Abstract
The larvae of scarab beetles, known as “white grubs” and belonging to the genera Phyllophaga and Anomala (Coleoptera: Scarabaeidae), are regarded as soil-dwelling pests in Mexico. During a survey conducted to find pathogenic bacteria with the potential to control scarab larvae, a native Serratia sp. (strain Mor4.1) was isolated from a dead third-instar Phyllophaga blanchardi larva collected from a cornfield in Tres Marías, Morelos, Mexico. Oral bioassays using healthy P. blanchardi larvae fed with the Mor4.1 isolate showed that this strain was able to cause an antifeeding effect and a significant loss of weight. Mortality was observed for P. blanchardi, P. trichodes, and P. obsoleta in a multidose experiment. The Mor4.1 isolate also caused 100% mortality 24 h after intracoelomic inoculation of the larvae of P. blanchardi, P. ravida, Anomala donovani and the lepidopteran insect Manduca sexta. Oral and injection bioassays were performed with concentrated culture broths of the Mor4.1 isolate to search for disease symptoms and mortality caused by extracellular proteins. The results have shown that Mor4.1 broths produce significant antifeeding effects and mortality. Mor4.1 broths treated with proteinase K lost the ability to cause disease symptoms and mortality, in both the oral and the injection bioassays, suggesting the involvement of toxic proteins in the disease. The Mor4.1 isolate was identified as a putative Serratia entomophila Mor4.1 strain based on numerical taxonomy and phylogenetic analyses done with the 16S rRNA gene sequence. The potential of S. entomophila Mor4.1 and its toxins to be used in an integrated pest management program is discussed.
Larvae of the scarab beetles (Coleoptera: Scarabaeidae) known as “white grubs” are important soil-dwelling insect pests that feed on the roots of plants; this habitat is pernicious to several important crops worldwide. In some regions of Mexico, losses due to these insects may reach 50% of the corn production (35). “White grubs” belonging to the genera Phyllophaga and Anomala are responsible for major crop damage in Mexico (25, 29). Chemical control is the usual way to cope with these insects, which has negative effects on the environment and induces the development of insect resistance. There are about 68 different species of “white grubs” reported as potential pests in Mexico (25), and to date, no effective biological control agent for scarab larvae has been developed in this country. Only three commercial bacterial products are available worldwide to control “white grubs.” One of them, named Doom, is based on Paenibacillus popilliae, formerly known as Bacillus popilliae, the causal agent of milky disease. This bacterium has been used for more than a decade for the suppression of the Japanese beetle (Popillia japonica Newman) populations in the United States (22). However, because the sporulation of this bacterium is difficult to obtain in vitro (21), it has been manufactured by the production of spores in vivo, using scarab larvae, and therefore its production is limited. The second product, based on the Buibui strain of Bacillus thuringiensis (known as Buihunter) is available in Japan. The B. thuringiensis Buibui strain is active against A. donovani, P. japonica Newman (14, 31), and Exomala orientalis Waterhouse (1). However, the “white grubs” of Cyclocephala hirta LeConte and Cyclocephala pasadenae Casey are not completely susceptible to the Buibui strain. The third product has been developed as a commercial bioinsecticide in New Zealand, and it is based on the bacterium Serratia entomophila (strain A1MO2) (family Enterobacteriaceae), which causes “amber disease” in Costelytra zealandica (11). S. entomophila A1MO2 colonizes the larval gut of the scarab beetle, which causes a reduction in food consumption (the antifeeding effect [AFE]), a clearing of the gut, and the development of an amber coloration (20). At the end of the disease, 1 to 3 months after inoculation, the bacterium invades the hemocoel and causes the death of the insect (20). The molecular mechanism of pathogenicity is unclear. However, the pathogenic determinants seem to be coded in a 155-kb plasmid (16). Sequencing data and genetic evidence have shown that the antifeeding component is part of a large gene cluster that forms a defective prophage (17) containing three DNA regions, (i) the amb2 locus, (ii) the intact lysis cassette, and (iii) the antifeeding prophage cluster. Several genes located in these regions (17, 28) and upstream of the prophage cluster (18) have been implicated in the AFE. It has been proposed that the afp18 gene might encode an active protein responsible for antifeeding activity in the grass grub larvae (17). Also, the sepA, sepB, and sepC genes have been shown to be associated with the disease (18) and to be responsible for a “less stable antifeeding effect” (17). Products of the sepABC genes are considered homologues of the insecticidal toxin complex (Tc) (18) produced by the nematode-associated bacterium Photorhabdus luminescens (4). It is thought that the defective prophage might form a virus-like structure able to cause the AFE and mortality in C. zealandica larvae (15).
On the other hand, it has been suggested that extracellular toxin proteins are involved in the disease, since the AFE and amber coloration are mimicked by concentrated culture broth (CCB) from pathogenic S. entomophila strains (27). However, to date, no protein has been reported in the culture broths of pathogenic S. entomophila.
Amber disease, caused by S. entomophila A1MO2, seems to be specific to C. zealandica (18), and therefore the bacteria and/or the proteins involved in the disease are unable to cause disease in other scarab larvae. Therefore, the discovery and development of entomopathogenic bacteria to reduce larval populations and crop damage caused by a wide spectrum of scarab larvae are very important goals in Mexico and other countries. However, multiple factors have contributed to the delay in the development of microbial agents for the control of scarab larvae in Mexico. One factor is the high diversity of scarab species coexisting in the same soil area, mainly in tropical and subtropical regions. The insects are not reared efficiently under laboratory conditions, and research is almost fully supported for field-collected larvae. Coping with 68 different potential pest species, several of which coexist in the same area at the same time, is an extremely hard task.
In this paper, we report the isolation of a Mexican strain (Mor4.1) putatively identified as a Serratia entomophila strain that is pathogenic for several species of scarab larvae. Also, we report a toxin-like activity in the CCB developed with this strain that causes toxic activity and mortality when administered per os to the larvae of P. blanchardi and mortality when administered by injection to the P. blanchardi and A. donovani bacteria. In addition, we showed the presence of several proteins in the CCB of Mor4.1 that might be associated with toxin-like activity. The finding of a putative S. entomophila bacterial strain pathogenic to Mexican “white grubs” is an important finding for the future development of biotechnological strategies to reduce crop damage by this important complex of soil-dwelling pests.
MATERIALS AND METHODS
Culture media and strains.
Bacteria were grown in nutrient broth or nutrient broth agar (24) at 30°C and maintained in Luria-Bertani broth and 25% glycerol at −70°C. For isolating the Mor4.1 strain, the larvae were first externally washed with 15% sodium hypochlorite and sterile H2O for asepsis. Isolation was done by puncturing the larval body and extracting 20 μl from the body fluid. This aliquot was used as an inoculum, which was grown on nutrient broth agar. Single colonies were propagated and used for bioassays and for further biochemical tests of taxonomic identity of the bacteria. S. entomophila strains A1MO2 and UC9 were kindly provided by Trevor Jackson (AgResearch, Lincoln, New Zealand) and H. K. Mahanty (University of Canterbury, Christchurch, New Zealand), respectively. The S. marcescens S-48 isolate was obtained from the strain collection located at the Facultad de Química (Universidad Nacional Autónoma de México); Serratia marcescens MAC and Escherichia coli DH5α were obtained from the Facultad de Ciencias Agropecuarias (Universidad Autónoma del Estado de Morelos).
Biological material.
Larvae for bioassays were collected mainly from cornfield soils located in different regions of Mexico. P. blanchardi larvae were collected from the community of Buena Vista del Monte (northern Morelos) within remnants of a pine forest. P. ravida and A. donovani larvae were collected from the surroundings of Cuernavaca, Morelos, also within the remnants of a pine forest. P. trichodes larvae were obtained from Joya de Salas, from the Biosphere Reserve El Cielo, Tamaulipas, and P. obsoleta larvae were collected from the surroundings of San Cristobal de las Casas, Chiapas. All larvae were maintained individually under laboratory conditions in trays containing soil from the same locality and small pieces of fresh carrot as food.
Taxonomic identification of “white grub” species associated with cornfields was carried out using adult beetles emerging from field-collected larvae following the recommendations of Najera et al. (26). Taxonomic identification of the larval instars collected randomly from cornfield soil samples was made as described by Villalobos (34).
Larvae of Manduca sexta (Lepidoptera: Sphingidae) were obtained from the Instituto de Biotecnología, Universidad Nacional Autónoma de Mexico (Cuernavaca, Morelos, Mexico). Larvae of Spodoptera frugiperda (Lepidoptera: Noctuidae) were reared at the Centro de Investigación en Biotecnología, Universidad Autónoma del Estado de Morelos (Cuernavaca, Morelos, Mexico).
CCB.
Bacterial cultures were centrifuged at 5,000 × g for 10 min, and supernatants were filter sterilized. CCB samples were obtained by culture supernatant ultrafiltration with a 30-kDa-molecular-mass-cutoff membrane (Centricon-plus-20; Millipore Corp., Bedford, MA) as suggested by the manufacturer. Unless otherwise stated, proteins were diluted finally in sterile water for oral bioassays and in 10 mM Tris buffer, pH 7.4, for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Bioassays with insect larvae.
Oral inoculation tests with bacteria were done basically as reported by Nuñez-Valdez and Mahanty (28) by feeding the larvae with small cylinders of fresh carrot (approximately 16 mm3 each) coated with a single dose of 108 bacteria per larva. Control larvae were fed with uncoated pieces of carrot. A similar multidose experiment was done by giving larvae daily doses of 108 bacteria for 6 consecutive days. After this period was completed, larvae were fed with uncoated carrot. For bioassays using bacteria, parallel tests were run with carrot coated with 108 bacteria of a nonpathogenic E. coli (DH5α) strain, a nonpathogenic Serratia plymuthica ATCC 15928 strain, or a nonpathogenic Enterobacter sp. For toxicity tests done with CCB, larvae were fed for a period of 10 days with small pieces of nutrient broth agar of 16 mm3 containing approximately 700 ng of protein per piece; control larvae were fed with nutrient broth agar pieces prepared with the identical amount of a nontoxic protein (bovine serum albumin). Tests were also done with nutrient broth agar pieces containing CCB from E. coli DH5α culture. After a 10-day period, the larvae were maintained with small pieces of carrot until the end of the bioassays. The AFE was defined as the percentage of larvae that consumed less than 30% of the piece of food. Differences in the numbers of larvae showing amber disease symptoms were assessed by a hypothesis test with two-way frequency tables, using the χ2 statistical test and Student's t test for larval weight.
Bioassays by injection were done by administering 50 μl of bacterial suspension (101 to 107 bacteria per larva) or 50 μl of CCB (30 μg of protein per larva in H2O or nutrient broth) directly into the hemocoel, while control larvae were injected with 50 μl of nutrient broth or bovine serum albumin (30 μg protein per larva in H2O or nutrient broth) or 50 μl of CCB from an E. coli DH5α culture at a similar protein concentration. Mortality was recorded at 24-h intervals. The 50% lethal dose (LD50) and confidence limits were obtained by probit analysis (8).
Treatments of larvae using CCB with proteinase K (Fermentas) were done by adding the enzyme to aliquots of CCB at 200 μg ml−1 and then incubating the culture for 1 h at 37°C. After the CCB aliquots were treated with proteinase K, the samples were heated at 80°C for 15 min to inactivate the enzyme before the corresponding bioassays were performed. Control CCB samples using nutrient broth for injection or oral inoculation tests were also incubated for 1 h at 37°C. Proteinase K hydrolyzes proteins and cleaves peptide bonds adjacent to the carboxyl group of aliphatic and aromatic amino acid residues (7).
All bioassays were done at least twice using 10 to 20 larvae per treatment, according to the availability of larvae. The insects used for oral or injection bioassays were healthy second- and/or third-instar larvae, as indicated above. In all cases, similar results were obtained for replicate experiments.
An oral bioassay with Spodoptera frugiperda (Lepidoptera: Noctuidae) was done with neonate larvae, as described by Aranda et al. (2). The bacterial suspension (107 bacteria per larva) of Mor4.1 was applied to the diet surface.
Bacterial identification.
The characteristics described by Grimont and Grimont (10), by Bergey's Manual of Systematic Bacteriology (13), and by Grimont et al. (11) were used as taxonomical tests for Serratia spp. Similarities among different strains were determined using the matching coefficient (Ss) method based on the percentage of the characteristics that are common to two organisms, that is, characteristics that are present or absent in both organisms (23, 30). Ss was calculated according to the formula Ss = a + d/a + b + c + d, where a is the number of characteristics present in both organisms, b is the number of characteristics present in organism 1 and absent in organism 2, c is the number of characteristics absent in organism 1 and present in organism 2, and d is the number of characteristics absent in both organisms.
For molecular identification, amplification of the 16S rRNA gene of the Mor4.1 isolate was done using Taq DNA polymerase (Fermentas) from Mor4.1 genomic DNA, and the 1.5-kb DNA fragment was purified from a 1% agarose gel, using the commercial product for DNA purification, PureLink quick extraction kit (Invitrogen). The PCR product was sequenced in the automated sequencing facilities of the Instituto de Biotecnología, UNAM (Cuernavaca, México). Universal primers directed toward the 16S rRNA gene were modified from primers reported previously (12). The forward primer used was 5′ CGG AAT TCA GAA GTT TGA TCM TGGMCTC AG 3′, and the reverse primer was 5′ CGG GAT CCA AGG AGG TGA TCC ANC CRC A 3′.
RESULTS
Isolation of the Mor4.1 strain and oral pathogenicity tests with bacteria.
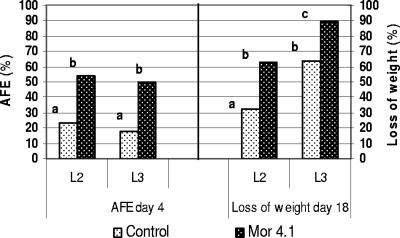
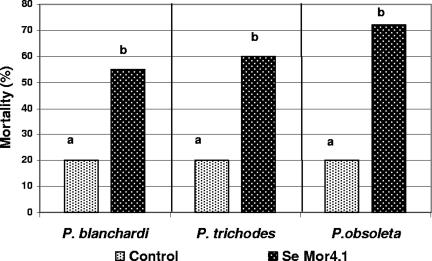
During a general survey conducted for pathogenic bacteria in Mexico with the potential to control larvae and to reduce crop damage caused by “white grubs,” a native bacterial strain was isolated from the hemocoel of a dead third-instar larva of P. blanchardi that showed previous cessation of feeding and an amber appearance. The larva was collected from the soil of a cornfield located at Tres Marias, Morelos, Mexico. Oral bioassays were done to test the ability of the Mor4.1 isolate to cause pathogenic symptoms: AFE, development of amber coloration, and reduction in larval weight. Healthy larvae of P. blanchardi were fed with small pieces of carrot coated with the Mor4.1 isolate, as indicated in Materials and Methods. Larvae in the control treatment group were fed with uninoculated carrot. The Mor4.1 strain caused AFEs (54% and 50%, respectively, for second- and third-instar larvae; P < 0.05) after 4 days from the beginning of the test and a significant reduction in larval weight (63.7% and 89.5%, respectively, for second- and third-instar larvae; t test, P < 0.05) after 18 days (Fig. 1). No significant AFE was observed for control larvae fed with uninoculated carrot (23% and 18% for second- and third-instar larvae, respectively) or for larvae fed with nonpathogenic bacteria (20% for S. plymuthica, Enterobacter sp., or E. coli DH5α; data not shown). Significant mortality was not observed after using a single dose of bacteria; however, when multidose experiments were performed with three Phyllophaga spp. (Fig. 2), significant mortality rates of 55% (P < 0.01), 60% (P < 0.01), and 72% (P < 0.05) were observed after 27, 20, and 37 days from the beginning of the assay for P. blanchardi, P. trichodes, and P. obsoleta, respectively. A mortality rate of 20% was observed for P. blanchardi larvae fed in a similar way with the nonpathogenic bacterium S. plymuthica ATCC 15928 after 27 days from the beginning of the test. Similarly, a mortality rate of 20% was observed for P. trichodes larvae (after 20 days) and for P. obsoleta larvae (after 37 days) fed with a nonpathogenic Enterobacter sp. Development of an amber coloration was not observed for any of these treatments.
FIG. 1.
Oral bioassays done with a single inoculation of S. entomophila Mor4.1 bacteria. The AFE and loss of weight in second-instar (L2; n = 13) and third-instar (L3; n = 12) P. blanchardi larvae are shown. The AFE was recorded after 4 days and the loss of weight after 18 days from inoculation. Statistical differences among treatments and controls were evaluated by χ2 and Student t tests for larval weight (P < 0.05). Lack of significant difference is indicated by the same letter (a, b, or c) above the bars.
FIG. 2.
Multidose experiments were done by orally inoculating Phyllophaga spp. with S. entomophila Mor4.1. Mortality was recorded after 27 days from inoculation for third-instar larvae of P. blanchardi (n = 20), after 20 days for P. trichodes larvae (n = 20), and after 37 days for P. obsoleta larvae (n = 11). P. blanchardi control larvae were fed with the nonpathogenic bacterium S. plymuthica ATCC 15928, or P. trichodes and P. obsoleta were fed with Enterobacter spp. in independent bioassays. A lack of significant differences is indicated by the same letter above the bars (χ2, P < 0.01 for P. blanchardi and P. trichodes; P < 0.05 for P. obsoleta).
The potential of the Mor4.1 isolate to cause mortality in the lepidopteran S. frugiperda larvae was also evaluated. No mortality was observed with oral inoculation bioassays (results not shown).
Pathogenicity tests by intracoelomic inoculation of bacteria.
To test the ability of the Mor4.1 isolate to cause mortality by injection, healthy third-instar larvae of P. blanchardi, P. ravida, and A. donovani were injected with 107 bacteria per larva. This concentration of bacteria killed 100% of the larvae 24 h after injection (P < 0.001). The same amount of E. coli DH5α injected into the larvae did not cause significant mortality (Table 1). Then, to evaluate the Mor4.1 virulence and to estimate the Mor4.1 LD50, healthy third-instar larvae of P. blanchardi were injected with different concentrations of bacteria, ranging from 101 to 106 bacteria per larva. The LD50 was 994 bacteria per larva (95% confidence interval, 89 to 10,206). All larvae injected with 106 and 105 bacteria died 24 and 48 h, respectively, after inoculation, while larvae injected with doses of <105 bacteria died gradually within a time period of 16 days postinoculation.
TABLE 1.
Bioassay of intracoelomic inoculation of insect larvae with bacteria and CCB
| Pathogen treatment | Incubation time (h) | Insect species (instar no.) | Mortality (%) | Mortality occurrence and significance level
|
|
|---|---|---|---|---|---|
| No. of days to mortality (larva n) | P value | ||||
| Bacteriaa | |||||
| S. entomophila Mor4.1 | P. blanchardi (L3) | 100 | 1 (20) | <0.001 | |
| S. entomophila Mor4.1 | P. ravida (L3) | 100 | 1 (10) | <0.001 | |
| S. entomophila Mor4.1 | A. donovani (L3) | 100 | 1 (10) | <0.001 | |
| S. entomophila Mor4.1 | M. sexta (L5) | 100 | 1 (10) | <0.001 | |
| Control | |||||
| E. coli DH5α | |||||
| E. coli DH5α | P. blanchardi (L3) | 0 | 1 (20) | ||
| E. coli DH5α | P. ravida (L3) | 22 | 1 (10) | ||
| E. coli DH5α | A. donovani (L3) | 0 | 1 (10) | ||
| E. coli DH5α | M. sexta (L5) | 0 | 1 (10) | ||
| CCBb | |||||
| S. entomophila Mor4.1 | 72 | P. blanchardi | 80 | 2 (14) | <0.001 |
| S. entomophila Mor4.1 | Anomala spp. (L3) | 80 | 6 (10) | <0.001 | |
| S. entomophila Mor4.1 with proteinase K | 72 | P. blanchardi (L3) | 0 | 2 (14) | |
| S. entomophila Mor4.1 with proteinase K | A. donovani (L3) | 0 | 6 (10) | ||
| Controls | |||||
| Bovine serum albumin | P. blanchardi (L3) | 0 | 2 (14) | ||
| Bovine serum albumin | A. donovani (L3) | 0 | 6 (10) | ||
| E. coli DH5α | 72 | P. blanchardi (L3) | 0 | 2 (14) | |
| E. coli DH5α | A. donovani (L3) | 0 | 6 (10) | ||
107 bacteria larva−1.
30 μg of protein larva−1.
The ability of the Mor4.1 isolate to cause mortality by injecting it into larvae belonging to the insect order Lepidoptera was also tested. Then, similar amounts of Mor4.1 and E. coli DH5α (107 bacteria per larva) were injected into fifth-instar larvae of the tobacco hornworm, Manduca sexta. Twenty-four hours after the larvae were injected with Mor4.1, 100% of them were dead (P < 0.001). On the contrary, 100% of the larvae injected with E. coli DH5α survived (Table 1).
Identification of toxic activity in Mor4.1 culture broth.
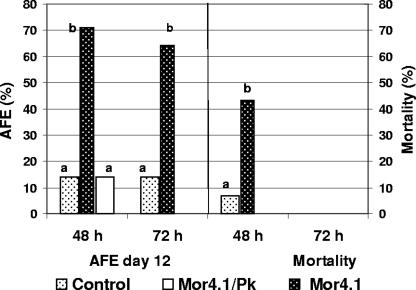
It has been suggested that some extracellular toxin proteins might be involved in amber disease caused by the S. entomophila infection of C. zealandica larvae (27). To test the ability of the Mor4.1 extracellular proteins to produce disease symptoms such as AFE and/or mortality, oral bioassays were done with CCB from the Mor4.1 isolate grown for 48 and 72 h. CCB samples were fed to P. blanchardi larvae as indicated in Materials and Methods. CCB samples of 48 and 72 h, respectively, caused 71% (P < 0.01) and 64% (P < 0.05) of AFE at day 12 after the beginning of the test (Fig. 3). The CCB samples from the 48-h culture also caused a significant mortality rate of 43% (P < 0.05) 30 days after the beginning of the test. No mortality was observed for the CCB samples grown for 72 h. Development of amber coloration was not observed for any of the treatments. Control larvae fed with bovine serum albumin and larvae fed with CCB from Mor4.1 previously treated with proteinase K showed no significant AFE (15%) or mortality (Fig. 3). Tests done with larvae fed with CCB from E. coli DH5α showed no significant activity (data not shown).
FIG. 3.
Bioassay done with CCB grown with S. entomophila Mor4.1 showing the AFE and mortality in third-instar P. blanchardi larvae. Broth samples cultured with S. entomophila Mor4.1 for 48 h and 72 h were evaluated (n = 14). Control larvae were fed with nutrient broth agar containing bovine serum albumin. Broth samples from S. entomophila Mor4.1 (48-h culture) were treated with proteinase K (PK), added to nutrient broth agar, and fed to the larvae. The AFE was recorded after 12 days and the mortality after 30 days from the beginning of the test. The lack of significant difference is indicated by the same letter above the bars (χ2 P < 0.01 for the AFE at 48 h; P < 0.05 for the AFE at 72 h and for mortality at 48 h).
To evaluate the potential of CCB samples to cause mortality by injection, bioassays were done by injecting 72-h CCB grown with the Mor4.1 isolate into third-instar P. blanchardi and third-instar A. donovani larvae (Table 1). A parallel experiment was performed with both species by injecting similarly grown CCB with Mor4.1 previously treated with proteinase K and CCB grown with E. coli DH5α as a control. Results showed that the Mor4.1 CCB caused a significant mortality of 80% (P < 0.001) 2 and 6 days after injection for P. blanchardi and A. donovani, respectively. On the contrary, larvae injected with Mor4.1 CCB treated with proteinase K and E. coli DH5α CCB showed no mortality at all (Table 1).
SDS-PAGE analysis of the CCB.
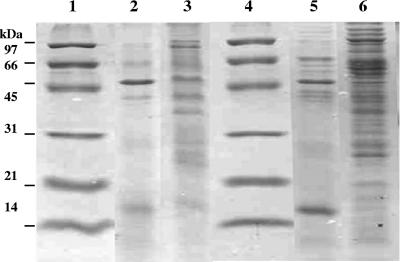
SDS-PAGE analyses of 48- and 72-h cultures were done to correlate the toxin-like activity with the presence of proteins in the CCB grown with the Mor4.1 isolate. To compare, we also analyzed CCB from the S. entomophila UC9 (a derivative of A1MO2) that caused amber disease in C. zealandica larvae. The results of this analysis are shown in Fig. 4. A series of proteins are shown that range in estimated molecular mass from approximately 17 kDa to more than 97 kDa in both strains. However, there are strong differences between the patterns of proteins of the CCB samples of 48 and 72 h. While there are about 7 proteins at 48 h, at 72 h of culture, there are at least 11 and 17 different protein bands for Mor4.1 and UC9, respectively. Two main proteins of ∼17 and ∼54 kDa were observed with the Mor4.1 isolate protein profile at the 48-h culture. For UC9, there are also two main bands of ∼17 and ∼54 kDa. Similar bands also appear in the patterns of the proteins of the two strains cultured for 72 h, though to a lesser extent, almost unnoticed for UC9. Besides, high-molecular-mass proteins (>97 kDa) are present, noticeable mainly in the CCB of UC9. No proteins were observed after the CCB samples were treated with proteinase K (data not shown).
FIG. 4.
SDS-PAGE analysis of CCB cultured with S. entomophila Mor4.1 and S. entomophila UC9. CCB was obtained by ultrafiltration (30-kDa-molecular-mass-cutoff membrane) of culture broths after centrifugation and filter sterilization by a 0.22-μm membrane. CCBs were concentrated 100-fold. Lanes 1 and 4, molecular mass markers of standard proteins; lane 2, S. entomophila Mor4.1 at 48 h; lane 3, S. entomophila Mor4.1 at 72 h; lane 5, S. entomophila UC9 at 48 h; lane 6, S. entomophila UC9 at 72 h.
Taxonomic identification of bacterial species.
Results of the phenotypic and biochemical tests used for Serratia taxonomy are presented in Table 2. All tests were also done for S. entomophila A1MO2 as a reference. Since S. marcescens is phenotypically the species closest to S. entomophila (11), all tests were also done for comparison of three different isolates of S. marcescens.
TABLE 2.
Phenotypic and biochemical characteristics of bacterial isolates
| Characteristic | Response to S. entomophila strain
|
Response to S. marcescens strain
|
|||
|---|---|---|---|---|---|
| Mor4.1 | A1MO2 | S-48 | MAC | ATCC 13082 | |
| Catalase reaction | + | + | + | + | + |
| Gram stain reaction | − | − | − | − | − |
| Nitrate reduction | + | + | + | + | + |
| Acid production when grown on: | |||||
| Adonitol | + | + | + | + | + |
| Arabinose | − | − | − | − | − |
| Citrate | + | + | + | + | + |
| Glucose | + | + | + | + | + |
| Inositol | + | + | + | + | + |
| Lactose | − | − | − | − | − |
| Maltose | + | + | + | + | + |
| Mannitol | − | + | + | + | + |
| Melibiose | − | − | − | − | − |
| Raffinose | − | − | − | − | − |
| Rhamnose | − | − | − | − | − |
| Sorbitol | − | − | + | + | + |
| Sucrose | + | + | + | + | + |
| Xylose | + | + | − | − | − |
| Arginine dihydrolase | − | − | − | − | − |
| Gelatin hydrolyzed | + | + | + | + | + |
| Esculin hydrolyzed | + | + | + | + | + |
| ONPG hydrolyzed | + | + | + | − | + |
| Urea hydrolyzed | − | − | − | − | − |
| DNase production | + | + | + | + | + |
| H2S production | − | − | − | − | − |
| Indole production | − | − | − | − | − |
| Oxidase production | − | − | − | − | − |
| Lysine decarboxylase | − | − | + | + | + |
| Ornithine decarboxylase | − | − | + | + | + |
| Acetamide test | − | − | − | − | − |
| Malonate test | − | − | − | − | − |
| Methyl red test | − | − | − | − | − |
| Voges-Proskauer test | + | + | + | + | + |
| Tartrate test | − | − | − | − | − |
| Caprylate-thallous | + | + | + | + | + |
| Glucose | + | + | + | + | + |
| Indoxyl-β-d-glucoside | + | + | + | + | − |
| Itaconate | − | − | − | − | − |
| Triclosana | + | + | + | + | + |
| P-Cumaric acid | + | + | + | + | + |
| Peptone-tryptophan | + | + | + | + | + |
| Polymyxin B | + | + | + | + | + |
| Tryptophan deaminase | − | − | − | − | − |
| Motility | + | + | + | + | + |
Triclosan, 2,4,4′-trichloro-2′-hydroxydiphenyl ether.
The Mor4.1 isolate proved to be a motile, rod-shaped, gram-negative, aerobic, catalase-positive organism, able to grow well after 24 h at 30°C and 37°C but weakly at 4°C. No growth was observed at or above 42°C. Results of the 43 different tests showed that the Mor4.1 isolate is very similar to S. entomophila A1MO2. The matching coefficient between the Mor4.1 isolate and the S. entomophila A1MO2 strain was 97.67%, and the matching coefficient between the Mor4.1 isolate and the S. marcescens ATCC 13082 strain was 86.04%. The matching coefficients among S. marcescens ATCC 13082 and those of S. marcescens S-48 and S. marcescens MAC were 97.67% and 95.34%, respectively. Similar to S. entomophila A1MO2, Serratia Mor4.1 resembled S. marcescens in its incapacity to ferment arabinose, rhamnose, melibiose, and raffinose but differed from S. marcescens in its incapacity to ferment sorbitol and its deficiency of lysine and ornithine decarboxylase. However, the Mor4.1 isolate differed from S. entomophila A1MO2 and S. marcescens in its inability to ferment mannitol. Mor4.1 is similar to the S. entomophila A1MO2 strain in its inability to ferment itaconate (Table 2). However, these data differed from those reported for the S. entomophila A1MO2 isolate (11). Our results show that the closest relative to Mor4.1 seems to be the S. entomophila A1MO2 type strain, with a matching coefficient of 97.67%. Therefore, both strains might be considered in the same species group.
To better define the species of the Mor4.1 isolate, the 16S rRNA gene was amplified and sequenced as described in Materials and Methods. The sequence was deposited in the GenBank database (accession number EU250329) and compared with other sequences, using the BLAST sequence homology searches at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
The sequence produced a significant alignment to the S. entomophila 16S rRNA gene of the DSM 12358 strain (GenBank accession number AJ233427.1), showing 99% identity. The BLAST phylogenetic analysis showed that the closest relative to the Mor4.1 isolate was the S. entomophila DSM 12358 strain (see the supplemental material).
DISCUSSION
A putative S. entomophila strain pathogenic for several Phyllophaga spp. and A. donovani was isolated from the hemocoel of a dead larva. The strain showed moderate pathogenic activity against second- and third-instar P. blanchardi larvae by the oral test, causing a significant AFE very soon after the onset of inoculation and also a loss of weight and mortality at a late period after the inoculation. The possibility that the AFE might be caused by taste aversion to a large dose of bacteria is discarded since larvae fed with large doses of nonpathogenic bacteria showed no significant AFE (results not shown). Besides, the AFE is also observed when larvae are fed with S. entomophila Mor4.1 CCBs free of bacteria (Fig. 3). Although a considerable loss of weight was also observed for the control larvae, probably associated with stress under laboratory conditions, the differences observed with larvae fed with the S. entomophila Mor4.1 bacteria was statistically significant. The development of amber coloration was not observed for the S. entomophila Mor4.1 test, suggesting the participation of different virulence factors in this strain compared with that of S. entomophila A1MO2. The S. entomophila Mor4.1 isolate administrated per os in a multidose experiment caused a significant mortality in the larvae of P. blanchardi, P. trichodes, and P. obsoleta. This fact suggests a wide spectrum of action of the S. entomophila Mor4.1 isolate among members of the genus Phyllophaga. Mortality was observed at a late period after inoculation, similar to the mortality caused by S. entomophila A1MO2 in C. zealandica (20). However, several doses of S. entomophila Mor4.1 were needed to cause significant mortality in the larvae, suggesting that a large amount of bacteria might be required to surpass the gut barriers, suppress the insect's defensive response, and then kill the larvae. We have observed that larvae fed with S. entomophila Mor4.1 reduced their food ingestion very soon after oral inoculation, and therefore, the larvae ingest only a small portion of the bacteria. It is possible then, that a constant inoculation of small amounts of bacteria would be enough to cause mortality. No pathogenic activity was observed by oral bioassay when S. entomophila Mor4.1 was tested with the lepidopteran insect S. frugiperda, suggesting that oral activity might be restricted to Phyllophaga spp.
On the other hand, it has been reported that larvae of M. sexta are able to survive when as many as 106 to 107 bacterial cells of either S. marcescens, E. coli D31, or Pseudomonas aeruginosa ATCC 9027 are injected into the hemocoel (6). Therefore, to evaluate the potential of S. entomophila Mor4.1 to kill larvae by injection, we tested the concentration of 107 bacteria per larva injected into the hemocoels of the three Phyllophaga species studied. One hundred percent of the larvae were dead in 24 h; in contrast, no mortality was observed after 24 h for the larvae injected with E. coli DH5α, indicating that S. entomophila Mor4.1 produces virulence factors that provide it with the ability to survive in the hemocoel and kill the larvae very soon after inoculation. The estimated LD50 of S. entomophila Mor4.1 for P. blanchardi (994 bacteria per larva) was close to the LD50s of Serratia proteamaculans strains 2746 and 142 (880 and 802 bacteria per larva, respectively) associated with amber disease reported for C. zealandica (32), but it was higher than the LD50 of the amber disease-associated S. entomophila 154 and S. plymuthica 590 strains (<5 and 22 bacteria per larva, respectively). It is possible that the virulence observed for S. entomophila Mor4.1 after bacterial coelomic injection might be related to a generalized Serratia toxin(s), as suggested by Tan et al. (32), since injections of similar amounts of E. coli DH10B in C. zealandica (32) or E. coli DH5α into the three Phyllophaga species and the M. sexta larvae studied in this work did not cause mortality. On the contrary, S. plymuthica 590 and S. marcescens 363, strains not associated with amber disease and presumably nonpathogenic, also caused mortality in C. zealandica after intracoelomic inoculation (32). Therefore, it is possible that S. entomophila Mor4.1 and other Serratia spp. may produce multiple toxins and other virulence factors that act at different sites, some of which have toxic activity in the insect gut after oral inoculation and others that have toxic activity once they have reached the insect hemocoel.
To evaluate whether the potential for the S. entomophila Mor4.1 isolate to kill larvae by intracoelomic inoculation was restricted to Phyllophaga spp. or whether it might be broadened to other insect orders, M. sexta larvae from the insect order Lepidoptera were also injected with S. entomophila Mor4.1 (Table 1). Since the bacteria also killed 100% of larvae 24 h after injection, it is possible that the associated toxin(s) might have a broad spectrum of action, at least for Phyllophaga spp. and some lepidopteran insects.
CCB from the S. entomophila Mor4.1 isolate caused disease symptoms and mortality when administrated per os to larvae of P. blanchardi and by injection to P. blanchardi and A. donovani, suggesting that toxic products were present in the S. entomophila Mor4.1 CCB. The toxic activity was abolished when the CCB was previously treated with proteinase K. Since the samples were heated at 80°C for 15 min to inactivate the enzyme before the corresponding bioassays, this result shows that the toxic factor(s) might be inactivated either by heat or by proteinase K hydrolysis. This result also suggests that a toxin-like protein might be involved in the disease. The possibility that other nonprotein factors might participate in toxicity is not discarded, since recent evidence has shown that bacterial wall components such as lipopolysaccharides might play an important role in pathogenicity to insects (3).
The results are in agreement with the hypothesis that the S. entomophila Mor4.1 isolate might produce some extracellular toxin factors that cause disease symptoms, similar to the S. entomophila A1MO2 strain and other entomopathogenic bacteria such as Photorhabdus luminescens (37). The results suggested the production of extracellular toxin-like factors by the Mor4.1 isolate at 48 and 72 h of culture. On the other hand, CCB from the 48-h culture caused mortality in third-instar P. blanchardi larvae. On the contrary, no significant mortality was observed for CCB from 72 h. This result suggests that CCB from 48 h might contain a factor responsible for the observed mortality that was not present in CCB from 72 h. At this time, some proteolysis might degrade the active factors. However, 72-h CCB administrated by injection caused mortality to P. blanchardi and to A. donovani larvae (Table 1), suggesting the presence of different toxic proteins acting at different levels in the process of pathogenicity: at the level of the insect gut, causing AFE as a first step, and also as a second step at the level of the hemocoel, causing mortality. Previous genetic evidence has suggested that toxin-like proteins were involved in amber disease caused by S. entomophila A1MO2 infection of C. zealandica (28, 18, 17). Analysis of the S. entomophila Mor4.1 and UC9 CCB by SDS-PAGE has shown the presence of a series of proteins ranging in estimated molecular mass from approximately 17 kDa to more than 97 kDa in both strains, which might be associated with AFE and mortality. Although CCBs were obtained by ultrafiltration with a 30-kDa-molecular-mass cutoff membrane, the SDS-PAGE profile has proteins smaller than the 30-kDa cutoff. We have observed that UC9 and S. entomophila Mor4.1 CCB analyzed by native PAGE renders several protein bands, apparently larger than 30 kDa (M. E. Nuñez-Valdez, M. A. Calderon, and F. J. Villalobos, unpublished observation), that separate in the series of proteins observed for Fig. 4. It is possible that the toxin-like proteins may derive from individual gene products of different sizes, which are exported, and then fold themselves or attach together, forming a complex of proteins larger than 30 kDa. The proteins could represent various stages of processing of a larger protein(s). The insecticidal protein complex of P. luminescens W14 shows this characteristic (4). Unfortunately, it was not possible at this stage to correlate any protein in particular with the toxic activity, since many proteins were present in the supernatants. However, it is interesting to observe that the 48-h CCB from both S. entomophila Mor4.1 and UC9 shares similar patterns of protein bands, while those of CCBs at 72 h look very different. There are several high-molecular-mass proteins (>97 kDa), noticeable mainly in the CCB of UC9. The presence of these proteins in the case of UC9 might correlate with the predicted high-molecular-weight proteins SepA, SepB, and SepC (18) and the named effector protein Afp18 (17), which are essential for the production of amber disease symptoms. The genes related to these proteins and to the insecticidal Tc from P. luminescens (4, 33) might be absent in S. entomophila Mor4.1, as judged by the lack of associated DNA products in a PCR directed toward these genes (see the supplemental material), correlating with the lack of marked bands of high-molecular-weight proteins observed with the CCB from S. entomophila Mor4.1. Also, the strong differences in the full pattern of protein bands between UC9 and S. entomophila Mor4.1 at 72 h suggest the participation of different protein components in the disease caused by S. entomophila A1MO2 and that caused by S. entomophila Mor4.1. This suggestion is supported by the fact that the virulence genes in S. entomophila A1MO2 are plasmid borne (9), and no plasmid was identified in S. entomophila Mor4.1 (Nuñez-Valdez et al., unpublished observations). Amber disease caused by S. entomophila A1MO2 is a multifactorial event, and multiple virulence factors might be involved; therefore, multiple virulence factors are hypothesized for S. entomophila Mor4.1. A recent study of S. entomophila A1MO2 has shown the induction of a phage tail-like bacteriocin that causes AFE, amber coloration, and mortality of C. zealandica larvae (15). The production and active role in disease of similar structures or the presence of homologues to the sepABC genes or homologues to the insecticidal toxin complex genes by S. entomophila Mor4.1 is not discarded, but the fact that S. entomophila Mor4.1 does not cause amber coloration in bioassays, which seems to be associated with the SepABC toxins (19), suggests also that different virulence factors might be implicated in S. entomophila Mor4.1 pathogenicity.
Phenotype tests and numerical taxonomy analysis showed that the Mor4.1 isolate is very closely related to the S. entomophila A1MO2 strain, with a matching coefficient of 97.67%. Numerical taxonomy eliminates the biases common with other taxonomic analysis approaches and provides an objective comparison among organisms. Using this method, the matching coefficients of S. marcescens ATCC 13082 with S. marcescens S-48 and S. marcescens MAC were 97.67% and 95.34%, respectively. Therefore, the matching coefficient of 97.67% observed between Mor4.1 and S. entomophila A1MO2 suggests that the Mor4.1 isolate is a putative member of the S. entomophila species. Even though itaconate utilization is a special characteristic of S. entomophila species (11), our results showed that both S. entomophila A1MO2 and Mor4.1 were unable to use it as carbon source. On the contrary, S. entomophila UC9, a derivative of S. entomophila A1MO2, was able to use it in a parallel test done in our laboratory (result not shown). Therefore, the ability to ferment itaconate (11) might be an unstable characteristic and should not be considered as a definitive test for S. entomophila sp., as it has been suggested (11).
On the other hand, the sequence of the Mor4.1 isolate 16S rRNA gene showed 99% identity with the Serratia entomophila 16S rRNA gene of the type strain DSM 12358. Therefore, based on the numerical taxonomy analysis and the BLAST phylogenetic analysis done with the 16S rRNA gene sequence, the Mor4.1 isolate was identified as a putative S. entomophila strain.
Since S. entomophila Mor4.1 is a moderate pathogen with relative low virulence, it is unlikely that it could be used in a classical augmentative biological control program. However, the approximate 50% AFE caused by the bacteria in second- and third-instar larvae very soon after the bacteria are ingested represents a significant reduction in food consumption, since second- and third-instar larvae are the most destructive for the crop in the insect life. This suggests that the potential use of these bacteria under an integrated pest management program would, hypothetically, contribute to a crop damage reduction in a significant proportion. Besides, the fact that toxin-like proteins in the CCB cause higher AFE levels and also mortality than the bacteria (70%, CCB versus 50%, bacteria) offers the opportunity to isolate and characterize the genes and proteins for future biotechnological applications. Similar studies have been done to characterize the insecticidal toxins of P. luminescens (5).
We have observed that S. entomophila Mor4.1 is able to colonize the roots of lettuce and also to work as a plant growth-promoting rhizobacterium (Nuñez-Valdez et al., unpublished observations). Hypothetically, as a potential model, S. entomophila Mor4.1 might colonize the roots of the plants growing in the soil, and then, once the “white grubs” start to eat the root, they would be discouraged by S. entomophila Mor4.1-associated AFE. Since the root damage would be reduced and the plant growth would be promoted by the presence of S. entomophila Mor4.1, crop yields might be increased. Also, since a large amount of bacteria might be necessary to kill the larvae in the model described above and since the larvae suffering S. entomophila Mor4.1-associated AFE might not be able to ingest enough bacteria to die, then the larvae would remain alive, and in this way the development of secondary pests might be limited. This model would lead to a more ecological and sustainable management of “white grub” populations (36). Therefore, the potential of the S. entomophila Mor4.1 and its toxin(s) to be used under an integrated pest management program to reduce crop damage caused by “white grubs” should be investigated.
The ability of the S. entomophila Mor4.1 isolate or its CCB to cause pathogenicity symptoms in three different species of Phyllophaga and in A. donovani suggests a wide spectrum of action among species of Scarabaeidae. Therefore, the potential of this bacterium to cope with the numerous species of “white grubs” in Mexico is promising. The pathogenic oral activity might be restricted for scarab species, since no pathogenic activity was observed for the lepidopteran S. frugiperda in oral bioassays. S. entomophila Mor4.1 also represents a potential source of novel virulence genes and proteins that could help in the future to reduce crop damage or control insect pests. We are currently analyzing the virulence factors involved in the disease to clarify the molecular mechanism of pathogenicity and to identify the protein(s) responsible for the AFE and for the mortality observed for oral and injection bioassays. We are following a genomic approach by constructing an S. entomophila Mor4.1 fosmid library in E. coli. We are screening the library clones that carry virulence factors by injecting individual clones into Phyllophaga sp. larvae and selecting those able to kill the larvae. Then, we will test the insecticidal clones for oral toxicity. To date, we have isolated five different insecticidal clones. The characterization of these insecticidal clones will be the matter of another publication. This approach will define the nature and number of toxins and virulence genes involved in the pathogenicity of S. entomophila Mor4.1. Also, it will allow the cloning and expression of the virulence genes, to improve their production and activity by genetic manipulation.
Supplementary Material
Acknowledgments
We thank AgResearch, Lincoln, New Zealand, as well as T. A. Jackson and H. K. Mahanty for providing type strains of S. entomophila. We also thank A. Bravo of the Instituto de Biotecnología, Universidad Nacional Autónoma de Mexico, for providing M. sexta larvae and Adriana Castro for providing larvae of P. obsoleta for bioassays. The kind help offered by corn farmers from the studied sites to carry out this research is greatly appreciated. We thank Paul Gaytán and Eugenio López for primer synthesis and Arturo Yañez for sequencing.
This research was supported by grants from SEP-PROMEP and CONACYT (grant 61816), Mexico.
Footnotes
Published ahead of print on 14 December 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alm, S. R., M. G. Villani, T. Yeh, and R. Shutter. 1997. Bacillus thuringiensis serovar japonensis strain Buibui for control of Japanese and oriental beetle larvae (Coleoptera: Scarabaeidae). Appl. Entomol. Zool. 32:477-484. [Google Scholar]
- 2.Aranda, E., J. Sanchez, M. Peferoen, L. Güereca, and A. Bravo. 1996. Interaction of Bacillus thuringiensis crystal protein with the midgut epithelial cells of Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 68:203-212. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, H. P. J., and D. J. Clarke. 2005. The pbgPE operon in Photorhabdus luminescens is required for pathogenicity and symbiosis. J. Bacteriol. 187:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen, D. J., and J. C. Ensign. 1998. Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Appl. Environ. Microbiol. 64:3029-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daborn, P. J., N. Waterfield, C. P. Silva, C. P. Au, S. Sharma, and R. H. ffrench-Constant. 2002. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc. Natl. Acad. Sci. USA 99:10742-10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn, P. E., and D. R. Drake. 1983. Fate of bacteria injected into naïve and immunized larvae of the tobacco horn worm Manduca sexta. J. Invertebr. Pathol. 41:77-85. [Google Scholar]
- 7.Ebeling, W., N. Hennrich, M. Klockow, H. Metz, H. D. Orth, and H. Lang. 1974. Proteinase K from Tritirachium album Limber. Eur. J. Biochem. 47:91-97. [DOI] [PubMed] [Google Scholar]
- 8.Finney, D. J. 1971. Probit analysis. Cambridge University Press, Cambridge, England.
- 9.Glare, T. R., G. E. Corbett, and A. J. Sadler. 1993. Association of a large plasmid with amber disease of the New Zealand grass grub, Costelytra zealandica, caused by Serratia entomophila and Serratia proteamaculans. J. Invertebr. Pathol. 62:165-170. [Google Scholar]
- 10.Grimont, P. A. D., and F. Grimont. 1981. The genus Serratia, p. 1187-1203. In M. P. Starr, H. Stolp, H. G. Truper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes. A handbook on habitats, isolation and identification of bacteria. Springer, New York, NY.
- 11.Grimont, P. A. D., T. A. Jackson, E. Ageron, and M. J. Noonan. 1988. Serratia entomophila sp. nov. associated with amber disease in the New Zealand grass grub Costelytra zealandica. Int. J. Syst. Bacteriol. 38:1-6. [Google Scholar]
- 12.Hogg, J. C., and M. J. Lehane. 1999. Identification of bacterial species associated with the sheep scab mite (Psoroptes ovis) by using amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 65:4227-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt, J. G., N. R. Krieg, P. H. Sneath, J. T. Staley, and S. T. Williams (ed.). 1994. Facultative anaerobic gram-negative rods. Group 5, p. 175-289. In Bergey′s manual of determinative bacteriology, 9th ed. Williams and Wilkins, Baltimore, MD.
- 14.Hori, H., N. Suzuki, K. Ogiwara, M. Himejima, L. S. Indrasith, S. Minami, R. Sato, M. Ohba, and H. Iwahana. 1994. Characterization of larvicidal toxin protein from Bacillus thuringiensis serovar Japonensis strain Buibui specific for Scarabaeidae beetles. J. Appl. Bacteriol. 76:307-313. [DOI] [PubMed] [Google Scholar]
- 15.Hurst, M. R., S. S. Beard, T. A. Jackson, and S. M. Jones. 2007. Isolation and characterization of the Serratia entomophila antifeeding prophage. FEMS Microbiol. Lett. 270:42-48. [DOI] [PubMed] [Google Scholar]
- 16.Hurst, M. R., and T. R. Glare. 2002. Restriction map of the Serratia entomophila plasmid pADAP carrying virulence factors for Costelytra zealandica. Plasmid 47:51-60. [DOI] [PubMed] [Google Scholar]
- 17.Hurst, M. R., T. R. Glare, and T. A. Jackson. 2004. Cloning Serratia entomophila antifeeding genes—a putative defective prophage active against the grass grub Costelytra zealandica. J. Bacteriol. 186:5116-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurst, M. R., T. R. Glare, T. A. Jackson, and C. Ronson. 2000. Plasmid-located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J. Bacteriol. 182:5127-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst, M. R., S. M. Jones, B. Tan, and T. A. Jackson. 2007. Induced expression of the Serratia entomophila Sep proteins shows activity towards the larvae of the New Zealand grass grub Costelytra zealandica. FEMS Microbiol. Lett. 275:160-167. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, T. A., A. M. Huger, and T. R. Glare. 1993. Pathology of amber disease in the New Zealand grass grub Costelytra zealandica (Coleoptera: Scarabaeidae). J. Invertebr. Pathol. 61:123-130. [Google Scholar]
- 21.Klein, M. G. 1981. Advances in the use of Bacillus popilliae for pest control, p. 183-192. In H. D. Burges (ed.), Microbial control of pests and plant diseases 1970-1980. Academic Press, Inc., New York, NY.
- 22.Klein, M. G. 1988. Pest management of soil-inhabiting insects with microorganisms. Agric. Ecosyst. Environ. 24:337-349. [Google Scholar]
- 23.Logan, N. A. 1994. Bacterial systematics. Blackwell Scientific, Boston, MA.
- 24.Maniatis, T., E. F. Fritsch, and J. Sambrook (ed.). 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Morón, M. A. 1994. Aspectos bioecológicos sobre Scarabaeidae (sensu lato) (Insecta: Coleoptera), p. 151-158. In Memorias XXI Congreso Soc. Colombiana de Entomología. Medellin, Socolen, Colombia.
- 26.Najera, M. B., F. J. Villalobos, and M. A. Morón. 1994. Conclusiones y recomendaciones. Relatoría, p. 1-6. In IV Mesa Redonda Sobre Plagas Subterráneas. Xalapa, Veracruz, Mexico.
- 27.Nuñez-Valdez, M. E. 1994. Identification and analysis of the virulence factors in Serratia entomophila causing amber disease to the grass grub Costelytra zealandica. Ph.D. thesis. University of Canterbury, Christchurch, New Zealand.
- 28.Nuñez-Valdez, M. E., and H. K. Mahanty. 1996. The amb2 locus from Serratia entomophila confers anti-feeding effect on larvae of Costelytra zealandica (Coleoptera: Scarabaeidae). Gene 172:75-79. [DOI] [PubMed] [Google Scholar]
- 29.Nuñez-Valdez, M. E., A. A. Romero-López, R. Arzuffi-Barreda, L. Aldana-Llanos, M. E. Valdés-Estrada, R. Figueroa-Brito, and F. J. Villalobos. 2002. Agrobiotecnología aplicada al manejo integrado de plagas subterráneas: un enfoque promisorio para el manejo de la gallina ciega (Coleoptera: Scarabaeidae) y el picudo negro del nardo y agave (Coleoptera: Curculionidae), p. 139-157. In G. A. Aragón, J. F. López-Olguín, and C. M. Tornero (ed.), Métodos para la generación de tecnología agrícola de punta. Publicación especial de la Benemérita Universidad Autónoma de Puebla, Puebla, Mexico.
- 30.Struffi, P., V. Corich, A. Giacomini, A. Benguedouar, A. Squartini, S. Casella, and M. P. Nuti. 1998. Metabolic properties, stress tolerance and macromolecular profiles of rhizobia nodulating. J. Appl. Microbiol. 84:81-89. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, N., H. Hori., K. Ogiwara, S. Asano, R. Sato, M. Ohba, and H. Iwahana. 1992. Insecticidal spectrum of a novel isolate of Bacillus thuringiensis serovar Japonensis. Biol. Control 2:136-142. [Google Scholar]
- 32.Tan, B., T. A. Jackson, and M. R. Hurst. 2006. Virulence of Serratia strains against Costelytra zealandica. Appl. Environ. Microbiol. 72:6417-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tennant, S. M., N. A. Skinner, A. Joe, and R. M. Robins-Browne. 2005. Homologues of insecticidal toxin complex genes in Yersinia enterocolitica biotype 1A and their contribution to virulence. Infect. Immun. 73:6860-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villalobos, F. J. 1991. The community structure of soil Coleoptera (Melolonthidae) from a tropical grassland in Veracruz, Mexico. Pedobiologia 35:225-238. [Google Scholar]
- 35.Villalobos, F. J. 1992. The potential of entomopathogens for the control of white grubs pests in Mexico, p. 253-260. In T. A. Jackson and T. R. Glare, (ed.), Use of pathogens in scarab pest management. Intercept, Andover, Hampshire, United Kingdom.
- 36.Villalobos, F. J. 2003. El manejo sustentable: un paradigma alternativo para lograr una solución pragmática al problema causado por la gallina ciega (Coleoptera: Scarabaeidae), p. 139-157. In C. M. Tornero, J. F. López-Olguín, and A. G. Aragón (ed.), Agricultura, ambiente y desarrollo sustentable. Publicación especial de la Benemérita Universidad Autónoma de Puebla, Puebla, Mexico.
- 37.Waterfield, N., A. Dowling, S. Sharma, P. J. Daborn, U. Potter, and R. H. Ffrench-Constant. 2001. Oral toxicity of Photorhabdus luminescens W14 toxin complexes in Escherichia coli. Appl. Environ. Microbiol. 67:5017-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.