Abstract
Bombardier beetles, when physically assaulted, eject a hot quinonoid spray from the tip of the abdomen. Photographic evidence is presented demonstrating that the African bombardier beetle, Stenaptinus insignis, can aim its spray in virtually any direction. It can target its individual legs, and even the individual segments of its legs. Moreover, in aiming at a leg, it takes into account the postural orientation of that leg. The beetle is able even to target sites on its back. It is postulated that the ability to aim helps the beetle mainly in defense against ants.
Keywords: chemical defense, quinones, Carabidae, Formicidae
Beetles have a problem. Unlike flies, butterflies, dragonflies, and many other insects, they cannot as a rule take instantly to the air. To activate the wings they must first unfurl these from beneath the wing covers (the elytra), and this takes time. Delays are unaffordable in emergencies, and it should come as no surprise that many beetles have evolved means for “buying time” when under attack. The species of the family Carabidae, the so-called ground beetles, are a case in point. Living at soil level, carabids are in constant danger from ants, against which they are protected by their dischargeable defensive glands (1–3). Paired devices, these glands take the form of more or less capacious sacs, lying side by side in the abdomen and opening on the abdominal tip (4, 5). Diverse toxicants are produced by these glands, often at high concentrations, including acids, aldehydes, phenols, and quinones (2, 3). Most carabids are able to eject these fluids forcibly, in the form of sprays (5–9).
Ants can attack from virtually any direction and, for maximal effectiveness, need to be targeted to be repelled. Not surprisingly, many carabids have the capacity to aim their spray in different directions (5–9). None are perhaps better marksmen than the so-called bombardier beetles, as we document here photographically for one species, the African Stenaptinus insignis.
The spray of bombardier beetles contains p-benzoquinones (10), compounds well known for their irritant properties (11). A single bombardier beetle can discharge upward of 20 times before depleting its glands (6). The discharges are accompanied by audible detonations, and they have been shown to be potently deterrent to a number of predators, including ants (6, 12–15).
The spray of bombardier beetles is ejected at 100°C (13). This is because the quinones are generated explosively at the moment of ejection by the mixture of two sets of chemicals ordinarily stored separately in the glands. Each gland consists of two confluent compartments. The larger of these (storage chamber or reservoir) contains hydroquinones and hydrogen peroxide while the smaller one (reaction chamber) contains special enzymes (catalases and peroxidases). To activate the spray, the beetle mixes the contents of the two compartments, causing oxygen to be liberated from hydrogen peroxide and the hydroquinones to be oxidized by the freed oxygen. The oxygen also acts as the propellant, causing the mixture to “pop” out (16–18). The heat that accompanies the formation of the spray is perceptible (13) and contributes to the defensive effectiveness of the secretion (14, 15). An early explorer, reporting on large bombardier beetles from the neotropics, commented that when these “play off their artillery” they are so hot to the touch “that only few (can) be captured with the naked hand” (19). Although it was known that bombardier beetles can aim their spray by revolving the abdominal tip (6), the degree of precision with which they target their ejections had escaped notice.§
MATERIALS AND METHODS
The Beetles.
The S. insignis were sent to us from Kenya. They were maintained in cages bearing a bottom layer of moist sand and were offered freshly cut up mealworms (larvae of Tenebrio molitor) and water (soaked cotton wad). Individuals survived for up to 4 years. Females outlived males. The photos shown here are all of females.
Preparation of Beetles.
For photographic purposes, each beetle was outfitted with a wire hook, fastened to its elytra with a droplet of wax. The hook provided a handle by which the beetle could be picked up (without being caused to spray) and held at any desired orientation relative to the camera. The hook also could be coupled, by way of a small link of plastic tubing, to the end of a firmly held brass rod, thereby providing a means for setting the beetle in place in a normal stance (as in Fig. 1). To affix the hook, the beetle was first submerged in ice-cold water. This resulted in its virtual immobilization and prevented it from spraying when the droplet of melted wax was placed on its elytra.
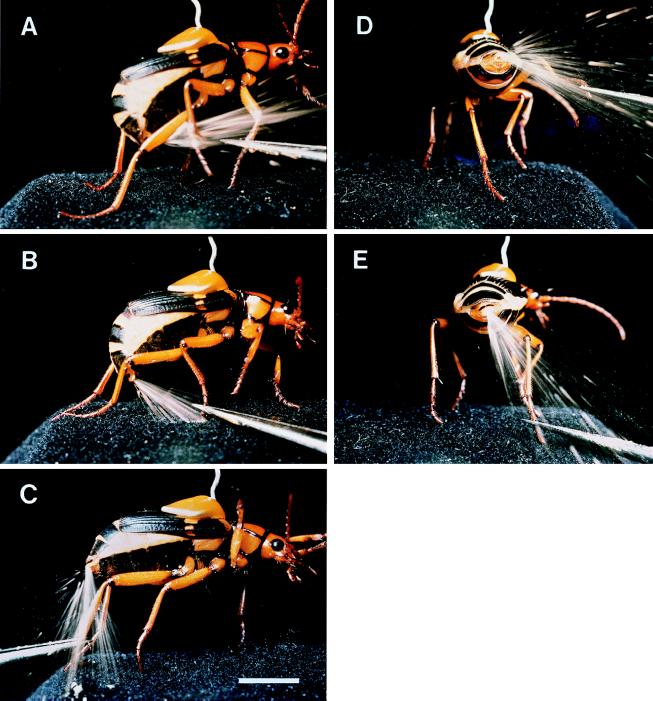
Figure 1.
Pinching of right foreleg (A), midleg (B), and hindleg (C) and pinching of tarsus of right hindleg (beetle in rear-end view), with leg in raised position (D) and in downward position (E). [Bar (C) = 0.5 cm.]
Elicitation of Ejections.
To induce a beetle to spray, fine-tipped forceps were used to pinch its individual appendages or to poke parts of its body. Upward of 10 consecutive discharges could be elicited as a matter of routine from individual S. insignis, provided these had been kept undisturbed for at least 2 weeks beforehand. The pinchings and pokings inflicted no noticeable injury on the beetles.
Photographic Technique.
The pictures were taken by electronic flash illumination. Two flash units were used, one triggered by the sound of the beetle’s ejection, the other (slave unit) by the discharge of the first unit. The microphone that sensed the beetle’s detonation and relayed this sound by electronic circuitry to the first flash unit was positioned directly above the beetle, just outside the viewing field of the camera.
Positioning of the beetle in front of the camera was effected in dim light. After opening the shutter, the beetle was stimulated, causing it to spray and the flashes to be triggered, after which the shutter was promptly closed again. The combined duration of the flashes, which discharged in virtual synchrony, was in the order of 10 msec. The background illumination, during the time the shutter was kept open, was of insufficient intensity to register on the film.
Photos (Kodachrome 25 PKM 135 film, Kodak) were taken with 35-mm cameras (Fig. 1), and with a Wild (Heerbrugg, Switzerland) M400 Photomakroskop (Figs. 2 and 3 A–E). The scanning electronmicrograph (Fig. 3F) was of a specimen prepared by critical point drying.
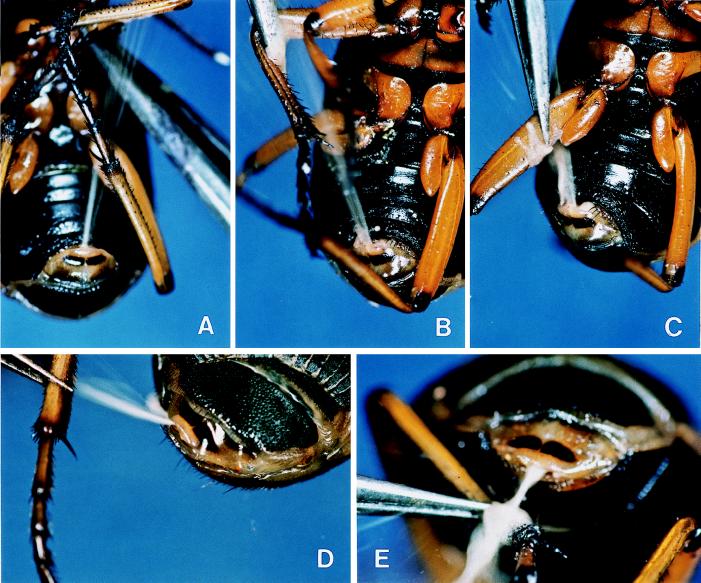
Figure 2.
Pinching of base of left midleg (A), of distal portion of femur of right midleg (B), and of base of femur of right hindleg (C) and pinching of distal portion of left femur, with femur positioned beside body (D) and positioned behind abdominal tip (E). (A–C) Ventral view of abdomen. (D) Dorsal view of abdominal tip. (E) End-on view of abdominal tip.
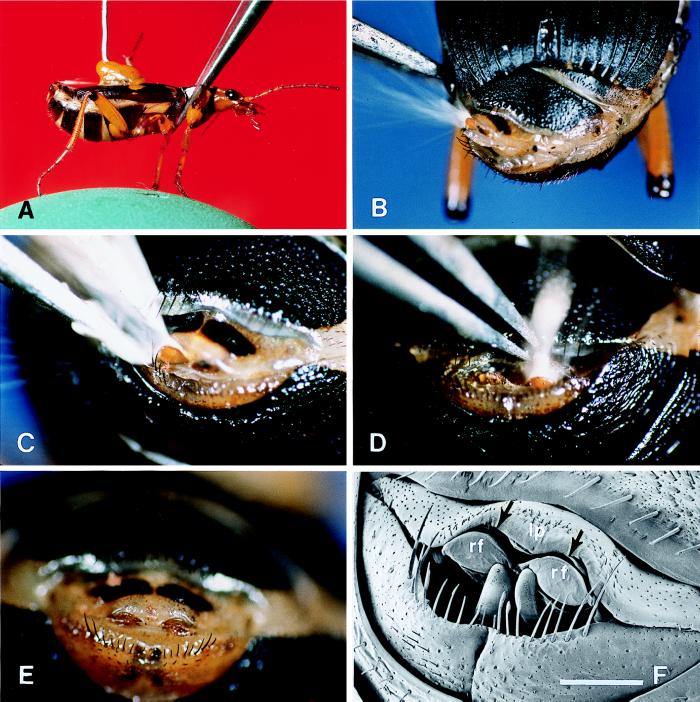
Figure 3.
(A) Pinching of prothorax from above. A faint trace of the discharge is seen in white, emerging forward from the abdominal tip. (B) Poking of left flank of abdomen. Note that the abdominal tip (dorsal view) is rotated to the left and that the spray is bouncing off the left reflector. (C) Poking of abdominal tip just to the left of glandular opening. The secretion is bouncing off the left reflector and has almost drenched the tip of the forceps. (D) Poking of the abdominal tip, just to the right of the midline, above the gland opening. The secretion is bouncing off the right reflector and is hitting the tip of the forceps. (E) Undisturbed abdominal tip, showing the two sclerotized reflectors (light brown) in their unexposed position. (F) Comparable to preceding (scanning electronmicrograph). The arrows denote the curved slit-like opening through which the spray is expelled. The two reflectors (rf), shown in their unexposed position, form the lower “lip” of this opening. The upper lip (lp) is a membranous fold. [Bar (E) = 0.5 mm.]
RESULTS
Targeting of Legs.
Pinching of individual legs of a beetle resulted in an accurate targeting of these legs (Fig. 1 A–C). The beetle aims the spray by deflecting the abdominal tip forward beneath the body. The deflection is maximal when the beetle is spraying furthest forward, as toward a foreleg (Fig. 1A) and minimal when it sprays toward a hindleg (Fig. 1C).
Targeting of Leg Segments.
Discharges toward a leg are aimed, not broadly in the general direction of that leg, but with considerable precision toward the particular leg segment, or portion of a segment, that is stimulated. Fig. 2 A–C shows three discharges elicited by pinching, respectively, the coxal region of a midleg, the distal portion of the femur of a midleg, and the basal portion of the femur of a hindleg. Note that, in Fig. 2 B and C, the forceps have been visibly wetted with secretion.
Positional Tracking.
In aiming toward a given portion of a leg, the beetle takes into account the postural orientation of that leg. In Fig. 1 D and E, the same stimulus (pinching of tarsus of right hindleg) is twice applied to a beetle, once when the leg is in a raised position and then again when it is in a downward position. The beetle sprayed with accuracy in each case, indicating that it is able to keep track of an intended bodily target site as this is taken through its range of motions. Comparable accuracy is revealed in closeup view in Fig. 2 D and E, in which the distal end of a hindleg femur is stimulated, first when the femur is positioned to the side of the beetle and then when it is positioned directly behind the abdominal tip.
Forward Ejections, Over the Back.
When stimulating various sites along the back of the beetle, we noted that the insect is able to direct its spray toward such regions as well. Thus, for example, when the pronotum was pinched from above, the beetle responded by spraying forward over the back, in parallel to the back’s surface (Fig. 3A). In achieving such directionality, the beetle rotates the abdominal tip upward and forward, but not so far as to bring the tip to point directly ahead. In fact, to achieve dorsal forward ejections, the beetle resorts to the stratagem of bouncing the secretory jet off a pair of cuticular reflectors that it deploys specifically for the purpose. Indeed, closeup views of the abdominal tip during such forward ejections show clearly how the reflectors operate (Fig. 3 B–D). They appear to be used one at a time, the spray in any one “shot” being deflected off the reflector closest to the site stimulated (Fig. 3 C and D). Sideways rotation of the abdominal tip also may contribute to the overall aiming of such dorsal discharges (Fig. 3B).
Deployment of the reflectors would appear to require special action on the part of the beetle. Ordinarily, the reflectors (Fig. 3E; rf in Fig. 3F) are kept unexposed, in such fashion that they form a lower lip beneath the curved slit-like opening (arrows in Fig. 3F) from which the spray emerges. The upper lip of that opening is a membranous fold (lp in Fig. 3F) that presumably can be retracted, in such manner as to expose the reflectors. We envision the beetle having the capacity to retract the upper lip asymmetrically, thereby achieving the unilateral exposure of the reflectors. One can also envision the upper lip being projected outward over the reflectors, to cause the emergent spray to be deflected downward. In Fig. 2E, the upper lip seems to be effecting precisely such deflection.
DISCUSSION
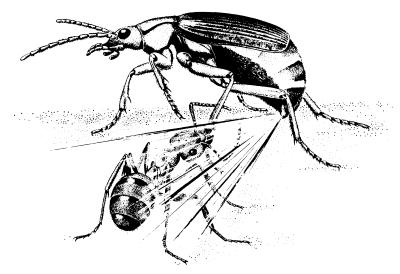
The assault depicted in Fig. 4 must be of routine occurrence in the life of S. insignis. Yet one can imagine the beetle usually surviving such attacks. Its hot quinonoid spray, in itself formidably repellent (6, 12–15), can only be rendered more effective by being aimed. And as indicated by our photos, that aiming is precise. There is virtually no site on the beetle’s body where an ant could inflict a bite without entailing the risk of being sprayed in return.
Figure 4.
Schematic representation of an encounter between an ant and a bombardier beetle (reprinted from ref. 24 by permission).
Other bombardier beetles related to Stenaptinus (subfamily Brachininae), such as those of the large genus Brachinus, probably aim their spray in much the same manner as Stenaptinus. Indeed, we found the abdominal tip of Brachinus to resemble that of Stenaptinus in every structural detail.
Aiming is achieved differently in bombardier beetles of another subfamily (Paussinae), which also eject hot quinones (20, 21). Paussines lack the reflective devices of Stenaptinus. Instead, when “shooting” forward, paussines direct their ejections along a pair of lateral elytral grooves that serve as launching guides for anteriorly aimed ejections (22, 23).
Given that so many insects play off chemical “artillery” in defense, there can be no question that diverse spray-aiming mechanisms remain to be discovered among insects. Photography and cinematography could prove helpful in elucidating how these mechanisms operate. Questions remain, however, even about S. insignis itself. Although we know that the males of this species also aim their discharges, they appear to do so with an apparatus that differs somewhat from that of the female. Thus, for instance, for ejecting forward over the back, males make use of a single broad reflective shield, instead of the pair of devices used by the female (data not shown). We are also ignorant about whether S. insignis always discharges from both glands simultaneously or whether it does so from one gland at a time. And, of course, there is the vexing problem of how the beetle, which inevitably drenches itself when discharging, withstands the heat and irritancy of its own spray.
Acknowledgments
We dedicate this paper affectionately to the memory of Professor Dr. Max Renner of the University of Munich, exemplary naturalist and friend. We thank Maria Eisner for help with the manuscript, for preparing the illustrations, and for providing the scanning electronmicrograph. Andrés González and Jerrold Meinwald provided helpful comments on the manuscript. This study was supported by National Institutes of Health Grant AI02908.
Footnotes
This paper is no. 162 in the series “Defense Mechanisms in Arthropods.” Paper no. 161 is ref. 25.
References
- 1.Eisner T. In: Chemical Ecology. Sondheimer E, Simeone J B, editors. New York: Academic; 1970. pp. 157–217. [Google Scholar]
- 2.Weatherston J, Percy J E. In: Handbook of Experimental Pharmacology: Arthropod Venoms. Bettini S, editor. Vol. 48. New York: Springer; 1978. pp. 511–554. [Google Scholar]
- 3.Blum M S. Chemical Defenses of Arthropods. Orlando, FL: Academic; 1981. [Google Scholar]
- 4.Forsyth D J. Trans Zool Soc London. 1972;32:249–309. [Google Scholar]
- 5.Rossini C, Attygalle A B, González A, Smedley S R, Eisner M, Meinwald J, Eisner T. Proc Natl Acad Sci USA. 1997;94:6792–6797. doi: 10.1073/pnas.94.13.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisner T. J Insect Physiol. 1958;2:215–220. [Google Scholar]
- 7.Eisner T, Swithenbank C, Meinwald J. Ann Entomol Soc Am. 1963;56:37–41. [Google Scholar]
- 8.Eisner T, Hurst J J, Meinwald J. Psyche. 1963;70:94–116. [Google Scholar]
- 9.Attygalle A B, Meinwald J, Eisner T. J Chem Ecol. 1992;18:489–498. doi: 10.1007/BF00994247. [DOI] [PubMed] [Google Scholar]
- 10.Schildknecht H. Angew Chem. 1957;69:62–63. [Google Scholar]
- 11.Thomson R H. Naturally Occurring Quinones. New York: Academic; 1971. [Google Scholar]
- 12.Eisner T, Dean J. Proc Natl Acad Sci USA. 1976;73:1365–1367. doi: 10.1073/pnas.73.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aneshansley D J, Eisner T, Widom J M, Widom B. Science. 1969;165:61–63. doi: 10.1126/science.165.3888.61. [DOI] [PubMed] [Google Scholar]
- 14.Dean J. J Comp Physiol. 1980;135:41–50. [Google Scholar]
- 15.Dean J. J Comp Physiol. 1980;135:51–59. [Google Scholar]
- 16.Schildknecht H, Holoubek K. Angew Chem. 1961;73:1–7. [Google Scholar]
- 17.Schildknecht J, Maschwitz E, Maschwitz U. Z Naturforsch. 1968;23b:1213–1218. [PubMed] [Google Scholar]
- 18.Dean J, Aneshansley D J, Edgerton H E, Eisner T. Science. 1990;248:1219–1221. doi: 10.1126/science.2349480. [DOI] [PubMed] [Google Scholar]
- 19.Westwood J O. An Introduction to the Modern Classification of Insects. Vol. 1. London: Longman; 1839. [Google Scholar]
- 20.Roach B, Dodge K R, Aneshansley D J, Wiemer D, Meinwald J, Eisner T. Coleopterists Bull. 1979;33:17–20. [Google Scholar]
- 21.Aneshansley D J, Jones T H, Alsop D, Meinwald J, Eisner T. Experientia. 1983;39:366–368. [Google Scholar]
- 22.Eisner T, Aneshansley D J. Science. 1982;215:83–85. doi: 10.1126/science.215.4528.83. [DOI] [PubMed] [Google Scholar]
- 23.Eisner T, Attygalle A B, Eisner M, Aneshansley D J, Meinwald J. Chemoecology. 1991;2:29–34. [Google Scholar]
- 24.Jacobs W, Renner M. Biologie und Ökologie der Insekten: Ein Taschenlexikon. Stuttgart: Fischer; 1988. [Google Scholar]
- 25.Iyengar, V. K. & Eisner, T. (1999) Proc. Natl. Acad. Sci. USA, in press.