Abstract
A phenological study of springtime events was made over a 61-year period at one site in southern Wisconsin. The records over this long period show that several phenological events have been increasing in earliness; we discuss evidence indicating that these changes reflect climate change. The mean of regressions for the 55 phenophases studied was −0.12 day per year, an overall increase in phenological earliness at this site during the period. Some phenophases have not increased in earliness, as would be expected for phenophases that are regulated by photoperiod or by a physiological signal other than local temperature.
Keywords: climate warming, phenology, flowering, bird migration, photoperiod
Phenology is the study of the cycling of biological events throughout the year—a reading of the “pulse of life.” The cycling of phenological events such as flowering, fruiting, bird migration, or animal reproduction is frequently used to define annual seasonal sequences. Phenological studies have also proved useful in predicting the production stages of certain crops (1) and in measuring the response of plant systems to changes in temperature (2).
Climatic warming would be expected to have an impact on some phenological sequences (3, 4). If phenological records are continued over a sufficient length of time, they may reflect climate change, as has been suggested by Beaubien and Johnson (5). With widespread evidence that climate warming has occurred over the past 40 years (6–8), long-term phenological records may reflect such climate warming. We report here such a record of phenological events at a site in southern Wisconsin. This record offers an unusual opportunity to observe long-term changes by various phenophases (seasonal biological events).
METHODS
Phenological data have been collected at a farm in Fairfield Township, Sauk County, in southern Wisconsin during two intervals of time. From 1936 to 1947, Aldo Leopold (9) maintained records of spring events. After a lapse of 29 years, similar records were kept by Nina Leopold Bradley at the same farm for a subsequent 22 years, from 1976 to 1998, spanning a total of 61 years. The record includes 74 phenophases, focusing especially on arrival dates for migratory birds and dates of first bloom of spring flowers. We estimate the accuracy during the first 11-year period to be ±4 days and during the later 22-year interval to be ±2 days.
In this work, we refer to climate warming as a rise in analogous temperatures over the 61-year period, not as seasonal warming within a single year.
To limit our analysis to phenophases that can be identified with the spring season, we report only those events that occur before the end of June (Julian calendar day 181). We used only phenophases for which there were at least six yearly records in each of the two recording periods. We analyzed 55 phenophases within these parameters for long-term changes in the dates of springtime events. Regression analysis (10) performed on the yearly records for each phenophase yielded an approximation of the slope of the data for the six-decade period. For each phenophase we report the average date of occurrence across the entire data collection period, the number of yearly observations recorded for that phenophase, and the slope of the linear regression plot for those observations (Table 1). In addition, for each phenophase we report the t value (Student’s t distribution), an estimate of the deviation from the population mean, and the p value, a measure of the statistical probability of fit to the regression line.
Table 1.
Regression analyses of the changes in phenophases over a 61-year period.
| Julian day (avg) | Date (avg) | Phenophase (species) | No. of observations | Regression
|
||
|---|---|---|---|---|---|---|
| Slope* | t† | P‡ | ||||
| 46 | 15 Feb | Cardinal first song (Cardinalis cardinalis) | 29 | −0.365 | −2.75 | 0.01§ |
| 68 | 9 Mar | Geese arrival (Branta canadensis) | 27 | −0.476 | −5.42 | 0.00§ |
| 73 | 14 Mar | Bluebird arrival (Sialia sialis) | 18 | 0.024 | 0.26 | 0.79¶ |
| 74 | 15 Mar | Redwinged blackbird arrival (Agelaius phoeniceus) | 30 | −0.166 | −2.21 | 0.04§ |
| 74 | 15 Mar | Robin arrival (Turdus migratorius) | 25 | −0.159 | −2.71 | 0.01§ |
| 79 | 20 Mar | Meadowlark arrival (Sturnella magna) | 25 | −0.121 | −1.50 | 0.15 |
| 80 | 21 Mar | Woodcock first peent (Scolopax minor) | 27 | −0.156 | −2.04 | 0.05§ |
| 80 | 21 Mar | Fox sparrow arrival (Passarella iliaca) | 18 | 0.000 | 0.00 | 0.00¶ |
| 88 | 29 Mar | Phoebe arrival (Sayornis phoebe) | 28 | −0.299 | −3.99 | 0.09 |
| 91 | 1 Apr | Great blue heron arrival (Ardea herodias) | 13 | −0.185 | −1.87 | 0.09 |
| 95 | 5 Apr | Kingfisher arrival (Ceryle alcyon) | 23 | −0.144 | −1.43 | 0.17 |
| 101 | 11 Apr | Hepatica first bloom (Hepatica acutiloba) | 31 | −0.170 | −2.13 | 0.04§ |
| 103 | 11 Apr | Cowbird arrival (Monothrus ater) | 14 | 0.231 | 2.12 | 0.05§ |
| 105 | 15 Apr | Pasque flower first bloom (Anemone patens) | 19 | −0.188 | −1.70 | 0.11 |
| 108 | 18 Apr | Pussytoes first bloom (Antennaria neglecta) | 24 | −0.036 | −0.44 | 0.67¶ |
| 110 | 20 Apr | Dutchman’s britches first bloom (Dicentra cucullaria) | 28 | −0.142 | −1.36 | 0.19 |
| 110 | 20 Apr | Towhee arrival (Pipilio erythrophthalamus) | 22 | 0.100 | 0.89 | 0.38¶ |
| 112 | 22 Apr | Brown thrasher arrival (Toxostomum rufum) | 28 | −0.037 | −0.76 | 0.46¶ |
| 116 | 26 Apr | House wren arrival (Troglodytes aedon) | 24 | −0.293 | −3.33 | 0.00§ |
| 116 | 26 Apr | Marsh marigold first bloom (Caltha palustris) | 22 | −0.133 | −1.71 | 0.10 |
| 119 | 29 Apr | Bellwort first bloom (Uvularia grandiflora) | 19 | −0.116 | −1.27 | 0.22 |
| 120 | 30 Apr | Amelanchier first bloom (Amelanchier laevis) | 25 | −0.071 | −0.99 | 0.33¶ |
| 122 | 2 May | Forest phlox first bloom (Phlox divaricata) | 22 | −0.242 | −3.04 | 0.01§ |
| 122 | 2 May | Rose-breasted grosbeak arrival (Pheucticus ludovicianus) | 26 | −0.128 | −4.04 | 0.00§ |
| 123 | 3 May | Birdsfoot violet first bloom (Viola pedata) | 15 | 0.062 | 0.66 | 0.52¶ |
| 124 | 4 May | Wood anemone first bloom (Anemone quinquefolia) | 10 | −0.040 | −0.34 | 0.74¶ |
| 125 | 5 May | Northern oriole arrival (Icterus galbula) | 30 | −0.074 | −1.67 | 0.11 |
| 125 | 5 May | Whip-poor-will arrival (Caprimulgus vociferus) | 17 | −0.197 | −2.52 | 0.02§ |
| 126 | 6 May | Large trillium first bloom (Trillium grandiflorum) | 25 | −0.105 | −1.32 | 0.20 |
| 127 | 7 May | Hoary puccoon first bloom (Lithospermum canescens) | 16 | 0.244 | 2.08 | 0.06 |
| 127 | 7 May | Wood thrush arrival (Hylocicla mustelina) | 20 | −0.110 | −1.28 | 0.22 |
| 132 | 12 May | Choke cherry first bloom (Prunus virginiana) | 18 | 0.136 | 1.70 | 0.11 |
| 132 | 12 May | Columbine first bloom (Aquilegia canadensis) | 25 | −0.213 | −2.83 | 0.01§ |
| 133 | 13 May | Wild geranium first bloom (Geranium maculatum) | 24 | −0.064 | −0.63 | 0.51¶ |
| 134 | 14 May | Blue-eyed grass first bloom (Sisryinchium campestre) | 23 | −0.081 | 0.94 | 0.36¶ |
| 136 | 16 May | Lupine first bloom (Lupinus perennis) | 23 | −0.081 | −1.45 | 0.16 |
| 136 | 16 May | Violet wood-sorrel first bloom (Oxalis violacea) | 11 | 0.030 | 0.24 | 0.82¶ |
| 137 | 17 May | Shooting star first bloom (Dodecatheon media) | 22 | −0.163 | −2.44 | 0.02§ |
| 143 | 23 May | Pink prairie phlox first bloom (Phlox philosa) | 21 | −0.145 | −1.72 | 0.10 |
| 149 | 29 May | Canadian anemone first bloom (Anemone canadensis) | 23 | −0.135 | −2.10 | 0.05§ |
| 150 | 30 May | Spiderwort first bloom (Tradescantia ohiensis) | 26 | −0.110 | −1.80 | 0.08 |
| 152 | 1 Jun | Rose first bloom (Rosa carolina) | 23 | 0.031 | 0.49 | 0.63¶ |
| 153 | 2 Jun | Slender pentstemon first bloom (Pentstemon gracilis) | 13 | 0.012 | 0.14 | 0.39¶ |
| 156 | 5 Jun | Baptisia first bloom (Baptisia leucantha) | 25 | −0.295 | −3.96 | 0.00§ |
| 158 | 7 Jun | Yarrow first bloom (Achillea millefolium) | 15 | 0.142 | 0.47 | 0.16¶ |
| 163 | 12 Jun | Daisy fleabane first bloom (Erigeron striigosus) | 12 | 0.020 | 0.31 | 0.77¶ |
| 163 | 12 Jun | Harebell first bloom (Campanula rotundifolia) | 19 | 0.037 | 0.47 | 0.64¶ |
| 167 | 16 Jun | Flowering spurge first bloom (Euphorbia corollata) | 20 | 0.087 | 0.75 | 0.47¶ |
| 169 | 18 Jun | Rudbeckia first bloom (Rudbeckia hirta) | 24 | −0.132 | −2.15 | 0.04§ |
| 174 | 23 Jun | Dogbane first bloom (Apocynum androsaemifolium) | 10 | −0.046 | −0.41 | 0.69¶ |
| 176 | 25 Jun | Butterfly weed first bloom (Asclepias tuberosa) | 25 | −0.300 | −4.43 | 0.00§ |
| 176 | 25 Jun | St. Johns wort first bloom (Hypericum perforatum) | 16 | −0.012 | −0.15 | 0.89¶ |
| 177 | 26 Jun | Common milkweed first bloom (Asclepias syriaca) | 17 | −0.206 | −2.49 | 0.02§ |
| 180 | 29 Jun | Butter-and-eggs first bloom (Linaria vulgaris) | 12 | 0.041 | 0.23 | 0.23¶ |
| 181 | 30 Jun | Marsh milkweed first bloom (Asclepias incarnata) | 15 | −0.213 | −3.69 | 0.00§ |
The slopes of the regression plot are reported in days/year.
t values are given as Student’s t distribution.
P values are probability.
Values with a 95% probability of significance.
Values with no apparent change (t between +1 and −1).
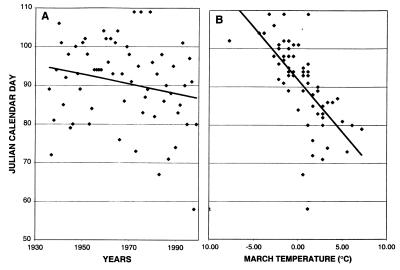
An effective component of this study would be a record of actual temperatures indicating the increase over the past decades. However, the scatter of daily, weekly, and monthly temperatures encompasses a vast range, and a significant drift in local temperatures over the decades is difficult to define. The data on planetary warming indicate a very small total increase, less than 1°C over 50 years (6–8, 11). For a natural integrator of seasonal temperature changes, we have selected the date of ice-melt in Lake Mendota, located in the adjoining county. A regression analysis of the melt dates for the succession of years is presented in Fig. 1A; it indicates an overall increase in earliness over the 61-year period, with a slope of −0.124 days per year, and with a 97% probability of significance (P = 0.031). For evidence that the regression of Lake Mendota ice-melt is driven by temperature, we plotted the melt dates against the average March temperature (Fig. 1B); we find a regression of −2.72 days in earliness per °C.
Figure 1.
Regression analysis of the date of ice-melt from Lake Mendota over the years of phenological records. (A) The Julian calendar day of ice-melt is plotted against the year. The regression indicates a change in earliness of −0.124 day per year (R2 = 0.046). (B) the Julian calendar day of ice-melt is plotted against the average temperature for the month of march. The regression in this case indicates a change in earliness of −2.719 days per degree of March temperature (R2 = 0.453). Data on ice break-up are from State Climatology Office, 1999: Lake Mendota Ice Summary, 1853–1999, Electronic database appearing at http://www.uwex.edu/sco/icemend.html, Wisconsin Geological and Natural History Survey, Madison. Data for Madison temperatures are from National Climatic Data Center, Local Climatological Data for Madison, WI, at the Environmental Data and Information Service, National Oceanic and Atmospheric Administration, Asheville, NC.
RESULTS
The long-term phenological record at one site presents an opportunity to examine changes in the dates of occurrence of various phenophases over a period of climate change. In Table 1 we present springtime phenophases in the order of their occurrence. For example, the average date of the first song of cardinals occurred on day 46 of the Julian calendar; there were 29 year-records of the date of that event. These records yielded a regression with a slope of −0.365 day in earliness per year. The t test yielded −2.75; and the p value was 99% (P = 0.01).
Seventeen phenophases [indicated by section marks (§) in Table 1] show significant advances in springtime occurrence. The data also indicate that 20 phenophases [indicated by paragraph marks (¶) in Table 1] do not appear to be increasing in earliness. These are phenophases with t values between +1 and −1, thus with minimal divergence from the average date of occurrence. The remaining 18 phenophases show intermediate regressions, and are statistically not assignable to either the responder or the nonresponder class.
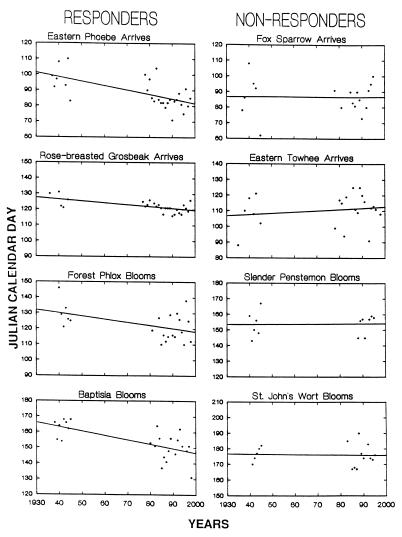
Examples of responders and nonresponders are illustrated in Fig. 2; individual plots of four phenophases with increasing earliness are compared with four phenophases without statistical increases in earliness. Each datum point in the graphs indicates the date for that event in a single year.
Figure 2.
On the left are regressions of four selected springtime phenophases that do show significant increases in earliness during the 61-year period of record: arrival dates of migrating eastern phoebe (Sayornis phoebe) and rose-breasted grosbeak (Pheucticus ludovicianus) and first-bloom date of forest phlox (Phlox divaricata) and baptisia (Baptisia leucantha). These are compared with four selected phenophases that do not show significant increases in earliness: arrival dates of fox sparrow (Passarella iliaca) and eastern towhee (Pipilio erythrophthalamus) and first-bloom dates of slender pentstemon (Pentstemon gracilis) and St. John’s wort (Hypericum perforatum). The regression values are recorded in Table 1.
In Table 2, the numbers of phenophases are clustered for each of the five springtime months. The averages of the regression slopes are given for each month. The number and percentage of phenophases that show significant increases in earliness are recorded with the number and percentage of phenophases that qualify as nonresponders. Only one phenophase in February is represented. The regression averages for the eight phenophases in March yielded a value of −0.169 day/year. The regression averages for all phenophases lessened in the subsequent months from March through June. As springtime advanced, the number (or percentage) of phenophases increasing in earliness diminished, whereas the number (or percentage) of phenophases not changing in earliness increased. The mean regression for all 55 phenophases was −0.12 day/year, comparable to the regression for the ice-melt data in Fig. 1A.
Table 2.
Comparison of 55 phenophases for springtime months in response to climate warming
| Month | No. of phenophases | Avg regression slope, day/year | Increasing in earliness
|
Not
increasing in
earliness
|
||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| February | 1 | −0.365 | 1 | 100 | — | |
| March | 8 | −0.169 | 4 | 50 | 2 | 25 |
| April | 13 | −0.091 | 4 | 31 | 4 | 31 |
| May | 19 | −0.074 | 6 | 31 | 5 | 27 |
| June | 14 | −0.060 | 4 | 28 | 9 | 64 |
| Mean = −0.12 | ||||||
Those increasing in earliness had >95% probability of significance (P < 0.05). Those not increasing in earliness had t values between +1 and −1.
DISCUSSION
Of the 55 phenophases reported here for the 61-year period in southern Wisconsin, 19 showed statistically significant increases in earliness. Twenty phenophases were considered nonresponders, based on the range of t values. Thus, roughly one-third of the phenophases appeared to advance in earliness over the period, one-third appeared not to advance, and the remaining third were statistically intermediate.
Within the 61-year span of our observations, the surface temperatures of the planet have warmed (5, 6, 8, 12). Climate warming has frequently been reported to have resulted in increases in earliness of some phenophases (11, 13). Experimental applications of heat have also been shown to result in phenological advances in plants (2, 14–20). It seems reasonable to expect that climate warming can induce advances in some phenological events.
Our examples of phenophases showing increases in earliness may be responding to climate warming. Because the range of seasonal temperature changes is magnified at higher latitudes (11, 12) and may be almost imperceptible at tropical or even subtropical sites (21), phenological responses may be accentuated at more polar latitudes and minimal or even absent near the equator. Studies of phenological processes in Alaska and other near-polar sites have shown dramatic changes in various plant phenophases in response to increases in temperature (13, 22, 23). Satellites have recorded measurements of regional changes in photosynthesis showing increased earliness associated with climate warming in northern latitudes (17). In this study, maximal increases in earliness of photosynthetic activity were observed for latitudes between 45° and 65° N. Our site in Wisconsin at 43.5° N lies just below the range of the maximal photosynthesis response.
Several reports have shown that phenological responses to temperature in colder, northern climates can be simulated at sites along an altitudinal gradient (24, 25). Our preliminary evidence in Table 2 suggests that phenological advances may be more frequent in the colder months of early spring; phenological responses to warming may be more substantial in colder sites or seasons.
The fact that some phenophases respond to a drift toward climate change and other phenophases do not raises some questions about phenological adaptability and its possible relation to species survival during extended climate change. Is there a survival advantage for species having phenological adaptability to climate change? The checkerspot butterfly (Euphrydryas editha) is one documented species that has shifted its range as an adaptation to climate warming (26). Species lacking phenological adaptability, such as the amelanchier (A. laevis; see Table 1) may require a stronger signal or may be unable to adapt to climate warming. We speculate that species without phenological adaptability may experience greater stress or even extinction during extended climate change.
Among the species that do not show phenological adaptability are the many organisms in which seasonal developments are regulated by photoperiod or other genetic regulatory systems. Many seasonal biological events have been found to be controlled by photoperiod. These include dormancy, growth rates, and flowering in plants; diapause in insects; reproductive activity in vertebrates; and migration in birds (27). An abundance of literature describes phenological controls by photoperiod. We would expect that photoperiodic responders may fail to show changes in earliness in response to climate warming. For example, extensive literature describes photoperiodic regulation of bird reproduction and migration (28, 29). Our records include four bird migration phenophases that show no apparent change with climate warming: the arrival dates for bluebird (Sialia sialis), fox sparrow (Pasaella iliaca), towhee (Pipilio erythrophthalamus), and brown thrasher (Toxostomum rufum) (Table 1). These may be expected to be regulated by photoperiods.
A comparison of bird migration dates that are correlated with temperature and others that are not has been made by Temple and Cary (30). Their evidence indicates that short-distance migrants may usually be correlated with temperatures, whereas long-distance migrants may not. The onset of flowering in plants serves as a contrast; the temperature-responding and the nonresponding species grow in the same locality and have presumably developed different strategies of floral regulation.
From our data, we suggest that some organisms may be facile in changing their seasonal progressions in relation to climate changes, whereas others are less able to respond. Differences in phenological adaptability may be expected to bear on the ability of species to adapt to climate warming; species with poor phenological adaptability may face increasing stress during prolonged climate changes.
Acknowledgments
We thank John Cary and David Weinstein for assistance with the statistics.
References
- 1.Leith H, editor. Phenology and Seasonality Modeling. New York: Springer; 1976. p. 369. , 401. [Google Scholar]
- 2.Suzuki S, Kudo G. Global Change Biol. 1997;3:108–115. [Google Scholar]
- 3.Oettingen A J. Phanologie der Dorpater Lignosum. Dorpot, Estonia: Heinrich Laakmann; 1879. [Google Scholar]
- 4.Waggoner P E. In: Phenology and Seasonality Modeling. Leith M, editor. New York: Springer; 1974. pp. 401–405. [Google Scholar]
- 5.Beaubien E G, Johnson D L. Int J Biometeorol. 1994;38:23–28. [Google Scholar]
- 6.Jones P. In: Analysis of Climate Variability. Stoich H V, Navarra A, editors. New York: Springer; 1995. pp. 53–75. [Google Scholar]
- 7.Monastersky R. Sci News. 1997;153:38. [Google Scholar]
- 8.Kerr R A. Science. 1998;279:315–316. [Google Scholar]
- 9.Leopold A, Jones E. Ecol Monogr. 1947;17:81–122. [Google Scholar]
- 10.Draper N R, Smith H. Applied Regression Analysis. New York: Wiley; 1966. [Google Scholar]
- 11.Hansen J, Johnson D, Lacis A, Lebedoff S, Lee P, Rind D, Russell D. Science. 1981;213:957–966. doi: 10.1126/science.213.4511.957. [DOI] [PubMed] [Google Scholar]
- 12.Smith R C, Ainley D, Baker K, Domack E, Emslie S, Fraser B, Kennett J, Leventer A, Mosely-Thompson E, Stammerjohn S, et al. BioScience. 1999;49:393–404. [Google Scholar]
- 13.Callaghan T V, Carlsson B A, Tyler N J C. J Ecol. 1989;77:823–827. [Google Scholar]
- 14.Podolsky A S. New Phenology: Elements of Mathematical Forecasting in Ecology. New York: Wiley; 1985. [Google Scholar]
- 15.Inouye D W, McGuire A D. Am J Bot. 1991;78:997–1001. [Google Scholar]
- 16.Farnsworth E J, Nunez-Farfan J, Caeaga S A, Bazzaz F A. J Ecol. 1995;83:967–977. [Google Scholar]
- 17.Henry G H R, Molau U M. Global Change Biol. 1997;3:1–9. [Google Scholar]
- 18.Wookey P A, Parsons N A, Walker J M. Oikos. 1993;76:490–502. [Google Scholar]
- 19.Myneni R B, Keeling C D, Tucker C J, Asrar G, Nemai R R. Nature (London) 1997;386:698–702. [Google Scholar]
- 20.Price M V, Waser N M. Ecology. 1998;79:1261–1271. [Google Scholar]
- 21.Reich P B. Am J Bot. 1995;73:164–174. [Google Scholar]
- 22.Chapin F S, Shave G R, Gibbin A E, Nadelhoffer K J, Laundre J A. Ecology. 1995;76:94–111. [Google Scholar]
- 23.Saccheri I, Koussaari M, Kanakara M, Vikman P, Fortelius W, Hanskki I. Nature (London) 1998;392:491–494. [Google Scholar]
- 24.Farquhar G D. Science. 1997;278:1411. [Google Scholar]
- 25.Street-Perrott F A, Huang R A, Perrott R A, Eglinton G, Barker P, Ben Khelifa L. Science. 1997;278:1422–1426. doi: 10.1126/science.278.5342.1422. [DOI] [PubMed] [Google Scholar]
- 26.Parmesan R. Nature (London) 1996;382:765–766. [Google Scholar]
- 27.Withrow R B, editor. Photoperiodism and Related Phenomena in Plants and Animals. Washington, DC: AAAS; 1959. [Google Scholar]
- 28.Wolfson A. In: Photoperiodism and Related Phenomena in Plants and Animals. Withrow R B, editor. Washington, DC: AAAS; 1959. pp. 679–716. [Google Scholar]
- 29.Berthold P. Control of Bird Migration. London: Chapman & Hall; 1996. [Google Scholar]
- 30.Temple S A, Cary J R. Passenger Pigeon. 1987;46:70–75. [Google Scholar]