Abstract
Leptin exerts its weight-reducing effects by binding to its receptor and activating signal transduction in hypothalamic neurons and other cell types. To identify the components of the leptin signal transduction pathway, an approach was developed in which bacterially expressed phosphorylated fragments of Ob receptor b (Ob-Rb) were used as affinity agents. Leptin binding to the Ob-Rb form of the leptin receptor leads to tyrosyl phosphorylation of the cytoplasmic domain of its receptor. Two of the three cytoplasmic tyrosines of Ob-Rb, at positions 985 and 1138, are phosphorylated after leptin treatment. Affinity chromatography using a tyrosine-phosphorylated fragment spanning Tyr 985 of Ob-Rb was used to identify proteins that bind to this site. The SH2 domain containing protein tyrosine phosphatase 2 (SHP-2) was isolated from bovine and mouse hypothalamus by using this method. After cotransfection of Ob-Rb, Janus kinase 2 (JAK2), and SHP-2 into 293T cells, leptin results in direct binding of SHP-2 to the phosphorylated Tyr 985. The bound SHP-2 is itself tyrosine phosphorylated after leptin treatment. SHP-2 is not phosphorylated after leptin treatment when a Y→F 985 receptor mutant is cotransfected. In the absence of SHP-2 phosphorylation, the level of JAK2 phosphorylation was increased. Tyrosyl phosphorylation of the leptin receptor and signal transducer and activater of transcription 3 (STAT3) are not affected by phosphorylation of SHP-2. These data suggest that activation of SHP-2 by the leptin receptor results in a decreased phosphorylation of JAK2 and may act to attenuate leptin signal transduction. The method used in this report can in principle be used to isolate additional components of the leptin, or other, signal transduction pathway.
Leptin is a 16-kDa hormone that has potent weight reducing effects in vivo (1, 2). Available data indicate that leptin functions as a signal in a negative feedback loop regulating food intake and body weight. The leptin receptor is a member of the cytokine receptor family (3). Leptin’s effects depend on binding to the Ob receptor b (Ob-Rb) isoform of its receptor (4, 5). The Ob-Rb form of the leptin receptor encodes a long intracytoplasmic domain of 302 amino acids that includes several motifs for protein–protein interaction. The other forms of this receptor have short cytoplasmic regions (Ob-Ra, c, d) or are secreted (Ob-Re). A mutation that specifically ablates Ob-Rb expression in db (diabetic) mice results in obesity and complete leptin resistance. Ob-Rb is highly expressed in the hypothalamus, suggesting that this brain region is an important site of leptin action (6, 7). The importance of the hypothalamus as a target of leptin action also is suggested by the high potency of intrathecally administrated leptin and the fact that hypothalamic lesions lead to leptin resistance (8, 9).
Signal transduction by cytokine receptors generally depends on ligand induced phosphorylation of soluble receptor tyrosine kinases such as Janus kinase 1 (JAK1), JAK2, JAK3, and Tyk2 (10). These kinases in turn phosphorylate tyrosine residues on the receptor, which serve as docking sites for SH2 domain containing proteins. Binding of proteins containing SH2 domains to the activated receptor and/or phosphorylation of such proteins initiates signal transduction.
The identification of SH2 domain containing proteins activated by the leptin receptor is likely to have important implications for an understanding of the leptin signal transduction pathway and the molecular mechanisms that control body weight. To identify the components of the leptin signal transduction pathway, tyrosine-phosphorylated cytoplasmic fragments of the leptin receptor were expressed in bacteria and were used as affinity reagents to isolate proteins that interact with the leptin receptor. In this fashion, SH2 domain containing protein tyrosine phosphatase 2 (SHP-2), a phosphotyrosine phosphatase, was isolated and found to bind Tyr 985 of the leptin receptor (11–15). The STAT3 transcription factor also was found to bind to a phosphopeptide spanning Tyr 1138.
SHP-2 is tyrosine phosphorylated after leptin treatment of cells transfected with the leptin receptor. Tyrosyl phosphorylation of SHP-2 was associated with decreased phosphorylation of JAK2 but not leptin receptor or STAT3 in transfected cells. These data suggest that SHP-2 is a component of the leptin signal transduction pathway and may indicate that it attenuates leptin signaling by decreasing the level of phosphorylation of JAK2. These data also indicate that protein fragments that are phosphorylated in bacteria can be successfully used as affinity reagents to identify proteins that interact with activated receptors.
MATERIALS AND METHODS
Eukaryotic Expression Vectors.
cDNAs encoding Ob-Ra and Ob-Rb were subcloned into the eukaryotic expression vector pcDNA3.1(−)/MycHisA (Stratagene). SHP-2 was cloned by using overlapping PCR. To mutagenize Cys463 of SHP-2 into Ser, QuikChange Site-Directed Mutagenesis Kit (Stratagene) was used.
Antibodies.
Monoclonal antibodies against phosphotyrosine (pY20) and SHP-2 were from Transduction Laboratories (Lexington, KY); antibody against JAK2 was from Santa Cruz Biotechnology; SHP-2 antibody also was generated by Covance Research Products (Denver, PA); antibody against SHP-1 was from Matt Thomas of Washington University (St. Louis); antibody against the leptin receptor was as described (16).
Transfection of 293T Cells.
Cells were seeded in 6-well plates 1 day before transfection. One microgram of receptor DNA and 0.5 μg each of SHP-2, JAK2, and STAT3 DNA were transfected. Forty-eight to seventy-two hours posttransfection, cells were starved for five hours, and leptin was added directly to a final concentration of 0.2 μg/ml (10 nM). Cells were washed and lysed with RIPA buffer (50 mM Tris, pH 8.0/150 mM NaCl/1.0% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS). Supernatant was used for immunoprecipitation or Western blotting. RIPA buffer was supplemented with tyrosine phosphatase inhibitor Na3VO4 and protease inhibitors before use.
Glutathione S-Transferase (GST) Fusion Proteins.
Three Ob-Rb cytoplasmic fragments containing individual tyrosines were subcloned into the bacterial expression vector pGEX-KG (17). The boundaries are GST-Tyr1, amino acids 965–1,001 (37 residues); GST-Tyr2, 1,040–1,093 (54 residues); and GST-Tyr3, 1,079–1,162 (84 residues). Constructs were transformed into the TKX1 strain of Escherichia coli (Stratagene) for protein generation.
Binding of GST Fusion Proteins to Hypothalamic Extracts.
Mature bovine hypothalami (Pel-Freeze Biologicals) were homogenized in buffer A (10 mM Hepes, pH 7.4/150 mM NaCl/1% Nonidet P-40/3 mM MgCl2/3 mM CaCl2) supplemented with protease inhibitors. Supernatant was obtained by centrifugation at 100,000 × g, was adjusted to 4 mg/ml protein with buffer A, and was sequentially passed through a screening column and empty glutathione agarose beads. Fifty micrograms of fusion protein on glutathione agarose beads was incubated with 50 ml of above extract (≈200 mg protein) at 4°C for 12 hours. Beads were washed three times and were reconstituted directly into 1 × SDS sample buffer.
RESULTS
Leptin Receptor Is Tyrosine Phosphorylated After Leptin Treatment of Transfected 293T Cells.
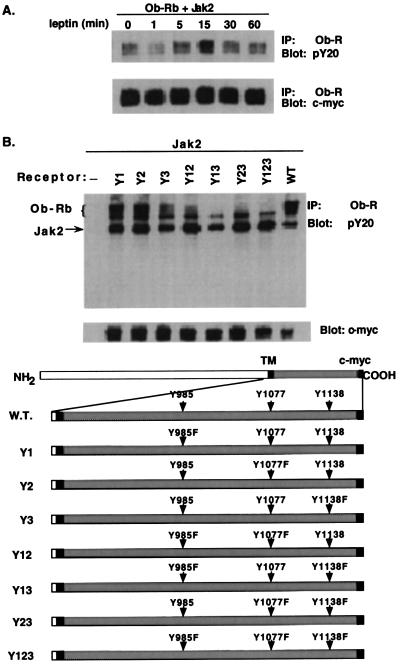
The Ob-Rb form of the leptin receptor encodes three tyrosines in its carboxyl terminus at positions 985, 1,077, and 1,138. Although previous data have shown that Ob-Rb is phosphorylated after leptin treatment, the specific residues that are phosphorylated have not been identified (18). Wild-type and mutant cDNAs of the leptin receptor were cloned into the expression vector pcDNA3.1(−)/Myc-HisA and were transfected into 293T cells. The phosphorylation state of the wild-type and mutant receptors was scored by immunoprecipitating the receptor with an antireceptor polyclonal antibody followed by immunoblotting with an antiphosphotyrosine (pY20) antibody. The amount of receptor immunoprecipitated in each lane was subsequently detected by using an anti-c-myc antibody.
Phosphorylation of the wild-type or mutant leptin receptor required cotransfection of JAK2. Previous studies demonstrated that JAK2 constitutively associates with the leptin receptor (19). When JAK2 was cotransfected in the absence of leptin, a low level of receptor phosphorylation was seen (data not shown). However, when JAK2 was present, there was an increase in leptin-induced receptor tyrosyl phosphorylation, which was maximal at ≈15 min. The level of phosphorylation then decreased to baseline by 60 min (Fig. 1A). This observation is in agreement with earlier results (21). The level of receptor protein was equivalent at all time points, as shown by immunoblotting using a monoclonal antibody that recognizes the c-myc tag fused to Ob-Rb.
Figure 1.
Tyrosyl phosphorylation of wild-type and Tyr→Phe Ob-Rb constructs in 293T cells. (A) Tyrosyl phosphorylation of wild-type Ob-Rb after leptin binding in transfected 293T cells. Cells were treated with leptin and were lysed, and Ob-Rb was immunoprecipitated. The immunoprecipitate was blotted with an antiphosphotyrosine monoclonal antibody (pY20). Total receptor expression was scored by immunoblotting of the same filter with an anti c-myc monoclonal antibody that recognizes the c-myc tag fused in frame to Ob-Rb. (B) Tyrosyl phosphorylation of Tyr→Phe Ob-Rb mutants. 293T cells transfected with JAK2 and different Ob-Rb mutants were treated with leptin (as above). Receptor phosphorylation and expression was confirmed with pY20 and the anti c-myc monoclonal antibody. A schematic diagram of wild-type and Tyr→Phe Ob-Rb constructs is shown below.
These studies were repeated by using constructs in which phenylalanine(s) replaced either one of the three tyrosines in the carboxyl terminus of Ob-Rb (Y1 = Y985, Y2 = Y1077, and Y3 = Y1138) or combinations of them (Fig. 1B). The phosphorylation state of these receptors was determined by transfecting each Ob-Rb construct with JAK2 and treating with leptin. A background level of anti pY20 immunoreactivity was seen in the Y123 receptor, a mutant in which each of the three tyrosines was changed to phenylalanine. An increased level of phosphorylation relative to the Y123 mutant was seen in both the Y23 and Y12 mutants. These data indicate that Tyr985 and Tyr1138 are phosphorylated after leptin treatment. The signal intensity of the Y13 mutant was not different from that of the Y123 mutant, indicating that Tyr1077 is either weakly phosphorylated or is not phosphorylated by leptin in vitro. In each of these three mutants, the signal intensity was considerably lower than that of the wild-type receptor. This is in contrast to the receptors in which either Tyr985 alone (Y1) or Tyr1077 (Y2) alone was replaced by phenylalanine. In these two cases, the level of phosphorylation was equivalent to the wild-type receptor. The Y3 mutant (Y1138→F) was only weakly phosphorylated. In aggregate, these data indicate that Tyr985 and Tyr1138 are phosphorylated by leptin and raise the possibility that there is cooperativity for phosphorylation among these tyrosines.
Tyrosine Phosphorylated Receptor Fragments Bind to SH2 Domain-Containing Proteins.
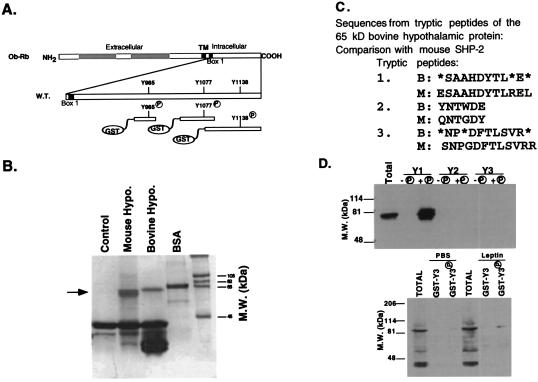
These data suggested that, when phosphorylated, Tyr985 and Tyr1138 then can serve as binding sites for SH2 domain-containing proteins. Previous studies have shown that the STAT3 transcription factor is activated by leptin treatment in vivo (20). To isolate other proteins involved in leptin signaling, GST was fused to three polypeptides spanning each of the three tyrosine residues encoded in the cytoplasmic region of Ob-Rb. The receptor domains expressed in each of these three constructs is indicated (Fig. 2A). Each of these peptides encodes a single tyrosine. The three GST Ob-Rb fusion proteins were introduced into a TKX1 strain of bacteria also expressing the elk tyrosine kinase. Expression of the elk kinase leads to the specific tyrosyl phosphorylation of the Ob-Rb fragments (data not shown). Treatment of the fusion proteins with thrombin followed by immunoblotting with an antiphosphotyrosine antibody confirmed that the tyrosine residue within the Ob-Rb peptide sequence was the only residue that was phosphorylated (data not shown).
Figure 2.
Binding of a 64-kDa protein to phosphotyrosine 985 from the Ob-Rb cytoplasmic region. (A) The structure of three receptor-GST constructs, each containing an individual tyrosine from the cytoplasmic domain of Ob-Rb, is shown. (B) The GST fusion protein with phosphorylated Tyr985 binds to a 64-kDa protein in mouse and bovine hypothalamus. The GST-Ob-Rb peptide spanning Tyr 985 was incubated with protein extracts from bovine or mouse hypothalamus. After precipitation, bound proteins were eluted, were resolved on SDS/PAGE, and were stained with Coomassie blue. A 64-kDa protein was observed. (C) Microsequencing of three internal tryptic fragments of the 64-kDa protein yielded peptide sequences identical to that of SHP-2, SH2 domain-containing protein tyrosine phosphatase 2. (D) Immunoblots of the protein precipitate of Ob-Rb fragments. The phosphorylated and unphosphorylated GST fusion proteins for fragments 1, 2, and 3 were incubated with protein extracts of mouse or bovine hypothalamus. The precipitated proteins were immunoblotted by using specific antibodies to SHP-2. When extracts from PBS- or leptin-treated mice were incubated with GST fusion proteins for fragment 3, STAT3 was detected.
Each of the three fusion proteins was coupled to glutathione agarose beads and was incubated with protein extracts from bovine and mouse hypothalamus. The bound proteins were precipitated, eluted, and fractionated by SDS/PAGE. A single protein band of 64 kDa was observed after Coomassie blue staining of protein precipitated after incubation with the fusion protein spanning the first tyrosine at position 985 (Fig. 2B). Precipitation of this protein depended on the presence of a phosphotyrosine at position 985 because the fusion protein without a phosphotyrosine did not precipitate the 64-kDa protein. The 64-kDa protein was transferred to poly(vinylidene difluoride) membrane, and both N-terminal and internal sequencing analyses were performed. The amino acid sequences obtained from three peptides were all found to match SHP-2 (Fig. 2C). SHP-2 is a SH2 domain containing phosphotyrosine phosphatase (13–17). The 64-kDa protein was confirmed to be SHP-2 by using specific monoclonal and polyclonal anti-SHP-2 antisera. SHP-2 binding to the GST fusion protein depends on the presence of a phosphotyrosine at position 985 (data not shown).
The relative efficiency of binding of SHP-2 to GST Ob-Rb fragment 1 was compared to the binding of STAT3 to the GST fusion protein spanning tyrosine 1138 (fragment 3). A STAT3 binding motif, YMPQ, is found in the region of tyrosine 1138 (21). Ob-Rb fragments 1 and 3 were incubated with hypothalamic extracts and were immunoblotted by using anti-STAT3 and anti-SHP-2 antisera. SHP-2 immunoreactivity was found only in the precipitate using the Ob-Rb fusion protein spanning Tyr 985. When the GST Ob-Rb fragment spanning Tyr 1138 was incubated with hypothalamic extract from PBS- or leptin-treated mice, binding of STAT3 also was detected (Fig. 2D). The intensity of the SHP-2 signal was much greater than that of STAT3. These data establish that SHP-2 binds to Tyr 985 of Ob-Rb and that STAT3 binds to Tyr1138, both in a sequence- and phosphotyrosine-dependent manner.
SHP-2 Is Phosphorylated After Treatment by Leptin and Directly Associates with the Leptin Receptor.
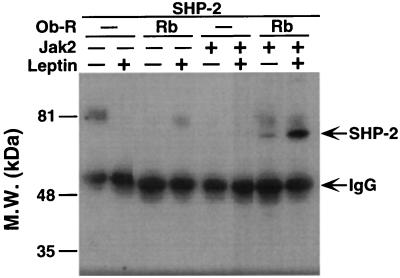
To assess whether SHP-2 plays a role in leptin signal transduction, cDNA clones encoding Ob-Rb, JAK2, and SHP-2 were transfected in various combinations into 293T cells. The transfected cells were treated with either PBS or leptin. In each case, SHP-2 was immunoprecipitated by using a specific antibody and was blotted by using an antiphosphotyrosine antibody. Leptin-inducible phosphorylation of SHP-2 was observed only in the cells that were cotransfected with Ob-Rb and JAK2 (Fig. 3). In the untreated Ob-Rb-transfected cells, there was a low level of phosphorylation of SHP-2 that increased severalfold when leptin was added. These data indicate that leptin binding to Ob-Rb leads to the phosphorylation of SHP-2.
Figure 3.
Binding of leptin to Ob-Rb leads to the inducible phosphorylation of SHP-2. 293T cells were transfected by using combinations of Ob-R, JAK2, and SHP-2 as indicated. The state of SHP-2 tyrosyl phosphorylation was assayed with and without leptin treatment. SHP-2 was immunoprecipitated and blotted with pY20. Leptin treatment of cells transfected with Ob-Rb, JAK2, and SHP-2 led to a several-fold increase of SHP-2 phosphorylation.
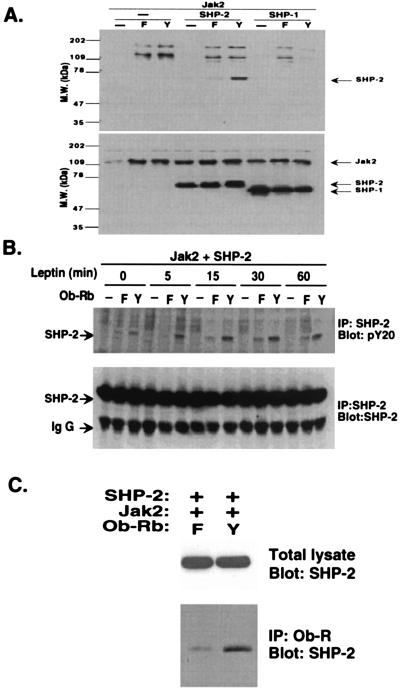
The specificity of SHP-2 tyrosyl phosphorylation by the leptin receptor was confirmed by performing analogous experiments with SHP-1. SHP-1 is a related phosphatase that is activated by the hematopoietic growth factor erythropoietin and other ligands (22). Leptin treatment of cells transfected with Ob-Rb, JAK2, and SHP-1 did not result in the phosphorylation of SHP-1. SHP-1 was expressed in the transfected cells, as evidenced by the fact that high levels of the protein were seen after immunoblotting with an anti-SHP-1 antisera (Fig. 4A). However, although the phosphorylation of SHP-1 is not induced by leptin, Jak2 phosphorylation is reduced in samples transfected with SHP-1, raising the possibility that JAK2 could still serve as a substrate for SHP-1.
Figure 4.
Specific activation of SHP-2 by leptin. (A) 293T cells were cotransfected with Ob-Rb and JAK2. In addition, either SHP-1 or SHP-2 also was transfected. Tyrosyl phosphorylation of SHP-1 or SHP-2 was scored by analyzing the total cellular lysate after leptin treatment. SHP-2 was phosphorylated in Ob-Rb-expressing cells whereas SHP-1 was not. The level of expression of SHP-1 and SHP-2 is equivalent. (B). 293T cells were transfected with wild-type Ob-Rb or a Y→F 985 mutant receptor together with JAK2 and SHP-2. The level of SHP-2 phosphorylation was scored at various times. SHP-2 phosphorylation was increased only in the leptin-treated cells transfected with the wild-type receptor. (C) SHP-2 phosphorylation is the result of direct binding to Tyr 985 of Ob-Rb. Immunoprecipitation of Ob-Rb followed by immunoblotting with an anti-SHP-2 antibody in leptin treated cells confirmed that SHP-2 binds directly to the leptin receptor but not to the Y→F mutant. The total level of SHP-2 in the cells from these two experiments was identical.
The time course of SHP-2 tyrosyl phosphorylation by leptin in Ob-Rb-transfected cells was compared between wild-type and a Y→F 985 mutant receptor (Fig. 4B). Leptin induced SHP-2 phosphorylation in the cells transfected with the wild-type receptor within 5 min, and SHP-2 remained tyrosine-phosphorylated after 1 hour. The level of SHP-2 phosphorylation was substantially reduced in cells transfected with the Y→F 985 mutant receptor at all time points. This agrees with the in vitro data indicating that SHP-2 binds to Y985 of the leptin receptor in a phosphotyrosine-dependent manner.
SHP-2 binds directly to the full length leptin receptor, as shown by immunoprecipitation of Ob-Rb followed by blotting with an anti-SHP-2 antibody in leptin-treated cells (Fig. 4C). The amount of SHP-2 that is directly associated with the leptin receptor is considerably higher in cells transfected with the wild-type leptin receptor compared with the background level seen in cells transfected with the Y→F 985 mutant.
SHP-2 Phosphorylation Leads to the Dephosphorylation of JAK2.
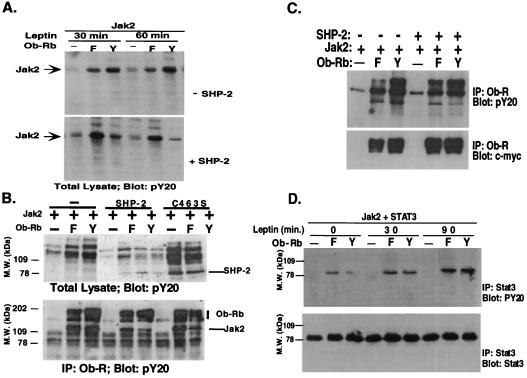
The possible function of SHP-2 in signal transduction was further assessed by scoring the level of phosphorylation of the various components of the leptin signal transduction pathway in cells transfected with the wild-type or Y→F 985 mutant receptor. The possible role of SHP-2 in decreasing the level of JAK2 was first tested. The leptin-dependent phosphorylation of JAK2 was decreased when SHP-2 was cotransfected into cells also transfected with the wild-type receptor (Fig. 5A). The level of JAK2 phosphorylation was not decreased when SHP-2 was transfected with the Y→F 985 mutant. In the absence of SHP-2, JAK2 was highly phosphorylated in response to leptin after 30 and 60 min of treatment. In this case, the level of JAK2 phosphorylation of the wild-type receptor was greater than that of the Y→F 985 mutant. However, when SHP-2 was added, this relationship was reversed, and the level of JAK2 phosphorylation was much greater in the cells that were transfected with the Y→F 985 mutant form of the receptor. These data suggest that the activation of SHP-2 after binding to phosphotyrosine 985 leads to decreased phosphorylation of JAK2. To determine whether the decrease in JAK2 tyrosyl phosphorylation requires the enzymatic activity of SHP-2, a point mutation was introduced that changed Cysteine 463 to Serine. This abolishes the phosphatase activity of SHP-2 without affecting its other actions (23). Leptin induced a greater tyrosyl phosphorylation of JAK2 in wild-type Ob-Rb-expressing cells with mutant SHP-2, suggesting that the dephosphorylation of JAK2 requires the catalytic activity of SHP-2 (Fig. 5B Upper). We also observed that the mutant SHP-2 (C463S) is more strongly tyrosine phosphorylated than wild-type SHP-2 even in Y→F Ob-Rb-expressing cells. This is consistent with earlier reports that SHP-2 can modulate its own state of tyrosyl phosphorylation, a property that is lost when the C463S mutation is introduced (24). The tyrosyl phosphorylation of Ob-Rb-bound JAK2 is greatly reduced in wild-type receptor-expressing cells in the presence of catalytically active SHP-2, but not in the C463S mutant (Fig. 5B Lower). The expression level of SHP-2, JAK2, and Ob-Rb was also equivalent in all lanes by blotting with respective antibodies (data not shown).
Figure 5.
Activation of SHP-2 leads to decreased phosphorylation of JAK2 but not Ob-Rb or STAT3. (A) SHP-2 decreases phosphorylation of JAK2. The level of phosphorylation of JAK2 was assayed after leptin treatment in cells transfected with the wild-type or Y→F 985 mutant. After leptin treatment, the level of JAK2 phosphorylation was severalfold greater in cells transfected with the wild-type receptor. When SHP-2 was cotransfected, the level of JAK2 phosphorylation was now greater in cells transfected with the Y→F 985 mutant. These data indicate that binding of leptin to Ob-Rb and activation of SHP-2 leads to a decrease in the level of phosphorylation of JAK2. (B) JAK2 dephosphorylation is a direct action of SHP-2. Cells were transfected as indicated and were treated with leptin. Total cellular lysate was blotted with pY20 (Upper), or Ob-Rb was first immunoprecipitated, followed by pY20 blotting (Lower). SHP-2 became hyperphosphorylated when C463S mutation was introduced. Tyrosyl phosphorylation of receptor-associated JAK2 was greatly reduced when wild-type receptor and SHP-2 were cotransfected, not when SHP-2 is replaced with SHP-2 C463S. (C) SHP-2 does not appreciably affect Ob-Rb tyrosyl phosphorylation. Ob-Rb Y→F (F) or wild-type (Y) receptor was transfected into 293T cells together with JAK2 with or without SHP-2. Cells were treated with leptin, and Ob-Rb was immunoprecipitated. PY20 and anti c-myc monoclonal antibodies were used to examine receptor tyrosyl phosphorylation and expression. (D) SHP-2 does not appreciably affect STAT3 tyrosyl phosphorylation. STAT3 was cotransfected into 293T cells together with the wild-type or Y→F receptor mutant. STAT3 phosphorylation was analyzed by immunoprecipitation and pY20 blotting. The amount of STAT3 protein was measured with a monoclonal antibody.
The phosphorylation state of the Y→F 985 mutant and wild-type leptin receptors was compared with and without cotransfection of SHP-2 (Fig. 5C). Although the level of receptor phosphorylation was lower in the Y→F 985 mutant than wild type, the phosphorylation state of Ob-Rb was unchanged when SHP-2 was cotransfected. These data suggest that tyrosyl phosphorylation of SHP-2 does not lead to the dephosphorylation of Tyr 1138 of the leptin receptor. Similarly, the level of tyrosyl phosphorylation of STAT3 was indistinguishable in cells transfected with the Y→F 985 mutant and wild-type Ob-Rb constructs at all times up to 90 min after leptin addition. A time-dependent increase in STAT3 phosphorylation was found after transfection of both the wild-type and mutant receptor (Fig. 5D).
DISCUSSION
In this report, a potentially general method was used to confirm that SHP-2 is a component of the leptin signal transduction pathway. The identification of SHP-2 directly from extracts of hypothalamus supports this conclusion, as does the data from studies of transfected cells. The method used here is validated by independent studies that suggested a possible role for SHP-2 in leptin signal transduction based solely on empirical studies using cultured cells (25).
SHP-1 and SHP-2 are members of the family of SH2 domain-containing protein tyrosine phosphatases (26). Both contain tandem SH2 domains at their amino terminus, followed by a carboxyl terminal catalytic domain that has phosphotyrosine phosphatase activity. SHP-1 and SHP-2 associate with many types of activated receptors and can act to increase and/or decrease signal transduction. For example, SHP-1 is specifically recruited to the activated erythropoietin receptor and causes the termination of proliferative signals (22). On the other hand, SHP-2 can be a positive or negative regulator of receptor signaling (27, 28).
It is as yet unclear whether SHP-2 plays a positive and/or negative role in leptin signal transduction in vivo. The data here and in a previous report suggest that tyrosine-phosphorylated SHP-2 can lead to decreased phosphorylation of JAK2 and thus may play a role in the termination of leptin signal transduction (25). This putative role is analogous to that played by SHP-1, which dephosphorylates JAK2 in hematopoietic cells (22). This possibility is also consistent with data indicating that SHP-2 decreases expression of a leptin-inducible reporter gene in transfected cells (25).
The data also suggest that the dephosphorylation of JAK2 is a direct action of SHP-2. Thus, a point mutation that ablates SHP-2 phosphatase activity also ablates its effects on the state of JAK2 phosphorylation. Although SHP-2 does have intrinsic phosphatase activity, it also could lead to dephosphorylation of JAK2 indirectly by functioning as an adapter protein. For example, binding of SHP-2 to the activated platelet-derived growth factor receptor leads to its own phosphorylation at position Tyr 584, which in turn leads to binding of Grb2. Grb2 then activates ras and the mitogen-activated protein kinase signaling pathway. Previous studies have shown that leptin can activate mitogen-activated protein kinase (29). Indeed the available data are consistent with the possibility that SHP-2 could both decrease JAK2 phosphorylation and stimulate signaling via the mitogen-activated protein kinase or other pathways.
A mechanism for the induction of SHP-2 phosphatase activity has been proposed from an analysis of its crystal structure (30). The structure predicts that, when the SH2 domains of SHP-2 are bound to a receptor, the phosphatase domain is exposed and thus activated. Biochemical evidence from studies of SHP-2 association with platelet/endothelial cell adhesion molecule 1 agrees very well with this prediction (31, 32). The SH2 domains of SHP-2 generally binds simultaneously to two phosphorylated tyrosine residues, and SHP-2 is likely to bind to the two Tyr 985 residues on an Ob-Rb homodimer, similar to the binding of SHP-2 to the platelet-derived growth factor receptor (30). Thus, binding of SHP-2 to an Ob-Rb homodimer could lead to phosphatase activation and direct JAK2 dephosphorylation. This possibility is supported by the effect of C463S mutation of SHP-2.
The functional significance of tyrosyl phosphorylation of SHP-2 in leptin signalling is as yet unclear. When activated, multiple sites on SHP-2 become phosphorylated on Ser, Thr, and Tyr residues (33). Previous studies have indicated that the protein tyrosine phosphatase activity of SHP-2 is similar in the unphosphorylated and phosphorylated forms. Binding of SHP-2 to Ob-Rb could lead to activation of its phosphatase activity as well as serving as a binding site for other SH2 proteins.
The data presented here do not exclude the possibility that other proteins, such as the recently isolated SOCS proteins, also might act to modulate leptin signal transduction (34). If dephosphorylation of JAK2 is an important action of SHP-2, inhibition of its activity could enhance leptin action. This would require that the dominant role of SHP-2 in leptin signal transduction in vivo is to inhibit rather than activate leptin signal transduction, a possibility that can be tested by mutating Tyr 985 in embryonic stem cell-derived mice. However, SHP-2 function is not likely to be limited to leptin action because it is expressed in hypothalamus and most other tissues in mice. Moreover, mice with induced mutations in SHP-2 are not viable and die after ≈10.5 days of development (35). It is as yet unknown which roles SHP-2 plays in adult animals, and it is formally possible that inhibition of its activity could modulate leptin action in vivo. Experiments are under way to introduce the Y→F 985 mutation into mice and to induce mutations in SHP-2 in leptin receptor-positive cells. These in vivo studies should clarify the role of SHP-2 in leptin signal transduction.
Acknowledgments
We thank Susan Korres for expert assistance in preparing this manuscript, Naseem Fidahusein for excellent technical assistance, and Dr. Matt Thomas (Washington University) and Dr. James Darnell (The Rockefeller University) for reagents. Recombinant leptin was provided by Amgen, Inc. Protein sequence determination was performed by the Protein/DNA Technology Center of The Rockefeller University.
Footnotes
Abbrevation: GST, glutathione S-transferase, SHP-2, SH2 domain containing protein tyrosine phosphatase 2; Ob-R, Ob receptor, JAK, Janus kinase; STAT, signal transducer and activator of transcription.
References
- 1.Zhang Y, Proenca P, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 3.Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, et al. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, et al. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 6.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim M H, Skoda R. Proc Natl Acad Sci USA. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fei H, Okano H J, Li C, Lee G-H, Zhao C, Darnell R, Friedman J M. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maffei M, Fei H, Lee G W, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman J M. Proc Natl Acad Sci USA. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halaas J L, Boozer C, Blair-West J, Fidahusein N, Denton D, Friedman J M. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darnell J E. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 11.Adachi M, Sekiya M, Miyachi T, Matsuno K, Hinoda Y, Imai K, Yachi A. FEBS Lett. 1992;314:335–339. doi: 10.1016/0014-5793(92)81500-l. [DOI] [PubMed] [Google Scholar]
- 12.Freeman R M J, Plutzky J, Neel B G. Proc Natl Acad Sci USA. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel W, Lammers R, Huang J, Ullrich A. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 14.Feng G S, Hui C C, Pawson T. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad S, Banville D, Zhao Z, Fischer E H, Shen S H. Proc Natl Acad Sci USA. 1993;90:2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Ioffe E, Fidahusein N, Connolly E, Friedman J M. J Biol Chem. 1998;273:10078–10082. doi: 10.1074/jbc.273.16.10078. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Ullrich B, Zhang J Z, Anderson R G, Brose N, Sudhof T C. Nature (London) 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 18.Bjorbaek C, Uotani S, da Silva B, Flier J S. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 19.Ghilardi N, Skoda R C. Mol Endocrinol. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 20.Vaisse C, Halaas J L, Horvath C M, Darnell J E, Jr, Stoffel M, Friedman J M. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 21.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Jr, Yancopoulos G D. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 22.Klingmuller U, Lorenz U, Cantley L C, Neel B G, Lodish H F. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 23.Yu Z, Ahmad S, Schwartz J, Banville D, Shen S. J Biol Chem. 1997;272:7519–7524. doi: 10.1074/jbc.272.11.7519. [DOI] [PubMed] [Google Scholar]
- 24.Stein-Gerlach M, Kharitonenkov A, Vogel W, Ali S, Ullrich A. J Biol Chem. 1995;270:24635–24637. doi: 10.1074/jbc.270.42.24635. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter L R, Farruggella T J, Symes A, Karow M L, Yancopoulos G D, Stahl N. Proc Natl Acad Sci USA. 1998;95:6061–6066. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonks N K, Neel B G. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 27.Ali S, Chen Z, Lebrun J J, Vogel W, Kharitonenkov A, Kelly P A, Ullrich A. EMBO J. 1996;15:135–142. [PMC free article] [PubMed] [Google Scholar]
- 28.Servidei T, Aoki Y, Lewis S E, Symes A, Fink J S, Reeves S A. J Biol Chem. 1998;273:6233–6241. doi: 10.1074/jbc.273.11.6233. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Y, Yasuhiho O, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. J Biol Chem. 1997;272:12897–12900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- 30.Hof P, Pluskey S, Dhe-Paganon S, Eck M J, Shoelson S E. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 31.Jackson D E, Kupcho K R, Newman P J. J Biol Chem. 1997;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- 32.Jackson D E, Ward C M, Wang R, Newman P J. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 33.Vogel W, Ullrich A. Cell Growth Differ. 1996;7:1589–1597. [PubMed] [Google Scholar]
- 34.Bjorbaek C, Elmquist J K, Frantz J D, Shoelson S E, Flier J S. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 35.Arrandale J M, Gore-Willse A, Rocks S, Ren J M, Zhu J, Davis A, Livingson J N, Rabin D U. J Biol Chem. 1996;271:21353–21358. doi: 10.1074/jbc.271.35.21353. [DOI] [PubMed] [Google Scholar]