Abstract
The aim of this paper was to validate the published UCSF Oral Cancer Pain Questionnaire. To test for validity of the questionnaire 16 oral cancer patients completed the 8-item questionnaire immediately prior to and following treatment (surgical resection) of their oral cancer. For all 8 questions the difference between mean preoperative and mean postoperative responses were statistically significant (p<0.05) confirming the validity of the questionnaire to measure oral cancer pain. Internal consistency of the questionnaire was evaluated using Cronbach’s alpha which provides an estimate of reliability based on all correlations between the items (questions) of the instrument (questionnaire). In the oral cancer pain questionnaire questions 1, 3, and 5 evaluate the intensity, sharpness and throbbing nature of pain when the patient is not engaged in oral function (talking, eating and drinking). Questions 2, 4, and 6 measure the intensity, sharpness and throbbing nature of pain during oral function. Cronbach’s alpha for questions 1, 3, and 5 is 0.87 and Cronbach’s alpha for questions 2, 4, and 6 is 0.94, values greater than 0.7 indicate reliability. In this study we have validated the UCSF Oral Cancer Pain Questionnaire as an effective tool in quantifying pain from oral cancer. Perspective: The study validates an oral cancer pain questionnaire. The questionnaire can be used to reliably measure oral cancer patients’ pain levels before and after surgical resection.
Keywords: oral cancer, cancer pain, quality of life, questionnaire, validation
INTRODUCTION
Outside of survival, pain is the primary concern for oral cancer patients 3, 8, 11, 12. Pain is rated as the worst symptom by oral cancer patients 8 and impairs a patient’s speech, swallowing, eating, drinking, interpersonal relations and quality of life 3, 12. Furthermore, the intensity of oral cancer pain becomes worse with disease progression 2. In an attempt to improve management of cancer pain the World Health Organization and the Agency for Health Care Policy recommends characterizing cancer pain as mild, moderate or severe and re-evaluating pain levels throughout treatment 9, 15. Despite this recommendation there has not been an instrument available to quantify and characterize oral cancer pain. To address the need for such an instrument in 2004 we published the UCSF Oral Cancer Pain Questionnaire to quantify patients’ pain and to identify the functions that lead to oral cancer pain 6. The questionnaire consisted of 8 items on a visual analogue scale, with a scale 0–100 mm, rated by the patient. The design of the 8 questions was aimed at differentiating function-related and spontaneous pain, as well as, determining the quality of pain. The functional restrictions of eating, drinking and swallowing were also evaluated. The questionnaire was constructed to be short and easily self-administered. We previously demonstrated that patients experienced significant pain that was characterized as “sharp” and “intense” when performing oral functions such as talking, eating or drinking. We hypothesized that surgical resection of the oral cancer would significantly reduce the magnitude of pain, as well as restore function, and that the questionnaire would be a valid instrument to measure oral cancer pain. The purpose of the current study was to test these hypotheses and validate the UCSF Oral Cancer Pain Questionnaire.
MATERIALS AND METHODS
The development of the pain questionnaire and its distribution were approved by the Committee on Human Research at the University of California, San Francisco. The UCSF Oral Cancer Pain Questionnaire was administered to 16 patients with biopsy proven oral squamous cell carcinoma.
Inclusion criteria for patients in the study were:
Untreated, biopsy proven oral squamous cell carcinoma.
Comprehension of the questionnaire after reading it.
Exclusion criteria were:
Having a diagnosed psychiatric condition.
Being addicted to pain medications or recreational drugs.
Having taken pain medications since the diagnosis of oral cancer.
Patients completed the pain questionnaire at their first visit, prior to being prescribed analgesics for their oral pain and prior to any treatment. The published form of the UCSF Oral Cancer Pain Questionnaire was completed by patients 6. Demographic information was collected for each patient including age, sex, ethnicity (white, Hispanic, African-American, Asian), oral SCC location (tongue, floor of mouth, buccal mucosa, gingival, palate), tumor size (greatest dimension based on clinical exam) and evidence of metastasis (metastasis, no metastasis). A similar consultation room was used for all patients to complete the questionnaire. Once patients consented to participate in the study, the appropriate tumor and demographic data were collected. Standard instructions for completing the questionnaire were given verbally to the patient. Patients were told that the questionnaire consisted of eight questions. The visual analog scale was explained to the patient by showing that the far left portion of the scale represented no pain and the far right portion represented the highest pain level imaginable. Patients were instructed to place a vertical line along the scale to approximate their pain level. Investigators were not present in the room during completion of the questionnaire but were available to answer questions. Oral cancer patients are treated primarily with surgery, followed by radiotherapy with or without chemotherapy if indicated based on clinical and histopathologic criteria. The same patients were asked to complete the questionnaire after the cancer resection, once they had healed and once they did not require analgesics for pain associated with the surgery for at least one week. Patients completed the questionnaire during a postoperative visit in the same consultation room that they completed the questionnaire prior to surgery. If a patient required radiation therapy or chemotherapy he or she completed the questionnaire prior to beginning adjuvant therapy.
Reliability of the questionnaire was assessed by internal consistency. Internal consistency of the questionnaire was evaluated by Cronbach’s alpha value. Values greater than 0.7 were considered significant 1, 4, 13, 14. T-test was used to evaluate for statistical difference of the responses to the same question pre and post treatment. We considered p values < 0.05 statistically significant.
RESULTS
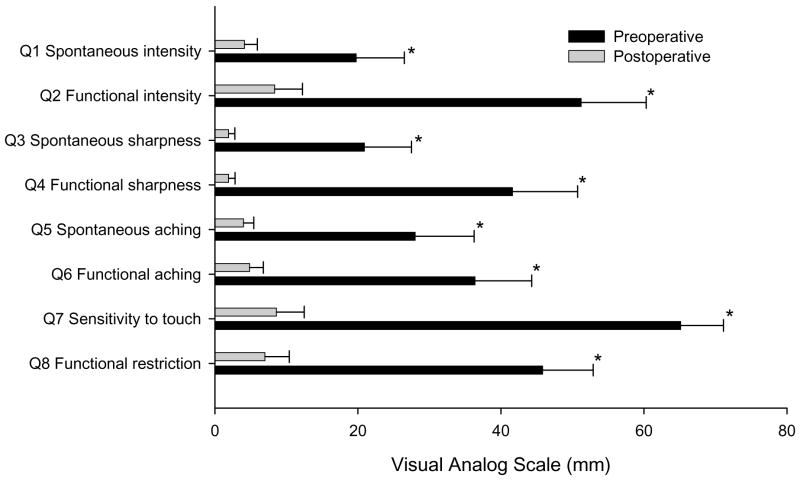
Sixteen patients met the inclusion criteria and were able to complete the questionnaire both before and after surgical resection of the cancer. Patient and tumor information are presented in Table 1. For questions 1, 3 and 5 that are designed to evaluate spontaneous sharpness, intensity and throbbing nature of pain, the Cronbach’s alpha was found to be 0.87. For questions 2, 4 and 6 that evaluate sharpness, intensity and throbbing pain during function, Cronbach’s alpha was 0.94. Statistically significant differences were found to the responses of all 8 questions prior to and following cancer resection with p values < 0.05 (Figure 1, Table 2).
Table 1.
Patients Demographics, Tumor Location and Staging
| Patient | Age (Y) | Race | Gender | Tumor Location | TNM Tumor Staging |
|---|---|---|---|---|---|
| Patient 1 | 50 | Caucasian | M | Tongue | T1N0M0 |
| Patient 2 | 68 | Caucasian | M | Tongue | T1N0M0 |
| Patient 3 | 52 | Caucasian | M | Tongue | T1N0M0 |
| Patient 4 | 70 | Caucasian | F | Tongue | T1N0M0 |
| Patient 5 | 45 | Caucasian | F | Tongue | T3N0M0 |
| Patient 6 | 55 | Caucasian | F | Tongue | T1N0M0 |
| Patient 7 | 71 | Caucasian | M | Tongue | T1N0M0 |
| Patient 8 | 90 | Caucasian | F | Gingiva | T4aN0M0 |
| Patient 9 | 39 | Caucasian | M | Palate | T2N0M0 |
| Patient 10 | 63 | Caucasian | F | Floor of mouth | T2N0M0 |
| Patient 11 | 40 | Caucasian | F | Tongue | T1N0M0 |
| Patient 12 | 84 | Asian | F | Palate | T2N2bM0 |
| Patient 13 | 57 | African American | M | Tongue | T1N0M0 |
| Patient 14 | 55 | Asian | M | Tongue | T1N1M0 |
| Patient 15 | 70 | Asian | M | Tongue | T1N0M0 |
| Patient 16 | 79 | Caucasian | F | Gingiva | T4aN0M0 |
Figure 1.
Mean visual analogue scale pain scores with standard error for each of the eight questions pre and post oral cancer resection. Significant differences for the mean preoperative and postoperative scores are indicated by *. P values for each set of questions are listed in Table 2.
Table 2.
Student’s t-test p-values for Comparison of Mean Preoperative and Postoperative Pain Scores.
| Preoperative-Postoperative Questions | p-values |
|---|---|
| Q1 | 0.0128 |
| Q2 | <0.0001 |
| Q3 | 0.0126 |
| Q4 | 0.0002 |
| Q5 | 0.032 |
| Q6 | 0.0006 |
| Q7 | <0.0001 |
| Q8 | <0.0001 |
DISCUSSION
In the current study we have demonstrated that the UCSF Oral Cancer Pain Questionnaire is a valid and reliable instrument for quantifying oral cancer pain. Validity evaluates the degree to which an instrument measures what it is designed to measure. Questionnaire validity is comprised of face, content, construct, and criterion validity. We proposed that if the Oral Cancer Pain Questionnaire is valid then the responses at two different time points should reflect anticipated differences based on surgical treatment. If valid, the Oral Cancer Pain Questionnaire should demonstrate:
Face Validity: the instrument should include items that subjects perceive relevant and important. For oral cancer patients relevant items are spontaneous or function related pain, quantity of pain and functional restriction as a result of pain 3.
Content Validity: the instrument should reflect the domains of interest. For the Oral Cancer Pain Questionnaire functional and spontaneous oral cancer pain and impact on daily activities are the domains of interest to patients and investigators 1, 4, 13, 14, 15.
Construct Validity: the instrument should be able to discriminate between people who would be expected to have different levels of pain. In addition, if the pain is due to oral cancer, then once the cancer is treated, pain should be eliminated or at least significantly diminished. We tested for construct validity based on the hypothesis that after surgical resection of the cancer the magnitude of patient responses would be significantly reduced. Of the four aspects of validity (face, content, construct and criterion), construct validity is the only aspect that can be tested statistically. We have demonstrated statistically significant construct validity in the current study.
Criterion Validity: the instrument should correlate well with a known gold standard. In cancer related pain there are multiple questionnaires, but for oral cancer pain there is no such gold standard. The University of Washington Quality of Life Questionnaire is the most commonly used questionnaire in the United States for head and neck cancer patients 10. This questionnaire consists of 14 questions but has only a single question that simply asks whether the patient is having pain. The European Organization for the Research and Treatment of Cancer (EORTC) Head and Neck Questionnaire is another commonly used questionnaire. This questionnaire consists of 35 questions with only three questions that specifically address head and neck pain. We wished to validate the Oral Cancer Pain Questionnaire specifically for measuring oral cancer pain, as well as the impact pain has on oral function. The other two questionnaires are not appropriate instruments for addressing the character of oral cancer pain or the impact on oral function.
Reliability of an instrument, which is the proportion of the observed variance attributable to the true score difference between subjects, is ideally assessed by internal consistency. If the instrument is reliable, related questions should correlate. With the UCSF Oral Cancer Pain Questionnaire the subgroups of questions focused on the intensity, sharpness and aching quality of spontaneous pain (Q1, Q3 and Q5) and function related pain (Q2, Q4 and Q6) should correlate. We evaluated these subgroups for internal consistency and found that questions within each subgroup correlated significantly with each other.
Our finding of significant reduction in pain following surgical resection of the cancer also provides insight into the etiology and potential neural mechanisms underlying oral cancer pain. While the etiology of cancer pain remains unknown, we and others hypothesize that nociceptive (pain-producing) mediators secreted by the cancer leads to sensitization of pain sensory fibers in the tumor microenvironment 17–19. For example, endothelin (ET-1), a vasoactive peptide that is produced by a number of different malignancies, sensitizes peripheral afferent sensory nerve fibers and contributes to both soft tissue and bone cancer pain, as well as pain due to oral squamous cell carcinoma 7, 16, 19, 20. We recently demonstrated that the site of action for ET-1 in producing cancer pain in a mouse model is in the periphery within the tumor microenvironment 19. The finding that a peripheral nociceptive mediator is responsible for cancer pain supports our current finding in humans that surgical resection leads to a significant reduction in pain since the cellular source of the mediator would be removed. In cases where the head and neck cancer is nonoperable, patients experience debilitating, intractable and progressive pain at the primary site of carcinoma. Terminal cancer pain is often refractory to narcotics or tolerance rapidly develops 5. For such patients regular assessment of their pain with an appropriate instrument should be performed at regular intervals and the analgesic regimen and dose adjusted to provide comfort.
Acknowledgments
NIH/NIDCR DE14609, WEORC-K12 DE014609-03, Tobacco-related disease research program grants 12KT-0166.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aggarwal VR, Lunt M, Zakrzewska JM, Macfarlane GJ, Macfarlane TV. Development and validation of the Manchester orofacial pain disability scale. Community Dentistry and Oral Epidemiology. 2005;33:141–149. doi: 10.1111/j.1600-0528.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 2.Bjordal K, Ahlner-Elmqvist M, Hammerlid E, Boysen M, Evensen JF, Biorklund A, Jannert M, Westin T, Kaasa S. A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope. 2001;111:1440–52. doi: 10.1097/00005537-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Bjordal K, Kaasa S. Psychological distress in head and neck cancer patients 7–11 years after curative treatment. Br J Cancer. 1995;71:592–7. doi: 10.1038/bjc.1995.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bland JM, Altman DG. Statistics notes: Cronbach’s alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasseur L. Review of current pharmacologic treatment of pain. Drugs. 1997;53:10–7. doi: 10.2165/00003495-199700532-00005. [DOI] [PubMed] [Google Scholar]
- 6.Connelly ST, Schmidt BL. Evaluation of pain in patients with oral squamous cell carcinoma. J Pain. 2004;5:505–10. doi: 10.1016/j.jpain.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9:2279–83. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- 8.Hammerlid E, Bjordal K, Ahlner-Elmqvist M, Boysen M, Evensen JF, Biorklund A, Jannert M, Kaasa S, Sullivan M, Westin T. A prospective study of quality of life in head and neck cancer patients. Part I: at diagnosis. Laryngoscope. 2001;111:669–80. doi: 10.1097/00005537-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Jacox A, Carr DB, Payne R. Management of cancer pain. Clinical practice guideline no. 9. (AHCPR no. 94–0592) Agency for Health Care Policy and Research; Rockville, MD: 1994. [Google Scholar]
- 10.Kanatas AN, Rogers SN. A national survey of health-related quality of life questionnaires in head and neck oncology. Ann R Coll Surg Engl. 2004;86:6–10. doi: 10.1308/003588404772614605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.List MA, Stracks J, Colangelo L, Butler P, Ganzenko N, Lundy D, Sullivan P, Haraf D, Kies M, Goodwin W, Vokes EE. How Do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol. 2000;18:877–84. doi: 10.1200/JCO.2000.18.4.877. [DOI] [PubMed] [Google Scholar]
- 12.Morton RP. Life-satisfaction in patients with head and neck cancer. Clin Otolaryngol. 1995;20:499–503. doi: 10.1111/j.1365-2273.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 13.Mystakidou K, Mendoza T, Tsilika E, Befon S, Parpa E, Bellos G, Vlahos L, Cleeland C. Greek Brief Pain Inventory: Validation and Utility in Cancer Pain. Oncology. 2001;60:35–42. doi: 10.1159/000055294. [DOI] [PubMed] [Google Scholar]
- 14.Mystakidou K, Parpa E, Tsilika E, Kalaidopoulou O, Georgaki S, Galanos A, Vlahos L. Greek McGill Pain Questionnaire: Validation and Utility in Cancer Patients. Journal of Pain and Symptom Management. 2002;24:379–387. doi: 10.1016/s0885-3924(02)00495-5. [DOI] [PubMed] [Google Scholar]
- 15.Cancer pain relief. World Health Organization; Geneva: 1986. [Google Scholar]
- 16.Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, Mantyh PW. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience. 2004;126:1043–52. doi: 10.1016/j.neuroscience.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Sabino MA, Ghilardi JR, Jongen JL, Keyser CP, Luger NM, Mach DB, Peters CM, Rogers SD, Schwei MJ, de Felipe C, Mantyh PW. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002;62:7343–9. [PubMed] [Google Scholar]
- 18.Sabino MA, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer. 2003;104:550–8. doi: 10.1002/ijc.10999. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, Jordan RC. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. 2007;11:406–14. doi: 10.1016/j.ejpain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Yuyama H, Koakutsu A, Fujiyasu N, Fujimori A, Sato S, Shibasaki K, Tanaka S, Sudoh K, Sasamata M, Miyata K. Inhibitory Effects of a Selective Endothelin-A Receptor Antagonist YM598 on Endothelin-1-induced Potentiation of Nociception in Formalin-induced and Prostate Cancer-induced Pain Models in Mice. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S479–82. doi: 10.1097/01.fjc.0000166309.63808.5f. [DOI] [PubMed] [Google Scholar]