Abstract
In the yeast Saccharomyces cerevisiae, the synthesis of endogenous trehalose is catalyzed by a trehalose synthase complex, TPS, and its hydrolysis relies on a cytosolic/neutral trehalase encoded by NTH1. In this work, we showed that NTH2, a paralog of NTH1, encodes a functional trehalase that is implicated in trehalose mobilization. Yeast is also endowed with an acid trehalase encoded by ATH1 and an H+/trehalose transporter encoded by AGT1, which can together sustain assimilation of exogenous trehalose. We showed that a tps1 mutant defective in the TPS catalytic subunit cultivated on trehalose, or on a dual source of carbon made of galactose and trehalose, accumulated high levels of intracellular trehalose by its Agt1p-mediated transport. The accumulated disaccharide was mobilized as soon as cells entered the stationary phase by a process requiring a coupling between its export and immediate extracellular hydrolysis by Ath1p. Compared to what is seen for classical growth conditions on glucose, this mobilization was rather unique, since it took place prior to that of glycogen, which was postponed until the late stationary phase. However, when the Ath1p-dependent mobilization of trehalose identified in this study was impaired, glycogen was mobilized earlier and faster, indicating a fine-tuning control in carbon storage management during periods of carbon and energy restriction.
Trehalose is a nonreducing disaccharide of α(1,1)-linked glucose present in many organisms, including bacteria, fungi, insects, and plants (12). Fungal cells can accumulate this disaccharide to up to 15% of the cell dry mass depending on growth conditions and environmental stress (for a review, see reference 15). Genetic and metabolic studies led to the proposal that trehalose plays two distinct functions in living cells. On the one hand, it acts as a stress protectant of proteins and biological membranes against adverse conditions (39). On the other hand, it may play a role as a storage carbohydrate in the yeast Saccharomyces cerevisiae. This latter function was suggested by the rapid mobilization of intracellular trehalose upon the resumption of growth of starved cells on a fresh glucose medium and during spore maturation and germination, as well as during oscillatory events in continuous or batch yeast cultures (22, 28). Trehalose is also consumed very slowly when cells are maintained in nongrowing conditions, and this breakdown often follows that of glycogen, the major storage carbohydrate in yeast (26, 32), although the mobilization pattern of one glucose store before the other can be dependent on the growth conditions (17, 34, 37).
In the yeast S. cerevisiae, the intracellular level of trehalose is the result of a well-regulated balance between enzymatic synthesis and degradation. The synthesis of trehalose is catalyzed by an UDP-glucose-dependent trehalose synthase (TPS) protein complex encoded by four genes. TPS1 and TPS2 encode the trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase, respectively, and TPS3 and TSL1 code for two regulatory subunits of the TPS complex (for a review, see reference 15). The tps1 mutants not only lack the ability to synthesize trehalose but also exhibit several pleiotropic defects including an inability to grow on glucose (3, 20), alterations in glycogen synthesis (6, 42) and in respiration (18), and a sporulation deficiency (9). Altogether, these phenotypic traits support the idea that Tps1p plays a key regulatory role in the control of yeast physiology (15, 16, 41). Hydrolysis of trehalose can be carried out by two enzymatic systems: a neutral trehalase encoded by NTH1 (24a) and an acid trehalase encoded by ATH1 (10). A third gene, namely, NTH2, a paralog of NTH1 (77% identity at the protein level) exists in the yeast genome, but no trehalase activity has been associated with its product so far. Moreover, only Nth1p is known to catalyze the intracellular mobilization of trehalose (31), and the apparent lack of Ath1p to carry out this function could be attributed to its exclusion from the cytosolic compartment (23).
Trehalose is also a potential carbon source for many yeast species (2) including the yeast S. cerevisiae. Recently, we demonstrated that the assimilation of exogenous trehalose takes place by two different enzymatic pathways (23). The first one relies on Ath1p, whose major activity was measured extracellularly and thereby hydrolyzes at the cell surface the disaccharide into two glucose units, which are thereafter taken up by hexose transporters. The second pathway consists of a coupling between the Agt1p-mediated trehalose transport (36) and the Nth1p-dependent hydrolysis of the imported disaccharide (23). It is noteworthy that the expression of AGT1, which encodes a high-affinity H+-disaccharide symporter and belongs to the major facilitator superfamily (for a review, see reference 8), is dependent on the MAL system (19). This feature explains why the Agt1-Nth1p pathway is defective in mal-negative strains, as for instance in the BY background (4).
During our investigation of the physiological properties of S. cerevisiae tps1 mutants cultivated on trehalose or on a dual carbon source, i.e., galactose and trehalose, we observed that the Agt1p-dependent accumulation of intracellular trehalose was followed by a rapid mobilization of this intracellular store. This mobilization occurred when the exogenous carbon source was no longer available, and surprisingly, before that of glycogen. Our work led to the finding that the major degradation of endogenous trehalose takes place in the absence of Nth1p and Nth2p. We here report that the mobilization of intracellular trehalose involved its export and cleavage into glucose at the cell surface by Ath1p.
MATERIALS AND METHODS
Media and culture conditions.
Yeast cells were routinely cultured in shake flasks (1-liter Erlenmeyer flasks with a working volume of 200 ml) at 30°C in YN synthetic medium (1.7 g liter−1 yeast nitrogen base without amino acids and without ammonium sulfate [Difco Laboratories]) and 5 g liter −1 ammonium sulfate. The carbon source was either 2% trehalose or 2% galactose plus 1% trehalose (wt/vol). YN medium was buffered at pH 4.8 by the addition of 14.3 g liter−1 succinic acid and 6 g liter−1 NaOH (23). The growth was followed by measuring the absorbance at 600 nm with an Easyspec IV spectrophotometer (Safas, Monaco, France). Optical density at 600 nm (OD600) values could be converted to cell dry mass by use of a calibration curve that was previously established for the CEN.PK113-7D strain under these growth conditions, i.e., 0.41 g liter−1 of dry cell for one OD600 unit. The maximal specific growth rate (μmax) of the cultures was calculated by fitting an exponential regression over the experimental data points as described in reference 32. These points were selected so that they led to a correlation coefficient (r2) higher than 0.998.
All experiments reported in this work have been performed at least three times, starting from independent precultures and independent medium preparation. For the sake of clarity, the figures report data from one representative experiment only.
Cell transfer and drug treatment.
For transfer of (pre)stationary-phase cells, yeast culture was divided into equal parts and centrifuged for 2 min at 4,000 × g. The pellets were rinsed once with sterile water to discard extracellular trehalose traces. They were resuspended in either the initial YN trehalose culture medium or a fresh YN medium without a carbon source buffered at pH 5 or 7. By use of the same procedure, yeast cells were also suspended in a trehalose-depleted YN medium which was obtained from stationary-phase Ath1+ cells. The inhibition of sugar uptake was carried out by the addition of NaF (final concentration, 50 mM) as described previously (23). Samples for the measurement of extracellular sugar and intracellular trehalose and glycogen levels were taken over a period of at least 200 h.
Strain and plasmid construction.
The prototrophic MAL constitutive CEN.PK113-7D strain (MATa MAL2-8c SUC2 [45]) was used as the wild type and the host strain for mutant constructions (Table 1). Constructions of tps1, nth1, nth2, ath1, nth1 nth2, and nth1 nth2 ath1 null mutants have been described in previous works (18, 23). The construction of the atg1 mutant was carried out by homologous recombination with a PCR product that was obtained as follows. A 2.8-kb fragment bearing the atg1::kanMX4 allele was amplified by PCR using genomic DNA from BY agt1::Kanr from the Euroscarf mutant collection (http://web.uni-frankfurt.de/fb15/mikro/euroscarf/index.html) (4) and primers ATG1-600 (5′-TTCCCGTTAATCATCTTTTC-3′) and ATG1+600 (5′-CGGATCCTAATACCAATTCT-3′). Correct replacement of the gene in the mutant strain was analyzed by PCR. Strains that combined tps1, nth1, nth2, and atg1 mutations were obtained by use of classical genetics procedures, i.e., crosses, sporulation, and marker-based selection (Table 1). The construction of the NTH2-overexpressing plasmid (pNTH2) used in this work has been previously described, and this plasmid was referred to as pGRSd-NTH2 (23).
TABLE 1.
Strains used in this study
| Strain name/mutation | Genotype | Reference or source |
|---|---|---|
| CEN.PK113-7D | aMAL2-8cSUC2 | 45 |
| CEN.PK113-1A | α MAL2-8cSUC2 | 45 |
| tps1 | aMAL2-8cSUC2 ura3-52 tps1Δ::URA3 | 18 |
| ath1 | aMAL2-8cSUC2 ath1Δ::kanMX4 | 23 |
| nth1 | aMAL2-8cSUC2 nth1Δ::kanMX4 | 23 |
| nth1 nth2 | aMAL2-8cSUC2 leu2 nth1Δ::kanMX4 nth2Δ::LEU2 | 23 |
| ath1 nth1 | aMAL2-8cSUC2 ath1Δ::kanMX4 nth1Δ::kanMX4 | 23 |
| ath1 nth1 nth2 | aMAL2-8cSUC2 leu2 ath1Δ::kanMX4 nth1Δ::kanMX4 nth2Δ::LEU2 | 23 |
| tps1 nth1 | aMAL2-8cSUC2 ura3-52 tps1Δ::URA3 nth1Δ::kanMX4 | This study |
| tps1 nth2 | aMAL2-8cSUC2 ura3-52 leu2 tps1Δ::URA3 nth2Δ::LEU2 | This study |
| tps1 nth1 nth2 | aMAL2-8cSUC2 ura3-52 leu2 tps1Δ::URA3 nth1Δ::kanMX4 nth2Δ::LEU2 | This study |
| tps1 nth1 nth2 ath1 | aMAL2-8cSUC2 ura3-52 leu2 tps1Δ::URA3 nth1Δ::kanMX4 nth2Δ::LEU2 ath1Δ::kanMX4 | This study |
| tps1 ath1 | aMAL2-8cSUC2 ura3-52 tps1Δ::URA3 ath1Δ::kanMX4 | This study |
| tps1 nth1 apg1 | aMAL2-8cSUC2 ura3-52 leu2 tps1Δ::URA3 nth1Δ::kanMX4 nth2Δ::LEU2 apg1Δ::kanMX4 | This study |
| tps1 nth1 nth2 apg1 | aMAL2-8cSUC2 ura3-52 leu2 tps1Δ::URA3 nth1Δ::kanMX4 nth2Δ::LEU2 apg1Δ::kanMX4 | This study |
Determination of intracellular trehalose and glycogen and extracellular sugars.
Cell samples corresponding to 15 to 30 OD600 were quickly harvested and centrifuged for 2 min at 4,000 × g in screw-cap microfuge tubes, and the pellet was used for glycogen and trehalose level determination (33). Trehalose in the culture medium was measured by adding 0.6 ml of 0.2 M Na-acetate, pH 5.2, to 0.4 ml of supernatant, followed by digestion with trehalase as described before. Glucose in the medium was directly measured using a glucose oxidase assay (kit 540; Sigma-Aldrich). The values for glycogen and trehalose were given as equivalent glucose per OD600 unit. One OD600 unit corresponds to 1.4 × 106 cells or 0.4 mg cell dry mass.
Preparation of extracts and enzyme assays.
Yeast cells (equivalent dry mass, 30 mg) were harvested by centrifugation (3,000 × g, 5 min, 4°C) and resuspended in 0.5 ml of extraction buffer (20 mM HEPES, pH 7.1, 1 mM EDTA, 100 mM KCl, completed just before use with 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride). The cell suspension was vigorously disrupted by vortexing in the presence of 1 g of glass beads for four 30-s periods at 4°C. After centrifugation (16,000 × g for 10 min), the supernatant was used as a crude extract for enzyme assays. Acid trehalase activity was measured as previously described (23) in a total volume of 0.5 ml containing 315 mM sodium citrate, pH 4.5, 1.4 mM EDTA, and 55 mM trehalose. Unless otherwise stated, the neutral trehalase was assayed as previously described (29) in a total volume of 0.5 ml containing 20 mM HEPES (pH 7.1), 2.5 mM CaCl2, and 50 mM trehalose. One unit is defined as the amount of enzyme that catalyzes the hydrolysis of 1 μmol of trehalose per minute under the conditions of the assay.
RESULTS
Rapid mobilization of intracellular trehalose store in tps1 mutants cultivated on trehalose.
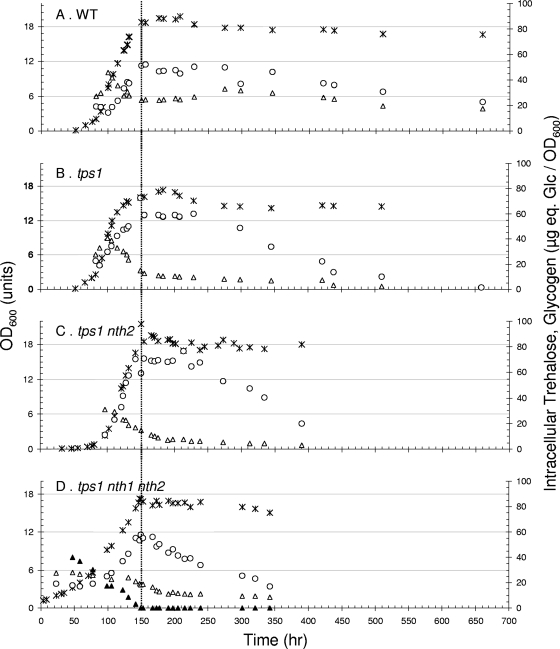
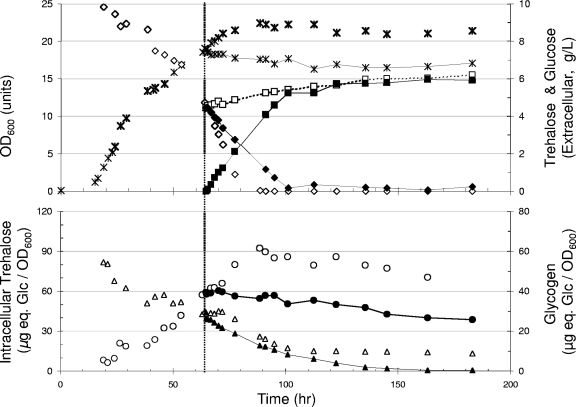
In recent work, we showed that the growth of S. cerevisiae on trehalose as the sole carbon source was strictly respiratory, with a maximal growth rate of about 0.07 h−1 (22, 23). Under this condition, wild-type cells accumulated intracellular trehalose to up to 10% of the dry mass (Fig. 1A). The disaccharide was then partially mobilized (a drop from 10 to ∼6% of dry mass) during a transition phase preceding the entrance of cells into stationary phase. It then remained at this level for about 150 to 200 h and was further degraded very slowly without being completely exhausted even after 700 h of culture. Glycogen also accumulated with the growth and reached a maximum of 12% of dry mass at the end of the growth phase on trehalose (Fig. 1A). It remained at this high level for about 100 h in the stationary phase, which was followed by a very slow mobilization to drop by only 30% over the next 200 h of the culture.
FIG. 1.
The loss of TPS1 triggered the breakdown of intracellular trehalose independently of neutral trehalases. Cultures were performed on YN medium set at pH 4.8 with 2% (wt/vol) trehalose. Wild-type (WT) CEN.PK113-7D strain (A) and its derivative tps1 (B), tps1 nth2 (C), and tps1 nth1 nth2 (D) mutants are shown. Symbols: , OD; ▴, extracellular trehalose; ▵, intracellular trehalose; ○, glycogen. For the sake of clarity, the time scales were arbitrary arranged so that all the strains enter the stationary phase simultaneously, at the time when exogenous trehalose has been completely consumed (vertical dashed line).
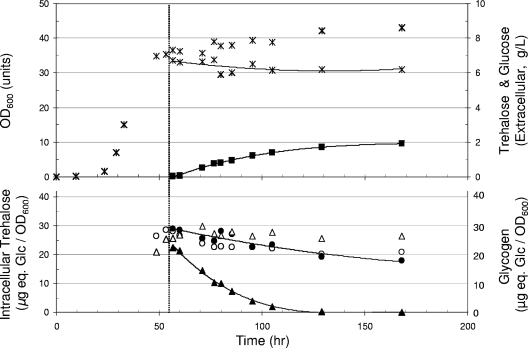
The loss of TPS1 function did not modify the specific growth rate on trehalose, although the biomass yield was about 10% lower than that for the wild type (Fig. 1B). Also, this genetic defect did not prevent the accumulation of intracellular trehalose, which reached a maximal level comparable to that in the wild-type cells at the end of the exponential phase of growth. This result favored the idea that in wild-type cells, the accumulation of trehalose arose mainly from the active uptake of exogenous trehalose by the AGT1-encoded transporter (36). In contrast to what was seen for the wild-type cells, however, the mobilization of trehalose was faster in tps1 cells. Most of the disaccharide was consumed during the short transition phase that preceded the entrance into the stationary phase, while the remaining part was slowly mobilized and fully depleted in less than 300 h (Fig. 1B). The deletion of TPS1 also significantly modified the pattern of glycogen degradation, which started earlier than in the wild-type strain and was almost complete after 300 h of growth in the stationary phase (Fig. 2B). Taken together, these results revealed a notable impact of TPS1 deletion that promoted a rapid trehalose mobilization and reversed the usual observation that trehalose degradation follows that of glycogen during the stationary phase of growth (14, 26, 32).
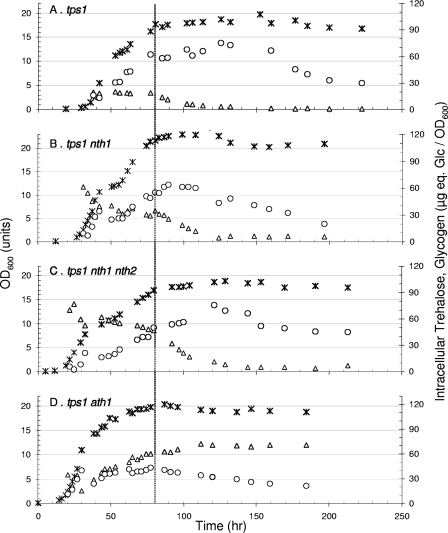
FIG. 2.
Ath1p is indispensable for the complete mobilization of intracellular trehalose in a tps1 mutant. Cells were cultivated in YN medium set at pH 4.8 containing 2% (wt/vol) galactose and 1% (wt/vol) trehalose. Symbols are as in Fig. 1.
The tps1-promoted intracellular trehalose mobilization is independent of neutral trehalases.
To know whether NTH1 and/or NTH2 was implicated in this rapid mobilization of intracellular trehalose, we carried out similar experiments with tps1 mutants from which either one or both of the NTH1 and NTH2 genes were further deleted. The growth of the tps1 nth1 mutant on this carbon source was twofold lower than that for the wild type or the tps1 mutant (0.03 versus 0.07 h−1), due to the absence of the Agt1p-Nth1p-dependent pathway (23). In spite of this growth rate reduction, the deletion of NTH1 did not modify the pattern of trehalose accumulation and breakdown (data not shown). The deletion of NTH2 in the tps1 strain had no notable effect on growth rate and trehalose accumulation. We nevertheless repeatedly found that the mobilization of the accumulated trehalose was lower than that in the tps1 cells during the transition period and extended beyond the onset of the stationary phase (Fig. 1C). The nth1 nth2 tps1 mutant exhibited the combined phenotypes mentioned for nth1 and nth2 mutants (Fig. 1D), which indicated that rapid and sustained trehalose mobilization in a tps1 mutant was largely independent of both Nth1p and Nth2p.
The mobilization of cytosolic trehalose is dependent on Ath1p.
The finding of intracellular trehalose mobilization in a tps1 mutant lacking both NTH1 and NTH2 prompted us to investigate whether this phenotype could be attributed to the trehalase encoded by ATH1. Since Ath1p is essential for growth on trehalose in the absence of the Agt1p-Nth1p pathway (23), it was not possible to investigate this question using a tps1 nth1 nth2 mutant that was further defective in Ath1p. We circumvented this difficulty by cultivating the quadruple tps1 nth1 nth2 ath1 mutant in a galactose medium containing trehalose, since this medium is permissive for the growth of tps1 mutants and enables trehalose accumulation by Agt1p-mediated transport (35). Under this condition, the culture of the tps1 strain followed a biphasic growth (Fig. 2A), i.e., a first phase corresponding to the growth on galactose followed by a second purely oxidative growth phase on the remaining trehalose and on the ethanol produced from galactose catabolism. At the time when the latter two carbon sources were depleted, cells entered the stationary phase, and trehalose that had accumulated during the growth to ∼25 μg eq Glc/OD600 (6% of dry mass) was readily degraded. As already observed in Fig. 1, the mobilization of the disaccharide occurred prior to that of glycogen. The deletion of NTH1 in the tps1 mutant did not alter the growth kinetic but resulted in a twofold increase of trehalose content in the postdiauxic phase of growth (Fig. 2B). Quite interestingly, the tps1 nth1 nth2 mutant accumulated even more trehalose than the tps1 nth1 mutant (Fig. 2C). Altogether, these results provided additional genetic evidence that NTH2 may encode a functional trehalase.
This experimental setup described above then allowed study of the role of acid trehalase by deletion of ATH1 from a tps1 nth1 nth2 strain. As can be seen in Fig. 2D, this mobilization was actually dependent on Ath1p, since the deletion of ATH1 completely prevented this degradation. In this mutant, the trehalose level remained at about 17% of the dry mass in the stationary phase, and it was even higher in the tps1 ath1 nth1 nth2 mutant defective in all three trehalases, reaching the exceptional level of 25% of the cell dry mass (data not shown). It can also be seen from this figure that glycogen was mobilized in ath1 mutants earlier than in the Ath1+ cells and as soon as cells entered the stationary phase. It is thus possible that this degradation of glycogen compensated for the lack of trehalose mobilization to supply the cells with carbon and energy during stationary phase.
The mobilization of intracellular trehalose by Ath1p requires the export of the disaccharide.
Recently, we showed that a significant part of Ath1p activity was measured extracellularly (23). We therefore assumed that the remaining intracellular, most probably vacuolar, trehalase activity (1, 24) could be responsible for trehalose catabolism, similarly to the vacuolar glucoamylase-dependent degradation of glycogen during the late stationary phase (46, 47). Since this later process is dependent on ATG1, which encodes a serine/threonine protein kinase required for autophagy (27, 40), we evaluated whether trehalose degradation followed the same fate as glycogen by disrupting ATG1 in both tps1 nth2 and tps1 nth1 nth2 mutants. Contrary to expectation, these mutants were still able to mobilize trehalose with the same kinetic observed for their isogenic control strains (data not shown), while glycogen mobilization was altered as previously reported (46). Altogether, these data showed that macroautophagy did not bring intracellular trehalose to the vacuole for subsequent cleavage, even though this pathway was functional under our growth conditions and actively participated in glycogen catabolism.
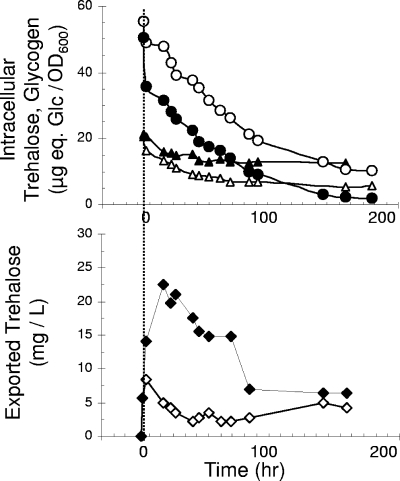
We therefore examined the possibility that the mobilization of intracellular trehalose relied on the extracellular pool of Ath1p (23). As a first step to evaluate this mechanism, we took advantage of the pH sensitivity of Ath1p, since we previously showed that Ath1p-dependent trehalose assimilation was optimal at pH 5.0 and was severely impaired at neutral pH (23). Thus, the tps1 nth1 nth2 mutant was cultivated on a trehalose medium at pH 4.8 until it had accumulated enough disaccharide (onset of stationary phase) (Fig. 1D). These trehalose-loaded tps1 nth1 nth2 cells were then transferred to a carbon-free minimal medium set at pH 5 or 7. As shown in Fig. 3, the mobilization of intracellular trehalose was faster in yeast cells resuspended in a pH 5.0 medium, whereas the converse was found for glycogen degradation. This result is an additional evidence of a close interaction between these two glucose stores in the management of carbon and energy during poor growth conditions. More importantly, we detected the presence of trehalose in the resuspension culture medium, indicating that the disaccharide was exported out of the cells. In both culture media, a transient burst of trehalose that was about five times higher in the culture medium at pH 7.0 than at pH 5.0 was observed, in agreement with a higher Ath1p activity at this latter pH (23). After the peak, trehalose declined and remained at very low but detectable levels of approximately 0.05 mM. Altogether, these results favored the idea that the mobilization of an intracellular trehalose store in a tps1 mutant cultivated on trehalose requires the export of the disaccharide and its hydrolysis at the cell surface by Ath1p.
FIG. 3.
Intracellular trehalose mobilization and its export into the medium. Trehalose-loaded tps1 nth1 nth2 cells cultivated on YN trehalose were centrifuged, washed once with water, resuspended in a fresh medium free of any carbon source, and set at pH 5.0 (empty symbols) or 7.0 (full symbols). (Top) Mobilization of intracellular trehalose (▵, ▴) and glycogen (○, •). (Bottom) Trehalose exported from intracellular stores and measured in the culture medium (⋄, ⧫).
The trehalase Ath1p exerts a pull effect on the export of intracellular trehalose.
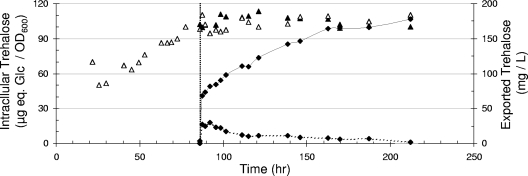
To further demonstrate the occurrence of a trehalose export, we transferred trehalose-loaded tps1 nth1 nth2 ath1 mutant cells in a carbon-free medium set at pH 5.0. Following this transfer, the disaccharide was immediately expulsed from the medium and accumulated to 100 mg/liter after 200 h of incubation, without a significant decrease of the intracellular store (Fig. 4). This result indicated that the equilibrium between trehalose export and Agt1p-mediated import was in favor of the intracellular location of the disaccharide. The export of trehalose and the partition between intracellular and extracellular disaccharide were not modified when yeast cells were transferred in a medium set at pH 7.0 (data not shown), indicating that the effect of increasing the pH (Fig. 3) merely affected the activity of Ath1p. Very interestingly, the presence of extracellular Ath1p shifted this equilibrium and resulted in a net mobilization of the intracellular trehalose. This was shown by transferring trehalose-loaded tps1 nth1 nth2 ath1 (Ath1−) cells in a filtered, carbon-depleted growth medium obtained from a culture of the tps1 nth1 nth2 (Ath1+) strain grown to stationary phase. According to our previous data (23), free secreted Ath1p (approximately 5% of the total activity) can be found in the supernatant of stationary-phase culture on trehalose. As indicated in Fig. 4, the presence of this “exogenous” Ath1p activity in the resuspension medium partially prevented the accumulation of trehalose in the medium while promoting the complete intracellular depletion of the disaccharide (not shown). Altogether, these results indicated that extracellular trehalose cleavage into glucose by Ath1p is necessary for the efficient export of the disaccharide.
FIG. 4.
Lack of Ath1p results in a weak trehalose accumulation in the medium. Trehalose-loaded tps1 nth1 nth2 ath1 mutant cells cultivated on YN containing galactose-trehalose were transferred into carbon-free medium (vertical dashed line). Intracellular trehalose (▵, control; ▴, after transfer) and trehalose exported in the culture medium after the transfer (⧫) in fresh medium set at pH 5.0 with no carbon source (continuous line) or in a filtered, trehalose-exhausted medium from Ath1+ strain cultivated to the stationary phase (dashed line).
To further validate the fact that the trehalose was expulsed and cleaved at the cell surface by Ath1p, we repeated the experiment in the presence of NaF, as this drug is reported to inhibit carbon metabolism and hence indirectly block the consumption of extracellular glucose (23, 38). As expected, the exposure of tps1 nth1 nth2 (Ath1+) cells to 50 mM NaF before the onset of stationary phase suddenly stopped the assimilation of extracellular trehalose and caused growth cessation (Fig. 5). Moreover, this drug treatment enhanced the mobilization of intracellular trehalose, with a complete depletion after 100 h of incubation. Concomitantly, glucose increased in the culture medium to up to 6 g/liter as a result of the Ath1p-dependent hydrolysis of both the residual trehalose from the medium (∼4 g/liter at the time when NaF was added) and the exported intracellular trehalose store. As a control, we carried out the same experiment with tps1 nth1 nth2 (Ath1+) cells cultivated in galactose as the sole carbon source so it could not accumulate any intracellular trehalose. The addition of NaF to these cells did not cause any release of glucose into the culture medium. This result confirmed that the production of glucose in trehalose-loaded tps1 nth1 nth2 cells after NaF treatment was strictly dependent on the disappearance of its intracellular trehalose store.
FIG. 5.
Addition of NaF to Ath1+ cells provoked a complete depletion of intracellular trehalose, which was recovered in the culture medium as free glucose. Trehalose-loaded cells of the tps1 nth1 nth2 strain cultivated on galactose-trehalose (empty symbols) were treated with 50 mM NaF (vertical dashed line; full symbols). Symbols: , OD; ▵ and ▴, intracellular trehalose; ○ and •, glycogen; ⋄, exogenous trehalose as the substrate. Free glucose in the medium (▪) as measured by glucose oxidase assay after NaF treatment. This glucose arose from Ath1p-dependent hydrolysis of both the residual exogenous trehalose (⧫) and the exported intracellular trehalose store. The total carbon after NaF treatment as given in glucose equivalents (□) includes free glucose in the medium (see above) plus glucose resulting from in vitro trehalase treatment of the culture medium.
The export of trehalose also takes place in Tps1+cells.
The experiments presented above relied on the finding that yeast cells can accumulate a large amount of trehalose through the Agt1p transporter when this sugar is present in the medium. Under this condition, we found that a tps1 mutant exhibited a rapid mobilization of its intracellular store, which seemed to occur by the export of trehalose and its extracellular cleavage by Ath1p. The question was therefore whether this singular event was truly specific to the lack of TPS1. When we studied nth1 nth2 ATH1 cells cultivated on glucose or galactose as the sole carbon source, we found that trehalose accumulated through de novo synthesis. It is shown in Fig. 6 that the treatment of these yeast cells with NaF caused a sudden depletion of the trehalose store concomitantly with the accumulation of free glucose in the culture medium. In addition, when this strain was cultivated in glucose or galactose medium supplemented with 10 g·liter−1 trehalose as previously done for the tps1 mutant, the glucose that was released in the medium arose from the cleavage of trehalose that remained in the growth medium and the one that was exported from the cells (data not shown). Altogether, these results indicate that the export of trehalose in the medium was neither triggered by the loss of TPS1 function nor induced by the presence of extracellular trehalose.
FIG. 6.
Addition of NaF to Tps1+ cells provoked a depletion of intracellular trehalose store, which was recovered in the culture medium as free glucose. The nth1 nth2 (Tps1+ Ath1+) strain was grown on galactose as the sole carbon source. Details and symbols are as for Fig. 5.
The fact that an export of trehalose could be seen for Tps1+ cells allowed us to verify whether the export of the disaccharide was dependent upon the H+/trehalose transporter encoded by AGT1. To this end, the wild type and an agt1 mutant were cultivated on galactose until the stationary phase and were then treated with 50 mM NaF as described above. In both cases, glucose appeared in the medium (data not shown), indicating that the export of trehalose was not due to the reversal of its uptake by Agt1p.
NTH2 encodes a functional trehalase.
Data in Fig. 1 and 2 suggested that NTH2, the paralog of NTH1, may encode a functional trehalase. To gain additional evidence for a role for NTH2 in trehalose metabolism, we tested if the overexpression of NTH2 could rescue the growth defect of an ath1nth1 mutant on trehalose. This experiment was unsuccessful, suggesting that NTH2 encodes a weakly active or nonfunctional trehalase. We therefore carried out enzymatic measurement using various trehalase mutants. As indicated in Table 2, the deletion of NTH1 in either the wild type or the tps1 or ath1 mutant led to a 75% decrease in neutral trehalase activity measured at pH 7.0. The residual 25% activity could be attributed to the product of NTH2, since it was abolished by the deletion of this gene in nth1, ath1 nth1, or tps1 nth1 mutants. In contrast, the overexpression of NTH2 in an nth1 mutant resulted in an approximately 10-fold increase of trehalose-hydrolyzing activity. From data in Table 2, it can be proposed that the NTH2-dependent trehalase activity is dependent on the growth medium, since the “residual neutral trehalase activity” nth1 was about fivefold higher in cells cultivated on trehalose than in stationary-phase cells on glucose. Moreover, this activity is undetectable during growth on glucose and began to be detected at the onset of the diauxic shift to reach a maximum of about 10 milliunits/mg protein (or 0.2 units/g cell dry mass) in the stationary phase. While further biochemical and genetic analyses are currently under way, we verified that Nth2p was optimally active at a neutral pH (Table 2) and at a temperature of 30 to 35°C and displayed an apparent Km of about 10 mM for trehalose (data not shown).
TABLE 2.
Assay of extracellular (acid) and cytosolic (neutral) trehalases with different mutants and growth conditions
| Medium | Strain/genotype | Activity (units/g dry mass) ofa:
|
|
|---|---|---|---|
| Cytosolic (neutral) trehalase | Extracellular (acid) trehalase | ||
| YPD | Wild type | 0.79 (2.51b) | ND |
| ath1 | 0.82 (3.07b) | ND | |
| nth1 | 0.18 | ND | |
| ath1 nth1 | 0.15 0.03 (pH 8.0), 0.06 (pH 6.0) | ND | |
| nth1 nth2 | 0.0 | ND | |
| ath1 nth1 nth2 | 0.0 | ND | |
| nth1 pNTH2 | 1.41 | ND | |
| YN + trehalose | Wild type | 3.81 | 1.81 |
| nth1 | 1.14 | 1.89 | |
| tps1 nth1 | 0.93 | 1.68 | |
| tps1 nth1 nth2 | 0.0 | 1.04 (0.13c) | |
| YN Gal + trehalose | Wild type | 3.80 | 1.80 |
| tps1 nth1 nth2 | 0.0 | 1.55 | |
Neutral and acid trehalases were assayed at pH 7.1 and 4.8, respectively, in crude extracts from stationary-phase cells. Values given are means of three independent experiments. ND, not determined.
Activity measured after a preincubation for 15 min with 1 mM MgATP and 0.1 mM cyclic AMP.
Value of acid trehalase activity measured at pH 7.1.
DISCUSSION
Despite the fact that trehalose has been the focus of many studies over decades, several issues remained ambiguous with respect to the enzymatic machinery devoted to this disaccharide. In this work, we provided two major results that clarify some of these long-standing enquiries. First, we showed that NTH2, a paralog of NTH1, encodes a functional neutral trehalase. Second, we demonstrated that the extracellular acid trehalase encoded by ATH1 can be implicated in the mobilization of endogenous trehalose, and we showed that this process required an export of the disaccharide. On top of these findings, we reinforced previous findings (6, 15, 26) on the existence of an interconnection between trehalose and glycogen in yeast cell carbon storage management.
No trehalase activity has ever been attributed to Nth2p, in spite of a strong sequence similarity with Nth1p, which led some authors to suggest that this protein might be a regulator of Nth1p (30). In this work, we provided genetic and biochemical evidence that NTH2 encodes a functional cytosolic/neutral trehalase with a measurable activity in the stationary phase on glucose or during growth on trehalose. This result is in accordance with the expression level of NTH2, which is low during the exponential phase of growth on glucose, increases in the late exponential phase, and peaks at the onset of the stationary phase (31). Curiously, a similar pattern of expression has been also found for NTH1 (32), although the enzymatic activity of Nth1p followed an opposite trend (13). This opposite profile is explained by a posttranscriptional control of Nth1p by a cyclic AMP-dependent protein kinase phosphorylation that takes place during growth on glucose and causes its activation (30). Whether Nth2p is also regulated by this type of mechanism is an open question. Moreover, while a weak intervention of Nth2p in trehalose mobilization has been uncovered in the experimental context of this work, it still remains to identify other situations that may support its physiological function in yeast.
In a recent work, we demonstrated that the bulk of acid trehalase activity encoded by ATH1 was localized at the cell surface and that this localization accounted for the growth of yeast on trehalose (23). However, we did not exclude the possibility that the residual fraction of Ath1p could be located in the vacuoles (1), thus enabling an autophagy-dependent trehalose mobilization. Autophagy is indeed a self-eating, starvation-induced process, in which various cytoplasmic components such as organelles and cytoplasm are delivered into the vacuole for degradation. The outcome of this process is probably to allow basic building blocks to be regenerated. Such turnover of a large amount of cytoplasm is essential for survival under nutrient-depleted conditions, a condition that de facto matched with the timing and putative role of trehalose consumption observed at the end of growth on trehalose medium. We explored this hypothesis by use of tps1 mutants with deletion of ATG1. This mutation blocks early steps of double-membrane vesicle formation, not only in macroautophagy (autophagosomes) but also in the cytoplasm-to-vacuole targeting biosynthetic pathway. Since the latter appears to exclude bulk cytoplasm and occurs during vegetative conditions to deliver hydrolases into the vacuole, the atg1 mutant was expected in our study to prevent cytosolic trehalose sequestration into the autophagosomes. Contrary to expectation, this mutation did not alter the intracellular trehalose pattern, although a clear effect of the atg1 mutation on glycogen mobilization was observed in total, in agreement with results from Roach's group (46, 47). We concluded that this pathway did not contribute to trehalose catabolism. Other forms of autophagy processes, such as microautophagy that involves the direct engulfment of cytoplasm at the surface of the vacuole (11, 25) or a yet unknown sugar transport system from cytoplasm to vacuole, could not be totally excluded. However, our experimental data strongly support the notion that the intracellular degradation of trehalose involved its export and cleavage into glucose at the cell surface. In favor of this mechanism, the deletion of ATH1 in the tps1 mutant resulted in a moderate accumulation of trehalose in the medium. In addition, the efficiency of trehalose export was promoted by the activity of Ath1p, which indicated a pull effect of this enzyme on the export of the disaccharide.
Very little is known about sugar exporters in the yeast S. cerevisiae, despite a growing number of characterized hexose transporters in this microorganism (43). The physiological significance of most of the 20 hexose transporter proteins has not been elucidated so far, and some of them could be important under atypical growth conditions. As an example, an in vivo expulsion of internal glucose was observed when yeast cells growing in maltose-limiting continuous conditions were exposed to an excess of exogenous maltose (21). This expulsion was explained as a means to prevent a massive entry of glucose into glycolysis and the dissipation of metabolic energy. With respect to trehalose, an export of this disaccharide has been already reported for germinating yeast cells (7). In a previous work, we showed that the Agt1-H+/trehalose symporter also called Mal11p allows the import of trehalose into yeast cells (36). Whether this transporter can also catalyze the reverse reaction, i.e., the export of trehalose, remains an open question, but the possibility is attractive. Using isolated plasma membrane vesicles, it was indeed shown that nonconcentrative maltose transport is possible in the absence of an electrochemical gradient and that maltose transport is in principle reversible (5, 44). Here, we found that this Agt1p/Mal11p transporter cannot mediate the export of trehalose in vivo under the experimental conditions described herein.
Expression studies with wild-type cells showed that ATH1 is regulated by the carbon source: Ath1p activity is low in the presence of fermentable sugars, including galactose, and high during the stationary phase of growth on glucose or during growth on respiratory substrates (24, 38). Our finding that trehalose was readily mobilized during stationary phase in tps1 mutants was originally considered as a singular trait associated with the loss of TPS1. Taking into account the expression data presented above, this active mobilization was apparently quite logical. However, the export system was shown to be active whatever the carbon source and TPS1 allele, raising the question of why such mobilization did not occur in wild-type Tps1+ cells during the stationary phase. A hypothesis to account for this preservation of the trehalose store in wild-type cells is the existence of a “sugar in-out cycling” due to the concomitant and compartmentalized activities of Ath1p and of the trehalose synthase complex, respectively. Accordingly, as for ATH1, genes encoding the TPS complex are derepressed and Tps1p activity is also increased when cells enter stationary phase (3, 15), allowing de novo synthesis of the disaccharide. The absence of Tps1p may break down this cycling, revealing a potent degradation of trehalose in this tps1 mutant context. Based on our study, Ath1p likely plays the leading role in this cycling, favoring outward migration of the disaccharide with the immediate, extracellular cleavage of trehalose into glucose moieties that can be assimilated by the cells.
Finally, these experiments revealed that tps1 mutants present increased energy demand as soon as they face starvation conditions. We also showed in this work that this mutant cultivated on a medium that contains trehalose delayed its glycogen mobilization to after that of trehalose. In this context, earlier and faster glycogen degradation was triggered when trehalose mobilization was prevented. At this stage, our data do not allow for a molecular mechanism that explains the compensatory mechanism between the two glucose stores to be proposed. From a physiological viewpoint, these results definitively pose trehalose as an important energy store in yeast and reveal a fine-tuning control in carbon storage management under starvation conditions.
Acknowledgments
This work was supported in part by the ACI program “Microbiology and Pathogenicity” of the French Ministry of Education. G.B. was supported by a 1-year Marie Curie Young Training Fellowship (no. HPMT-EC-2000-00135) as part of her Ph.D. thesis in J.F.'s laboratory. M.J. was supported by a doctoral grant from the French Ministry of Education and Research.
Footnotes
Published ahead of print on 7 December 2007.
This paper is dedicated to Carlos and Juana Maria Gancedo, friends and colleagues, for their outstanding contributions to the field of yeast biochemistry.
REFERENCES
- 1.Alizadeh, P., and D. J. Klionsky. 1996. Purification and biochemical characterization of the ATH1 gene product, vacuolar acid trehalase, from Saccharomyces cerevisiae. FEBS Lett. 391:273-278. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, J. A. 1997. Sugar utilization by Saccharomyces cerevisiae, p. 35-43. In F. K. Zimmermann and K. D. Entian (ed.), Yeast sugar metabolism. Technomic Publishing Co. Inc., Lancaster, PA.
- 3.Blazquez, M. A., and C. Gancedo. 1995. Mode of action of the qcr9 and cat3 mutations in restoring the ability of Saccharomyces cerevisiae tps1 mutants to grow on glucose. Mol. Gen. Genet. 249:655-664. [DOI] [PubMed] [Google Scholar]
- 4.Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 5.Brondijk, T. H., W. N. Konings, and B. Poolman. 2001. Regulation of maltose transport in Saccharomyces cerevisiae. Arch. Microbiol. 176:96-105. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, J. F., J. R. Pringle, A. Fiechter, and M. Khalil. 1994. Characterization of glycogen-deficient glc mutants of Saccharomyces cerevisiae. Genetics 136:485-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuber, R., E. C. Eleutherio, M. D. Pereira, and A. D. Panek. 1997. The role of the trehalose transporter during germination. Biochim. Biophys. Acta 1330:165-171. [DOI] [PubMed] [Google Scholar]
- 8.De Hertogh, B., F. Hancy, A. Goffeau, and P. V. Baret. 2006. Emergence of species-specific transporters during evolution of the hemiascomycete phylum. Genetics 172:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Silva-Udawatta, M. N., and J. F. Cannon. 2001. Roles of trehalose phosphate synthase in yeast glycogen metabolism and sporulation. Mol. Microbiol. 40:1345-1356. [DOI] [PubMed] [Google Scholar]
- 10.Destruelle, M., H. Holzer, and D. J. Klionsky. 1995. Isolation and characterization of a novel yeast gene, ATH1, that is required for vacuolar acid trehalase activity. Yeast 11:1015-1025. [DOI] [PubMed] [Google Scholar]
- 11.Dubouloz, F., O. Deloche, V. Wanke, E. Cameroni, and C. De Virgilio. 2005. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol. Cell 19:15-26. [DOI] [PubMed] [Google Scholar]
- 12.Elbein, A. D., Y. T. Pan, I. Pastuszak, and D. Carroll. 2003. New insights on trehalose: a multifunctional molecule. Glycobiology 13:17-27. [DOI] [PubMed] [Google Scholar]
- 13.Francois, J., P. Eraso, and C. Gancedo. 1987. Changes in the concentration of cAMP, fructose 2,6-bisphosphate and related metabolites and enzymes in Saccharomyces cerevisiae during growth on glucose. Eur. J. Biochem. 164:369-373. [DOI] [PubMed] [Google Scholar]
- 14.Francois, J., M. J. Neves, and H. G. Hers. 1991. The control of trehalose biosynthesis in Saccharomyces cerevisiae: evidence for a catabolite inactivation and repression of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase. Yeast 7:575-587. [DOI] [PubMed] [Google Scholar]
- 15.Francois, J., and J. L. Parrou. 2001. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:125-145. [DOI] [PubMed] [Google Scholar]
- 16.Gancedo, C., and C. L. Flores. 2004. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 4:351-359. [DOI] [PubMed] [Google Scholar]
- 17.Gimeno-Alcaniz, J. V., J. E. Perez-Ortin, and E. Mattallana. 1999. Differential pattern of trehalose accumulation in wine yeast during the microvinification process. Biotechnol. Lett. 21:271-274. [Google Scholar]
- 18.Guillou, V., L. Plourde-Owobi, J. L. Parrou, G. Goma, and J. Francois. 2004. Role of reserve carbohydrates in the growth dynamics of Saccharomyces cerevisiae. FEMS Yeast Res. 4:773-787. [DOI] [PubMed] [Google Scholar]
- 19.Han, E. K., F. Cotty, C. Sottas, H. Jiang, and C. A. Michels. 1995. Characterization of AGT1 encoding a general alpha-glucoside transporter from Saccharomyces. Mol. Microbiol. 17:1093-1107. [DOI] [PubMed] [Google Scholar]
- 20.Hohmann, S., M. J. Neves, W. de Koning, R. Alijo, J. Ramos, and J. M. Thevelein. 1993. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr. Genet. 23:281-289. [DOI] [PubMed] [Google Scholar]
- 21.Jansen, M. L., J. H. de Winde, and J. T. Pronk. 2002. Hxt-carrier-mediated glucose efflux upon exposure of Saccharomyces cerevisiae to excess maltose. Appl. Environ. Microbiol. 68:4259-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jules, M., J. Francois, and J. L. Parrou. 2005. Autonomous oscillations in Saccharomyces cerevisiae during batch cultures on trehalose. FEBS J. 272:1490-1500. [DOI] [PubMed] [Google Scholar]
- 23.Jules, M., V. Guillou, J. Francois, and J. L. Parrou. 2004. Two distinct pathways for trehalose assimilation in the yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 70:2771-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller, F., M. Schellenberg, and A. Wiemken. 1982. Localization of trehalase in vacuoles and of trehalose in the cytosol of yeast (Saccharomyces cerevisiae). Arch. Microbiol. 131:298-301. [DOI] [PubMed] [Google Scholar]
- 24a.Kopp, M., H. Muller, and H. Holzer. 1993. Molecular analysis of the neutral trehalase gene from Saccharomyces cerevisiae. J. Biol. Chem. 268:4766-4774. [PubMed] [Google Scholar]
- 25.Kunz, J. B., H. Schwarz, and A. Mayer. 2004. Determination of four sequential stages during microautophagy in vitro. J. Biol. Chem. 279:9987-9996. [DOI] [PubMed] [Google Scholar]
- 26.Lillie, S. H., and J. R. Pringle. 1980. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J. Bacteriol. 143:1384-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuura, A., M. Tsukada, Y. Wada, and Y. Ohsumi. 1997. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192:245-250. [DOI] [PubMed] [Google Scholar]
- 28.Munch, T., B. Sonnleitner, and A. Fiechter. 1992. The decisive role of the Saccharomyces cerevisiae cell cycle behaviour for dynamic growth characterization. J. Biotechnol. 22:329-351. [DOI] [PubMed] [Google Scholar]
- 29.Neves, M. J., and J. Francois. 1992. On the mechanism by which a heat shock induces trehalose accumulation in Saccharomyces cerevisiae. Biochem. J. 288:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nwaka, S., and H. Holzer. 1998. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 58:197-237. [DOI] [PubMed] [Google Scholar]
- 31.Nwaka, S., M. Kopp, and H. Holzer. 1995. Expression and function of the trehalase genes NTH1 and YBR0106 in Saccharomyces cerevisiae. J. Biol. Chem. 270:10193-10198. [DOI] [PubMed] [Google Scholar]
- 32.Parrou, J. L., B. Enjalbert, L. Plourde, A. Bauche, B. Gonzalez, and J. Francois. 1999. Dynamic responses of reserve carbohydrate metabolism under carbon and nitrogen limitations in Saccharomyces cerevisiae. Yeast 15:191-203. [DOI] [PubMed] [Google Scholar]
- 33.Parrou, J. L., and J. Francois. 1997. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal. Biochem. 248:186-188. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Torrado, R., J. V. Gimeno-Alcaniz, and E. Matallana. 2002. Wine yeast strains engineered for glycogen overproduction display enhanced viability under glucose deprivation conditions. Appl. Environ. Microbiol. 68:3339-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plourde-Owobi, L., S. Durner, G. Goma, and J. Francois. 2000. Trehalose reserve in Saccharomyces cerevisiae: phenomenon of transport, accumulation and role in cell viability. Int. J. Food Microbiol. 55:33-40. [DOI] [PubMed] [Google Scholar]
- 36.Plourde-Owobi, L., S. Durner, J. L. Parrou, R. Wieczorke, G. Goma, and J. Francois. 1999. AGT1, encoding an alpha-glucoside transporter involved in uptake and intracellular accumulation of trehalose in Saccharomyces cerevisiae. J. Bacteriol. 181:3830-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roustan, J. L., and J. M. Sablayrolles. 2004. Role of trehalose and glycogen in alcoholic fermentation in wine-making conditions. J. Wine Res. 15:189-202. [Google Scholar]
- 38.Silveira, M. C., E. Carvajal, and E. P. Bon. 1996. Assay for in vivo yeast invertase activity using NaF. Anal. Biochem. 238:26-28. [DOI] [PubMed] [Google Scholar]
- 39.Singer, M. A., and S. Lindquist. 1998. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotechnol. 16:460-468. [DOI] [PubMed] [Google Scholar]
- 40.Straub, M., M. Bredschneider, and M. Thumm. 1997. AUT3, a serine/threonine kinase gene, is essential for autophagocytosis in Saccharomyces cerevisiae. J. Bacteriol. 179:3875-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thevelein, J. M., and S. Hohmann. 1995. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem. Sci. 20:3-10. [DOI] [PubMed] [Google Scholar]
- 42.Torija, M. J., M. Novo, A. Lemassu, W. Wilson, P. J. Roach, J. Francois, and J. L. Parrou. 2005. Glycogen synthesis in the absence of glycogenin in the yeast Saccharomyces cerevisiae. FEBS Lett. 579:3999-4004. [DOI] [PubMed] [Google Scholar]
- 43.Van Belle, D., and B. Andre. 2001. A genomic view of yeast membrane transporters. Curr. Opin. Cell Biol. 13:389-398. [DOI] [PubMed] [Google Scholar]
- 44.van der Rest, M. E., A. H. Kamminga, A. Nakano, Y. Anraku, B. Poolman, and W. N. Konings. 1995. The plasma membrane of Saccharomyces cerevisiae: structure, function, and biogenesis. Microbiol. Rev. 59:304-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Dijken, J. P., J. Bauer, L. Brambilla, P. Duboc, J. M. Francois, C. Gancedo, M. L. Giuseppin, J. J. Heijnen, M. Hoare, H. C. Lange, E. A. Madden, P. Niederberger, J. Nielsen, J. L. Parrou, T. Petit, D. Porro, M. Reuss, N. van Riel, M. Rizzi, H. Y. Steensma, C. T. Verrips, J. Vindelov, and J. T. Pronk. 2000. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb. Technol. 26:706-714. [DOI] [PubMed] [Google Scholar]
- 46.Wang, Z., W. A. Wilson, M. A. Fujino, and P. J. Roach. 2001. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol. Cell. Biol. 21:5742-5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson, W. A., Z. Wang, and P. J. Roach. 2002. Systematic identification of the genes affecting glycogen storage in the yeast Saccharomyces cerevisiae: implication of the vacuole as a determinant of glycogen level. Mol. Cell. Proteomics 1:232-242. [DOI] [PubMed] [Google Scholar]