Abstract
Colletotrichum graminicola is a filamentous ascomycete that causes anthracnose disease of maize. While the fungus can cause devastating foliar leaf blight and stalk rot diseases, little is known about its ability to infect roots. Previously published reports suggest that C. graminicola may infect maize roots and that root infections may contribute to the colonization of aboveground plant tissues, leading to disease. To determine whether C. graminicola can infect maize roots and whether root infections can result in the colonization of aboveground plant tissues, we developed a green fluorescent protein-tagged strain and used it to study the plant root colonization and infection process in vivo. We observed structures produced by other root pathogenic fungi, including runner hyphae, hyphopodia, and microsclerotia. A mosaic pattern of infection resulted from specific epidermal and cortical cells becoming infected by intercellular hyphae while surrounding cells were uninfected, a pattern that is distinctly different from that described for leaves. Interestingly, falcate conidia, normally restricted to acervuli, were also found filling epidermal cells and root hairs. Twenty-eight percent of plants challenged with soilborne inoculum became infected in aboveground plant parts (stem and/or leaves), indicating that root infection can lead to asymptomatic systemic colonization of the plants. Many of the traits observed for C. graminicola have been previously reported for other root-pathogenic fungi, suggesting that these traits are evolutionally conserved in multiple fungal lineages. These observations suggest that root infection may be an important component of the maize anthracnose disease cycle.
Anthracnose of maize is caused by the filamentous fungus Colletotrichum graminicola (Ces.) G. W. Wils. The disease can affect most plant tissues, though the stalk rot and seedling blight forms of the disease are the most economically damaging. It has become recognized as one of the predominant stalk rot pathogens of maize in North America during the past few decades (28, 32, 39). During the leaf blight form of the disease (anthracnose leaf blight [ALB]), visible lesions form first on the lower leaves and progressively move upward as the plant matures (27, 33). The consensus from the literature is that conidia formed on infected crop residue from the previous season are the primary source of inoculum for ALB (4, 5). Anthracnose stalk rot disease may begin from wounds, senescent stalk tissues, or root infections (5). It has been suggested previously that C. graminicola can infect maize roots, although it is generally not considered to be a root disease-causing pathogen, and very little is known about this phase of the disease (5, 63). However, root infection and systemic colonization may be important contributors to the initiation and development of ALB.
Much of our knowledge of maize anthracnose comes from study of the leaf blight phase of the disease. From ultrastructural studies, it is known that following appressorium formation a penetration peg is formed that invades the epidermal cell through the cuticle. Within the epidermal cell a large-diameter, irregular primary hypha (also known as an infection hypha) forms (36, 46). The hypha grows biotrophically and can form one or more branches that spread to adjacent epidermal or mesophyll cells. The fungus grows between the plant plasma membrane and plant cell wall and spreads from cell to cell in this manner for approximately 36 to 48 h. By 48 to 72 h, a switch to necrotrophic growth occurs, which is typified by the production of large numbers of smaller-diameter hyphae that extensively colonize the inter- and intracellular spaces of the tissue, causing the death of host cells prior to colonization (36, 44, 46). After the onset of tissue browning, narrow secondary hyphae branch from the primary hyphae within dead host cells and radiate into the surrounding tissue. This two-stage lifestyle is termed hemibiotrophy and is found in many species of Colletotrichum as well as a number of pathogenic ascomycetes (1, 44).
Colletotrichum spp. employ widely varying modes of obtaining nutrition, which change depending on the host species, the developmental stage of the host, and other factors. There are numerous reports of pathogenic Colletotrichum spp. that form endophytic and epiphytic associations with plants (2, 45, 52). Some, such as C. gloeosporioides, C. acutatum, and C. magna, are described as pathogens of one host and asymptomatic endophytes of other species (15, 44, 48). Thus, the pathogen is seemingly able to adopt different lifestyles, depending on the host it is colonizing. Reports by Freeman and Rodriguez (17) and Redman et al. (49) have also shown that single-gene mutations in C. magna can mediate a change in lifestyle from that of a necrotrophic pathogen to an endophyte, indicating that regulation of lifestyle is not simply controlled by the environment in which the fungus is growing (48). An interesting outcome of these experiments is that loss-of-function mutations in C. magna pathogenicity factors do not always result in the loss of the ability of the fungus to colonize its host (17, 49). Thus, the ability to cause disease and the ability to colonize host tissue seem to be separate traits with independent genetic control (24, 30).
A recent study demonstrated that the rice blast fungus Magnaporthe oryzae, typically thought of as a foliar pathogen, could infect roots (10, 53). In fact, many fungi that are commonly regarded as causal agents of foliar diseases can also infect their hosts by invading roots (10, 18, 53, 59). In the case of the M. oryzae-rice interaction, the initial stages of foliar and root infection have been shown to be quite different. However, once inside the root, colonization appears to be similar to the process that occurs in leaves (53).
Previous reports suggest that maize roots may become infected by C. graminicola, leading to top dieback and stalk rot diseases in mature plants, and that systemic colonization can be asymptomatic until the onset of disease (3, 5). However, root infections followed by systemic colonization of the plant have not been shown, and no studies have described how the fungus can attach, penetrate, and colonize plant roots. Such knowledge would be helpful for understanding the development of the disease and in the development of disease management strategies. We developed a green fluorescent protein (GFP)-tagged strain of C. graminicola and used it to test the hypothesis that C. graminicola can infect maize roots and systemically colonize aerial plant tissues. We found that C. graminicola can infect maize seedling roots and asymptomatically colonize aerial plant tissues, a developmental program that is significantly different from the foliar infection process. We also found that when C. graminicola colonized roots, it formed structures that are commonly found in root pathogens, including hyphopodia, runner hyphae, and microsclerotia (MS), that are not found during foliar infections. The importance of the direct infection of roots from infested, buried plant debris in the life cycle of C. graminicola has not been previously considered, and these results may therefore have important implications for the disease cycle of this pathogen.
MATERIALS AND METHODS
Fungal strains and culture conditions.
Wild-type strain M1.001-BH (also known as CgM2) (13) and its derivative transgenic strains were used in all the experiments. Cultures were maintained at 25°C on potato dextrose agar medium (PDA; Difco Laboratories, Detroit, MI) with continuous illumination from a fluorescent light source. Long-term storage at −80°C involved suspending conidia in 7% skim milk mixed with silica gel (unless specified otherwise, all reagents were obtained from Sigma, St. Louis, MO) (57).
Construction of a GFP-containing strain of C. graminicola.
Several GFP-tagged strains of C. graminicola were obtained by transforming fungal protoplasts with plasmid pGFP (21, 35), which contains the GFP gene driven by the promoter from the Aspergillus nidulans gdp gene as well as the hph gene, which confers resistance to hygromycin B (35). Protoplast preparation and transformation were performed as described by Thon et al. (56), except that 100 mg of lysing enzymes per ml of 0.7 M NaCl was used to digest conidial cell walls. Protoplasts were released after 4 to 5 hours of digestion. Selection for transformants was performed using PDA amended with 100 mg/liter hygromycin B (PDA+hyg).
Analysis of transformant stability and pathogenicity.
Transformants were genetically purified by preparing a dilute suspension of conidia in sterile H2O and spreading the suspension on PDA. After incubating for 24 h, a single colony was selected. This process was repeated once. The transformants were assayed for colony morphology, growth rate, and virulence. Transformants were assayed for GFP activity by fluorescence microscopy using techniques and equipment described below. Overall colony morphology and color were compared to the wild-type strain, while growth rate was measured on PDA in 150-mm petri dishes by measuring colony radius daily for seven consecutive days after inoculation. Foliar virulence was assayed using the method described by Thon et al. (56), and root virulence was assayed using the methods described below. The transformants were also tested for mitotic stability by subculturing for several weeks on PDA in the absence of hygromycin and then transferring them to PDA+hyg and assaying them for GFP activity.
Root pathogenicity assays.
Maize inbred lines B73 (susceptible to ALB [13]) and H99 (highly resistant to ALB [62]) were inoculated with C. graminicola, and infection was followed over a time course. To study systemic colonization, we infected maize lines B73, H99, and Mo940 (also susceptible to leaf blight [13, 61, 62]). Prior to being planted, all seeds were treated by coating them with the fungicide trifloxystrobin (Flint 50; Bayer CropScience) and allowing them to dry at room temperature for 24 h. The seeds were washed in 70% ethanol for 5 min and immediately rinsed with water to remove the fungicide before being inoculated. We noted no adverse effect on the development of C. graminicola after this treatment (data not shown). Three root inoculation techniques were employed. For studies of early root colonization, we inoculated maize roots from seeds that had been germinated in petri dishes. Seeds were placed in petri dishes in sets of four on wet, autoclaved filter paper and watered every 2 days with a solution of 0.5 g liter−1 Peter's fertilizer (Peter's 20-20-20; AG Spectrum, IA). Seedlings were inoculated 3 days postgermination using either a root soak method or an agar plug method. For the root soak procedure, conidia were collected from 14- to 21-day-old cultures of M1.001-BH-gfp or M1.001-BH. The conidia were filtered through three layers of cheesecloth and then washed with two rounds of resuspension in sterile distilled water (dH2O) followed by centrifugation (3,000 rpm, 3 min, room temperature). The roots were soaked for 1 hour in a suspension of 107 conidia ml−1. For the agar plug inoculation method, cultures of M1.001-BH-gfp were grown on PDA for 14 days. Agar plugs (approximately 10 mm in diameter, cut from the leading edge of a 14-day-old colony) were placed directly on exposed seedling roots. Once inoculated, the seedlings were observed daily under a microscope starting 1 day postinoculation (dpi).
The late stages of root colonization, including the colonization of upper plant parts, were studied by inoculating plants with the protocol described by Dufresne and Osbourn (10). Briefly, five agar plugs from 14-day-old M1.001-BH-gfp cultures were placed in polycarbonate 50-ml centrifuge tubes containing 35 ml of autoclaved vermiculite clay. Another 5 ml of vermiculite was placed over the agar plugs, one maize seed was added, and an additional 5 ml of vermiculite was added to create approximately 1 cm of vermiculite cover. Ten seedlings were prepared for each trial. For a control, one set of five seedlings was prepared for each trial in tubes inoculated with sterilized PDA plugs. Each tube was watered with sterile dH2O and sealed with Parafilm. Once the seedlings germinated, the Parafilm was removed, and the seedlings were watered every other day using 0.5 g liter−1 Peter's fertilizer (20-20-20). The experiment was performed four times.
For experiments requiring plants more than 3 weeks old, seedlings were first grown in vermiculite and inoculated with agar plugs as described above. The plants were transferred to 3- or 10-liter pots containing a mixture of 40% peat, 40% loam, 10% vermiculite, and 10% compost. The greenhouse temperature was 25 to 27°C, and daylight was supplemented with light from fluorescent tubes to provide 14 h of continuous light. The light intensities in the greenhouse during the day were between 120 and 200 microeinsteins m−2. The plants used in greenhouse experiments were irrigated daily with water and twice a week with the Peter's fertilizer solution.
Isolation of C. graminicola from infected plants.
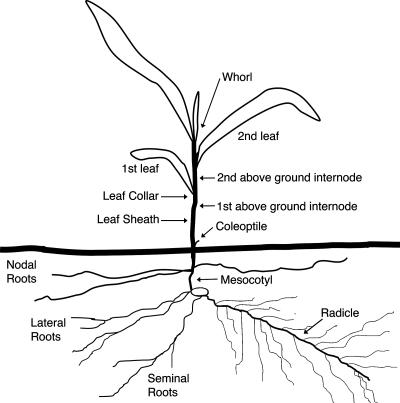
Tissue samples were dissected from inoculated and mock-inoculated plants at 42 dpi. Two- to 3-mm-diameter sections were cut from leaves (first to third leaf), stems (first and second aboveground internodes), and roots (mesocotyl, radicle, and nodal root) (Fig. 1). The dissected tissues were surface sterilized for 45 seconds in 10% sodium hypochlorite and rinsed three times in sterile dH2O. Each dissected tissue section was divided into 10 pieces and placed on one-half-strength PDA containing 1.5 ml liter−1 of 80% lactic acid, 100 μg ampicillin/ml, and 100 μg hygromycin B/ml. The samples were incubated at 25°C for 10 days and examined daily by bright-field and fluorescence microscopy. This experiment was replicated three times, with each replicate consisting of seven inoculated plants and seven mock-inoculated plants. From each plant, 10 samples from each tissue were cultured and examined for the presence of C. graminicola. The tissue was considered infected if C. graminicola was found in any of the 10 samples.
FIG. 1.
Schematic representation of a maize plant. Adapted from the work of Ritchie et al. (50).
Tissue processing and microscopy.
The images shown in Fig. 2 to 5 (with exceptions noted below) were acquired with an Olympus BX60 microscope outfitted with 10×, 40×, and 100× objectives (Olympus America Inc., Melville, NY). Observation of GFP fluorescence employed an Endow GFP long-pass filter with excitation and emission wavelengths of 470 and 500 nm, respectively, and a dichroic mirror at 495 nm (model no. 41018; Chroma Technology, Rockingham, VT). Images were captured with a Q-FIRE digital camera (Q-Imaging, Burnaby, BC, Canada) using Q-Capture software version 2.56. Alternatively, the image shown in Fig. 3C was acquired using an Olympus BX51 microscope outfitted with a DP70 camera (Olympus America Inc., Melville, NY). A detailed description of the features of this system has been described earlier (54). The images in Fig. 4E to G and that in Fig. 5J were acquired with an Olympus SZX9 stereomicroscope outfitted with an Olympus DP70 camera. Adobe Photoshop CS2 version 9.02 (Adobe, Mountain View, CA) was used to prepare images for publication.
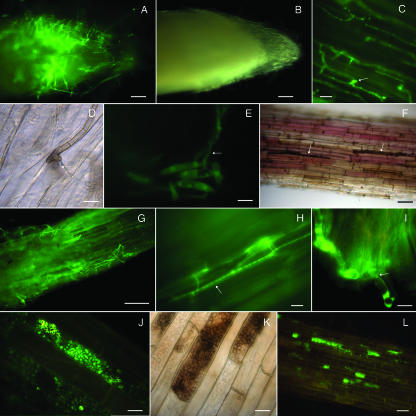
FIG. 2.
Early colonization stages of maize roots by C. graminicola M1.001-BH-gfp. Roots were excised from 3-day-old sterile maize seedlings grown in vitro and inoculated with agar plugs containing C. graminicola vegetative mycelium (A to D and F to H) or with a spore suspension (E). Roots were excised from maize seedlings grown in sterile vermiculite and artificially inoculated with agar plugs containing M1.001-BH-gfp vegetative mycelium (I to L). (A and B) Colonization of the root surface observed with fluorescence (A) and dark-field (B) illumination at 1 dpi. (C) Hyphae growing along the epidermis parallel to the longitudinal axis of the root and forming lateral hyphopodia (arrow); 2 dpi. (D) A penetration hypha (arrow) is visible under a melanized hyphopodium at the junction between epidermal cells; 3 dpi. (E) Falcate conidia on the root epidermis germinate and give rise to vegetative hyphae (arrow) at 2 dpi. (F) Melanized runner hyphae (arrow) extensively colonize the root surface; 3 dpi. (G) Younger green fluorescent hyphae extensively colonize the root surface, forming a network around the root; 3 dpi. (H) Hyphae (arrow) colonize intercellular spaces of epidermal maize roots cells; 3 dpi. (I) Hyphae spread intracellularly through the cell wall of epidermal and cortical cells. Hyphae are constricted (arrow) at these penetration points; 5 dpi. (J) Hyphae proliferate within root epidermal cells, filling most of the intracellular space; 6 dpi. (K) Hyphae melanize as they mature; 7 dpi. (L) Root cells become colonized by hyphae in a mosaic pattern, leaving some cells uninfected; 10 dpi. Bars = 100 μm (A, B, F, and G), 20 μm (C, H, and L), and 10 μm (D, E, I, J, and K).
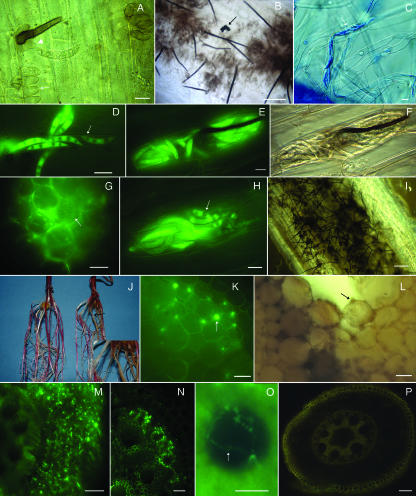
FIG. 5.
Colletotrichum graminicola spreads systemically from infected roots to aerial plant parts. Plants were from maize seeds grown in a sterile vermiculite system and inoculated with agar plugs of M1.001-BH-gfp mycelium. Photographs were obtained using bright-field microscopy (C, G, and H), using fluorescence microscopy (A to B, D to F, and I), or under a stereomicroscope (J). (A) Adventitious root of maize systemically colonized by C. graminicola; 28 dpi. (B and C) Senescent coleoptile colonized by C. graminicola; 28 dpi. Viewed with fluorescence (B) and bright-field (C) illumination. (D) Colonization of the third leaf sheath from a root-infected plant; 28 dpi. (E) Hyphae growing inside of a leaf trichome (arrow). (F) C. graminicola growing from individual vascular bundles (arrow) of a stem cut in transverse section and cultured on isolation medium. (G) Five-micrometer cross section of a maize leaf from a root-inoculated plant; 28 dpi. Hyphae are indicated by arrows. (H) Lobed hyphopodia on an infected, senescent leaf sheath; 28 dpi. (I) Hyphopodia begin to accumulate melanin, though GFP fluorescence within the hyphae is still observed in the hypha leading to the hyphopodium. (J) Lobed hyphopodia produced in liquid culture; 3 dpi. Bars = 100 μm (A and F), 20 μm (B, C, and G), and 10 μm (D, E, H, I, and J).
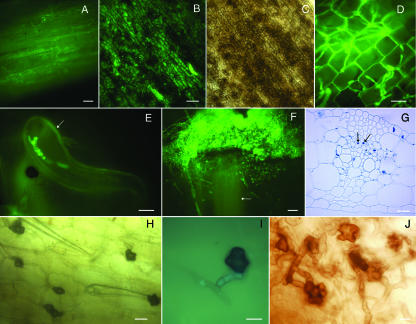
FIG. 3.
Advanced colonization stages of maize root by C. graminicola expressing GFP. Roots were excised from maize seeds grown in a sterile vermiculite system artificially inoculated with agar plugs containing vegetative mycelium of C. graminicola expressing GFP. All the photographs were obtained with a fluorescence microscope unless otherwise indicated. (A) Initiation of an acervulus on the root; 5 dpi. Hyphae elongate into conidiophores, which produce the mature primary conidia. Note immature conidiophores (arrow). Mature conidiophores (arrowhead) produce falcate conidia and setae. (B) Acervuli on the root surface with abundant setae and an MS (arrow); 6 dpi. (C) Falcate conidia fill root hairs (arrow); 10 dpi. (Stained with cotton blue.) (D) Falcate conidia fill root hairs (arrow); 14 dpi. (E and F) Falcate conidia fill a root epidermal cell; 20 dpi. Viewed with fluorescence (E) and bright-field (F) illumination. Setae can form inside the cell. (G) Cross section of maize root; 14 dpi. Cortical cells are colonized by hyphae and oval conidia (arrow). (H) Root epidermal cell filled with falcate conidia. Some conidia are germinating (arrow); 20 dpi. (I) Root covered with acervuli; 20 dpi. (J) Root inoculated with C. graminicola (right) and mock inoculated (left); 28 dpi. Brown discoloration (arrow, inset) can be observed for infected roots, but no necrosis is seen. Red pigmentation is characteristic of inbred line B73. (K) Hand-cut cross section of a root; 7 dpi. Hyphae colonize the cortical cells inter- and intracellularly (arrow). (L) Cross section of a root; 14 dpi. Intercellular thick melanized hyphae (arrow) colonizing the cortex of the root. (M) Cross section of a root at 21 dpi. Colonization of the root cortex. Hyphae heavily colonize epidermis and cortex and reach the endodermis. (N) Cross section of a root; 24 dpi. Hyphae colonize the endodermis, phloem, and xylem. (O) Cross section of a root; 28 dpi. Xylem vessels colonized by hyphae (arrow). (P) Cross section of an uninfected 16-day-old maize root. Yellow autofluorescence can be seen in the maize epidermis, endodermis, phloem, and xylem. Bars = 10 μm (A, C, D, E, F, G, H, K, L, and O), 20 μm (B, M, and N), and 100 μm (I and P).
FIG. 4.
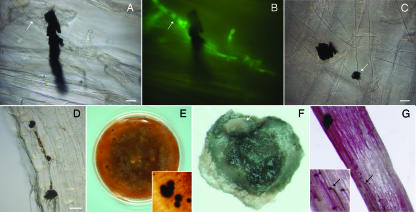
MS of Colletotrichum graminicola. Roots were excised from maize seeds grown in sterile vermiculite inoculated with agar plugs colonized with vegetative mycelium of C. graminicola expressing GFP (A to D). MS of C. graminicola M1.001-BH-gfp were produced in vitro (E to G). Photographs were obtained by bright-field microscopy (A, C, and D), by fluorescence microscopy (B), or under a stereomicroscope (E to G). (A and B) MS of C. graminicola attached to hyphae (arrow) on the maize root surface at 10 dpi, viewed with bright-field (A) and fluorescent (B) illumination. (C) MS of C. graminicola (arrow) on the maize root; 7 dpi. Notice the variation in MS size and shape. (D) Many MS on the root; 20 dpi. (E) MS produced in vitro. (F) Cleaned MS produced in vitro. Masses of salmon-orange conidia (arrow) can be observed on the surface of the MS. (G) Germination of MS on roots. Melanized hyphae and hyphopodia (arrow, inset) can be observed colonizing the root surface; 7 dpi. Bars = 10 μm (A, B, and C) and 20 μm (D).
Between 1 and 28 dpi, infected roots were monitored for fungal colonization. For each time point examined, at least 20 inoculated and 5 mock-inoculated roots were assayed from each of four independent experiments. The tissue samples were placed on a microscope slide, submerged in a water droplet, covered with a glass coverslip, and visualized as described above.
Tissue samples for Fig. 5G were prepared using previously described methods (37). Briefly, the tissues were fixed in Methacarn (55) for 24 h at 4°C, dehydrated in a graded ethanol series, and embedded in paraffin. Five-micrometer sections were cut with a microtome, stained with lactophenol aniline blue (0.01% [wt/vol]), and examined with bright-field microscopy.
MS induction and isolation and inoculation of plant roots.
MS production was induced in vitro using the protocol described by Sanogo and Pennypacker (51). Briefly, agar plugs from 7- to 10-day-old cultures were transferred to petri dishes containing PDA. The petri dishes were sealed with Parafilm and incubated in darkness at 28°C for 6 weeks. The MS were collected with a sterile toothpick and cleaned of medium, mycelia, and spores by rolling them on celite medium (3% agar and 1% diatomaceous earth as a mild abrasive) (26). Following the celite treatment, the MS were surface sterilized by agitating them gently for 45 seconds in 10% sodium hypochlorite. The MS were collected in a Büchner funnel fitted with filter paper and washed three times by alternately filling the funnel with 100 ml sterile dH2O and applying a vacuum. Individual cleaned MS were used to inoculate 3-day-old maize seeding roots. Seedlings were watered every 2 days with 0.5 g liter−1 Peter's 20-20-20 fertilizer. The root tissue was observed microscopically between 2 and 10 days postinfection.
RESULTS
Development of C. graminicola strains expressing GFP.
We used 6 μg of plasmid DNA to transform 107 protoplasts and obtained 38 hygromycin-resistant transformants. Thirty-five of the transformants exhibited GFP expression, though variation in expression levels was detected among the transformants. Ten transformants with high levels of fluorescence were selected for further analysis and were purified by single-spore isolation. Microscopic analysis confirmed that in all of these strains, high levels of fluorescence were observed in the mycelium, falcate conidia, and oval conidia (data not shown). Transformants showed phenotypes indistinguishable from that of the wild type with respect to colony morphology, pigmentation, spore production, and growth rate (data not shown). Foliar pathogenicity assays performed with these transformants resulted in lesion development that was indistinguishable from that of the wild-type strain, and transformants were stable in media with or without hygromycin B (data not shown). A single strain was randomly selected for use in further experiments.
Assays of infected plants showed that GFP fluorescence could be detected in infected plants at least 18 weeks after infection. GFP fluorescence was observed for all nonmelanized fungal structures during maize leaf and root colonization, consistent with observations of axenic cultures.
Colonization of maize roots by C. graminicola is similar to that of root pathogens.
To determine whether C. graminicola could infect maize roots, we performed infection assays followed by a time course of microscopic observations. The infection assays were performed with the ALB-susceptible maize line B73 as well as the ALB-resistant line H99. Throughout the time course study, no differences in infection patterns between leaf blight-resistant and -susceptible lines were observed.
Early infection and colonization events were observed by means of germinating maize seeds in petri dishes and inoculating the 3-day-old seedlings with colonized agar plugs. As soon as 1 day postinfection, hyphae colonized the radicle surface (Fig. 1 and 2A and B). At 2 dpi, hyphae grew along the epidermis parallel to the longitudinal axis of the root (Fig. 2C). At this point, lateral, swellings could be seen at the junction between plant cells (Fig. 2C and D). Shortly thereafter, the lateral swellings became melanized, and hyphae could be observed within the subtending epidermal cells (Fig. 2D). We refer to these swellings as hyphopodia, following the definition provided by Howard (22), which describes appressoria as structures that develop from swellings at the tips of conidial germ tubes and hyphopodia as structures that arise from mature vegetative hyphae. No appressoria, structures typical of C. graminicola foliar infections, were observed on the root surface, even when spore suspensions were used as the inoculum. Instead, conidia germinated to form hyphae (Fig. 2E).
At 3 dpi, the hyphae became thick and melanized, resembling microhyphae or runner hyphae (20), and could be observed extensively colonizing the root surfaces (Fig. 2F). At this stage, fluorescence was generally not observed because the accumulation of melanin blocked the light, although less mature fluorescent hyphae could be found in association with the melanized hyphae. The hyphae extensively colonized the root surface, forming a network around the root (Fig. 2G). At 3 dpi, hyphae were also observed invading the intercellular spaces of epidermal roots cells (Fig. 2H), although no visible disease symptoms could be found on the roots. Once inside the root, the hyphae swelled and spread intracellularly into the cytosol of the cell through specific points of contact with other epidermal and cortical cells. Hyphae became greatly constricted as they passed through the cell wall (Fig. 2I). Hyphae could also be observed growing intracellularly among the epidermal and cortical cells (Fig. 2J). Infected epidermal and cortical cells frequently became packed with hyphae (Fig. 2J to L). The hyphae proliferated within the cell, filling most of the intracellular space (Fig. 2J), and eventually became melanized (Fig. 2K). The hyphae appeared to be confined to the infected cell until the intracellular space was completely filled with hyphae (Fig. 2J and K). Only certain epidermal and cortical cells appeared to become colonized in this manner, while the surrounding cells remained uninfected, resulting in a mosaic pattern of plant cell colonization (Fig. 2L). This pattern of plant cell colonization continued for 4 to 25 dpi.
Many root pathogens are reported to preferentially infect their hosts through specific regions of the roots (18, 31, 42). For example, in the Fusarium verticillioides-maize root interaction, the main sites of penetration are the lateral roots (42). Specificity for certain regions of the roots may indicate that there is tissue-specific variation in factors that determine susceptibility to C. graminicola and thus may be helpful in identifying those factors. To determine if C. graminicola also has site of attachment or penetration preference, roots from 3-day-old plants were dipped in a spore suspension, transferred to a petri dish, and observed by fluorescence microscopy for 24 to 72 h. Hyphae could be seen colonizing the surfaces of mature roots, root caps, root elongation zones, and root hairs (data not shown), indicating that there was no root infection site preference.
The formation of acervuli began with the development of stromata within the epidermal cells. At 4 to 6 dpi, stromatic tissue formed inside epidermal cells, giving rise to conidiophores and setae (Fig. 3A and B). These structures subsequently expanded, resulting in mechanical rupture of the cuticle and the formation of masses of falcate conidia and characteristic setae. MS tended to form near acervuli (Fig. 3B). Interestingly, falcate conidia were formed in acervuli as soon as 5 dpi on the root surfaces but were also found filling epidermal cells and root hairs at 10 dpi (Fig. 3C to F). Cells that were filled with oval conidia could also be found (Fig. 3G). The conidia were viable and often they could be observed germinating within the epidermal cells (Fig. 3H) and the root hairs (not shown). Brown discoloration was evident on roots during the advanced stages of colonization (approximately 6 dpi and later). Microscopic examination of the roots indicated that the brown discoloration was due to the presence of plant cells that were densely packed with melanized hyphae and acervuli.
At 21 dpi, hyphae continued to spread intracellularly through specific points of contact with other cells (data not shown). At this time point, extensive root areas were covered with acervuli and setae (Fig. 3I) and large root areas were discolored. Despite the extensive colonization by melanized hyphae and the presence of acervuli, necrotic lesions were not observed on the roots at this time point. Microscopic observation of plants at 28 dpi revealed that the entire root system (Fig. 1), including the radicle, mesocotyl, and nodal, lateral, and seminal roots of both B73 and H99 were infected. Even root sections that showed no visible symptoms of colonization, such as brown discoloration, were colonized by the fungus (Fig. 3J). The red pigmentation of the root is characteristic of the maize line B73. After 42 dpi, some signs of root necrosis could be observed.
Sections from infected and uninfected roots of both leaf blight-susceptible and -resistant maize lines were examined at various time points to determine the rates and extents of colonization. At 7 dpi in both maize lines, the pathogen colonized the epidermis and the cortical cells, both intercellularly and intracellularly (Fig. 3K and L). At 14 dpi, the whole root cortex was heavily colonized, until the fungus reached the endodermis (Fig. 3L and M). The endodermis appeared to delay the progress of the hyphae, and it was not until 21 dpi that the fungus was observed colonizing the vascular cylinder (Fig. 3N and O), where it could be observed colonizing the endodermis, sieve cells (phloem) and xylem (Fig. 3N). While xylem vessels were colonized by hyphae (Fig. 3O), no blockage of the vascular system was observed, nor did the plants exhibit wilting symptoms, typically found with vascular diseases. Some yellow-green fluorescence was observed in the piths of the infected roots; however, the emission spectrum of this fluorescence was different from the GFP emission spectrum, indicating that the cells accumulated autofluorescing material, similar to what has been observed for the Fusarium verticillioides-maize root interaction (42). In the uninfected roots, yellow autofluorescence was observed in the maize epidermis, endodermis, phloem, and xylem (Fig. 3P).
MS produced in culture are able to infect plant roots.
As soon as 5 dpi, we observed MS attached to hyphae on root surfaces (Fig. 4A to C). Size and shape varied, but MS continued to increase in number and size over time (Fig. 4A to C and data not shown). The MS were generally small (5 to 20 μm) and appeared to be comprised of aggregated, compact, melanized hyphae. At 20 dpi, a large number of them could be observed on the root (Fig. 4D).
We investigated whether the MS were viable sources of inoculum. Because of their small size, isolation of MS from infected plant tissue was not feasible, so an in vitro MS induction system described previously for other Colletotrichum spp. was employed. The MS produced in vitro were numerous and had morphology similar to that found for infected plant tissue (Fig. 4E and F). Masses of salmon-orange conidia inside mucilage could be observed on the surfaces of the MS (Fig. 4F). The MS produced in culture were surface cleaned and sterilized (see Materials and Methods) to remove hyphal fragments and conidia from the surface before applying them to uninfected roots. The MS produced in culture were able to infect plant roots. Myceliogenic (Fig. 4G) and sporogenic (not shown) germinations of the MS were observed, and runner hyphae and hyphopodia could be observed colonizing the root surface (Fig. 4G).
Infection of plant roots leads to colonization of aerial plant organs.
To determine whether C. graminicola could colonize aboveground plant parts upon the infection of roots, we attempted to isolate the fungus from infected plants. Plants were infected with C. graminicola strain M1.001-BH-gfp. At 42 dpi, segments of above- and belowground plant parts were dissected, surface sterilized, and cultured on acidified PDA+hyg. The cultures exhibiting C. graminicola colony morphology were examined by fluorescence microscopy to confirm their identities. The number of successful C. graminicola isolations for each tissue type is shown in Table 1. Colletotrichum graminicola was not recovered from any part of the mock-inoculated plants but could be recovered from 97.8% of the roots and from 28.3% of the aboveground parts of root-inoculated plants. The fungus was recovered more than twice as often from the aerial parts of leaf blight-susceptible lines B73 (40.0%) and Mo940 (33.3%) as from the leaf blight-resistant line H99 (15.8%).
TABLE 1.
Incidence of C. graminicola isolated from infected maize plants at 42 dpi
| Maize inbred line | No. of plants testeda | No. (%) of plants from which hygromycin-resistant C. graminicola was recovered from:
|
|||
|---|---|---|---|---|---|
| Roots | Stem | Leaves | Aerial tissues (stem or leaves) | ||
| B73 | 15 | 15 (100) | 6 (40) | 3 (20) | 6 (40) |
| Mo940 | 12 | 12 (100) | 3 (25) | 2 (16.6) | 4 (33.3) |
| H99 | 19 | 18 (94.7) | 1 (5.2) | 2 (10.5) | 3 (15.8) |
| Total | 46 | 45 (97.8) | 10 (21.7) | 7 (15.2) | 13 (28.3) |
Sum of three experiments.
Examination of the aerial plant parts at 28 dpi under fluorescence microscopy revealed that the adventitious roots, senescent coleoptiles, and senescent first and second leaf sheaths were frequently colonized (Fig. 1 and 5 A to C). These results were consistently observed among all of the maize lines tested (B73, H99, and Mo940). Senescent coleoptiles were heavily colonized (Fig. 5B and C). Acervuli and conidia were also found on the aerial plant organs. Within the green, living leaf sheath tissue, hyphae were also occasionally observed inside plant cells (Fig. 5D) and leaf trichomes (Fig. 5E).
Often, the limiting step in the movement of plant pathogenic fungi from infected roots to the upper parts of the plant is the transition of the fungus from the seedling crown to the stalk, and the transition usually takes place through the vascular tissue (40, 42). Therefore, transverse sections of leaves and stems were used to determine whether C. graminicola colonized vascular tissue, parenchymatous tissue, or both tissue types. While the strong autofluorescence of the plant tissue made direct observation of C. graminicola in these sections difficult, culturing the stem transverse sections on isolation medium revealed the presence of hygromycin-resistant GFP-expressing C. graminicola from individual vascular bundles (Fig. 5F). To determine whether the fungus was localized within specific tissues within the leaves of infected plants, some samples were fixed, embedded, and stained with aniline blue and visualized with bright-field microscopy. Hyphae appeared to be restricted to the phloem tissue of the vascular bundle of the leaf (Fig. 5G). We also observed the production of lobed hyphopodia in the infected, senescent leaf sheaths (Fig. 5H to J). These structures originated from melanized hyphae and are attached to the plant leaves. Younger hyphopodia were fluorescent, but over time, melanin accumulation masked the fluorescence (Fig. 5H and I). At 6 to 8 weeks postinfection, necrotic, water-soaked lesions began to appear on the roots of infected plants, but no symptoms were observed on upper plant parts under the tested conditions (data not shown).
DISCUSSION
In this paper, we show that C. graminicola can infect maize roots by use of specialized structures and processes that are found in root pathogens. The growth of maize plants in the presence of C. graminicola soil inoculum led to the colonization of the roots by the fungus. During the colonization, we observed structures formed by C. graminicola during the infection of roots, including runner hyphae, hyphopodia, and MS. These specialized infection structures are commonly found for root pathogens such as Gaeumannomyces graminis (9, 14) but have not been previously reported for C. graminicola. Early in the infection process, runner hyphae enveloped the root surface and lateral hyphopodia and were frequently observed at the junction of adjacent epidermal cells. There are numerous structural similarities between hyphopodia and appressoria produced by plant pathogens. Hyphopodia and appressoria can be produced by the same organism, but generally only one or the other is found under specific environmental conditions (22, 38). Plant pathogens that rely upon epiphytic colonization of plant parts near or below the soil line usually form hyphopodia, and it is believed that they play a role in the penetration of the plant epidermis, in a manner similar to that seen for appressoria (22). It has been suggested that melanized hyphopodia might also function as structures for survival (11). Considering that we found hyphopodia on senescent leaves of plants that had been infected through their roots, it seems reasonable to hypothesize that in this case, the hyphopodia might also serve as survival structures and a source of inoculum for the subsequent spread of the fungus to adjacent plants during the same or the following growing season (11, 38).
Within the first 6 weeks, hyphae could be found colonizing the surfaces of the roots and invading the epidermis, cortex, and vascular tissues. These characteristics are consistent with reports of the rice blast fungus Magnaporthe oryzae, which follows a similar series of events (25). Unlike rice blast, however, the early stages of maize root infection by C. graminicola were characterized by extensive intercellular colonization and a mosaic pattern of intracellular colonization. Interestingly, a similar mosaic pattern of infection has been described for the pathogenic interactions of Fusarium oxysporum on tomato roots and F. verticillioides on maize roots (31, 42). This mosaic pattern of root colonization is also seen in the white clover-Glomus intraradices arbuscular mycorrhizal interaction (34, 60), indicating that this pattern of infection is not a trait that is unique to pathogenic fungi.
Colletotrichum graminicola normally forms two types of conidia. Falcate conidia are produced in acervuli on the surfaces of lesions and are associated with disease dispersion, while smaller oval conidia can be found within infected stems and leaves (43). It is thought that light and oxygen availability within leaves and stems plays a role in regulating the production of the different types of conidia (43). We found that both types of conidia were formed within root epidermal cells and root hairs, an environment that has low light and reduced air exchange. Conidium formation on root tissue has also been described for maize roots infected with Fusarium verticillioides (42). While the exact function of these conidia remains unknown, it is interesting to speculate that they may function as resting structures, enabling the fungus to remain within the root tissue in a metabolically inactive state that may prevent its recognition by the host. This hypothesis is somewhat reminiscent of the quiescent infection phase of C. gloeosporioides on avocado, in which conidia germinate and form appressoria and penetration hyphae on fruit but then stop development and remain latent until fruit ripening begins (6, 47). This infection strategy is common among fruit infecting Colletotrichum species, such as C. gloeosporioides, C. musae, and C. acutatum.
In roots, necrotrophic growth of C. graminicola at the infection site appears to be delayed until approximately 6 weeks after infection. In contrast, however, several reports describe stunting, rapid root necrosis, and 100% plant death within 3 to 4 weeks of strawberry plant infection by C. acutatum (16, 64). Roots of soybean inoculated with different isolates of C. truncatum also developed water-soaked lesions within 4 or 5 dpi (29). Like C. acutatum and C. truncatum, C. graminicola has the ability to colonize roots of its host, but it does so in a much less destructive manner. It has been suggested that the ability to live in close proximity with the plant without causing lesions that inhibit growth and development could be advantageous to soilborne fungal pathogens (19), and a similar case might be made for C. graminicola. If the primary means of overwintering is through infected aboveground plant parts, then premature stunting or killing of the plant may reduce the amount of inoculum that is available to infect plants in the next season.
In our study, MS were produced in infected roots. Sclerotia and MS are known to be formed by several other Colletotrichum spp. and are believed to serve as survival structures (29, 41). The tomato root and fruit rot pathogen C. coccodes forms MS freely in the soil that may survive and remain infective for several years (7, 12). There are also reports of C. truncatum forming MS on lentil and soybean plants (29, 41). In addition, the sclerotia of C. sublineolum in sorghum stalks residing on the soil surface are believed to be an important source of inoculum in the field (8). It is well known that infected plant residue remaining on the soil surface serves as a reservoir of primary inoculum for anthracnose infections (5). We observed the development of several fungal structures that may serve as survival structures that enable the fungus to overwinter. The MS produced in culture were able to infect plant roots, suggesting that MS found in infected plant tissues may also be viable and could serve as a source of inoculum. We also found hyphopodia produced on the root surface at the earliest stages of infection, where they may serve as infection structures. Hyphopodia were also observed late in the disease cycle on infected and senescent leaves, where they may also serve as survival and dispersal structures. In addition, the conidia produced within plant cells may also serve as another means for the fungus to survive winter conditions.
Perhaps one of the most important outcomes of this study is the discovery that upon successful colonization of the root system, C. graminicola can enter the vascular system and spread systemically to the aerial parts of the plant without causing widespread disease symptoms. The fate of a successful infection, i.e., whether it immediately becomes symptomatic or remains nonsymptomatic, may depend in part upon the tissue in which the infection begins. As has been suggested for the F. verticillioides-maize interaction (42), the nature of the interaction may result from a balance of environmental and/or physiological conditions between the plant and the fungus. Assuming that propagules formed on the aboveground plant parts are the primary form of dissemination of the fungus, it seems likely that the ability to asymptomatically colonize the host would present a selective advantage to the fungus. A fully mature but senescent host plant may be a better platform upon which to form propagules for dissemination.
Another notable outcome is that both the resistant and susceptible maize cultivars were susceptible to root infection, indicating that resistance is tissue specific. There are only a limited number of studies about the factors that condition the ability for pathogens to colonize different plant tissues (10, 23, 53). Deletion of the M. oryzae FOW1 homolog restricted colonization and symptom development in roots but did not affect leaf pathogenesis (53). These results, coupled with a previous report of tissue-specific mutants by Dufresne and Osbourn (10), show that in fungi, the genetic mechanisms underlying foliar and root pathogeneses are distinct. Further studies of tissue-specific plant responses to infection by C. graminicola will help to reveal the molecular nature of the resistance and susceptibility.
We have demonstrated that C. graminicola shares traits with other root-infecting fungi. Based on these observations, we hypothesize that this pattern of infection is an ancestral trait that has been maintained in several fungal lineages despite millions of years of divergent evolution. A corollary of this hypothesis is that root infection confers a selective advantage to the fungus and that it is common in field infections. Successful root infection is associated with specialized structures and processes, and it seems unlikely that a fungus would maintain the ability to perform this complex process if it were not advantageous (58). It is becoming clear that many fungi are able to infect both above- and belowground parts of their hosts. In fact the difference between what we regard as a “root pathogen” and a “foliar pathogen” may be due to the ability to produce symptoms and not to the ability to colonize these tissues.
Acknowledgments
This work was supported by funds from the Texas A&M University Department of Plant Pathology and Microbiology and the Texas Agricultural Experiment Station.
We thank Ane Sesma for kindly providing us with the root inoculation protocol, Lisa Vaillancourt for providing maize seed, Tania Cruz for her valuable technical assistance, and Thomas Isakeit for helpful comments and suggestions.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Bailey, J. A., R. J. O'Connell, R. J. Pring, and C. Nash. 1992. Infection strategies of Colletotrichum species, p. 88-120. In J. A. Bailey and M. J. Jeger (ed.), Colletotrichum: biology, pathology and control. CAB International, Wallingford, United Kingdom.
- 2.Bayman, P., L. L. Lebron, R. L. Tremblay, and D. J. Lodge. 1997. Variation in endophytic fungi from roots and leaves of Lepanthes (Orchidaceae). New Phytol. 135:143-149. [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom, F. B., and G. C. Bergstrom. 1987. Influence of maize growth stage on fungal movement, viability, and rot induction in stalks inoculated with Colletotrichum graminicola. Phytopathology 77:115. [Google Scholar]
- 4.Bergstrom, G. C., and R. L. Nicholson. 2000. The biology of Colletotrichum graminicola and maize anthracnose, p. 374-394. In D. Prusky, S. Freeman, and M. B. Dickman (ed.), Host specificity, pathology and host pathogen interaction of Colletotrichum. American Phytopathological Society, St. Paul, MN.
- 5.Bergstrom, G. C., and R. L. Nicholson. 1999. The biology of corn anthracnose—knowledge to exploit for improved management. Plant Dis. 83:596-608. [DOI] [PubMed] [Google Scholar]
- 6.Binyamini, N., and M. Schiffmann-Nadel. 1972. Latent infection in avocado fruit due to Colletotrichum gloeosporioides. Phytopathology 62:592-594. [PubMed] [Google Scholar]
- 7.Blackeman, J. P., and D. Hornby. 1966. The persistence of Colletotrichum coccodes and Mycosphaerella ligudicola in soil with special reference to sclerotia and conidia. Trans. Br. Mycol. Soc. 49:227-240. [Google Scholar]
- 8.Casela, C. R., and R. A. Frederiksen. 1993. Survival of Colletotrichum graminicola sclerotia in sorghum stalk residues. Plant Dis. 77:825-827. [Google Scholar]
- 9.Deacon, J. W. 2006. Fungal biology, 4th ed. Blackwell Publishing, Oxford, United Kingdom.
- 10.Dufresne, M., and A. E. Osbourn. 2001. Definition of tissue-specific and general requirements for plant infection in a phytopathogenic fungus. Mol. Plant-Microbe Interact. 14:300-307. [DOI] [PubMed] [Google Scholar]
- 11.Epstein, L. 1994. Production of hyphopodia by wild type and three transformants of Gaeumannomyces graminis var. graminis. Mycologia 86:72-81. [Google Scholar]
- 12.Farley, J. D. 1976. Survival of Colletotrichum coccodes in soil. Phytopathology 66:640-641. [Google Scholar]
- 13.Forgery, W. M., M. H. Blanco, and W. Q. Loegering. 1978. Differences in pathological capabilities and host specificity of Colletotrichum graminicola on Zea mays (maize). Plant Dis. Rep. 62:573-576. [Google Scholar]
- 14.Freeman, J., and E. Ward. 2004. Gaeumannomyces graminis, the take-all fungus and its relatives. Mol. Plant Pathol. 5:235-252. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, S., S. Horowitz, and A. Sharon. 2001. Pathogenic and nonpathogenic lifestyles in Colletotrichum acutatum from strawberry and other plants. Phytopathology 91:986-992. [DOI] [PubMed] [Google Scholar]
- 16.Freeman, S., and T. Katan. 1997. Identification of Colletotrichum species responsible for anthracnose and root necrosis of strawberry in Israel. Phytopathology 87:516-521. [DOI] [PubMed] [Google Scholar]
- 17.Freeman, S., and R. J. Rodriguez. 1993. Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science 260:75-78. [DOI] [PubMed] [Google Scholar]
- 18.Gunawardena, U., and M. C. Hawes. 2002. Tissue specific localization of root infection by fungal pathogens: role of root border cells. Mol. Plant-Microbe Interact. 15:1128-1136. [DOI] [PubMed] [Google Scholar]
- 19.Gunawardena, U., M. Rodriguez, D. Straney, J. T. Romeo, H. D. VanEtten, and M. C. Hawes. 2005. Tissue-specific localization of pea root infection by Nectria haematococca. Mechanisms and consequences. Plant Physiol. 137:1363-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henson, J. M., M. J. Butler, and A. W. Day. 1999. The dark side of the mycelium: melanins of phytopathogenic fungi. Annu. Rev. Phytopathol. 37:447-471. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz, S., S. Freeman, and A. Sharon. 2002. Use of green fluorescent protein-transgenic strains to study pathogenic and nonpathogenic lifestyles in Colletotrichum acutatum. Phytopathology 92:743-749. [DOI] [PubMed] [Google Scholar]
- 22.Howard, R. J. 1997. Breaching the outer barriers—cuticle and cell wall penetration, p. 43-60. In G. Carroll and P. Tudzynski (ed.), Plant relationships, vol. 5A. Springer-Verlag, New York, NY. [Google Scholar]
- 23.Inoue, I., F. Namiki, and T. Tsuge. 2002. Plant colonization by the vascular wilt fungus Fusarium oxysporum requires FOW1, a gene encoding a mitochondrial protein. Plant Cell 14:1869-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen, C., D. von Wettstein, W. Schäfer, K. H. Kogel, A. Felk, and F. J. Maier. 2005. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 102:16892-16897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen, M., A. Slusarenko, and U. Schaffrath. 2006. Competence of roots for race-specific resistance and the induction of acquired resistance against Magnaporthe oryzae. Mol. Plant Pathol. 7:191-195. [DOI] [PubMed] [Google Scholar]
- 26.Kaminskyj, S. G. W. 2001. Fundamentals of growth, storage, genetics and microscopy of Aspergillus nidulans. Fungal Genet. Newsl. 48:25-31. [Google Scholar]
- 27.Keller, N. P., and G. C. Bergstrom. 1988. Developmental predisposition of maize to anthracnose stalk rot. Plant Dis. 72:977-980. [Google Scholar]
- 28.Keller, N. P., G. C. Bergstrom, and R. I. Carruthers. 1986. Potential yield reduction in maize associated with an anthracnose European corn borer pest complex in New York. Phytopathology 76:586-589. [Google Scholar]
- 29.Khan, M., and J. Sinclair. 1992. Pathogenicity of sclerotia forming and nonsclerotia forming isolates of Colletotrichum truncatum on soybean plants and roots. Phytopathology 82:314-319. [Google Scholar]
- 30.Kogel, K. H., P. Franken, and R. Huckelhoven. 2006. Endophyte or parasite—what decides? Curr. Opin. Plant Biol. 9:358-363. [DOI] [PubMed] [Google Scholar]
- 31.Lagopodi, A. L., A. F. J. Ram, G. E. M. Lamers, P. J. Punt, C. A. M. J. J. Van den Hondel, B. J. J. Lugtenberg, and G. V. Bloemberg. 2002. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol. Plant-Microbe Interact. 15:172-179. [DOI] [PubMed] [Google Scholar]
- 32.Leonard, K. J. 1974. Foliar pathogens of corn in North Carolina. Plant Dis. Rep. 58:532-534. [Google Scholar]
- 33.Leonard, K. J., and D. L. Thompson. 1976. Effects of temperature and host maturity on lesion development of Colletotrichum graminicola on corn. Phytopathology 68:1336. [Google Scholar]
- 34.Lum, M. R., Y. Li, T. A. Larue, R. David-Schwartz, Y. Kapulnik, and A. Hirsch. 2002. Investigation of four classes of nonnodulating white clover (Melilotus alba annua Desr.) mutants and their responses to arbuscular-mycorrhizal fungi. Integr. Comp. Biol. 42:295-3003. [DOI] [PubMed] [Google Scholar]
- 35.Maor, R., M. Puyesky, B. Horwitz, and A. Sharon. 1998. Use of green fluorescent protein (GFP) for studying development and fungal-plant interaction in Cochliobolus heterostrophus. Mycol. Res. 102:491-496. [Google Scholar]
- 36.Mims, C. W., and L. J. Vaillancourt. 2002. Ultrastructural characterization of infection and colonization of maize leaves by Colletotrichum graminicola, and by a C. graminicola pathogenicity mutant. Phytopathology 92:803-812. [DOI] [PubMed] [Google Scholar]
- 37.Mitchum, M. G., S. Sukno, X. Wang, Z. Shani, G. Tsabary, O. Shoseyov, and E. Davis. 2004. The promoter of the Arabidopsis thaliana cel1 endo-1,4-β glucanase gene is differentially expressed in plant feeding cells induced by root-knot and cyst nematodes. Mol. Plant Pathol. 5:175-181. [DOI] [PubMed] [Google Scholar]
- 38.Money, N. P., T. C. Caesar-TonThat, B. A. Frederik, and J. M. Henson. 1998. Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in Gaeumannomyces graminis var. graminis. Fungal Genet. Biol. 24:240-251. [DOI] [PubMed] [Google Scholar]
- 39.Muimba-Kankolongo, A., and G. C. Bergstrom. 1992. Wound predisposition of maize to anthracnose stalk rot as affected by internode position and inoculum concentration of Colletotrichum graminicola. Plant Dis. 76:188-195. [Google Scholar]
- 40.Munkvold, G. P., D. C. McGee, and W. M. Carlton. 1997. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 87:209-217. [DOI] [PubMed] [Google Scholar]
- 41.O'Connell, R. J., A. B. Uronu, G. Waksman, C. Nash, J. P. R. Keon, and J. A. Bailey. 1993. Hemibiotrophic infection of Pisum sativum by Colletotrichum truncatum. Plant Pathol. 42:774-783. [Google Scholar]
- 42.Oren, L., S. Ezrati, D. Cohen, and A. Sharon. 2003. Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate. Appl. Environ. Microbiol. 69:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panaccione, D. E., L. Vaillancourt, and R. Hanau. 1989. Conidial dimorphism in Colletotrichum graminicola. Mycologia 8:876-883. [Google Scholar]
- 44.Perfect, S. E., H. B. Hughes, R. J. O'Connel, and J. R. Green. 1999. Colletotrichum—a model genus for studies on pathology and fungal-plant interactions. Fungal Genet. Biol. 27:186-198. [DOI] [PubMed] [Google Scholar]
- 45.Photita, W., J. W. Taylor, R. Ford, K. D. Hyde, and S. Lumyong. 2005. Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Divers. 18:117-133. [Google Scholar]
- 46.Politis, D. J., and H. Wheeler. 1973. Ultrastructural study of penetration of maize leaves by C. graminicola. Physiol. Plant Pathol. 3:456-471. [Google Scholar]
- 47.Prusky, D., and R. A. Plumbley. 1992. Quiescent infections of Colletotrichum in tropical and subtropical fruits, p. 289-307. In J. A. Bailey and M. J. Jeger (ed.), Colletotrichum: biology, pathology and control. CAB International, Wallingford, United Kingdom.
- 48.Redman, R. S., D. D. Dunigan, and R. J. Rodriguez. 2001. Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol. 151:705-716. [DOI] [PubMed] [Google Scholar]
- 49.Redman, R. S., J. C. Ranson, and R. J. Rodriguez. 1999. Conversion of the pathogenic fungus Colletotrichum magna to a nonpathogenic, endophytic mutualist by gene disruption. Mol. Plant-Microbe Interact. 11:969-975. [Google Scholar]
- 50.Ritchie, S. W., J. J. Hanway, and G. O. Benson. 1992. How a corn plant develops. University Extension publication SR 48. Iowa State University, Ames.
- 51.Sanogo, S., and S. P. Pennypacker. 1997. Factors affecting sporogenic and myceliogenic germination of sclerotia of Colletotrichum coccodes. Plant Dis. 81:333-336. [DOI] [PubMed] [Google Scholar]
- 52.Santamaria, J., and P. Bayman. 2005. Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microb. Ecol. 50:1-8. [DOI] [PubMed] [Google Scholar]
- 53.Sesma, A., and A. E. Osbourn. 2004. The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431:582-586. [DOI] [PubMed] [Google Scholar]
- 54.Shaw, B. D., and S. Upadhyay. 2005. Aspergillus nidulans swoK encodes an RNA binding protein that is important for cell polarity. Fungal Genet. Biol. 42:862-872. [DOI] [PubMed] [Google Scholar]
- 55.Shibutani, M., C. Uneyama, K. Miyazaki, K. Toyoda, and M. Hirose. 2000. Methacarn fixation: a novel tool for analysis of gene expressions in paraffin-embedded tissue specimens. Lab. Investig. 80:199-208. [DOI] [PubMed] [Google Scholar]
- 56.Thon, M. R., E. M. Nuckles, and L. J. Vaillancourt. 2000. Restriction enzyme-mediated integration used to produce pathogenicity mutants of Colletotrichum graminicola. Mol. Plant-Microbe Interact. 13:1356-1365. [DOI] [PubMed] [Google Scholar]
- 57.Tuite, J. 1969. Plant pathological methods: fungi and bacteria. Burgess Publishing Co., Minneapolis, MN.
- 58.Valent, B. 2004. Plant disease: underground life for rice foe. Nature 431:516. [DOI] [PubMed] [Google Scholar]
- 59.Vereijssen, J., H. J. H. M. Schneider, and A. A. J. Termorshuizen. 2004. Possible root infection of Cercospora beticola in sugar beet. Eur. J. Plant Pathol. 110:103-106. [Google Scholar]
- 60.Vierheilig, H., P. Schweiger, and M. Brundrett. 2005. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant. 125:393-404. [Google Scholar]
- 61.Warren, H. 1975. Field reaction of corn inbreds to Colletotrichum graminicola. Plant Dis. Rep. 59:767-769. [Google Scholar]
- 62.Warren, H. 1976. Relationship of Colletotrichum graminicola to foliar and kernel infection. Plant Dis. Rep. 60:1084-1086. [Google Scholar]
- 63.Warren, H. L., and R. L. Nicholson. 1975. Kernel infection, seedling blight, and wilt of maize caused by Colletotrichum graminicola. Phytopathology 65:620-623. [Google Scholar]
- 64.Wharton, P. S., and J. Diéguez-Uribeondo. 2004. The biology of Colletotrichum acutatum. An. Jard. Bot. Madr. 61:3-22. [Google Scholar]