like the pituitary, the developing teeth, lung mesenchyme, and limb buds (26) suggests that Figf/Vegf-D, in addition to playing a role in angiogenesis, could be involved in specific inductive signaling in these developing organs.
Figf/Vegf-D and VEGF-C share striking similarities in their primary sequence and posttranslational modifications, and most importantly, both factors are recognized by VEGFR-2 and VEGFR-3 present on vascular and lymphatic vessels (14, 23). By using porcine aortic endothelial cells selectively overexpressing VEGFR-2 or VEGFR-3, it was shown recently that VEGF-C could promote migration and proliferation independently of signaling through either receptor (21). In this study for the analysis of Figf/Vegf-D activity in vitro we used HUVECs and KS-IMM cells, which express both receptors. By immunoprecipitation experiments we showed that Figf/Vegf-D activates both VEGFR-2 and VEGFR-3 tyrosine phosphorylation on both HUVECs and KS-IMM cells. The activation of these receptors by Figf/Vegf-D stimulates a biological response that involves morphological, mitogenic, and motogenic responses. Thus, Figf/Vegf-D shows a direct activity on endothelial cells in vitro, confirming the in vivo data. Although both HUVECs and KS-IMM cells show a similar mitogenic response to Figf/Vegf-D they differ in motogenic responses because KS-IMM cells are more responsive than HUVECs to motogenic activation by Figf/Vegf-D. This discrepancy could be simply because of differences in the receptor levels or in intracellular signaling molecules, but it also could be the result of the presence of possible coreceptors that may modify the receptor affinity and modulate the response to Figf/Vegf-D. In line with this possibility, it recently has been shown in human endothelial cells that neuropilin-1 (48) and αvβ3 modulate the activity of VEGFR-2. Neuropilil-1 is a coreceptor for the VEGF-A165 isoform and the αvβ3 integrin associates with VEGFR-1 upon VEGF-A stimulation and regulates the level of tyrosine phosphorylation of the receptor (49).
Figf/Vegf-D differs from all other members of the VEGF family because it is the only angiogenic factor regulated by the nuclear oncogene c-fos (15). This unique regulation of Figf/Vegf-D may be relevant both during development and in tumor progression because c-fos is involved not only in transformation but also in the regulation of cell growth and differentiation of various tissues (50, 51). Tumors that develop in c-fos-deficient mice appear devoid of vascularization although, in these papillomas, VEGF-A expression is reduced but not absent (52). The role of Figf/Vegf-D in tumor progression is at the moment unclear, although the evidence that Figf/Vegf-D shows angiogenic activity on endothelial cells, as well as mitogenic and motogenic activity on tumor-derived KS-IMM cells, strongly suggest that Figf/Vegf-D can be a c-fos effector for tumor malignancy.
Acknowledgments
We thank Nicholas Valiante for the critical reading of the manuscript, Rino Rappuoli for hospitality in IRIS laboratories, Cesira Galeotti for yeast strains and helpful discussions, and Astrid Parenti and Beatrice Grandi for technical assistance. This work was supported by funds from Italian Association for Cancer Research, Chiron Vaccines and CEE N0 BMH4-CT98–3380 (S.O.); the Italian Association for Cancer Research, Istituto Superiore di Sanità (AIDS Project; Program on Tumor Therapy), Centro Nazionale delle Ricerche (Progetto Finalizzato Biotecnologie), and Ministero dell’Università e della Ricerca Scientifica e Tecnologica (60% and Programmi di Rilevante Interesse Nazionale) (F.B.), and the Italian Association for Cancer Research, Italian Ministry for University and for Scientific and Technological Research (Prot. 9706217225_06) and CEE PL 950669 (M.Z.). S.M. is supported by a grant from Centro Nazionale delle Ricerche (Progetto Biotecnologie).
ABBREVIATIONS
- Figf
c-fos-induced growth factor
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- HUVEC
human umbilical cord vein endothelial cell
- KS-IMM
Kaposi’s sarcoma-immortal
- CHO
Chinese hamster ovary
References
- 1.Ferrara N, Davis-Smyth T. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 2.Nicosia R F. Am J Pathol. 1998;153:11–16. doi: 10.1016/S0002-9440(10)65539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bussolino F, Mantovani A, Persico G. Trends Biochem Sci. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 4.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. EXS. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kiechens L, Gertsensein M, Faihrig M, Vandenhoech A, Harpal H, Ebherardt C, et al. Nature (London) 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Carver-More K, Chen H, Dowd M, Lu L, O’Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 8.Betsholtz C, Johnsson A, Heldin C-H, Westermark B, Lind P, Urdea M S, Eddy R, Shows T B, Philphott K, Mellor A L, et al. Nature (London) 1986;320:695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- 9.Keck P J, Hauser S D, Krivi G, Sanzo K, Warren T, Feder J, Connolly D T. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 10.Leung D W, Cachianes G, Kuang W, Goeddel D V, Ferrara N. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 11.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico M G. Proc Natl Acad Sci USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olofsson B, Pajusola K, Kaipanen A, von Euler G, Joukov V, Saksela O, Orpana A, Petterson R F, Alitalo K, Erikson U. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimmond S, Lagerkrantz J, Drinkwater C, Silins G, Towson S, Pollock P, Gotley D, Carson E, Rakar S, Nordenskjold M, et al. Genome Res. 1996;6:124–131. doi: 10.1101/gr.6.2.124. [DOI] [PubMed] [Google Scholar]
- 14.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lathinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 15.Orlandini M, Marconcini L, Ferruzzi R, Oliviero S. Proc Natl Acad Sci USA. 1996;93:11675–11680. doi: 10.1073/pnas.93.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocchigiani M, Lestingi M, Luddi A, Orlandini M, Franco B, Rossi E, Ballabio A, Zuffardi O, Oliviero S. Genomics. 1998;47:207–216. doi: 10.1006/geno.1997.5079. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. J Biol Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 18.Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin H G, Ziche M, Lanz C, Bettner M, Rziha H J, Dehio C. EMBO J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maglione D, Guerriero V, Viglietto G, Ferraro M G, Aprelikova O, Alitalo K, Del Vecchio S, Lei K-J, Chou J Y, Persico G G. Oncogene. 1993;8:925–931. [PubMed] [Google Scholar]
- 20.Cao Y, Chen H, Zhou L, Chiang M K, Anand-Apte B, Weatherbee J A, Wang Y, Fang F, Flanagan J G, Tsang M L. J Biol Chem. 1996;271:3154–3162. doi: 10.1074/jbc.271.6.3154. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi J-H, Claesson-Welsh L, Alitalo K. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain R K, Alitalo K. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 23.Achen M G, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks A F, Alitalo K, Stacker S A. Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada Y, Nezu J, Shimane M, Hirata Y. Genomics. 1997;42:483–488. doi: 10.1006/geno.1997.4774. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins N A, Woollatt E, Crawford J, Gilbert D J, Baldwin M E, Sutherland G R, Copeland N G, Achen M G. Chromosome Res. 1997;5:502–505. [PubMed] [Google Scholar]
- 26.Avantaggiato V, Acampora D, Orlandini M, Oliviero S, Simeone A. Mech Dev. 1998;73:221–224. doi: 10.1016/s0925-4773(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 27.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. Development (Cambridge, UK) 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 28.Enholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Jukov V, et al. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- 29.Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. J Biol Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- 30.Baldari C, Murray J A, Ghiara P, Cesareni G, Galeotti C. EMBO J. 1987;6:229–234. doi: 10.1002/j.1460-2075.1987.tb04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziche M, Alessandri G, Gullino P M. Lab Invest. 1989;61:629–634. [PubMed] [Google Scholar]
- 32.Ziche M, Morbidelli L, Choudhuri R, Zhang H T, Donnini S, Granger H J, Bicknell R. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bussolino F, Wang J M, Defilippi P, Turrini F, Sanavio F, Edgell C J S, Aglietta M, Arese P, Mantovani A. Nature (London) 1989;337:471–473. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- 34.Albini A, Paglieri I, Orengo G, Carlone S, Aluigi M G, DeMarchi R, Matteucci C, Mantovani A, Carozzi F, Donini S, Benelli R. AIDS. 1997;11:713–721. doi: 10.1097/00002030-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Kleinman H K, McGarvery M L, Hassel J R, Star V L, Cannon F B, Laurie G W, Martin G R. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 36.Keung K, Silfer E, Eppenberger V. Anal Biochem. 1989;182:16–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- 37.Dejana E, Languino L R, Polentarutti N, Balconi C, Ryckewaert J J, Larrieu M J, Mantovani A, Marguerie G. J Clin Invest. 1985;75:11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joukov V, Sorsa T, Kumar V, Jeltisch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinnnen N, Alitalo K. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morbidelli L, Birkenhaeger R, Roeckl W, Granger H J, Kaerst U, Weich H, Ziche M. Angiogenesis. 1997;1:117–130. doi: 10.1023/A:1018361217467. [DOI] [PubMed] [Google Scholar]
- 40.Montesano R, Orci L, Vassalli P. J Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant D S, Tashiro K, Segui-Real B, Yamada Y, Martin G R, Kleinman H K. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- 42.Basilico C, Moscatelli D. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 43.Ziche M, Maglione D, Ribatti D, Morbidelli L, Lago C T, Battisti M, Paoletti I, Barra A, Tucci M, Parise G, et al. Lab Invest. 1997;76:517–531. [PubMed] [Google Scholar]
- 44.Szebenyi G, Fallon J F. Int Rev Cytol. 1999;185:45–106. doi: 10.1016/s0074-7696(08)60149-7. [DOI] [PubMed] [Google Scholar]
- 45.Seghezzi G, Patel S, Ren C J, Gualandris A, Pintucci G, Robbins E S, Shapiro R L, Galloway A C, Rifkin D B, Mignatti P. J Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mima T, Ueno H, Fischman D A, Williams L T, Mikawa T. Proc Natl Acad Sci USA. 1995;92:467–471. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters K, Werner S, Liao X, Wert S, Whitsett J, Williams L. EMBO J. 1994;13:3296–3301. doi: 10.1002/j.1460-2075.1994.tb06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soker S, Takashima S, Miao H Q, Neufeld G, Klagsbrun M. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 49.Soldi R, Mitola S, Strasly S, Defilippi P, Tarone G, Bussolino F. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson R S, Spiegelman B M, Papaioannou V E. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z-Q, Ovitt C, Gregoriadis A E, Möhle-Steinlein U, Rüther U, Wagner E F. Nature (London) 1992;360:742–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- 52.Saez E, Rutemberg S E, Mueller E, Oppenheim H, Smoluk J, Yuspa S H, Spiegelman B M. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]