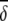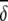Abstract
A high-throughput microbial profiling tool based on terminal restriction fragment length polymorphism was developed to monitor the poultry gut microbiota in response to dietary manipulations. Gut microbial communities from the duodena, jejuna, ilea, and ceca of 48 birds fed either a barley control diet or barley diet supplemented with exogenous enzymes for degrading nonstarch polysaccharide were characterized by using multivariate statistical methods. Analysis of samples showed that gut microbial communities varied significantly among gut sections, except between the duodenum and jejunum. Significant diet-associated differences in gut microbial communities were detected within the ileum and cecum only. The dissimilarity in bacterial community composition between diets was 73 and 66% within the ileum and cecum, respectively. Operational taxonomic units, representing bacterial species or taxonomically related groups, contributing to diet-associated differences were identified. Several bacterial species contributed to differences between diet-related gut microbial community composition, with no individual bacterial species contributing more than 1 to 5% of the total. Using canonical analysis of principal coordinates biplots, we correlated differences in gut microbial community composition within the ileum and cecum to improved performance, as measured by apparent metabolizable energy. This is the first report that directly links differences in the composition of the gut microbial community with improved performance, which implies that the presence of specific beneficial and/or absence of specific detrimental bacterial species may contribute to the improved performance in these birds.
The gut microbiota has an important role in animal health and production. Gut microbiota positively influence the host's gastrointestinal development, biochemistry, immunology, physiology, and nonspecific resistance to infection (31). In poultry, the microorganisms that colonize the gastrointestinal tract during the early posthatch period form a synergistic relationship with their host. Gastrointestinal microorganisms have a highly significant impact on the uptake and utilization of energy (17) and other nutrients (61, 62) and on the response of poultry to antinutritional factors (such as nonstarch polysaccharides [NSP]), pre- and probiotic feed additives and feed enzymes (12). Bacteria within the gut microbial community also interact with each other (22, 66), as well as with their host (40). Microorganisms can directly interact with the lining of the gastrointestinal tract (71), which may alter the physiology of the tract and immunological status of the bird (42).
The gastrointestinal microbiota has one of the highest cell densities for any ecosystem and in poultry ranges from 107 to 1011 bacteria per g of gut content (7). Earlier studies have predominantly used culture-dependent approaches for identifying the composition of the poultry gut microbiota (9, 51). However, a large number of bacteria remain unidentified due to lack in knowledge of appropriate culturing conditions. Furthermore, culturing and biochemical techniques have resulted in the misclassification of some bacteria (67).
Recent molecular studies targeting the bacterial DNA in poultry guts have yielded more detailed insight into the composition of the microbial community (2, 5, 29, 30, 35, 47, 50, 74, 75). From molecular studies it is estimated that the cecal microbiota consists of at least 640 species from 140 genera, of which 10% of the identified bacterial 16S rRNA gene sequences represent previously known bacterial species, and the remaining sequences belong to new species or even new genera (7). However, most of the molecular techniques currently used to study gut microbiota are unable to characterize the bacterial community in a single assay or are not conducive to high-throughput analysis.
To this end, we have developed a microbial profiling technique, based on terminal restriction fragment length polymorphism (T-RFLP) analysis (55), for examining the chicken intestinal microbiota based on high-throughput, high-resolution fingerprinting of bacterial 16S ribosomal gene regions. This technique enables a “snap-shot” view of the complex bacterial population at any particular time, making it ideal for comparative analysis. Furthermore, prior knowledge of particular bacterial species is not required to detect overall changes in microbial community composition. However, operational taxonomic units (OTUs), representing particular bacterial species or taxonomically related groups, characteristic of dietary treatments, can be identified and related to bird performance measures.
We investigate here the use of T-RFLP, in conjunction with multivariate statistical methods, to examine changes in gut microbial communities in response to the addition of an NSP-degrading enzyme product to a barley-based diet. Changes in gut microbiota composition were then related to bird performance. NSP-degrading additives are widely used in commercial poultry production (16); however, the manner by which they benefit their host is not completely understood (11). This is the first published study that directly links diet-induced changes in gut microbiota with subsequent improvement in poultry performance, as measured by energy metabolism.
MATERIALS AND METHODS
Chickens.
All experimental work with animals was done at the Pig and Poultry Production Institute Poultry Research Unit, Roseworthy Campus, University of Adelaide, with appropriate animal ethics approval. Cobb 500 broiler chickens were raised from hatch in two rearing pens in a controlled temperature room. Male and female chickens were reared separately. All birds were given commercial starter crumbles (Steg 600 starter; Ridley Agriproducts, Australia). At age day 13, a total of 48 chickens were placed, in single-sex pairs, in 24 metabolism cages located in a controlled-temperature room kept at 25 to 27°C, and continued to receive starter crumbles. Birds had free access to feed and water prior to and during the experimental period. At age day 15, chickens were transferred into individual metabolism cages for the start of the 7-day apparent metabolizable energy (AME) experiment. Barley-based diets, with or without the addition of an NSP-degrading feed enzyme product (Avizyme 1100 at a 1-kg/tonne inclusion rate), were given to 24 chickens each (12 male and 12 female). Avizyme 1100 (Feedworks, Australia) contains endo-1,3(4)-β-glucanase, 1,4-β-xylanase, and protease activities (according to the manufacturer). Each diet contained (per kg) 800 g of barley, 155 g of casein, 20 g of dicalcium phosphate, 11 g of limestone, 7 g of dl-methionine, 5 g of mineral and vitamin premix, 3 g of salt, and 2 g of choline chloride (60%). Both diets were cold pressed to form pellets approximately 6 mm in diameter and 6 mm in length.
Measurement of AME.
AME values of barley-based diets with or without feed enzyme product were determined in a classical energy balance study involving measurements of total feed intake and total excreta output and subsequent measurement of gross energy values of feed and excreta by bomb calorimetry (17). Diets were fed for 7 days. The first 3 days enabled chickens to adapt to the feeds. During the following 4 days, all excreta were collected daily and dried at 85°C and then pooled for each chicken. Moisture content of excreta voided over a 24-h period, and feed intake during the adaptation and collection phases of the study was measured. Birds were weighed at the start and end of the 7-day period. The dry matter contents of samples of pelleted and milled feeds were measured. Gross energy values of dried excreta and pelleted feeds were measured with a Parr isoperibol bomb calorimeter (Parr Instrument Company, Moline, IL). The AME of grain was calculated by subtracting from the total energy intake the energy contribution of casein, which is assumed to be 20.1 MJ/kg of dry matter (4).
Intestinal sample collection.
At age day 22, after completion of the metabolism study, all chickens were killed by intravenous injection of pentobarbitone sodium at 325 mg/ml at a dose rate of 0.5 ml/kg live weight (Lethobarb; Virbac Australia Pty., Ltd., Australia). The gastrointestinal tracts from the base of the gizzard down to the rectum were dissected, and sections approximately 3 cm long (including digesta) were cut from the mid regions of the duodenum, jejunum, ileum, and ceca. Samples were snap-frozen by immersion in liquid nitrogen and stored at −20°C prior to freeze-drying. Dissecting instruments were cleaned with 70% ethanol after use on each bird.
DNA extraction.
Total nucleic acid was extracted from chicken gut samples by a modification of a proprietary extraction method developed by the South Australian Research and Development Institute (64). Gut samples were incubated in an altered extraction buffer (1.5 M NaCl2, 1.3 M guanidine thiocyanate, 30 mM Tris-HCl [pH 7], 65 mM phosphate buffer [pH 8.0], 3.4% [wt/vol] N-lauroylsarcosine, 1.7% [wt/vol] polyvinylpyrrolidone) at 70°C for an hour prior to proceeding with the SARDI extraction method. An alternative extraction method, using a DNeasy tissue kit (Qiagen, Doncaster, Australia) according to the manufacturer's recommendations, was compared to the in-house method and found to give comparable results.
T-RFLP analysis.
Bacterial rRNA gene sequences were amplified with universal 16S bacterial primers 27F (49) and 907R (54). The forward primer was 5′ labeled with 6-carboxyfluorescein (FAM). PCRs were done in 50-μl volumes containing 1× PCR buffer II (Applied Biosystems, Scoresby, Australia), 1.5 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate (Invitrogen, Mulgrave, Australia), 0.2 μM concentrations of each primer (Sigma Proligo, Lismore, Australia), 1 U of AmpliTaq DNA polymerase LD (Applied Biosystems, Scoresby, Australia), and 50 to 100 ng of total nucleic acid template. All PCRs were done in duplicate and run in a MJ Research PTC-225 Peltier thermal cycler (GeneWorks, Adelaide, Australia) with the following amplification conditions: initial denaturation at 94°C for 5 min followed by 33 cycles of denaturation at 94°C for 45 s, annealing at 48°C for 45 s, and extension at 72°C for 1 min, with a final extension step at 72°C for 7 min. PCR products were quantified by fluorometry with Quant iT PicoGreen (Invitrogen, Mulgrave, Australia) according to the manufacturer's instructions, in a Wallac Victor2 1420 multilabel counter (Perkin-Elmer/Life Sciences, Australia). If duplicate PCRs varied by less than 20% in fluorescein counts they were pooled. The specificity of PCR products was analyzed by gel electrophoresis on a 1.5% agarose gel and visualized after staining with ethidium bromide. Approximately 200 ng of PCR product was digested with 2 U of MspI (Genesearch, Arundel, Australia) in the recommended enzyme buffer. Duplicate aliquots from the pooled PCR product obtained from each sample were digested in a final volume of 15 μl and incubated at 37°C for 4 h. After digestion, enzyme was inactivated by incubation at 65°C for 15 min. A single bacterial species positive control was used to check for complete digestion after gel electrophoresis on a 2% agarose gel and ethidium bromide staining. If digestion was complete, duplicate digestions were pooled. The length of fluorescently labeled terminal restriction fragments were determined from each pooled digestion in duplicate by comparison with an internal standard (GeneScan 1000 ROX; Applied Biosystems, Australia) after separation by capillary electrophoresis on a ABI 3700 automated DNA sequencer (Applied Biosystems, Australia) and data were analyzed by using GeneScan 3.7 software (Applied Biosystems, Australia).
Identification of OTUs.
A database was created for the data points generated by the GeneScan 3.7 software. Using queries built within the database the data points were validated, and outputs were generated for statistical analysis. Assumptions used to design queries in the database were modified from Dunbar et al. (24) and Egert et al. (25). Queries in the database were used to compare duplicate T-RFLP profiles (aliquots of a pooled digestion per sample) and identify synonymous fragment sizes (±2 bp). DNA quantity, as measured by total relative fluorescence between duplicates, was standardized and peaks that fell below the background threshold of 50 relative fluorescent units were excluded by using an iterative method described by Dunbar et al. (24). For each sample a derivative profile was then created from the average position and height of reproducible terminal restriction fragments. Terminal restriction fragments of ≥1.5% of the total relative peak height per sample were used in subsequent calculations. The resulting fragments were treated as OTUs, representing particular bacterial species or taxonomically related groups.
Statistical analysis.
Univariate analysis of variance (ANOVA) was used to determine effects of block, diet, and sex (fixed factors) on bird performance, as measured by AME, using the GLM model (Base SAS software; SAS Institute). Equal numbers of birds (n = 24) received either the barley control diet or the barley diet supplemented with enzyme. Each dietary group contained equal numbers of males and female chickens (n = 12).
OTUs obtained from the duodena, jejuna, ilea, and ceca of the 48 broiler chickens were also analyzed by using multivariate statistical techniques (PRIMER 6 and PERMANOVA+β1, PRIMER-E, Ltd., Plymouth, United Kingdom). These analyses were used to examine similarities in chicken gut microbial communities, identify OTUs accounting for differences observed in microbial communities, and examine correlations between the composition of the microbial community and bird performance data.
Bray-Curtis measures of similarity (13) were calculated to examine similarities between gut microbial communities of birds from the T-RFLP-generated (OTU) data matrices, following standardization and fourth root transformation. The Bray-Curtis similarity coefficient (13) is a reliable measure for biological data on community structure and is not affected by joint absences that are commonly found in microbial data (19). Two-way crossed analysis of similarity (ANOSIM) (19) was used to test whether gut microbial communities were significantly different between gut sections and diet, as well as between diet and sex, for each gut section. The R statistic value describes the extent of similarity between each pair in the ANOSIM, with values close to unity indicating that the two groups are entirely separate and a zero value indicating that there is no difference between the groups.
Similarity percentages (SIMPER) (19) analyses were done to determine which OTUs contributed most to the dissimilarity between dietary treatments. The overall average dissimilarity (δ̄) between gut microbial communities of birds on the two diets were calculated and the average contribution of the ith OTU (δ̄i) to the overall dissimilarity determined. The average abundance (ȳ) of important OTUs in each of the dietary groups was determined. OTUs contributing significantly to the dissimilarity between dietary treatments were calculated [δ̄i/SD(δi)>1]. The percent contributions of individual OTUs (δ̄i%) and the cumulative percent contribution (Σδ̄i%) to the top 50% of the average dissimilarities were also calculated.
Unconstrained ordinations were done to graphically illustrate the relationships between gut sections and/or diet by using nonmetric multidimensional scaling (nMDS) (45, 59) and principal coordinate analysis (PCO) (32). nMDS ordinations attempt to place all samples in an arbitrary two-dimensional space such that their relative distances apart match the corresponding pairwise similarities. Hence, the closer two samples are in the ordination the more similar are their overall gut bacterial communities. “Stress” values (Kruskal's formula 1) reflect the difficulty involved in compressing the sample relationship into the two-dimensional ordination. PCO ordinations also show the relationship among samples. However, nMDS uses the ranks of similarities, whereas PCO uses the actual similarity measures from the underlying Bray-Curtis similarity matrix.
Subsets of OTUs found to best represent results from nMDS ordinations on the full set of OTU data were also determined by using the BVSTEP procedure (20) on a random selection of starting variables. Matches of nMDS ordination produced from the subset of OTUs to the full set of OTUs were determined by Spearman rank correlation (ρ) of elements from the two underlying Bray-Curtis similarity matrices. A good correlation between underlying similarity matrices is determined when ρ ≥ 0.95. Both SIMPER and BVSTEP identify differences in community composition, but in slightly different ways. SIMPER identifies individual species (OTUs) contributing to the overall dissimilarity between treatments, whereas BVSTEP identifies sets of species (OTUs) summarizing the overall pattern differences in microbial community composition (21).
Constrained canonical analysis of principal coordinates (CAP) biplots (3) were constructed to investigate the relationship between OTUs associated with diet and AME. The a priori hypothesis that gut microbial communities were different between diets was tested in CAP by obtaining a P value using permutation procedures (999 permutations) on the canonical test statistic (squared canonical correlation, δ12). The number of PCO axes (m) was chosen to achieve the maximum proportion of correct allocations (% of trace [G]) of samples to diet. Pearson correlation (r) was calculated between the first canonical axis (CAP1) and AME.
RESULTS
Univariate analysis of classical growth and performance data showed that chickens fed a barley-plus-enzyme diet had a significantly higher (ANOVA, F1,11 = 46.47 P < 0.0001) AME (13.87 ± 0.22 SD MJ/kg dry matter) than chickens on the control barley diet (13.47 ± 0.29 SD MJ/kg dry matter). There was no effect of bird sex on AME (P > 0.05), and no interaction between diet and sex was detected (P > 0.05).
Multivariate statistical analysis showed that the composition of the gut bacterial community was significantly different between gut sections and diet. The global R value for differences among gut sections across diets was 0.440 (P < 0.001) and for differences between diets across all gut sections was 0.186 (P < 0.001). Bacterial community composition was significantly different among all gut sections, except between the duodenum and jejunum, regardless of diet (Table 1). This is graphically shown in the nMDS ordination as a separation into three groups; ileum, cecum, and duodenum-jejunum combined (Fig. 1A). However, when the samples are identified by diet (Fig. 1B) the influence of diet on gut microbial community composition becomes apparent, particularly within the caeca. It should be noted that the stress value for these two-dimensional ordinations is moderately high (0.24), indicating that it is not a good representation of the overall gut bacterial community differences. Stress is known to increase with reducing dimensionality and increasing quantity of data (21).
TABLE 1.
Two-way ANOSIM of gut microbial communities associated with gut sections and dieta
| Site |
R or P value
|
|||
|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Cecum | |
| Duodenum | 0.033 | 0.371 | 0.541 | |
| Jejunum | 0.066 | 0.405 | 0.625 | |
| Ileum | 0.001 | 0.001 | 0.631 | |
| Cecum | 0.001 | 0.001 | 0.001 | |
The R statistic (indicated in boldface) and significance (indicated in italics) are shown for comparisons between gut sections across both the barley control and the barley-plus-enzyme diets.
FIG. 1.
nMDS ordination of gut microbial communities identified by gut section and diet. (A) Gut microbial communities from the duodenum (triangles), jejunum (inverted triangles), ileum (circles), and ceca (squares) from all birds (n = 48) regardless of diet. (B) Same nMDS as in panel A. However, samples are identified by both gut section and diet. Gut microbial communities from the four gut sections of birds on the barley control diet are identified as duodenum (gray triangles), jejunum (inverted gray triangles), ileum (gray circles), and ceca (gray squares), while those of birds on the barley diet supplemented with enzyme are identified as duodenum (black triangles), jejunum (black inverted triangles), ileum (black circles), and ceca (black squares). The ordination is based on Bray-Curtis similarities calculated from fourth-root transformed OTU abundances (147 OTUs).
The effects of diet and sex were further investigated for each of the four gut sections. Significant differences in bacterial community composition associated with diet were only detected within the ileum and ceca (Table 2). No significant diet-associated differences in bacterial community composition were detected within the duodenum or jejunum. Furthermore, no significant differences were found in the gut bacterial community composition between male and female birds (Table 2).
TABLE 2.
Two-way ANOSIM of gut microbial communities associated with sex and diet for each of the four gut sections investigateda
| Parameter |
R and P values
|
|||
|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Cecum | |
| Sex | 0.038, 0.178 | 0.057, 0.117 | 0.052, 0.141 | 0.008, 0.394 |
| Diet | 0.007, 0.402 | 0.061, 0.090 | 0.197, 0.003 | 0.470, 0.001 |
The global R statistic (indicated in boldface) and significance (indicated in italics) are shown for each of the factors within each gut section.
The difference in ileal bacterial community composition associated with diet is shown in Fig. 2A. The relationship is represented in two-dimensional space, which has been reduced from a much higher-dimensional species (OTUs) space. This implies that many OTUs must be interchangeable in the way they characterize the samples and that an analysis of a small subset of the total number of OTUs may give a similar result to that of the full set. Hence, a subset of 17 OTUs was identified by the BVSTEP procedure, which showed a good correlation (ρ = 0.95) with the relationship generated from the full set of 91 OTUs. The nMDS shows a close match between the wider community pattern based on all 91 identified OTUs (Fig. 2A) and the pattern based on the subset of 17 OTUs (Fig. 2B).
FIG. 2.
nMDS ordination of gut microbial communities from the ileum of birds fed either a barley control diet (filled circles) or barley diet supplemented with enzyme (open squares) showing the extent to which the overall community pattern generated from the full set of 91 OTUs (A) is reproducible by a smaller subset of 17 OTUs (B) identified by the BVSTEP procedure. The matching coefficient (ρ) to panel A is shown in panel B. The subset of 17 OTUs identified consisted of OTUs 70, 96, 148, 182, 184, 190, 214, 224, 286, 288, 290, 300, 480, 522, 580, 590, and 880.
The difference in cecal bacterial community composition associated with diet is shown in Fig. 3A. A subset of 29 OTUs was identified by the BVSTEP procedure, which showed a good correlation (ρ = 0.95) with the relationship observed from the full set of 111 OTUs. Figure 3B shows the nMDS generated from the subset of 29 OTUs that is similar to the nMDS produced from the full set of OTUs (Fig. 3A). Of the subset of 17 and 29 diet-associated OTUs identified within the ileum and ceca, respectively, nine OTUs (70, 96, 184, 214, 286, 290, 300, 580, and 590) were common to both gut sections. The remaining eight (148, 182, 190, 224, 228, 480, 522, and 880) and 20 (92, 94, 146, 158, 188, 198, 212, 216, 222, 486, 488, 490, 496, 526, 528, 530, 536, 538, 546, and 548) diet-associated OTUs identified were unique to the ileum and ceca, respectively.
FIG. 3.
nMDS ordination of gut microbial communities from the ceca of birds fed either a barley control diet (filled circles) or a barley diet supplemented with enzyme (open squares) showing the extent to which the overall community pattern identified from the full set of 111 species (A) is reproducible by a smaller subset of 29 species (B) identified by the BVSTEP procedure. The matching coefficient (ρ) to panel A is shown in panel B. The subset of 29 OTUs identified were OTUs 70, 92, 94, 96, 146, 158, 184, 188, 198, 212, 214, 216, 222, 286, 290, 300, 486, 488, 490, 496, 526, 528, 530, 536, 538, 546, 548, 580, and 590.
Similarities in gut bacterial communities among birds on the same diet were calculated with SIMPER. Within the ileum, gut bacterial communities were 28% similar for birds on the barley control diet and 34% similar for birds on the barley diet supplemented with enzyme. Within the ceca, the similarities in gut bacterial community composition were higher and found to be 48 and 42% for birds on the barley control diet and the barley diet supplemented with enzyme, respectively. Dissimilarities in bacterial communities associated with diet were 73% in the ileum and 66% in the ceca. OTUs contributing to the top 50% of dissimilarity in bacterial community composition between diets were identified within the ileum (Table 3) and ceca (Table 4). Nine OTUs within the ileum and twenty-one OTUs within the ceca were identified as good discriminators between diets. Six of these diet-associated OTUs (286, 214, 590, 580, 96, and 224) were common to both the ileum and ceca.
TABLE 3.
OTU contribution to the dissimilarity in ileal microbial communities associated with dieta
| OTU | Avg abundance
|
|
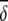 i/SD(δi) i/SD(δi) |
Individual contribution (%) | Cumulative contribution (%) | ||
|---|---|---|---|---|---|---|---|
| BE diet | B diet | ||||||
| 522 | 0.15 | 1.24 | 3.69 | 0.98 | 5.06 | 5.06 | |
| 190* | 1.47 | 1.49 | 3.44 | 1.21 | 4.72 | 9.78 | |
| 288* | 1.14 | 0.72 | 3.01 | 1.18 | 4.14 | 13.92 | |
| 286* | 1.23 | 0.50 | 2.98 | 1.26 | 4.09 | 18.01 | |
| 182* | 0.65 | 0.83 | 2.68 | 1.02 | 3.68 | 21.69 | |
| 214* | 0.86 | 0.96 | 2.55 | 1.20 | 3.50 | 25.19 | |
| 590* | 0.95 | 0.69 | 2.51 | 1.09 | 3.45 | 28.64 | |
| 290 | 0.73 | 0.29 | 2.44 | 0.72 | 3.35 | 31.99 | |
| 580* | 1.32 | 1.04 | 2.40 | 1.08 | 3.29 | 35.28 | |
| 96* | 0.65 | 0.62 | 2.20 | 1.06 | 3.01 | 38.29 | |
| 224* | 0.80 | 0.27 | 2.14 | 1.19 | 2.94 | 41.24 | |
| 880 | 0.68 | 0.00 | 1.86 | 0.98 | 2.55 | 43.79 | |
| 186 | 0.40 | 0.37 | 1.65 | 0.83 | 2.27 | 46.05 | |
| 184 | 0.38 | 0.26 | 1.65 | 0.66 | 2.26 | 48.32 | |
| 300 | 0.50 | 0.24 | 1.53 | 0.92 | 2.10 | 50.42 | |
Average abundances of important OTUs in ileal microbial communities of birds fed either a barley (B) diet or barley-plus-enzyme (BE) diet are shown. OTUs are listed in order of their contribution (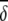 i) to the average dissimilarity δ (= 72.86%) between diets. The percent contribution of individual OTUs and the cumulative percent contribution to the top 50% of average dissimilarities are shown. OTUs identified as being good discriminators between diets are marked with an asterisk in column 1.
i) to the average dissimilarity δ (= 72.86%) between diets. The percent contribution of individual OTUs and the cumulative percent contribution to the top 50% of average dissimilarities are shown. OTUs identified as being good discriminators between diets are marked with an asterisk in column 1.
TABLE 4.
OTU contribution to the dissimilarity in cecal microbial communities associated with dieta
| OTU | Avg abundance
|
|
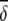 i/SD(δi) i/SD(δi) |
Individual contribution (%) | Cumulative contribution (%) | ||
|---|---|---|---|---|---|---|---|
| BE diet | B diet | ||||||
| 70* | 1.51 | 0.04 | 2.64 | 2.56 | 4.03 | 4.03 | |
| 96* | 1.15 | 2.36 | 2.43 | 1.52 | 3.70 | 7.73 | |
| 224* | 1.24 | 0.42 | 1.88 | 1.32 | 2.86 | 10.59 | |
| 212* | 0.58 | 1.08 | 1.71 | 1.17 | 2.61 | 13.20 | |
| 286* | 1.47 | 0.69 | 1.68 | 1.28 | 2.56 | 15.76 | |
| 548* | 0.46 | 1.13 | 1.62 | 1.30 | 2.47 | 18.23 | |
| 222* | 0.76 | 1.02 | 1.56 | 1.27 | 2.38 | 20.61 | |
| 188* | 1.21 | 0.61 | 1.54 | 1.26 | 2.35 | 22.96 | |
| 496* | 0.79 | 0.60 | 1.49 | 1.16 | 2.26 | 25.22 | |
| 94* | 0.31 | 0.79 | 1.43 | 1.03 | 2.18 | 27.40 | |
| 214* | 0.74 | 1.18 | 1.37 | 1.19 | 2.09 | 29.49 | |
| 198* | 0.80 | 0.51 | 1.34 | 1.14 | 2.05 | 31.54 | |
| 92 | 0.12 | 0.72 | 1.33 | 0.97 | 2.03 | 33.56 | |
| 522* | 0.05 | 0.72 | 1.27 | 1.07 | 1.94 | 35.50 | |
| 158* | 0.20 | 0.78 | 1.27 | 1.34 | 1.93 | 37.43 | |
| 146* | 0.76 | 0.26 | 1.22 | 1.20 | 1.86 | 39.29 | |
| 580* | 0.55 | 0.64 | 1.19 | 1.10 | 1.82 | 41.10 | |
| 300* | 0.52 | 0.50 | 1.17 | 1.02 | 1.78 | 42.88 | |
| 590* | 0.58 | 0.46 | 1.15 | 1.02 | 1.75 | 44.63 | |
| 318* | 0.97 | 0.60 | 1.11 | 1.09 | 1.69 | 46.32 | |
| 530* | 0.59 | 0.35 | 1.09 | 1.05 | 1.65 | 47.97 | |
| 290* | 0.60 | 0.29 | 1.08 | 1.02 | 1.65 | 49.62 | |
Average abundances of important OTUs in cecal microbial communities of birds fed either a barley (B) diet or barley-plus-enzyme (BE) diet are shown. OTUs are listed in order of their contribution (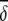 i) to the average dissimilarity δ (= 65.64%) between diets. The percent contribution of individual OTUs and the cumulative percent contribution to the top 50% of average dissimilarities are shown. OTUs identified as being good discriminators between diets are marked with an asterisk.
i) to the average dissimilarity δ (= 65.64%) between diets. The percent contribution of individual OTUs and the cumulative percent contribution to the top 50% of average dissimilarities are shown. OTUs identified as being good discriminators between diets are marked with an asterisk.
PCO was done on the subset of 17 OTUs identified within the ileum (Fig. 4) and 29 OTUs identified within the ceca (Fig. 5). The PCOs have been overlaid with the subset of OTUs identified by the BVSTEP procedure, indicating which OTUs are most strongly associated with a particular diet. These PCO ordinations confirm the separation in gut bacterial communities associated with diet. Table 5 summarizes common diet-associated OTUs identified within the ileum and ceca by both the SIMPER and the BVSTEP procedures. Within the ileum, nine diet-associated OTUs were confirmed by both the SIMPER and BVSTEP analyses, with five of these having a strong association with the barley diet supplemented with enzyme and one OTU having a strong association with the barley control diet (Table 5). OTUs 522 and 480 also had a strong association with the barley control diet, as illustrated by the PCO ordination (Fig. 4). However, these were not identified as good discriminators between diets (from the SIMPER analysis), even though OTU 522 was identified as contributing to the top 50% of dissimilarity between dietary treatments. Within the ceca, 18 diet-associated OTUs were confirmed by both SIMPER and BVSTEP analyses (Table 5). Seven of these OTUs were shown to have strong associations with the barley diet supplements with enzyme, and six OTUs had strong associations with the barley control diet (Table 5). Most of the diet-associated OTUs identified were specific to either the ileum or the ceca. However, OTUs 286 and 590 were common to both the ileum and ceca and associated with the barley-plus-enzyme treatment group.
FIG. 4.
PCO ordination of ileal microbial communities from birds fed either a barley control diet (filled circles) or a barley diet supplemented with enzyme (open squares). Overlaid onto the PCO are vectors of the subset of 17 OTUs identified by the BVSTEP procedure, indicating the association of OTUs with particular diets.
FIG. 5.
PCO ordination of cecal microbial communities from birds fed either a barley control diet (filled circles) or a barley diet supplemented with enzyme (open squares). Overlaid onto the PCO are vectors of the subset of 29 OTUs identified by the BVSTEP procedure, indicating an association of OTUs with particular diets.
TABLE 5.
Common diet-associated OTUs identified within the ileum and ceca by both the SIMPER and the BVSTEP procedures and identification of OTUs showing strong association with a particular diet
| Gut section | SIMPER/BVSTEP OTUs | OTUs with a strong diet association
|
|
|---|---|---|---|
| Barley plus enzyme | Barley | ||
| Ileum | 96, 182, 190, 214, 224, 286, 288,580, 590 | 224, 286, 288, 580 590 | 182 |
| Cecum | 70, 92, 94, 96, 146, 158, 188, 212, 214, 222, 286, 290, 300, 496, 530, 548, 580, 590 | 70, 146, 188, 286, 290, 530, 590 | 92, 96, 212, 214, 158, 548 |
A constrained CAP analysis done on gut bacterial communities from either the ileum or ceca produced biplots from the first canonical analysis of principal coordinates axis (CAP1) against bird performance as measured by AME. A good correlation was seen between ileal bacterial community composition and AME (r = 0.56) (Fig. 6A) and between cecal bacterial community composition and AME (r = 0.55) (Fig. 6B).
FIG. 6.
CAP of diet-associated gut microbial communities related to AME. CAP-versus-AME biplots for the ileum (A) and the ceca (B) are shown. CAP analysis was based on Bray-Curtis similarities calculated from fourth-root transformed species abundances. “m” achieves the maximum proportion of correct allocations (% of trace [G]) of samples to diet. Symbols: open squares, birds on the barley-plus-enzyme diet; filled circles, birds on the barley-only control diet.
DISCUSSION
T-RFLP is a robust and reproducible methodology for rapid analysis of microbial community structure (55, 68) and has become an extremely useful tool for comparing microbial communities in many biological systems (23, 25, 53). T-RFLP has also been shown to have higher resolution for detecting less-abundant species than other microbial profiling techniques, such as denaturing gradient gel electrophoresis (53). However, like all PCR-based technologies T-RFLP has inherent weaknesses, such as possible biased representation of microbial community structure due to PCR primer choice (74), DNA template concentration (15), and number of PCR cycles used (65). The latter two have been shown to result in differences in both the complexity and intensity of terminal restriction fragments in T-RFLP but not alter the presence of the most dominant peaks observed (55). In developing and assessing T-RFLP for microbial profiling of the poultry gut microbiota, we found that DNA extraction methodology and PCR primer choice affected the complexity of the resulting profiles (data not shown). Both the sensitivity and the specificity of our T-RFLP methodology was tested with a mixture of seven bacterial species (Bifidobacterium pseudolongum, Clostridium perfringens, Lactobacillus crispatus, L. reuterii, L. salivarius, L johnsonii, and an uncharacterized Lactobacillus species) and found to reproducibly detect and differentiate all bacterial species when present at ca. 103 to 104 bacterial cells per PCR (data not shown). However, detection was only semiquantitative. Some of this variation may have been due to PCR bias or the difference in 16S rRNA copies per bacterial cell for different bacterial species (68). Thus, T-RFLP is not a technique for the absolute characterization and identification of microbial communities but instead allows changes in community structure to be investigated. Indeed, any experimental approach has potential problems and biases associated with it, which makes it more or less appropriate for different applications.
Although parameters affecting T-RFLP have been widely studied and reported, methods for statistical analysis of the vast quantities of data potentially generated by the analysis have not. Thus, the potential of T-RFLP has not been fully utilized. Many studies using T-RFLP have based analysis on the visual interpretation of graphical T-RFLP profile outputs in order to identify the presence or absence of particular peaks indicative of bacterial species (27, 29, 30, 52). Such studies have also typically been based on small numbers of individual samples or even pooled samples per treatment. We have shown that there is individual variation in gut microbial communities among birds raised under identical dietary and environmental conditions. Similarity in bacterial community composition within the ilea and ceca of birds on a particular diet was low, ranging from 28 to 34% and 42 to 48%, respectively. This variation would have been masked if samples had been pooled. Furthermore, the high-throughput nature of T-RFLP makes it conducive to individual analysis of a large number of samples per treatment.
Several T-RFLP studies have used cluster analysis to depict the grouping of related samples (23, 28, 46, 48, 53, 57). However, a disadvantage of this method is that it groups samples into discrete clusters and does not display their inter-relations on a continual scale (21). Other studies have used principal component analysis (PCA) to examine community structure resulting from T-RFLP data (46, 56, 72). However, PCA is not appropriate where data contain many “zeros” or where observations (species) exceed the total number of samples (21), as is usually the case for T-RFLP data.
We have shown that T-RFLP in conjunction with several multivariate statistical techniques, such as unconstrained (nMDS and PCO) and constrained (CAP) ordinations, statistical tests of the hypothesis (ANOSIM), and characterization of the species responsible for the pattern differences (SIMPER and BVSTEP), are all useful tools for investigating the composition of the poultry gut microbial community. Using these combined techniques, we investigated gut microbial communities from the duodena, jejuna, ilea, and ceca of birds fed either a barley control diet or barley diet supplemented with exogenous enzyme and found these communities to be significantly different, with the exception of those in the duodenum and jejunum. This result is consistent with both culture-dependent and -independent studies showing that gut microbiota composition varies along the gastrointestinal tract of chickens (26, 30, 34, 50).
Diet-associated differences in the composition of the gut microbial community were only detected within the ileum and ceca. Diet has previously been shown to modify the overall cecal microbiota composition (6). We have shown that many bacterial species, not just a select few, are responsible for the overall difference in gut bacterial community composition associated with diet. Single OTUs identified as being good discriminators between diets (SIMPER) generally contributed 1 to 5% to the overall dissimilarity. Both SIMPER and BVSTEP have enabled potential OTUs (bacterial species) associated with a barley-based diet, either with or without supplementation of exogenous enzyme, to be identified. Most of the diet-associated OTUs identified were unique to either the ileum or ceca; however, a few were common to both gut sections. This suggests complex bacterial interactions within the microbiota, as well as between the host and the microbiota, are at play. Indeed, gut microbiota are suggested to communicate with each other through complex chemical signals and quorum sensing (22, 66), as well as with their host (40).
Classical growth and performance analysis showed, as expected, that chickens fed the barley-plus-enzyme diet had a significantly higher AME than chickens fed the control barley diet. Improvements in performance of birds on barley-based diets due to supplementation with NSP-degrading enzyme (β-glucanase) have been widely reported (1, 14, 36, 37). NSPs from cereal-based diets create a viscous environment within the intestinal lumen (17, 60) and are associated with low AME (8), poor nutrition absorption, and an increased incidence of wet and sticky droppings (60). Some degradation of NSP structural plant matter occurs due to gut microbial enzyme activity; however, the recovery of energy from total NSP is low in poultry since the short digesta transit times limit extensive fermentation (11). Furthermore, antibiotic supplementation of the poultry diet may also have a suppressive effect on the fermentative microbiota capable of NSP digestion (17).
Supplementation of wheat-based diets with the exogenous NSP-degrading enzyme, xylanase, has been shown to alter broiler gut microbiota by lowering the counts of enterobacteria, total gram-positive cocci (70), and C. perfringens (18). We show in the present study that the overall gut bacterial community composition is altered by exogenous enzyme supplementation of a barley-based diet. This is also the first report that directly correlates diet-associated changes in gut microbial community with improved performance. Constrained CAP (3) was used in the present study to correlate original performance variables (AME) with patterns in gut microbial community composition on canonical biplots. It is possible that the growth of beneficial bacteria, suppression of detrimental bacterial species, or both may be partially responsible for the improved AME. Indeed, it has recently been shown that in genetically predisposed obese mice versus lean mice that the gut microbiota differ in relative abundance of the Bacteroidetes and Firmicutes (69), indicating particular bacterial groups have increased capacity for energy harvest.
A limitation of T-RFLP analysis has been the inability to reliably assign OTUs to phylogenetic groups. However, the identity of OTUs can be predicted from in silico PCR amplification and restriction of 16S rRNA sequences found in a public database (http://mica.ibest.uidaho.edu/). Using this database, in conjunction with information gained from poultry gut-derived bacterial clones (data not shown), the identity of some of the diet-associated OTUs may be suggested. The barley plus enzyme diet-associated OTUs 580 and 188 identified within the ileum and ceca, respectively, may represent various Lactobacillus species. Furthermore, the barley control diet-associated OTUs 182 and 522 identified within the ileum may represent lactobacilli and C. perfringens (or other Clostridium species), respectively.
Indeed, lactobacilli (Firmicutes) are present throughout the gastrointestinal tract of poultry (33, 43, 47). Lactobacilli have various biochemical properties, including the production of antibacterial compounds (22, 63), β-glucanase (38), and bile salt hydrolase compounds (44). Lactobacilli with β-glucanase activity have been identified in the feces of piglets and linked to presence of β-glucans in the diet (38). Other bacteria containing 1,3-1,4-β-endoglucanase activities (Bacteroides ovatus, B. uniformis, B. capillosus, C. perfringens, and Streptococcus bovis) have recently been isolated from poultry (10). β-Glucanase activity attributed to these bacterial species may explain the association of lactobacilli (OTU 182) and C. perfringens (OTU 522) with the barley control diet. Alternatively, the incidence of C. perfringens has been shown to decrease in poultry fed a wheat-based diet supplement with xylanase (18).
However, such identification of OTUs can only be speculative since terminal restriction fragment size determination may not exactly match the fragment sizes determined by DNA sequencing (39) and phylogenetically disparate 16S rRNA gene sequences may yield identical terminal restriction fragment sizes (58). The latter difficulty may be addressed somewhat by using multiple restriction enzymes in the T-RFLP analysis (41). However, this will not lead to unequivocal identification of OTUs without genome sequence information.
Much information on the composition of the gut microbiota has been obtained through the generation of bacterial 16S rRNA clone libraries (47, 50, 74). However, the generation of clone libraries is laborious and expensive. Ideally, it would be best to screen for differences in microbial community composition with T-RFLP and then determine genome sequence of target OTUs only. A novel strategy to extract specific phylogenetic sequence information from community T-RFLP has recently been developed based on targeted isolation and cloning of terminal restriction fragments of interest (73). This will enable bacterial sequence information of interest to be determined, which may be used to develop specific tests for gut bacteria associated with poultry production traits. From there, it may be possible to develop dietary strategies to induce desirable changes in the gut microbiota for enhancement of growth and productivity of commercial chicken flocks.
In conclusion, multivariate statistical methods have demonstrated positive correlation of gut microbial communities and bird performance for the first time. We have identified several indicator bacterial species contributing to the diet-induced differences in the overall gut microbial community. The presence of specific beneficial bacterial species and/or the absence of specific detrimental bacterial species may contribute to the improved performance in these chickens.
Acknowledgments
This study was supported by the Australian Poultry Cooperative Research Centre (project 03-3a).
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Almirall, M., and E. Esteve-Garcia. 1994. Rate of passage of barley diets with chromium oxide: influence of age and poultry strain and beta-glucanase supplementation. Poult. Sci. 73:1433-1440. [DOI] [PubMed] [Google Scholar]
- 2.Amit-Romach, E., D. Sklan, and Z. Uni. 2004. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult. Sci. 83:1093-1098. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, M. J., and T. J. Willis. 2003. Canonical analysis of principal coordinates: a useful method of constrained ordinations for ecology. Ecology 84:511-524. [Google Scholar]
- 4.Annison, G., M. Choct, and R. J. Hughes. 1994. AME determination and its application to lupins. Proc. Aust. Poult. Sci. Symp. 6:92-96. [Google Scholar]
- 5.Apajalahti, J. H. A., L. K. Sarkilahti, B. R. E. Maki, J. P. Heikkinen, P. H. Nurminen, and W. E. Holben. 1998. Effective recovery of bacterial DNA and percent-guanine-plus-cytosine-based analysis of community structure in the gastrointestinal tract of broiler chickens. Appl. Environ. Microbiol. 64:4084-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apajalahti, J. H. A., A. Kettunen, M. R. Bedford, and W. E. Holben. 2001. Percent G+C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of boiler chickens. Appl. Environ. Microbiol. 67:5656-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apajalahti, J., A. Kettunen, and H. Graham. 2004. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World Poult. Sci. J. 60:223-232. [Google Scholar]
- 8.Austin, S. C., J. Wiseman, and A. Chesson. 1999. Influence of non-starch polysaccharide structure on the metabolisable energy of U.K. wheat-fed poultry. J. Cereal Sci. 29:77-88. [Google Scholar]
- 9.Barnes, E. M. 1979. The intestinal microflora of poultry and game birds during life and after storage. J. Appl. Bacteriol. 46:407-419. [DOI] [PubMed] [Google Scholar]
- 10.Beckmann, L., O. Simon, and W. Vahjen. 2006. Isolation and identification of mixed linked β-glucan degrading bacteria in the intestine of broiler chickens and partial characterization of respective 1,3-1,4-β-glucanase activities. J. Basic Microbiol. 46:175-185. [DOI] [PubMed] [Google Scholar]
- 11.Bedford, M. R. 2000. Exogenous enzymes in monogastric nutrition: their current value and future benefits. Anim. Feed Sci. Technol. 86:1-13. [Google Scholar]
- 12.Bedford, M. R., and J. Apajalahti. 2001. Microbial interaction in the response to exogenous enzyme utilization, p. 299-315. In M. R. Bedford and G. G. Partridge (ed.), Enzymes in farm animal nutrition. CABI Publishing, Wallingford, United Kingdom.
- 13.Bray, J. R., and K. R. Curtis. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27:325-349. [Google Scholar]
- 14.Brenes, A., J. Smith, W. Guenter, and R. R. Marquardt. 1993. Effect of enzyme supplementation on the performance and digestive tract size of broiler chickens fed wheat- and barley-based diets. Poult. Sci. 72:1731-1739. [DOI] [PubMed] [Google Scholar]
- 15.Chandler, D. P., J. K. Fredrickson, and F. J. Brockman. 1997. Effect of PCR template concentration on the composition and distribution of total community 16S rDNA clone libraries. Mol. Ecol. 6:475-482. [DOI] [PubMed] [Google Scholar]
- 16.Choct, M. 2006. Enzymes for the feed industry: past, present and future. World Poult. Sci. J. 62:5-15. [Google Scholar]
- 17.Choct, M., R. J. Hughes, J. Wang, M. R. Bedford, A. J. Morgan, and G. Annison. 1996. Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br. Poult. Sci. 37:609-621. [DOI] [PubMed] [Google Scholar]
- 18.Choct, M., M. Sinlae, R. A. M. Al-Jassim, and D. Pettersson. 2006. Effects of xylanase supplementation on between-bird variation in energy metabolism and the number of Clostridium perfringens in broilers fed a wheat-based diet. Aust. J. Agric. Res. 57:1017-1021. [Google Scholar]
- 19.Clarke, K. R. 1993. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18:117-143. [Google Scholar]
- 20.Clarke, K. R., and R. M. Warwick. 1998. Quantifying structural redundancy in ecological communities. Oecology 113:278-289. [DOI] [PubMed] [Google Scholar]
- 21.Clarke, K. R., and R. M. Warwick. 2001. Changes in marine communities: an approach to statistical analysis and interpretation, 2nd ed. PRIMER-E, Ltd., Plymouth, United Kingdom.
- 22.De Angelis, M., S. Siragusa, M. Berloco, L. Caputo, L. Settanni, G. Alfonsi, M. Amerio, A. Grandi, A. Ragni, and M. Gobbetti. 2006. Selection of potential probiotic lactobacilli from pig feces to be used as additives in pelleted feeding. Res. Microbiol. 157:792-801. [DOI] [PubMed] [Google Scholar]
- 23.Dunbar, J., L. O. Ticknor, and C. R. Kuske. 2000. Assessment of microbial diversity in four southwestern United States soils by 16S rRNA gene terminal restriction fragment analysis. Appl. Environ. Microbiol. 66:2943-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunbar, J., L. O. Ticknor, and C. R. Kuske. 2001. Phylogenetic specificity and reproducibility and new method for analysis of terminal restriction fragment profiles of 16S rRNA genes from bacterial communities. Appl. Environ. Microbiol. 67:190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egert, M., S. Marhan, B. Wagner, S. Scheu, and M. W. Friedrich. 2004. Molecular profiling of 16s rRNA genes reveals diet-related differences of microbial communities in soil, gut, and casts of Lumbricus terrestris L. (Oligochaeta: Lumbricidae). FEMS Microbiol. Ecol. 48:187-197. [DOI] [PubMed] [Google Scholar]
- 26.Engberg, R. M., M. S. Hedemann, T. D. Leser, and B. B. Jensen. 2000. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 79:1311-1319. [DOI] [PubMed] [Google Scholar]
- 27.Fairchild, A. S., J. L. Smith, U. Idris, J. Lu, S. Sanchez, L. B. Purvis, C. Hofacre, and M. D. Lee. 2005. Effects of orally administered tetracycline on the intestinal community structure of chickens and on the tet determinant carriage by commensal bacteria and Campylobacter jejuni. Appl. Environ. Microbiol. 71:5865-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez, E. d. V., J. L. Garland, and M. S. Roberts. 2004. Microbial structural diversity estimated by dilution-extinction of phenotypic traits and T-RFLP analysis along a land-use intensification gradient. FEMS Microbiol. Ecol. 49:253-259. [DOI] [PubMed] [Google Scholar]
- 29.Gong, J., R. J. Forster, H. Yu, J. R. Chambers, P. M. Sabour, R. Wheatcroft, and S. Chen. 2002. Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS Microbiol. Lett. 208:1-7. [DOI] [PubMed] [Google Scholar]
- 30.Gong, J., R. J. Forster, H. Yu, J. R. Chambers, R. Wheatcroft, P. M. Sabour, and S. Chen. 2002. Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol. Ecol. 41:171-179. [DOI] [PubMed] [Google Scholar]
- 31.Gordon, H. A., and L. Pesti. 1971. The gnotobiotic animal as a tool in the study of host-microbial relationships. Bacteriol. Rev. 35:390-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gower, J. C. 1966. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrics 53:325-338. [Google Scholar]
- 33.Guan, L. L., K. E. Hagen, G. W. Tannock, D. R. Korver, G. M. Fasenko, and G. E. Allison. 2003. Detection and identification of Lactobacillus species in crops of broilers of different ages by using PCR-denaturing gradient gel electrophoresis and amplified ribosomal DNA restriction analysis. Appl. Environ. Microbiol. 69:6750-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubener, K., W. Jahjen, and O. Simon. 2002. Bacterial responses to different dietary cereal types and xylanase supplementation in the intestine of broiler chicken. Arch. Anim. Nutr. 56:167-187. [DOI] [PubMed] [Google Scholar]
- 35.Hume, M. E., L. F. Kubena, T. S. Edrington, C. J. Donskey, R. W. Moore, S. C. Ricke, and D. J. Nisbet. 2003. Poultry digestive microflora biodiversity as indicated by denaturing gradient gel electrophoresis. Poult. Sci. 82:1100-1107. [DOI] [PubMed] [Google Scholar]
- 36.Jamroz, D., K. Jakobsen, J. Orda, J. Skorupinska, and A. Wilczykiewicz. 2001. Development of the gastrointestinal tract and digestibility of dietary fibre and amino acids in young chickens, ducks and geese fed diets with high amounts of barley. Comp. Biochem. Physiol. A 130:643-652. [DOI] [PubMed] [Google Scholar]
- 37.Jamroz, D., J. Orda, A. Wilczykiewicz, and J. Skorupinska. 2001. Efficacy of carbohydrases derived from Trichoderma longibrachiatum in wheat- and barley-based diets on performance and fermentation of carbohydrates in the intestine of broilers. J. Appl. Anim. Res. 19:61-72. [Google Scholar]
- 38.Jonsson, E., and S. Hemmingsson. 1991. Establishment in the piglet gut of lactobacilli capable of degrading mixed-linked β-glucans. J. Appl. Bacteriol. 70:512-516. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan, C. W., and C. L. Kitts. 2003. Variation between observed and true terminal restriction fragment length is dependent on true TRF length and purine content. J. Microbiol. Methods 54:121-125. [DOI] [PubMed] [Google Scholar]
- 40.Kelly, D., S. Conway, and R. Aminov. 2005. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 26:326-333. [DOI] [PubMed] [Google Scholar]
- 41.Kent, A. D., D. J. Smith, B. J. Benson, and E. W. Triplett. 2003. Web-based phylogenetic assignment tool for analysis of terminal restriction fragment length polymorphism profiles of microbial communities. Appl. Environ. Microbiol. 69:6768-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klasing, K. C., B. K. Johnston, and B. N. Benson. 1999. Implications of an immune response on growth and nutrition requirements of chicks, p. 35-47. In P. C. Garnsworthy and J. Wiseman (ed.), Recent developments in poultry nutrition, vol. 2. Nottingham University Press, Nottingham, United Kingdom. [Google Scholar]
- 43.Knarreborg, A., M. A. Simon, R. M. Engberg, B. B. Jensen, and G. W. Tannock. 2002. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 68:5918-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knarreborg, A., R. M. Engberg, S. K. Jensen, and B. B. Jensen. 2002. Quantitative determination of bile salt hydrolase activity in bacteria isolated from the small intestine of chickens. Appl. Environ. Microbiol. 68:6425-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruskal, J. B. 1964. Multidimensional scaling by optimizing a goodness of fit to a nonmetric hypothesis. Psychometrics 29:1-28. [Google Scholar]
- 46.Kuske, C. R., L. O. Ticknor, M. E. Miller, J. M. Dunbar, J. A. Davis, S. M. Barns, and J. Belnap. 2002. Comparison of soil bacterial communities in rhizospheres of three plant species and the interspaces in an arid grassland. Appl. Environ. Microbiol. 68:1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lan, P. T. N., H. Hayashi, M. Sakamoto, and Y. Benno. 2002. Phylogenetic analysis of cecal microbiota in chicken by the use of 16S rDNA clone libraries. Microbiol. Immunol. 46:371-382. [DOI] [PubMed] [Google Scholar]
- 48.Lan, P. T. N., M. Sakamoto, and Y. Benno. 2004. Effects of two probiotic Lactobacillus strains on jejunal and cecal microbiota of broiler chicken under acute heat stress condition as revealed by molecular analysis of 16S rRNA genes. Microbiol. Immunol. 48:417-929. [DOI] [PubMed] [Google Scholar]
- 49.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Inc., New York, NY.
- 50.Lu, J., U. Idris, B. Harmon, C. Hofacre, J. J. Maurer, and M. D. Lee. 2003. Diversity and succession of the intestinal bacterial communities of the maturing boiler chicken. Appl. Environ. Microbiol. 69:6816-6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mead, G. C. 1989. Microbes of the avian cecum: types present and substrates utilized. J. Exp. Zool. 1989(Suppl. 3):48-54. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto, T., M. Kawahara, and K. Minamisawa. 2004. Novel endophytic nitrogen-fixing clostridia for the grass Miscanthus sinensis as revealed by terminal restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 70:6580-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moeseneder, M., J. M. Arrieta, G. Muyzer, C. Winter, and G. J. Herndl. 1999. Optimization of terminal-restriction fragment length polymorphism analysis for complex marine bacterioplankton communities and comparison with denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 65:3518-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muyzer, G., A. Teske, C. O. Wirsen, and H. W. Jannasch. 1995. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch. Microbiol. 164:165-172. [DOI] [PubMed] [Google Scholar]
- 55.Osborn, A. M., E. R. B. Moore, and K. N. Timmis. 2000. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ. Microbiol. 2:39-50. [DOI] [PubMed] [Google Scholar]
- 56.Park, S., Y. K. Ku, M. J. Seo, D. Y. Kim, J. E. Yeon, K. M. Lee, S. C. Jeong, W. K. Yoon, C. H. Harn, and H. M. Kim. 2006. Principal component analysis and discriminant analysis (PCA-DA) for discriminating profiles of terminal restriction fragment length polymorphism (T-RFLP) in soil bacterial communities. Soil Biol. Biochem. 38:2344-2349. [Google Scholar]
- 57.Perez-Jimenez, J. R., and L. J. Kerkhof. 2005. Phylogeography of sulfate-reducing bacteria among disturbed sediments disclosed by analysis of the dissimilatory sulfite reductase genes (dsrAB). Appl. Environ. Microbiol. 71:1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidtt-Wagner, D., M. W. Friedrich, B. Wahner, and A. Brune. 2003. Axial dynamics, stability, and interspecies similarity of bacterial community structure in the highly compartmentalized gut soil feeding termites (Cubitermes spp.). Appl. Environ. Microbiol. 69:6018-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shepard, R. N. 1962. The analysis of proximities: multidimensional scaling with an unknown distance function: parts I and II. Psychometrics 27:125-140; 219-246. [Google Scholar]
- 60.Smits, C. H. M., and G. Annison. 1996. Non-starch plant polysaccharides in broiler nutrition: toward a physiologically valid approach to their determination. World Poult. Sci. J. 52:203-221. [Google Scholar]
- 61.Smits, C. H., A. Veldman, M. W. Verstegen, and A. C. Beynen. 1997. Dietary carboxymethyl cellulose with high instead of low viscosity reduces macronutrient digestion in broiler chickens. J. Nutr. 127:483-487. [DOI] [PubMed] [Google Scholar]
- 62.Steenfeldt, S., K. E. Bach Knudsen, C. F. Børsting, and B. O. Eggum. 1995. The nutritive value of decorticated mill fractions of wheat. 2. Evaluation with raw and enzyme treated fractions using adult cockerels. Anim. Feed Sci. Technol. 54:249-265. [Google Scholar]
- 63.Stern, N. J., E. A. Svetoch, B. V. Eruslanov, V. V. Perelygin, E. V. Mitsevich, I. P. Mitsevich, V. D. Pokhilenko, V. P. Levchuk, O. E. Svetoch, and B. S. Seal. 2006. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. 50:3111-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stirling, G. R., D. Griffin, K. Ophel-Keller, A. McKay, D. Hartley, J. Curran, A. M. Stirling, D. Monsour, J. Winch, and B. Hardie. 2004. Combining an initial risk assessment process with DNA assays to improve prediction of soilborne diseases caused by root-knot menatode (Meloidogyne spp.) and Fusarium oxysporum f. sp. lycopersici in the Queensland tomato industry. Aust. Plant Pathol. 33:285-293. [Google Scholar]
- 65.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swift, S., E. E. Vaughan, and W. M. De Vos. 2000. Quorum sensing within the gut ecosystem. Microb. Ecol. Health Dis. 2000(Suppl. 2):81-92. [Google Scholar]
- 67.Tellez, G., S. E. Higgins, A. M. Donoghue, and B. M. Hargin. 2006. Digestive physiology and the role of microorganisms. J. Appl. Poult. Res. 15:136-144. [Google Scholar]
- 68.Trotha, R., U. Reichl, F. L. Thies, D. Sperling, W. Konig, and B. Konig. 2002. Adaption of a fragment analysis technique to an automated high-throughput multicapillary electrophoresis device for the precise qualitative and quantitative characterization of microbial communities. Electrophoresis 23:1070-1079. [DOI] [PubMed] [Google Scholar]
- 69.Turnbaugh, P. J., R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis, and J. I. Gordon. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027-1031. [DOI] [PubMed] [Google Scholar]
- 70.Vahjen, W., K. Glaser, K. Schafer, and O. Simon. 1998. Influence of xylanase-supplemented feed on the development of selected bacterial groups in the intestinal tract of broiler chicks. J. Agric. Sci. 130:489-500. [Google Scholar]
- 71.van Leeuwen, P., J. M. Mouwen, J. D. van der Klis, and M. W. Verstegen. 2004. Morphology of the small intestinal mucosal surface of broilers in relation to age, diet formulation, small intestinal microflora and performance. Br. Poult. Sci. 45:41-48. [DOI] [PubMed] [Google Scholar]
- 72.Wang, M., S. Ahrne, M. Antonsson, and G. Molin. 2004. T-RFLP combined with principal component analysis and 16S rRNA gene sequencing: an effective strategy for comparison of fecal microbiota in infants of different ages. J. Microbiol. Methods 59:53-69. [DOI] [PubMed] [Google Scholar]
- 73.Widmer, F., M. Hartmann, B. Frey, and R. Kolliker. 2006. A novel strategy to extract specific phylogenetic sequence information from community T-RFLP. J. Microbiol. Methods 66:512-520. [DOI] [PubMed] [Google Scholar]
- 74.Zhu, X. Y., T. Z. Zhong, Y. Pandya, and R. D. Joerger. 2002. 16S rRNA-based analysis of microbiota from the cecum of boiler chickens. Appl. Environ. Microbiol. 68:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu, X. Y., and R. D. Joerger. 2003. Composition of microbiota in content and mucus from caeca of broiler chickens as measured by fluorescent in situ hybridization with group-specific, 16S rRNA-targeted oligonucleotide probes. Poult. Sci. 82:1242-1249. [DOI] [PubMed] [Google Scholar]