Abstract
Two hundred and two primarily African American/Caribbean children (classified by maternal report and infant meconium as 38 heavier, 74 lighter and 89 not cocaine-exposed) were measured repeatedly from birth to age 8 years to assess whether there is an independent effect of prenatal cocaine exposure on physical growth patterns. Children with fetal alcohol syndrome identifiable at birth were excluded. At birth, cocaine and alcohol exposures were significantly and independently associated with lower weight, length and head circumference in cross-sectional multiple regression analyses. The relationship over time of pre-natal exposures to weight, height, and head circumference was then examined by multiple linear regression using mixed linear models including covariates: child’s gestational age, gender, ethnicity, age at assessment, current caregiver, birth mother’s use of alcohol, marijuana and tobacco during the pregnancy and pre-pregnancy weight (for child’s weight) and height (for child’s height and head circumference). The cocaine effects did not persist beyond infancy in piecewise linear mixed models, but a significant and independent negative effect of pre-natal alcohol exposure persisted for weight, height, and head circumference. Catch-up growth in cocaine-exposed infants occurred primarily by 6 months of age for all growth parameters, with some small fluctuations in growth rates in the preschool age range but no detectable differences between heavier versus unexposed nor lighter versus unexposed thereafter.
Keywords: Cocaine, Alcohol, Pregnancy, Growth, Children
I. Introduction
Although the negative association of in utero exposure to tobacco, alcohol, and cocaine with size at birth has been well-documented [4,23,33,41], the effects of these substances on post-natal growth are not as well understood. As reviewed by Nordstrom-Klee, it appears unlikely that growth deficits attributable purely to tobacco exposure persist beyond infancy [41]. The effects of pre-natal alcohol exposure on height, however, may persist well into childhood, at least in low-income populations [41]. The relationship between pre-natal cocaine exposure and later growth is less clear.
Establishing whether there is an independent effect of prenatal cocaine exposure on growth is complicated by the need to control for intrauterine exposure to other substances in a dose-related manner [24] so that the effect of heavy exposure to other substances is not misattributed to cocaine. The studies evaluating the impact of intrauterine cocaine exposure reviewed here include only those in the literature which have controlled for intrauterine exposures to other substances, including tobacco, marijuana, and alcohol. Most [13,25,46,47], but not all [32,38], of these studies also controlled for maternal anthropometric data. Findings of these previous studies are not consistent, and do not extend beyond age 7 years.
The sample with the longest growth follow-up to date assessed children at age 7 years and found a negative cross-sectional effect of cocaine exposure (defined dichotomously as exposed or unexposed based on any cocaine use during pregnancy) only on height [13]. In another sample of about 300 children [46], about 20% of whom had been cocaine-exposed in utero, there was no detectable independent effect of cocaine exposure (defined dichotomously) on weight or length at ages 1 and 3 years. In cross-sectional adjusted analyses, children who had had any cocaine exposure in utero in the first trimester, however, had significantly smaller head circumferences at age 3 years (but not age 1 year). Notably, in this particular sample there was no detectable independent effect of cocaine on any anthropometric measure at birth, a finding not consistent with most other studies. In a separate sample from the same investigator evaluating 28 lightly- to moderately-cocaine-exposed children at age 6.5 years, cocaine exposure (defined dichotomously) had no independent cross-sectional effect on weight, height or head circumference [47], but the limited statistical power of this study may have failed to detect more subtle effects of cocaine exposure on growth. Minnes et al. evaluated growth parameters of about 150 cocaine-exposed children versus controls cross-sectionally at age 6 years using the log of cocaine metabolites in meconium to provide an assessment of dose effects. After controlling for demographic factors and other exposures, higher levels of cocaine exposure were related to lower height at 6 years, though lower levels of cocaine exposure appeared to have minimal effect. Cocaine exposure had no effect on weight or head circumference at 6 years, but greater intrauterine cocaine exposure was related to lower weight-for-height at age 6 years [38]. Finally, in a sample of about 140 cocaine-exposed infants with growth reported in repeated cross-sectional analyses up to 24 months, although birth weights differed between exposed and unexposed infants, attained weight at age 12 months did not differ by dichotomous exposure status. In this cohort while cocaine-exposed infants were significantly shorter at birth, length did not differ at age 24 months by exposure in boys. Cocaine-exposed girls, however, continued to be slightly shorter than unexposed at age 24 months. Finally, although cocaine-exposed infants had smaller head circumferences at birth, at age 24 months their head circumferences were slightly larger than those of the unexposed group [32].
These discrepant findings with regard to small differences in attained growth are nevertheless scientifically important given previously reported relationships between post-natal growth and children’s developmental and behavioral outcomes. Cocaine exposure in utero has recently been reported to have an indirect effect on development at age 3 years through its effect on head circumference at birth [7]. Persistent slow post-natal growth in head circumference is thought to reflect diminished brain growth [5,19]. In samples not selected to study intrauterine exposures, decreased post-natal somatic and head circumference growth have also been linked to a variety of adverse developmental [36], behavioral [45], and health outcomes [49]. Thus, consistent findings of depressed post-natal growth following intrauterine cocaine exposure would heighten concern about developmental and behavioral outcomes, although finding normal growth would not automatically obviate such concerns.
‘Catch-up growth’, first described more than 40 years ago, describes the rapid growth exceeding the normal rate that occurs after a period of growth inhibition [44]. Although the phenomenon of catch-up growth has now been well-described, its underlying mechanism remains poorly understood [9]. The timing of catch-up growth from neonatal deficits may have prognostic importance both for eventual intellectual functioning [10] and future growth potential [29]. Prior studies have demonstrated that small for gestational age, very low birth weight infants who have had catch-up growth in head circumference by 12 months of age show no difference in developmental outcomes compared to appropriate for gestational age infants [20]. The data regarding the effect of the timing of catch-up growth in very low birth weight, small for gestational age infants on future growth potential in other growth parameters are conflicting. Some studies have found no “critical period” for the timing of catch-up growth in length that can predict adult height in formerly very low birth weight infants [11], while others have found that catch-up growth in weight, length, and body mass index occurs by age 8 years and persists to age 20 years in girls, but not boys (who remain smaller than controls to age 20 years) [22]. Others have found that most healthy small for gestational age infants achieve catch-up growth by 6–12 months of age, and that of the few who do not, half have persistent short stature [28]. In short, prior work on the timing of catch-up growth and its relationship to future growth potential yields inconsistent findings. These studies are drawn from samples not directly comparable to children who have had cocaine exposure in utero.
A single study assessed the timing of catch-up growth following cocaine exposure in utero by evaluating growth-rates longitudinally. In this study, which focused only on the first 2 years of life, pre-natal cocaine exposure was found to be associated with faster post-natal weight gain up to 13 months of age (at which point cocaine-exposed infants were heavier than unexposed) but not with similar acceleration in gains in length or head circumference [25].
The first goal of the current analysis, therefore, was to assess whether there is an independent effect of level of pre-natal cocaine exposure on post-natal attained growth and growth patterns in weight, length/height, weight-for-length/BMI and head circumference in a sample followed up to approximately age 8 years, while controlling not only for exposure to other substances in a dose-related manner but for other potential confounds. Determining the timing of catch-up growth for each growth parameter, if it occurs, was of particular interest. The second goal of this analysis was to determine which variables, individually or in interaction with intrauterine cocaine exposure, may moderate such an association if it exists.
2. Methods
2.1. Sample selection criteria
The Institutional Review Board of the Boston University School of Medicine and Boston Medical Center (previously called Boston City Hospital) approved this study. Soon after delivery, all birth mothers gave informed consent to study participation, including the risk of incidental detection and reporting of child maltreatment during study assessments. If the child changed caregivers, similar informed consent was obtained for each new caregiver. A writ of confidentiality was obtained from the federal government to protect participants from having research data subpoenaed.
The sample was recruited by trained interviewer/recruiters who screened maternity and nursery records 7 days a week on the post-partum floor of Boston Medical Center from October 1990 to March 1993. Unexposed dyads comparable to cocaine-exposed mother–infant dyads in ethnicity (African American/Caribbean versus other) were preferentially approached for recruitment soon after delivery. All mother–infant dyads met the following criteria based on review of mother and infant medical records and confirmed by interviews, biological markers, and infant physical examinations obtained by study personnel: 1) Infant gestational age greater than or equal to 36 weeks; 2) No requirement for neonatal intensive care; 3) No obvious major congenital malformations; 4) No diagnosis of fetal alcohol syndrome in the neonatal record; 5) No history of human immunodeficiency virus seropositivity noted in the mother’s or infant’s medical record; 6) Mother’s ability to communicate fluently in English; 7) No indication by neonatal or maternal urine toxicology screen or history in medical record of mother’s use during pregnancy of illegal opiates, methadone, amphetamines, phencyclidine, barbiturates, or hallucinogens; and 8) Mother aged 18 years or older. These criteria were established to exclude infants with known major risk factors that might confound or obscure the effects, if any, of in utero cocaine exposure. Given that fetal alcohol syndrome is difficult to accurately detect in the newborn period, the subjects were monitored for the subsequent emergence of signs and symptoms of fetal alcohol syndrome. No children in the cohort have subsequently met criteria for the diagnosis.
Of the 192 known cocaine-using mothers invited to participate, 123 (64%) agreed to join the study. Of the 646 mothers who were not clinically known cocaine users, 134 (21%) agreed to join the study. Additional details about sample characteristics and recruitment are reported elsewhere [17,50].
2.2. Method of exposure classification
Mothers participating in the study were identified as either heavier, lighter, or non-users of cocaine by interview and by biological markers obtained by clinicians and study personnel. At intake on the post-partum floor, research assistants using the Addiction Severity Index [35] supplemented by study-specific questions interviewed the mothers about pregnancy and lifetime use of cigarettes, alcohol, and illicit drugs.
During the period of study recruitment at Boston Medical Center, urine testing for metabolites of illicit drugs was performed for clinical indications at the discretion of health care personnel, but was not universal. Clinical indications at the time included no pre-natal care, a jittery infant, unusual maternal behavior, or a known history of substance use. Results of the urine drug Enzyme Multiplied Immunoassay Technique (EMIT) assays obtained for clinical purposes during pre-natal care or labor and delivery from the mother or from the newborn after birth were recorded for the present study (when available in the medical record). Exposed newborns were targeted for recruitment on the basis of maternal self-report or positive clinical urine assays obtained from either mother or newborn. However, provisionally unexposed newborns were drawn from the nursery population as a whole, most of whom did not have maternal urine assays performed for drug metabolites for clinical purposes. Therefore, after recruitment and informed consent, additional urine samples were collected from all study mothers for analysis for benzoylecognine, opiates, amphetamines, benzodiazepines, and cannabinoids by radioimmunoassay using commercial kits (Abuscreen RIA, Roche Diagnostics Systems, Inc, Montclair, NJ). Meconium specimens were also sought from all enrolled newborns to be analyzed by radioimmunoassay for benzoylecognine (a cocaine metabolite), opiates, amphetamines, benzodiazepines, and cannabinoids. The radioimmunoassay used was a modification of the method of Ostrea et al. published in detail elsewhere [42,43].
All mother–infant dyads provided at least one biological marker, either urine from mother or infant or meconium that confirmed their exposure or lack of exposure to cocaine during pregnancy. To be classified in the “unexposed” group, mothers had to deny use of cocaine on interview and all available biological markers needed to be negative for cocaine use. Of the 257 participants who consented to enrollment in the study, 5 were excluded because their unexposed status could not be confirmed by biological assay. Of the remaining 252 dyads, 128 were provisionally considered unexposed, and 124 were considered provisionally exposed. Based on the biological assays, 15 unexposed dyads were reclassified as exposed, yielding a study sample of 139 exposed and 113 unexposed dyads. Of the 139 exposed, 124 had self-reported cocaine use, while 15 had denied cocaine use on self-report, but had at least one positive biological assay. Of the 139 exposed dyads, 49 were positive by meconium alone, 21 were positive by urine alone, and 69 were positive by both urine and meconium.
In this sample, the mean days of self-reported cocaine use during pregnancy was 20.6 days, with a range from 0 to 264. The mean meconium concentration of benzoylecognine/g was 1143 ng with a range from 0 ng to 17950 ng/g. Before data were analyzed, a composite measure of “heavier” use was a priori defined as the top quartile of meconium concentration for cocaine metabolites (>3314 ng of benzoylecognine/g meconium) and/or top quartile days of self-reported use (>61 days) during the entire pregnancy. All other use was classified as “lighter” [35]. This ordered classification scheme is comparable to that used by other investigators, where use of cocaine more than twice a week during pregnancy is considered “heavier” use [25].
Pragmatic as well as scientific considerations influenced this definition of exposure level. Because women are more likely to underreport rather than over report illicit substance use during pregnancy [42,53], women reporting days of use in the top quartile were considered heavier users a priori, even if the benzoylecognine levels in meconium were not in the top quartile. Not all infants exposed to cocaine in utero have positive meconium assays [31]. Moreover, meconium samples could not be obtained from 14% of study infants, whose exposure status was confirmed by maternal or infant urine assay. Therefore, whichever indicator (self-report or meconium assay) demonstrated higher exposure was used to define exposure category.
2.3. Outcome variables
At birth, a study pediatrician masked to exposure history measured recumbent length on a Holtain Infantometer (Holtain Ltd, Crymych Pembs, United Kingdom) and head circumference with a plastic-coated tape. The pediatrician also reviewed the infant’s medical record for birth weight (measured after delivery by nursery nurses using a Detecto scale [Jericho, NY]). Children’s weight, length or height, and head circumference were thereafter measured either during pediatric visits by clinic staff or, in most cases, during visits to the laboratory for developmental testing (scheduled at 6, 12, 24, 48, 72, and 96 months) by trained research assistants, masked to exposure history. Due to uneven compliance with schedules of pediatric or research visits in this study population, there was variability in the age at which these assessments were actually completed and the number of observations available at each age (Table 1). Z-scores for weight, length, head circumference and weight-for-length (to 24 months) or body mass index (BMI; from 24 months until the last available measurement), were calculated based on the child’s actual age on the date of measurement using National Center for Health Statistics growth charts [30]. Z-scores are expressed here in standard deviation units (SDU). One child was excluded from the analysis because of diagnosed growth hormone deficiency. Overweight and underweight were also evaluated as dichotomous categories for subjects with measurements obtained at the 96 month age window (n=128). Overweight was defined as a BMI greater than or equal to the 95th percentile, and underweight was defined as a BMI less than the 10th percentile based on age- and gender-based norms from the National Center for Health Statistics [30].
Table 1.
Scheduled assessments and actual age of child in age window
| Scheduled assessment age (months) | Actual age (months) | Number of observations | Number of unique subjects |
|---|---|---|---|
| Birth | Birth | 202 | 202 |
| 6 | 4–9 | 166 | 166 |
| 12 | 10–18 | 160 | 137 |
| 24 | 19–34 | 219 | 157 |
| 48 | 39–59 | 163 | 163 |
| 72 | 61–84 | 154 | 148 |
| 96 | 84–114 | 128 | 125 |
2.4. Candidate control variables
Within 8 to 72 h of birth (mean 48 h), a study pediatrician, trained to reliability and unaware of the infants’ cocaine exposure, assessed gestational age according to the method of Dubowitz et al. [16]. Pre-pregnancy weight was obtained by maternal report at birth. Birth mother’s height was obtained by self-report during post-partum interviews. Maternal alcohol and tobacco use were documented post-partum at enrollment in the study by trained interviewers using the Addiction Severity Index [35]. Birth mothers were asked for their estimate of the height of the baby’s father, but inadequate data were available for inclusion in the analysis.
Several covariates were tested in the models as potential confounders of the relationship between cocaine and growth, but not as independent predictors of growth. The manner of inclusion of these covariates is detailed below in the statistical analysis section. Lead levels and hemoglobin concentrations measured between 6 and 36 months of age were abstracted from the clinical record. Lead levels were obtained at a mean age of 33.2 months and hemoglobin levels at a mean age of 35.1 months. Per Centers for Disease Control guidelines, an elevated blood lead level was defined as a lead level >10 μg/dL [2] and anemia was defined as a hemoglobin <11.0 g/dL at any time point [1]. Identity of the current guardian was recorded at each research or clinical visit and caregiver was categorized as “birth mother”, “kinship care” or “unrelated foster care”. Although there are few and conflicting data regarding the effect of kinship care on child outcomes in prior literature [8,12], we used these categorizations as a proxy for the quality of the caregiving environment in the present study since in the cohort evaluated in this report, unrelated foster care was associated with more optimal and kinship care with less optimal developmental test scores among children in the first 2 years of life compared to children with or without intrauterine cocaine exposure living with impoverished birth mothers [18].
2.5. Statistical analysis
Univariate statistics were computed to describe the sample. Bivariate analyses were performed examining the relationship of potential confounding variables with the three previously described pre-natal cocaine exposure categories (unexposed, lighter, heavier).
2.5.1. Cross-sectional analyses
Cross-sectional bivariate analyses were performed to examine the relationship between three-level cocaine exposure and anthropometric z-scores (weight-for-age, height-for-age, weight-for-length or BMI, and head circumference-for-age) via one way analysis-of-variance (ANOVA) at each age. The association between cocaine exposure and children’s status as overweight or underweight was evaluated at the last time point measured for children with measurements in the 96-month age window via chi-square analysis. In addition, in order to provide context for our longitudinal analyses as well to enable comparisons with previously published findings, a cross-sectional multiple linear regression analysis was performed at birth to examine the relationship of cocaine exposure to the anthropometric z-scores adjusting in all analyses for the child’s gender, ethnicity, age at assessment, gestational age (GA), mother’s alcohol, marijuana and tobacco use during pregnancy, as well as for mother’s pre-pregnancy weight (for child’s birth weight) and maternal height (for child’s length and head circumference). Maternal height was used as a covariate for the child’s head circumference because an individual’s height and head circumference are correlated in adults and children [3,34], and data regarding maternal head circumference were not available. These covariates were selected a priori based on the literature.
2.5.2. Longitudinal analyses
Next, multivariate models were constructed to estimate the effect of pre-natal cocaine exposure on longitudinal growth adjusting for potential confounding variables in piecewise linear mixed models [15], assuming an unstructured working correlation structure for all growth parameters except for head circumference, for which a structure of compound symmetry was used. These working correlations structures were selected based on comparisons of Akaike’s Information Criterion (AIC) of models with the same covariates but different correlation structures. The mixed linear model, an accepted method of conducting comparison of groups, was selected. In these piecewise models, separate slopes were estimated for the differences in anthropometric measures between each age window described in Table 1: ages birth through 6 months; 6 months through 12 months; 12 months through 24 months; 24 months through 48 months; 48 months through 72 months; and 72 months through 96 months. Models for head circumference-for-age z-score employed separate slope parameters for these intervals up to the 24 month of age study window, by which time the period of most rapid head growth has ended [40]. Adjusted regression coefficients and their standard errors were estimated from these linear mixed models. As in the cross-sectional analyses, a base set of potential confounding variables was determined based on the literature. In these models, the effects of pre-natal cocaine exposure (heavier vs. unexposed; lighter vs. unexposed), age interval, and the following fixed covariates, selected a priori and described above, were examined: birth mother’s ethnicity, the natural logarithm of pre-natal alcohol average daily volume; the natural logarithm of pre-natal average daily cigarettes; pre-natal marijuana use (as a dichotomous variable of “yes” or “no”); gender and (for observations taken at age 24 months or younger) Dubowitz gestational age. Since marijuana is stored for long periods in body fat, marijuana metabolite concentration in meconium is considered a less valid indication of total dose than is that of cocaine metabolites [42]. We therefore could not reliably construct an index of marijuana dose, since meconium concentration is not entirely valid and a third of the marijuana users identified solely on the basis of meconium or urine assay denied use on interview in this cohort. In contrast, alcohol use and cigarette smoking were based on self-report only, and were therefore indexed as continuous variables. The natural log of pre-natal alcohol average daily volume and the natural log of pre-natal average daily cigarettes were used to stabilize the variances of the measures of daily use of these substances, given that their distributions were skewed. Maternal pre-pregnancy weight was controlled as a fixed covariate in analyses for weight-for-age and weight-for-length/BMI-for-age z-scores [52] and maternal height for length/height-for-age [21] and head circumference-for-age z-scores [51]. Several additional covariates (elevated lead levels (dichotomous), anemia (dichotomous), and identity of the current guardian (“birth mother”, “kinship care” or “unrelated foster care”) were tested and included in the models only if they substantially, i.e., by 10% or more, altered the observed associations of pre-natal cocaine exposure with anthropometric trajectories (according to the technique of Mickey and Greenland [37]). Interaction effects of pre-natal cocaine exposure with each pre-natal substance use variable and child’s age were further examined. Differences in the effect of other substances on growth by age were not evaluated due to sample size considerations and only the main effects of tobacco, marijuana, and alcohol on growth were examined in the models. Whether the caregiver was the birth mother, a kinship caregiver, or an unrelated foster parent was entered as a time-dependent covariate for each observation.
In addition, based on mixed linear models described below, adjusted mean z-scores between the cocaine exposure groups (heavier vs. unexposed; lighter vs. unexposed; heavier vs. lighter) at 48 months and 96 months of age were estimated and differences between pairs were statistically tested for all growth parameters except head circumference-for-age, which was analyzed in this fashion at 24 months of age. In addition, we performed diagnostic checks for our models by examining the standardized residuals, predicted values, Cook’s distances, and PRESS residuals for the model. We found no outliers or influence points based on these measures for which exclusion from our analyses were warranted.
The linear mixed models analyses were performed using PROC MIXED in SAS version 9.1(Cary, NC., 2003). In all analyses, two-tailed p-values are reported.
3. Results
3.1. Sample description
Two hundred two children were included (90 unexposed, 74 lighter, and 38 heavier), each of whom had at least one post-neonatal measurement. Seventy-six percent of mothers were African American. Fifty three percent of the infants were male. Maternal pre-pregnancy weight was available for 98% of the sample, and maternal heights for 80% of the sample (because the latter was assessed in post-natal interview, measurements for children not in birth mother’s care were not available). Analyses adjusting for maternal height were limited to subjects with non-missing maternal heights, though it should be noted that the proportion of missing heights differed by cocaine exposure group (14% missing in unexposed, 20% missing in lightly exposed, and 34% missing in heavily exposed, p=.04).
3.1.1. Attrition
Comparing the children who provided data for the longitudinal analyses to those who provided data at baseline (birth) only, there was no statistically significant difference in mean values for birth weight, (p=.73); birth length, (p=.88) or birth head circumference, (p=.44). To rule out later differential attrition of subjects by growth pattern, the analyses were repeated removing children who supplied data only up to 30 months of age. For anthropometric data collected up to 30 months of age, there was no significant difference in the growth patterns over time by pre-natal cocaine exposure group between children who were not examined past 30 months of age and those who were (data available from the authors upon request). Moreover, in multivariate analyses limited to children who were seen past 30 months of age, results with respect to differences in growth between pre-natal cocaine exposure groups were consistent with those observed in the full sample. There is thus no evidence of substantial selection bias in these growth data with respect to attrition after birth or before 30 months of age.
There were differences in the sample sizes used in models that employed maternal height as a covariate (height-for-age z-score, head circumference-for-age z-score) compared to those that used maternal pre-pregnancy weight (weight-for-age z-score, weight-for-length/BMI z-score) because of missing data on maternal heights for 41 mothers. The potential impact of this missingness was examined by imputing missing maternal height data based on a linear regression of maternal height on maternal pre-pregnancy weight and cocaine exposure group, because of concerns that cocaine-using mothers were lighter than non-using mothers (see below) and might have a different relationship of weight and height. The inclusion of the imputed maternal heights did not alter the results of the analyses that we present here.
3.1.2. Description of covariates by cocaine exposure status
Table 2 shows bivariate analyses of potential confounding variables by pre-natal cocaine exposure status. Statistically significant differences were found in the distributions of a number of these variables across the three cocaine exposure groups. For example, children in our sample in each of the prenatal cocaine-exposed groups were more likely to have mothers who described themselves as African-American than those who were unexposed. Mothers in the heavier and lighter cocaine exposure groups each had a significantly lower pre-pregnancy weight than the unexposed. Gestational age of the infants in each of the cocaine-exposed groups was slightly lower than that of the unexposed. Mothers in each of the cocaine-exposed groups were more likely than the unexposed to use marijuana, to drink more alcohol, and to smoke more cigarettes. Children of mothers in each of the cocaine-exposed groups were more likely than those unexposed to have been in kinship care or unrelated foster care at some point during childhood. No significant differences were found between the three cocaine exposure groups in gender, the frequency of elevated blood lead levels or anemia or in the self-reported heights of their mothers.
Table 2.
Subject characteristics by pre-natal cocaine exposure group (N=202)
| Unexposed (N=90) | Lighter (N=74) | Heavier (N=38) | P-value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal Ethnicity, n (%) | ||||
| White, Hispanic, Other | 8 (9%) | 14 (19%) | 3 (8%) | <.0001 |
| African Caribbean | 21 (23) | 1 (1) | 1 (3) | |
| African American | 61 (68) | 59 (80) | 34 (89) | |
| Pre-pregnancy weight (kg) (Mean (SD)) | 69.9 (18.0) | 63.4 (14.1) | 58.1 (10.4) | .0003 |
| Maternal height (cm) (Mean (SD))a | 162.5 (8.8) | 164.0 (7.1) | 165.5 (8.2) | .24 |
| Pre-natal marijuana use vs. non-use, n (%) | 8 (9) | 26 (35) | 14 (37) | <.0001 |
| Pre-natal average daily volume of alcohol (ounces)(Mean (SD)) | 0.002 (0.01) | 0.16 (0.53) | 0.37 (0.86) | <.0001 |
| Pre-natal average daily cigarettes (Mean (SD)) | 0.64 (1.77) | 2.97 (2.15) | 5.95 (1.94) | <.0001 |
| Child characteristics | ||||
| Dubowitz gestational age (weeks) (Mean (SD)) | 40.3 (1.2) | 39.8 (1.2) | 39.9 (1.2) | .05 |
| Male gender, n (%) | 51 (57) | 37 (51) | 19 (50) | .71 |
| Blood lead level between age 6 and 36 months ever > 10 mg/dL (%)b | 27 (30) | 19 (25) | 9 (23) | .78 |
| Hemoglobin between age 6 and 36 months ever <11 mg/dL (%)b | 30 (33) | 27 (36) | 14 (36) | .93 |
| Any kinship or unrelated foster care (time-dependent), n (%) | ||||
| Always in birth mother care | 85 (94) | 44 (59) | 18 (47) | <.0001 |
| Any kinship care | 3 (3) | 16 (22) | 11 (29) | |
| Any unrelated foster care | 2 (2) | 14 (19) | 9 (24) |
N=161.
N=140.
In post-hoc analyses comparing the two cocaine-exposed groups, no significant differences by level of exposure status (lighter versus heavier) were detected for ethnicity, maternal pre-pregnancy weight, marijuana or alcohol use, child’s gestational age, or frequency of placement in kinship care or unrelated foster care. Mothers who were heavier users of cocaine reported more cigarette use during pregnancy than mothers who were lighter users of cocaine.
3.2. Cross-sectional analyses
3.2.1. Unadjusted anthropometrics by cocaine exposure status at birth
Table 3 shows the unadjusted mean z-scores for each anthropometric parameter in cross-sectional analyses at each protocol age. At birth, unadjusted mean weight-for-age and length-for-age z-scores were lower among children in each of the cocaine exposure groups compared to the unexposed. At birth, unadjusted mean HC-for-age was lower in the heavier exposure group compared to the unexposed. In post-hoc comparisons, z-scores between the two cocaine-exposed groups (lighter versus heavier) at birth differed significantly only for length-for-age (p=.04), with infants in the heavier exposure group having significantly shorter lengths than infants in the light exposure group. Post-hoc comparisons (lighter versus heavier) did not reveal any statistically significant differences for any other birth anthropometric measure.
Table 3.
Unadjusted means and standard deviations of anthropometric z-scores with sample sizes by pre-natal cocaine exposure group and post-natal epoch of measurement (N=202) based on NCHS reference curves
| Unexposed |
Lighter |
Heavier |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | |
| Weight-for-age z-score | ||||||
| Birtha | −0.19 (0.92) | 90 | −0.75 (0.74)** | 74 | −0.91 (0.80)** | 38 |
| 6 months (3–9 m) | 0.21 (1.03) | 73 | 0.02 (0.97) | 62 | −0.01 (1.06) | 31 |
| 12 months (10–18 m) | −0.22 (0.97) | 67 | −0.31 (0.85) | 65 | −0.20 (1.04) | 28 |
| 24 months (19–36 m) | 0.06 (1.06) | 104 | −0.05 (1.02) | 74 | 0.07 (0.84) | 40 |
| 48 months (37–60 m) | 0.59 (1.05) | 78 | 0.23 (0.96) | 60 | 0.59 (0.88) | 25 |
| 72 months (61–84 m) | 0.90 (1.13) | 75 | 0.49 (1.02) | 54 | 0.78 (1.20) | 25 |
| 96 months (85 m or older)c | 1.04 (1.11) | 61 | 0.53 (0.95)** | 45 | 0.52 (1.17)** | 22 |
| Height-for-age z-score | ||||||
| Birtha | −0.11 (0.81) | 90 | −0.49 (0.68)** | 68 | −0.83 (0.79)** | 33 |
| 6 months (3–9 m) | 0.12 (0.92) | 73 | −0.18 (0.83) | 61 | 0.02 (0.88) | 31 |
| 12 months (10–18 m) | 0.18 (0.93) | 67 | −0.07 (0.93) | 65 | −0.14 (0.81) | 28 |
| 24 months (19–36 m) | 0.08 (1.01) | 104 | 0.12 (0.82) | 74 | 0.13 (0.77) | 41 |
| 48 months (37–60 m) | 0.51 (0.95) | 78 | 0.27 (0.92) | 60 | 0.31 (0.73) | 25 |
| 72 months (61–84 m) | 0.55 (1.01) | 75 | 0.29 (1.00) | 54 | 0.34 (0.81) | 25 |
| 96 months (85 m or older) | 0.45 (1.06) | 61 | 0.17 (0.94)* | 45 | −0.01 (0.94)* | 22 |
| Weight-for-height-for age z-score at age <24 months, BMI-for-age z-score at age > 24 months | ||||||
| Birth | 0.28 (0.89) | 89 | 0.20 (0.72) | 68 | 0.50 (0.61) | 33 |
| 6 months (3–9 m) | 0.51 (1.56) | 72 | 0.59 (1.62) | 62 | 0.15 (1.15) | 31 |
| 12 months (10–18 m) | 0.13 (0.94) | 67 | 0.20 (0.85) | 65 | 0.37 (1.19) | 28 |
| 24 months (19–36 m) | 0.30 (1.27) | 104 | 0.12 (1.07) | 74 | 0.47 (1.56) | 41 |
| 48 months (37–60 m) | 0.43 (1.01) | 78 | 0.08 (1.18) | 60 | 0.55 (1.24) | 25 |
| 72 months (61–84 m) | 0.82 (1.14) | 75 | 0.46 (1.05) | 54 | 0.77 (1.24) | 25 |
| 96 months (85 m or older) | 1.01 (1.16) | 61 | 0.60 (0.90)* | 45 | 0.51 (1.65)* | 22 |
| Head circumference-for-age z-score | ||||||
| Birthb | −0.42 (0.68) | 90 | −0.68 (0.76) | 73 | −0.89 (0.71)** | 38 |
| 6 months (3–9 m) | 0.34 (0.89) | 69 | 0.42 (0.92) | 60 | 0.20 (0.93) | 30 |
| 12 months (10–18 m) | 0.60 (0.90) | 65 | 0.61 (1.01) | 60 | 0.56 (1.11) | 27 |
| 24 months (19–36 m) | 0.51 (1.03) | 95 | 0.49 (0.92) | 70 | 0.72 (0.91) | 40 |
p<.05 vs. Unexposed by Tukey’s test.
p<.01 vs. Unexposed by Tukey’s test.
p<.0001 for overall ANOVA.
p<.05 for overall ANOVA.
p<.01 for overall ANOVA.
3.2.2. Adjusted anthropometrics by cocaine exposure status at birth
In multiple linear regression analyses at birth on the sample of children with post-natal follow-up, children with pre-natal cocaine exposure had lower mean z-scores for birth weight (heavier vs. unexposed by 0.33 standard deviation units (SDU), p=.06; lighter vs. unexposed by 0.39 SDU, p=.005; but, heavier vs. lighter higher on average by 0.06 SDU, p=.71), length (heavier vs. unexposed by 0.57 SDU, p=.003; lighter vs. unexposed by 0.32 SDU, p=.03; and heavier vs. lighter lower on average by 0.25 SDU, p=.17), and head circumference (heavier vs. unexposed by 0.31 SDU, p=.07; lighter vs. unexposed by 0.28 SDU, p=.03; and heavier vs. lighter lower on average by 0.03 SDU, p=.85) than children who were unexposed after adjusting for confounding variables.
3.2.3. Independent effect of other exposures on anthropometrics at birth
In the adjusted cross-sectional linear regression analyses at birth, children with pre-natal alcohol exposure had lower mean z-scores for birth weight (by 0.21 SDU per drink per day, p=.04), length (by 0.33 SDU per drink per day, p=.03) and HC (by 0.23 SDU per drink per day, p=.05) than children who were unexposed to alcohol after adjusting for confounding variables. No independent effect of marijuana or tobacco use on anthropometric parameters at birth was detected (data not shown).
3.2.4. Unadjusted anthropometrics by cocaine exposure status in the post-neonatal period
Table 3 shows that after birth, children with in utero cocaine exposure differed significantly in unadjusted cross-sectional analyses from the unexposed at only a single time point. At age 96 months, children in each of the cocaine exposure groups had significantly lower weight-for-age, height-for-age, and BMI-for-age compared to the unexposed group. There were no significant differences in any of these parameters at 96 months between the heavier and lighter exposure groups. There were no significant differences in any other anthropometric parameters at any other post-neonatal time point in these unadjusted analyses.
3.2.5. Adjusted anthropometrics by cocaine exposure status in the post-neonatal period
Table 4 shows, using mixed linear models, contrasts between pairs of adjusted mean z-scores of the cocaine exposure groups (i.e., heavier vs. unexposed, lighter vs. unexposed, heavier vs. lighter) at 48 months and 96 months of age for all parameters except for head circumference-for-age, which was analyzed in this fashion at 24 months of age. No statistically significant differences were detected between the adjusted mean z-scores at these specified ages except for head circumference at 24 months of age, where there was a significant difference between the adjusted mean z-scores of the heavier vs. the lighter cocaine-exposed groups such that the infants with heavier cocaine exposure had larger head circumferences than those with lighter cocaine exposure (p=.01).
Table 4.
Adjusted meansa (standard errors) from linear mixed models analysesb of anthropometric z-scores by pre-natal cocaine exposure group and post-natal time of measurement in months
| Unexposed N=90 | Lighter M=74 | Heavier N=38 | |
|---|---|---|---|
| Weight-for-age z-score | |||
| Birth | −0.25 (0.10) | −0.70 (0.10) | −0.65 (0.15) |
| 6* | 0.15 (0.12) | 0.11 (0.13) | 0.30 (0.18) |
| 12 | −0.28 (0.13) | −0.15 (0.14) | −0.03 (0.20) |
| 24 | −0.07 (0.12) | −0.09 (0.13) | 0.19 (0.20) |
| 48* | 0.45 (0.12) | 0.27 (0.13) | 0.40 (0.18) |
| 72 | 0.74 (0.13) | 0.60 (0.14) | 0.63 (0.19) |
| 96 | 0.94 (0.13) | 0.67 (0.15) | 0.71 (0.20) |
| Height-for-age z-score | |||
| Birth | 0.05 (0.11) | −0.41 (0.13) | −0.79 (0.19) |
| 6**** | 0.15 (0.12) | −0.04 (0.13) | 0.23 (0.20) |
| 12 | 0.31 (0.13) | −0.003 (0.14) | 0.15 (0.21) |
| 24*** | −0.07 (0.11) | 0.09 (0.13) | 0.47 (0.20) |
| 48** | 0.27 (0.12) | 0.11 (0.13) | 0.30 (0.21) |
| 72 | 0.36 (0.12) | 0.21 (0.14) | 0.25 (0.22) |
| 96 | 0.25 (0.12) | −0.07 (0.14) | 0.13 (0.22) |
| Weight-for-length/BMI z-score | |||
| Birth | 0.30 (0.09) | 0.20 (0.10) | 0.42 (0.13) |
| 6 | 0.50 (0.14) | 0.40 (0.14) | 0.62 (0.16) |
| 12 | 0.32 (0.14) | 0.23 (0.14) | 0.45 (0.16) |
| 24 | 0.21 (0.12) | 0.12 (0.12) | 0.34 (0.14) |
| 48 | 0.28 (0.13) | 0.19 (0.13) | 0.41 (0.16) |
| 72 | 0.74 (0.13) | 0.65 (0.13) | 0.86 (0.16) |
| 96 | 0.91 (0.13) | 0.81 (0.13) | 1.03 (0.16) |
| Head circumference-for-age z-score | |||
| Birth | −0.44 (0.08) | −0.69 (0.10) | −0.78 (0.18) |
| 6* | 0.34 (0.12) | 0.36 (0.13) | 0.54 (0.22) |
| 12 | 0.51 (0.12) | 0.66 (0.14) | 0.69 (0.25) |
| 24* | 0.67 (0.13) | 0.53 (0.15) | 1.14 (0.24) |
Change from adjusted mean at prior time point by pre-natal cocaine exposure group, p<.05.
Change from adjusted mean at prior time point by pre-natal cocaine exposure group, p<.01.
Change from adjusted mean at prior time point by pre-natal cocaine exposure group, p<.001.
Change from adjusted mean at prior time point by pre-natal cocaine exposure group, p<.0001.
Adjusted for child’s gender, ethnicity, age at assessment, gestational age (GA), mother’s pre-pregnancy weight (for child’s weight), maternal height (for child’s height and HC), and mother’s alcohol, marijuana and tobacco use during pregnancy; interaction terms for age-by-cocaine exposure included in all models except for weight-for-height.
1169 observations on 198 children for weight; 971 observations on 161 children for height (sample size reduced by missing data on maternal height); 1157 observations on 198 children for weight-for-height; 584 observations on 161 children for head circumference (sample limited to data up to 3 years of age).
3.2.6. Unadjusted prevalence of overweight and underweight at 96 months by cocaine exposure
Children who were not exposed to cocaine were more likely to be overweight (BMI greater than or equal to the 95th percentile) at 96 months than either children who were lightly cocaine-exposed or heavily-exposed (31% overweight in the unexposed group, 9% overweight in the lighter group, and 23% overweight in the heavier group; overall, p=.02; heavier vs. unexposed, p=.46; lighter vs. unexposed, p=.006; heavier vs. lighter, p=.12). The small number of overweight (N=28) children in the whole sample permitted only a bivariate, unadjusted assessment of the association of pre-natal cocaine exposure to childhood overweight. The small number of underweight (N=3) children in the whole sample did not permit analyses of the association of being underweight with cocaine exposure status.
3.3. Longitudinal analyses
3.3.1. Patterns in anthropometric growth by cocaine exposure
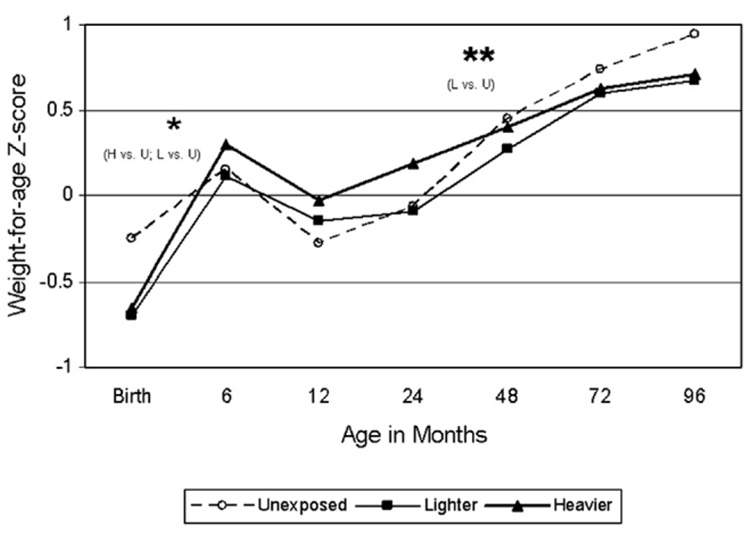
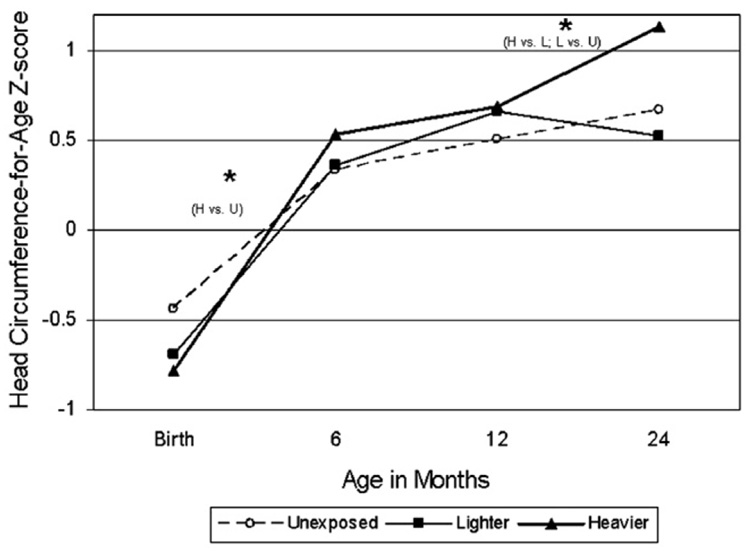
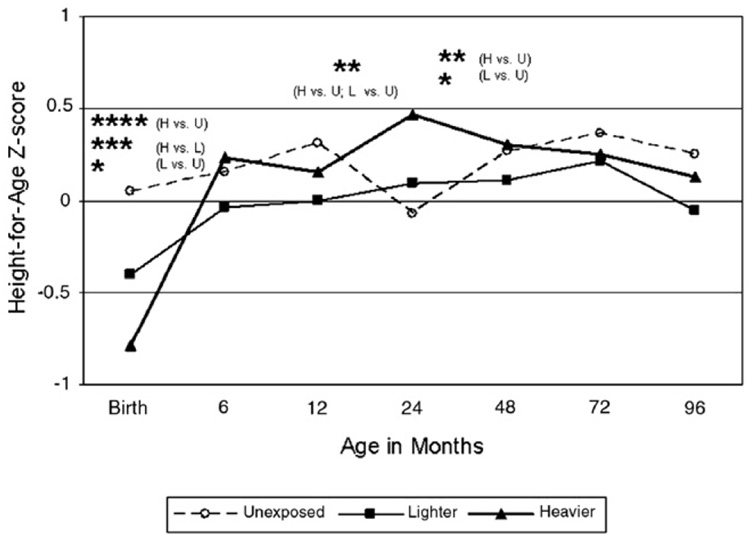
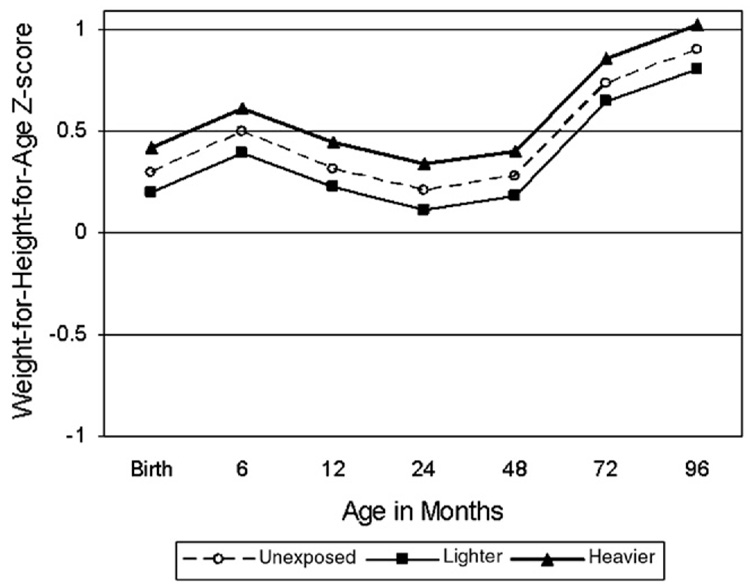
Table 4 and Fig. 1–Fig. 4 show differences in anthropometric growth patterns by cocaine exposure status between age windows estimated using mixed linear models and adjusted for covariates. Using dummy variables in these models, comparisons were made between those with heavier cocaine exposure and those unexposed, as well as between those with lighter exposure and those unexposed. Significant differences existed between certain age windows for rate of change in weight-for-age, height-for-age, and head circumference-for-age, but not weight-for-length/BMI-for-age. Specifically, Fig. 1 demonstrates that the cocaine-exposed groups (heavier versus unexposed and lighter versus unexposed) showed significantly more rapid gain in weight-for-age between birth and 6 months, but slower gain between 24 and 48 months. Fig. 2 demonstrates that the cocaine-exposed groups (both heavier versus unexposed and lighter versus unexposed) showed significantly more rapid gain in height-for-age (compared to unexposed) between birth and 6 months and between 12 and 24 months, but a significantly slower rate of gain between 24 and 48 months. Fig. 3 demonstrates that there were no differences between the cocaine-exposed groups (both heavier versus unexposed and lighter versus unexposed) in rate of gain in weight-for-length/BMI. Fig. 4 shows that when evaluating cocaine-exposed infants (lighter and heavier combined) versus unexposed, the rate of head circumference growth was significantly greater in the exposed versus unexposed infants between birth and 6 months and between 12 and 24 months. However, neither of the cocaine-exposed groups (neither the heavier nor lighter compared to the unexposed) individually showed significantly greater gain in head circumference between birth and 6 months, nor between 12 and 24 months.
Fig. 1.
Model-based longitudinal weight-for-age z-score by pre-natal cocaine exposure group (Adjusted for birth mother’s ethnicity, child’s gender, the natural logarithm of pre-natal alcohol average daily volume; the natural logarithm of pre-natal average daily cigarettes; pre-natal marijuana use; Dubowitz gestational age (for observations taken at age 24 months or younger), and maternal prepregnancy weight.). * p<.05 for slope within designated interval. ** p<.01 for slope within designated interval.
Fig. 4.
Model-based longitudinal head circumference-for-age z-score by pre-natal cocaine exposure group (Adjusted for birth mother’s ethnicity, child’s gender the natural logarithm of pre-natal alcohol average daily volume; the natural logarithm of pre-natal average daily cigarettes; pre-natal marijuana use; Dubowitz gestational age (for observations taken at age 24 months or younger), and maternal pre-pregnancy height.). * p<.05 for slope within designated interval.
Fig. 2.
Model-based longitudinal height-for-age z-score by pre-natal cocaine exposure group (Adjusted for birth mother’s ethnicity, child’s gender, the natural logarithm of pre-natal alcohol average daily volume; the natural logarithm of prenatal average daily cigarettes; pre-natal marijuana use; Dubowitz gestational age (for observations taken at age 24 months or younger), and maternal height.). * p<.05 for slope within designated interval. ** p<.01 for slope within designated interval. *** p<.001 for slope within designated interval. **** p<.0001 for slope within designated interval.
Fig. 3.
Model-based longitudinal weight-for-height-for-age z-score by pre-natal cocaine exposure group (Adjusted for birth mother’s ethnicity, child’s gender, the natural logarithm of pre-natal alcohol average daily volume; the natural logarithm of pre-natal average daily cigarettes; pre-natal marijuana use; Dubowitz gestational age (for observations taken at age 24 months or younger), and maternal pre-pregnancy weight.).
Lastly, based on these mixed linear models, contrasts between pairs of adjusted mean z-scores of the cocaine exposure groups at 48 months and 96 months of age were performed for all parameters except for head circumference-for-age, which was analyzed in this fashion at 24 months of age. There were no statistically significant differences between the adjusted mean z-scores at these specified ages except for head circumference at 24 months of age, where there was a significant difference between the adjusted mean z-scores of the heavier vs. the lighter cocaine-exposed groups (p=.01).
3.3.2. Effects of other pre-natal exposures on anthropometric growth patterns across age windows
In the mixed linear models including the cocaine by age interaction, the only noteworthy independent main effect of substances other than cocaine on growth was that the natural log of daily alcohol consumption was related to weight (p=.04), length (height) (p=.008), and head circumference (p=.06): the greater the pre-natal exposure to alcohol, the smaller the children were on each of these parameters overall from birth to last age of measurement. For example, mothers who consumed 1 drink per day compared to non-drinkers had children who had z-scores smaller (on average, across age windows) by 0.18 SDU on weight-for-age, 0.31 SDU on height-for-age, and 0.24 SDU on head circumference-for-age. There was no significant and independent effect of marijuana use on any growth parameter. The natural log of average daily number of cigarettes was also not significantly and independently related to any post-neonatal growth parameter.
3.3.3. Effects of other potential confounds on anthropometrics across age windows
In the mixed linear models, children of African-Caribbean mothers also had greater z-scores for head circumferences by 0.4 SDU (p=.03) than the children of African-American mothers. On average across age windows, maternal pre-pregnancy weight was positively associated with children’s weight-for-age (p=.005) but was not associated with weight-for-length/BMI-for-age z-scores (p=.37). Maternal height was positively associated with children’s height-for-age (p<.0001) and head circumference-for-age (p=.04), on average across age windows. Whether caregiver at time of measurement was the birth mother, a kinship caregiver, or an unrelated foster parent was not significantly associated with growth nor did it act as a time dependent confounder of the intrauterine cocaine exposure–growth relationships for any outcome including head circumference (detailed analyses available from authors on request).
4. Conclusions
This is the first study of which we are aware that documents longitudinal growth patterns after intrauterine cocaine exposure from birth until school age. Although at birth, the level of prenatal cocaine exposure correlates with statistically significant decrements in weight, length, and HC, these effects were not detected in longitudinal multivariate analyses beyond the neonatal period. The longitudinal analyses suggest that although the rate of change in anthropometric parameters of cocaine-exposed children (heavier versus unexposed and lighter versus unexposed) exceeded those of unexposed children in the first 2 years of life and then lagged up until age 4, the negative cocaine effects ultimately largely dissipated with age.
These findings are in concert with work of other investigators finding no differences in anthropometrics at school age [47]. Our data support this prior work and make it less likely that its negative findings were due to inadequate power or lesser exposure in that cohort, as we have presented growth data on the largest sample size to date of children followed to school age. It is noteworthy, however, that in at least two other cohorts followed to school age, a detrimental effect of in utero cocaine exposure on height was detected at age 6–7 years [13,38], and in one cohort a detrimental effect on weight-for-height [38]. Evaluation of the growth patterns of other cohorts, as well as the ongoing follow up of our own cohort and others, will continue to clarify the potential impact of in utero cocaine exposure on growth patterns to school age and beyond.
We also found that catch-up growth occurred primarily in the first 6–12 months. While there was some fluctuation in rates of growth in the preschool age range between cocaine exposure groups, any changes in rates of growth and attained growth had dissipated by school age in multivariate analyses. This is also in concert with others’ findings that catch-up growth, at least for weight, appears to occur by 12 months of age [32], but extends prior work by demonstrating that once the catch-up in growth among children with intrauterine cocaine exposure occurred, anthropometric parameters indistinguishable from unexposed children were maintained to school age.
The decrements in head circumference present at birth appeared to dissipate in the first 6–12 months, but we did detect a paradoxical but not unprecedented finding [32] that children with heavier cocaine exposure had larger head circumferences at 24 months than the unexposed. This finding must be interpreted with caution given the relatively small cell size for the heavily exposed. The significance of these findings remains unclear. Our group has previously demonstrated an increased prevalence of subependymal hemorrhage on neonatal cranial ultrasound in heavily cocaine-exposed infants [17]. The present finding that these same infants experience rapid head growth to eventually exceed head circumference of their non-exposed peers by 24 months may reflect recovery from early brain insult. However, synaptic pruning is also a critical component of normal development. It has been hypothesized that the macrocephaly observed in children with autism [14] may reflect deficient synaptic pruning. Although the heavily cocaine-exposed children in our cohort are not macrocephalic, the larger head circumference at age 24 months raises the possibility that the normal process of synaptic pruning may have been disrupted. The findings regarding head circumference growth are therefore not necessarily implicitly reassuring, but may or may not reflect ongoing disruption in normal brain growth first seen in infancy.
Our finding with regard to there being a higher prevalence of overweight in the unexposed, compared to the cocaine-exposed children at age 96 months raises several possibilities. First, it is possible that, although we did not detect differences in growth based on cocaine exposure over time, the finding represents an indication that some degree of restriction, at least with regard to weight gain, persists beyond infancy in the cocaine-exposed group. The effect may be subtle to the extent that it is only observable at the extreme of weight gain in the presently quite obesigenic environment of U.S. inner cities. Continuing to follow these children’s growth patterns until adult weight and height have been attained would help to answer this question. An equally plausible explanation is that some other confounder that we were unable to control for due to our limited sample size of overweight children accounts for the association. Potential confounders might include measures of food security, socioeconomic status, parental mental health, or child behavior, all of which may contribute to a child’s weight status.
Our findings with regard to tobacco and marijuana exposure also corroborate those of prior studies [41]: there does not appear to be a lasting effect of tobacco or marijuana exposure on post-natal growth. Exposure to alcohol in our sample had a statistically significant persistent negative effect on weight, height, and head circumference during the first 8 years of life, consistent with others’ data in low-income populations [39,41]. Analytic considerations limited our ability to determine if the effect of alcohol on growth changed over time (while also simultaneously evaluating the effect of our main effect of interest, cocaine, over time). The growth patterns of alcohol-exposed infants over time after control of multiple confounds, including cocaine exposure, is a question of interest for future research.
There are several limitations to our findings. The cell size for heavily exposed children (n=38) is relatively small, and therefore findings specifically with regard to the heavily exposed group must by necessity be interpreted with caution. Information on alcohol and cigarette use was obtained by retrospective self-report, and no biological markers were available against which to compare the accuracy of the information reported. Collecting self-report data and biological markers of cigarette and illicit substance use prospectively throughout pregnancy may have provided more reliable estimates of actual usage. Our definition of heavy cocaine exposure (top quartile for this sample, 61 or more days of reported use during pregnancy) differed from the definition used by others in other cohorts to define heavy cocaine use (2 times per week or more throughout pregnancy [26,27]), and it is therefore possible that our inability to detect an effect of heavy exposure on growth parameters may have been due to a cohort that was overall less heavily exposed than some other cohorts. Comparisons across cohorts are difficult since the potency and contamination of illicit substances, such as cocaine, vary across time and by geographic location so that number of days of use can only approximate the actual “dose” experienced by the fetus. We also did not have sufficient information on the heights of putative fathers. However, we believe it is unlikely that paternal anthropometric data differed by cocaine exposure status, and thus feel it is unlikely that it acts as a confounder. We did not have 20% of maternal heights, but imputing these missing data did not alter our findings. Moreover, the documented birth mothers’ heights did not differ by level of pre-natal cocaine use. We also did not have any data regarding the dietary history of our sample. However, high rates of anemia in early childhood did not differ between exposure groups, providing suggestive evidence that there were not clinically obvious differences in the overall poor dietary quality among the three groups. Blood lead levels also did not differ between groups and thus is an unlikely explanation for our findings.
We also excluded infants born prematurely, which may have omitted more infants with heavier exposure. Since prenatal cocaine exposure is associated with reduced gestational age, and an increased risk of premature birth [6], it is possible that the effect of cocaine exposure on long-term growth operates differently in pre-term, compared to term, infants. To our knowledge, there are no published reports to date evaluating a potential interaction, and this may be an area for future research.
There is converging evidence that pre-natal cocaine exposure at the levels measured in this and other studies does not have negative effects on either growth rates or attained growth beyond the neonatal period in term and near term infants. Our findings add to the body of research indicating that certain exposures which restrict fetal growth do not seem to alter the ability of the organism to recover normal growth patterns when removed from the exposure, while others, such as alcohol, may lead to persistent alteration in growth potential. Our findings also add to prior research by indicating that catch-up growth from pre-natal cocaine exposure occurs in the first 6–12 months of life, and growth continues at expected rates thereafter to school age. Further research will be needed to ascertain as research cohorts mature whether differences in growth patterns re-emerge with the pubertal growth spurt and in attained adult size.
From a clinical perspective, pre-natal exposure to tobacco, marijuana, or cocaine does not appear to be an adequate explanation for depressed post-natal growth in any parameter. Infants who have been cocaine-exposed in utero should be monitored carefully and provided with adequate nutritional support to ensure catch-up growth in the first 6–12 months of life. Pre-natal exposure to alcohol in children without fetal alcohol syndrome, however, is an important clinical correlate of depressed weight, height, and head circumference beyond the neonatal period, although causality cannot be inferred from an epidemiologic study such as this one. When not growing as expected from national norms, children with a history of any of these exposures, like any other child with post-natal growth failure, require a thorough evaluation with particular attention to human immunodeficiency virus [48], other treatable illnesses and adverse environmental conditions.
Acknowledgements
This work was supported by 5R01DA006532-14 to Dr. Frank, and NIH/NCRR M01 RROO533. The study sponsor had no role in the study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the paper for publication.
Footnotes
Conflict of interest statement
None of the authors have any financial, personal, or their relationships with other people or organizations within 3 years of beginning the work submitted that could inappropriately influence the work submitted.
References
- 1.Anonymous, Recommendations to prevent and control iron deficiency in the United States. MMWR. 1998;47 [PubMed] [Google Scholar]
- 2.Anonymous, Blood lead levels in young children: United States and selected states, 1996–1999. MMWR. 2000;49:1133–1137. [PubMed] [Google Scholar]
- 3.Bale SJ, Amos CI, Parry DM, Bale AE. Relationship between head circumference and height in normal adults and in the nevoid basal cell carcinoma syndrome and neurofibromatosis Type I. Am. J. Med. Genet. 1991;40:206–210. doi: 10.1002/ajmg.1320400217. [DOI] [PubMed] [Google Scholar]
- 4.Bandstra ES, Morrow CE, Anthony JC, Churchill SS, Chitwood DC, Steel BW, Ofir AY, Xue L. Intrauterine growth of full-term infants: impact of prenatal cocaine exposure. Pediatrics. 2001;108:1309–1319. doi: 10.1542/peds.108.6.1309. [DOI] [PubMed] [Google Scholar]
- 5.Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- 6.Bauer CR, Langer JC, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J. Acute neonatal effects of cocaine exposure during pregnancy. Arch. Pediatr. Adolesc. Med. 2005;159:824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 7.Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from prospective, longitudinal study of prenatal cocaine use: preschool development at age years. J. Pediatr. Psychol. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedict MI, Zuravin S, Stallings RY. Adult functioning of children who lived in kin versus nonrelative family foster homes. Child Welfare. 1996;75:529–550. [PubMed] [Google Scholar]
- 9.Boersma B, Wit JM. Catch-up growth. Endocr. Rev. 1997;18:646–661. doi: 10.1210/edrv.18.5.0313. [DOI] [PubMed] [Google Scholar]
- 10.Brandt I, Sticker EJ, Lentze MJ. Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. J. Pediatr. 2003;142:463–468. doi: 10.1067/mpd.2003.149. [DOI] [PubMed] [Google Scholar]
- 11.Brandt I, Sticker EJ, Gausche R, Lentze MJ. Catch-up growth of supine length/height of very low birth weight, small for gestational age preterm infants to adulthood. J. Pediatr. 2005;147:662–668. doi: 10.1016/j.jpeds.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter SC, Clyman RB. The long-term emotional and physical well-being of women who have lived in kinship care. Child Youth Serv. Rev. 2004:26. [Google Scholar]
- 13.Covington CY, Nordstrom-Klee B, Ager J, Sokol R, Delaney-Black V. Birth to age 7 growth of children prenatally exposed to drugs: a prospective cohort study. Neurotoxicol. Teratol. 2002;24:489–496. doi: 10.1016/s0892-0362(02)00233-7. [DOI] [PubMed] [Google Scholar]
- 14.Dementieva YA, Vance DA, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, DeLong GR, Abramson RK, Wright HH, Cuccaro ML. Accelerated head growth in early development of individuals with autism. Pediatr. Neurol. 2005;32:102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Diggle P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford UK: Clarendon Press; 1994. [Google Scholar]
- 16.Dubowitz L, Dubowitz A, Goldberg C. Clinical assessment of gestational age in the newborn infant. J. Pediatr. 1970;77:1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- 17.Frank DA, McCarten KM, Robson CD, Mirochnick M, Cabral HJ, Park H, Zuckerman B. Level of in utero cocaine exposure and neonatal ultrasound findings. Pediatrics. 1999;104:1101–1105. doi: 10.1542/peds.104.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank DA, Jacobs RR, Beeghly M, Augustyn M, Bellinger D, Cabral HJ, Heeren T. Level of prenatal cocaine exposure and scores on the Bayley Scale of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002;110:1143–1152. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisk V, Amsel R, Whyte HE. The importance of head growth patterns in predicting the cognitive abilities and literacy skills of small-for-gestational-age children. Dev. Neuropsychol. 2002;22:565–593. doi: 10.1207/S15326942DN2203_2. [DOI] [PubMed] [Google Scholar]
- 20.Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N. Engl. J. Med. 1991;325:231–237. doi: 10.1056/NEJM199107253250403. [DOI] [PubMed] [Google Scholar]
- 21.Hack M, Weissman B, Borawski-Clark E. Catch-up growth during childhood among very low-birth-weight children. Arch. Pediatr. Adolesc. Med. 1996;150:1122–1129. doi: 10.1001/archpedi.1996.02170360012002. [DOI] [PubMed] [Google Scholar]
- 22.Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003;112:e30–e38. doi: 10.1542/peds.112.1.e30. [DOI] [PubMed] [Google Scholar]
- 23.Hulse GK, English DR, Milne E, Holman CD, Bower CI. Maternal cocaine use and low birth weight newborns: a meta-analysis. Addiction. 1997;92:1561–1570. [PubMed] [Google Scholar]
- 24.Jacobson JL, Jacobson SW. Methodological considerations in behavioral toxicology in infants and children. Dev. Psychol. 1996;32:390–403. [Google Scholar]
- 25.Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol. Clin. Exp. Res. 1994;18:317–323. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. J. Pediatr. 1996;129:581–590. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev. Psychopathol. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- 28.Karlberg GJ, Albertssonwikland K. Growth in full-term small-for-gestational age infants from birth to final height. Pediatr. Res. 1995;38:733–739. doi: 10.1203/00006450-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Karlberg J, Albertsson-Wikland K, Kwan CW, Chan FY. Early spontaneous catch-up growth. J. Pediatr. Endocrin. Metabol. 2002;15:1243–1255. [PubMed] [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R. CDC Growth Charts. Adv. Data. 2000;314:1–28. [PubMed] [Google Scholar]
- 31.Lester BM, El Sohly M, Wright LL. The maternal lifestyle study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 32.Lewis MW, Misra S, Johnson HL, Rosen TS. Neurological and developmental outcomes of prenatally cocaine-exposed offspring from 12 to 36 months. Am. J. Drug Alcohol Abuse. 2004;30:299–321. doi: 10.1081/ada-120037380. [DOI] [PubMed] [Google Scholar]
- 33.Lutiger B, Graham K, Einarson TR, Koren G. Relationship between gestational cocaine use and pregnancy outcome: a meta-analysis. Teratology. 1991;44:405–414. doi: 10.1002/tera.1420440407. [DOI] [PubMed] [Google Scholar]
- 34.McCammon RW. Human Growth and Development. Springfield IL: Charles C. Thomas; 1970. pp. 104–150. [Google Scholar]
- 35.McLellan AT, Kushner H, Metzger D. The 4th Ed of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 36.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J. Nutr. 1999;129:1555–1562. doi: 10.1093/jn/129.8.1555. [DOI] [PubMed] [Google Scholar]
- 37.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 38.Minnes S, Robin NH, Alt AA, Kirchner HL, Satayathum S, Salbert B, Ellison L, Singer LT. Dysmorphic and anthropometric outcomes in 6-year-old prenatally cocaine-exposed children. Neurotoxicol. Teratol. 2006;28:28–38. doi: 10.1016/j.ntt.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore E, Ward RE, Jamison PL, Morris CA, Bader PI, Hall BD. The subtle facial signs of prenatal exposure to alcohol: an anthropometric approach. J. Pediatr. 2001;139:215–219. doi: 10.1067/mpd.2001.115313. [DOI] [PubMed] [Google Scholar]
- 40.Nelhaus G. Head circumference from birth to eighteen years/practical composite international and interracial graphs. Pediatrics. 1968;41 [PubMed] [Google Scholar]
- 41.Nordstrom-Klee B, Delaney-Black V, Covington C, Ager J, Sokol R. Growth from birth onwards of children prenatally exposed to drugs: a literature review. Neurotoxicol. Teratol. 2002;24:481–488. doi: 10.1016/s0892-0362(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 42.Ostrea EM, Brady M, Gause S, Raymundo AL, Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89:107–113. [PubMed] [Google Scholar]
- 43.Ostrea EM, Romero A, Knapp DK. Postmortem drug analysis of meconium in early-gestation human fetuses exposed to cocaine: clinical implications. J. Pediatr. 1994;124:477–479. doi: 10.1016/s0022-3476(94)70379-5. [DOI] [PubMed] [Google Scholar]
- 44.Prader A, Tanner JM, vonHarnack G. Catch-up growth following illness or starvation. An example of developmental canalization in man. J. Pediatr. 1963;62:646–659. doi: 10.1016/s0022-3476(63)80035-9. [DOI] [PubMed] [Google Scholar]
- 45.Puckering C, Pickle A, Skuse D, Heptinstall E, Dowdney L, Zur-Szpiro S. Mother–child interaction and the cognitive and behavioral development of four-year-old children with poor growth. J. Child Psychol. Psychiatry. 1995;36:573–595. doi: 10.1111/j.1469-7610.1995.tb02315.x. [DOI] [PubMed] [Google Scholar]
- 46.Richardson GA. Prenatal cocaine exposure: a longitudinal study of development. Ann. N.Y. Acad. Sci. 1998;846:144–152. [PubMed] [Google Scholar]
- 47.Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicol. Teratol. 1996;18:627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- 48.Saavedra JM, Henderson RA, Perman JA. Longitudinal assessment of growth in children born to mothers with human immunodeficiency virus infection. Arch. Pediatr. Adolesc. Med. 1995;149:497–502. doi: 10.1001/archpedi.1995.02170180027004. [DOI] [PubMed] [Google Scholar]
- 49.Strauss RS, Dietz WH. Growth and development of term children born with low birth weight: effects of genetic and environmental factors. J. Pediatr. 1998;133:67–72. doi: 10.1016/s0022-3476(98)70180-5. [DOI] [PubMed] [Google Scholar]
- 50.Tronick EZ, Frank DA, Cabral H, Mirochnick M, Zuckerman B. Late dose-response effects of prenatal cocaine exposure on newborn neurobehavioral performance. Pediatrics. 1996;98:76–83. [PMC free article] [PubMed] [Google Scholar]
- 51.Westwood M, Kramer MS, Munz D, Lovett JM, Watters GV. Growth and development of full-term non-asphyxiated small-for-gestational age newborns: follow-up through adolescence. Pediatrics. 1983;71:376–382. [PubMed] [Google Scholar]
- 52.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 53.Zuckerman B, Frank DA, Hingson R. Effects of maternal marijuana and cocaine use on fetal growth. N. Engl. J. Med. 1989;320:762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]