Abstract
We introduce a procedure for determining shear forces at the balance between attachment and detachment of bacteria under flow. This procedure can be applied to derive adhesion forces in weak-adherence systems, such as polymer brush coatings, which are currently at the center of attention for their control of bacterial adhesion and biofilm formation.
Flow displacement systems like the parallel plate flow chamber (PPFC) are powerful tools for studying the adhesion of colloidal particles, including bacteria, to surfaces under different hydrodynamic conditions (3). Experimental observables, i.e., the number of adhering bacteria and their distribution on surfaces, are used to derive attachment and detachment characteristics. A usual way to obtain qualitative information on the strength of the bacterium-surface bond in the PPFC is to simply pass an air bubble through the chamber and analyze the number of bacteria remaining on the surface; the force exerted by the air bubble on an adhering micrometer-sized particle is around 10−7 N (3). Therefore, this method is too insensitive to be used in systems with weak bacterium-surface interaction forces, such as polymer brush coatings.
One of the major advantages of the PPFC is the ability to adjust the shear rate and shear stress at the surface. These measures are related through the equation τ = F/A = ησ, where τ is the shear stress, F the force, A the area on which the force is exerted, η the absolute viscosity, and σ the shear rate. The wall shear rate is related to the flow rate, Q (8), according to the equation σ = 3Q/2b2w, with b being the half depth and w being the width of the chamber. The force on a single adhering bacterium can then be approximated as the product of wall shear stress times the bacterial surface area exposed to the shear.
Sufficiently high shear stresses cause adhering bacteria to slide and roll over a surface, which may lead to detachment. In order to characterize the attachment and detachment of bacteria with respect to wall shear, notions such as “shear to prevent adhesion” and “shear to remove adhered bacteria” are used (1, 3, 5, 6, 9, 10). Shear stresses in the range of 12 to 54 Pa have been reported to be necessary for the removal of different bacterial strains from regular surfaces, and usually a lower shear stress is required to prevent adhesion (17). These characteristic shear stresses, however, suffer from some ambiguity because the strength of adhesion can depend on the history of the contact between a bacterium and a substratum surface, i.e., its residence time and the shear stress applied during adhesion (11, 18). Moreover, the shear to detach adhered bacteria cannot be obtained for a wide range of adhesion forces within the laminar-flow regime.
Bacterial adhesion is the first step in the development of a biofilm and represents the onset of biomaterial implant-related infection, microbially induced corrosion, and the fouling of membranes and heat exchanger surfaces in food processing systems (4). Much attention has been directed toward the development of antifouling surfaces (14). Polymer brush coatings are currently considered the most promising of the nonfouling coatings, as they weaken the attractive interaction forces between the adhering bacteria and the underlying substratum (16, 19). Here, for the first time, we present a method that yields quantitative data on bacterium-surface affinity over a wide range of interaction forces, including the weak interaction forces that exist on nonfouling surfaces.
Staphylococcus epidermidis HBH276, Staphylococcus aureus ATCC 12600, and Pseudomonas aeruginosa #3 were precultured in 10 ml of tryptone soya broth at 37°C for 24 h from blood agar plates. These precultures were used to inoculate second cultures of 200 ml for 16 h. Subsequently, the bacteria were harvested and washed twice with demineralized water. To break up bacterial aggregates, the bacteria were sonicated three times for 10 s each time at 30 W. Finally, the bacteria were suspended in phosphate-buffered saline (PBS) solution (10 mM potassium phosphate, 150 mM NaCl, pH 6.8) to a concentration of 3 × 108 per ml for all experiments.
Implant grade silicone rubber sheets (Medin, Groningen, The Netherlands) were rinsed with ethanol and demineralized water, sonicated in 2% RBS35 detergent, rinsed thoroughly with demineralized water, washed in methanol, and rinsed with demineralized water again to remove oil contaminations and fingerprints. The silicone rubber was fixed to the bottom plate of the PPFC and exposed to a solution of 0.5 g liter−1 Pluronic F-127, a triblock copolymer of polyethylene oxide (PEO) and propylene oxide (PPO) with a PEO-PPO-PEO structure, in PBS for 20 min. Nonattached polymer chains were removed from the surface by washing with PBS. This has been proven to result in a brush layer of PEO chains on hydrophobic substrata (2, 12, 13).
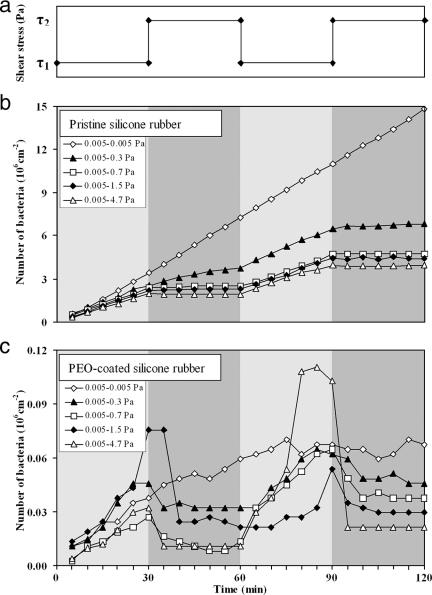
Our flow chamber and image analysis system have been previously described (3). The setup gives the possibility of having a steady flow and of being able to quickly switch from low to high flow rates. We used a fluctuating-flow protocol to study the attachment and detachment of different bacterial strains to pristine and PEO brush-coated silicone rubber. The protocol began with the flow of a bacterial suspension for 30 min at a wall shear stress of 0.005 Pa. Thereafter, the shear stress was either kept at 0.005 Pa or instantly adjusted to 0.3, 0.7, 1.5, or 4.7 Pa and maintained at that level for another 30 min. After each separate increase, the shear stress was reset to 0.005 Pa. This cycle was repeated twice (Fig. 1a).
FIG. 1.
Flow protocol to determine the critical shear stress at the balance between bacterial attachment and detachment. (a) Shear stress fluctuation between two values. The first shear stress, τ1, is always 0.005 Pa, while the second shear stress, τ2, is either 0.005, 0.3, 0.7, 1.5, or 4.7 Pa. (b and c) Adhesion profiles of S. epidermidis HBH276 bacteria on pristine silicone rubber and PEO-coated silicone rubber, respectively. These profiles are obtained from shear fluctuations from 0.005 Pa to X to 0.005 Pa to X (0.005 Pa ≤ X ≤ 4.7 Pa). Note the different scales along the y axis.
During the first 30 min at a shear stress of 0.005 Pa, bacteria attached to the surface. Increasing the flow rate implied not only a higher supply rate of bacteria to the surface but also an increased shear stress acting on adhering bacteria. A relatively small increase led to additional attachment of bacteria. Increasing the shear above a certain threshold would result in a net decrease in the number of adhering bacteria due to a dominant contribution of shear-induced detachment. We refer to the shear stress at which additional attachment and detachment balance each other as the “critical shear stress.”
The attachment and detachment results for S. epidermidis HBH276 bacteria on pristine and PEO-coated silicone rubber under a fluctuating shear are presented in Fig. 1. The deposition rate (slope of the curve for a time period) of S. epidermidis HBH276 bacteria on pristine silicone rubber was suppressed by increasing the shear stress, τ, because the high wall shear prevents newly arriving bacteria from attaching to the surface. The attachment and detachment results on PEO-coated surfaces were very different; the deposition rate at low shear was 1 to 2 orders of magnitude lower than the deposition rate at low shear for the pristine silicone rubber. Moreover, a noticeable portion of the bacteria detached after the application of the higher shear. The first and second cycles of shear fluctuation essentially yielded the same detachment results, indicating that the surfaces, particularly the PEO coatings, had not been affected by the high shear. At this stage, it is important to realize that all experiments were carried out in the initial phase of adhesion, i.e., at a time when the numbers of adhering bacteria were increasing linearly with time (Fig. 1). Therefore, all changes in attachment and subsequent detachment were due to the fluctuations in shear and not to the saturation of the surface. In Fig. 1, it can be seen that S. epidermidis HBH276 bacteria were much more loosely bound to the PEO coating than to the pristine silicone rubber surface.
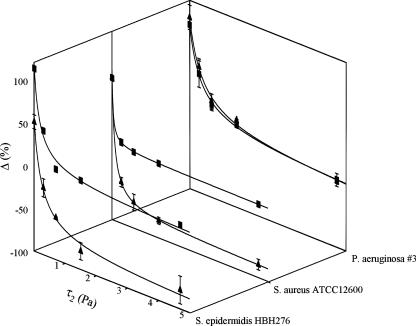
In order to determine the above-defined critical shear stress, we first calculated the net effect of increasing the shear, and then we plotted the percentage change in number of adhering bacteria after the application of the second shear stress as a function of the second shear stress (Fig. 2). This allowed us to determine the critical shear stress and an upper limit for shear-induced detachment. This change in the number of adhering bacteria upon increasing the shear showed a hyperbolic decay, as can be seen in Fig. 2. Zero change in the number of adhering bacteria, i.e., critical shear stress, occurred at 2.7 ± 1.1 Pa for S. epidermidis HBH276 bacteria on pristine silicone rubber and at only 0.2 ± 0.1 Pa on PEO-coated silicone rubber, corresponding with critical forces of 2.1 ± 0.9 pN and 0.1 ± 0.1 pN, respectively, for a single bacterium (assuming a staphylococcal radius of 0.5 μm and a radius of 0.6 μm for P. aeruginosa, which is the radius of a sphere whose volume is equal to that of this rod-shaped organism). The upper limit of shear-induced detachment amounted to 10% for S. epidermidis HBH276 bacteria on pristine silicone rubber, while over 90% of all adhering staphylococci could be detached from PEO brush-coated silicone rubber.
FIG. 2.
Percentage change in number of adhering bacteria after application of the second shear stress as a function of the second shear stress, τ2 {Δ = 100 × [(N2 − N1)/N1], where N is the number of adhering bacteria within a cycle of shear fluctuation}. The line represents a hyperbolic decay, from which the upper limit for shear-induced detachment (horizontal asymptote) and critical shear stress (shear stress for zero change) are derived. Each data point and vertical bar represent the average and range, respectively, for two experiments with separately grown bacteria on separately prepared surfaces. ▪, pristine silicone rubber; ▴, PEO-coated silicone rubber.
Table 1 compares the values of the critical shear stress and upper limits of shear-induced detachment for the three different bacterial strains. Clearly, different bacterial strains adhere to surfaces with different strengths. The adhesion strength of staphylococci is greatly decreased by the presence of a PEO coating, while the adhesion strength of P. aeruginosa #3 bacteria is hardly affected. This is in line with other findings, which show that PEO brush coatings are not effective against hydrophobic P. aeruginosa strains (16).
TABLE 1.
Critical shear stresses and forces at the balance between bacterial attachment and detachment and upper limits for shear-induced detachment of three bacterial strains on pristine and PEO-coated silicone rubbera
| Strain | Critical shear stress (Pa)
|
Critical shear force on a single bacterium (pN)
|
Upper limit of shear-induced detachment (%)
|
|||
|---|---|---|---|---|---|---|
| SR | PEO | SR | PEO | SR | PEO | |
| S. epidermidis HBH276 | 2.7 ± 1.1 | 0.2 ± 0.1 | 2.1 ± 0.9 | 0.1 ± 0.1 | 9.7 ± 3.5 | 91.1 ± 8 |
| S. aureus ATCC 12600 | 1.0 ± 0.2 | 0.1 ± 0.0 | 0.8 ± 0.1 | 0.1 ± 0.0 | 3.3 ± 0.5 | 86 ± 5.3 |
| P. aeruginosa #3 | 1.1 ± 0.2 | 1.3 ± 0.5 | 1.3 ± 0.3 | 1.6 ± 0.6 | 29.2 ± 3.8 | 31.8 ± 6.9 |
SR, pristine silicone rubber; PEO, PEO-coated silicone rubber. Values are means ± standard errors calculated for a series of five data points, each representing the results of two experiments with separately grown bacteria on separately prepared surfaces.
Atomic force microscopy (AFM) studies report adhesion forces mostly in the nN range for regular surfaces (7, 18), while the largest critical shear force in this study is 2.1 pN for S. epidermidis on pristine silicone rubber. However, it has to be realized that AFM measures the forces acting perpendicular to a surface, whereas tangential forces are measured under flow. Moreover, in flow displacement systems, the attachment and detachment of bacteria take place spontaneously, whereas in AFM studies, detachment is forced upon the system by retraction of the AFM tip. In AFM studies, adhesion forces have often been found to be either absent or too weak to measure when bacterial cell surfaces are separated from polymer brush coatings (15), as opposed to the method proposed here, which is based on the shear force balance between bacterial attachment and detachment under flow.
In conclusion, it can be stated that the method proposed provides better understanding of weak bacterium-surface interactions under controlled hydrodynamic conditions because it yields quantitative bond strength data not attainable with conventionally developed methods.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Anamelechi, C. C., G. A. Truskey, and W. M. Reichert. 2005. Mylar and Teflon-AF as cell culture substrates for studying endothelial cell adhesion. Biomaterials 26:6887-6896. [DOI] [PubMed] [Google Scholar]
- 2.Brandani, P., and P. Stroeve. 2003. Adsorption and desorption of PEO-PPO-PEO triblock copolymers on a self-assembled hydrophobic surface. Macromolecules 36:9492-9501. [Google Scholar]
- 3.Busscher, H. J., and H. C. van der Mei. 2006. Microbial adhesion in flow displacement systems. Clin. Microbiol. Rev. 19:127-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 5.Guillemot, G., G. Vaca-Medina, H. Martin-Yken, A. Vernhet, P. Schmitz, and M. Mercier-Bonin. 2006. Shear-flow induced detachment of Saccharomyces cerevisiae from stainless steel: influence of yeast and solid surface properties. Colloids Surf. B 49:126-135. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez, E., and A. Groisman. 2007. Quantitative measurements of the strength of adhesion of human neutrophils to a substratum in a microfluidic device. Anal. Chem. 79:2249-2258. [DOI] [PubMed] [Google Scholar]
- 7.Hinterdorfer, P., and Y. F. Dufrene. 2006. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 3:347-355. [DOI] [PubMed] [Google Scholar]
- 8.Hoek, E. M. V., and M. Elimelech. 2003. Cake-enhanced concentration polarization: a new fouling mechanism for salt-rejecting membranes. Environ. Sci. Technol. 37:5581-5588. [DOI] [PubMed] [Google Scholar]
- 9.Kurtis, M. S., T. A. Schmidt, W. D. Bugbee, R. F. Loeser, and R. L. Sah. 2003. Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheum. 48:110-118. [DOI] [PubMed] [Google Scholar]
- 10.Lorthois, S., P. Schmitz, and E. Angles-Cano. 2001. Experimental study of fibrin/fibrin-specific molecular interactions using a sphere/plane adhesion model. J. Colloid Interface Sci. 241:52-62. [DOI] [PubMed] [Google Scholar]
- 11.Marshall, B. T., K. K. Sarangapani, J. H. Lou, R. P. Mcever, and C. Zhu. 2005. Force history dependence of receptor-ligand dissociation. Biophys. J. 88:1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean, S. C., H. Lioe, L. Meagher, V. S. J. Craig, and M. L. Gee. 2005. Atomic force microscopy study of the interaction between adsorbed poly(ethylene oxide) layers: effects of surface modification and approach velocity. Langmuir 21:2199-2208. [DOI] [PubMed] [Google Scholar]
- 13.Norde, W., and D. Gage. 2004. Interaction of bovine serum albumin and human blood plasma with PEO-tethered surfaces: influence of PEO chain length, grafting density, and temperature. Langmuir 20:4162-4167. [DOI] [PubMed] [Google Scholar]
- 14.Nuzzo, R. G. 2003. Biomaterials: stable antifouling surfaces. Nat. Mater. 2:207-208. [DOI] [PubMed] [Google Scholar]
- 15.Razatos, A., Y. L. Ong, F. Boulay, D. L. Elbert, J. A. Hubbell, M. M. Sharma, and G. Georgiou. 2000. Force measurements between bacteria and poly(ethylene glycol)-coated surfaces. Langmuir 16:9155-9158. [Google Scholar]
- 16.Roosjen, A., H. J. Busscher, W. Norde, and H. C. van der Mei. 2006. Bacterial factors influencing adhesion of Pseudomonas aeruginosa strains to a poly(ethylene oxide) brush. Microbiology 152:2673-2682. [DOI] [PubMed] [Google Scholar]
- 17.Rutter, P. R., and B. Vincent. 1988. Attachment mechanisms in the surface growth of microorganisms, p. 87-107. In M. J. Bazin and J. I. Prosser (ed.), Physiological models in microbiology. CRC Press, Boca Raton, FL.
- 18.Xu, L. C., V. Vadillo-Rodriguez, and B. E. Logan. 2005. Residence time, loading force, pH, and ionic strength affect adhesion forces between colloids and biopolymer-coated surfaces. Langmuir 21:7491-7500. [DOI] [PubMed] [Google Scholar]
- 19.Zhao, B., and W. J. Brittain. 2000. Polymer brushes: surface-immobilized macromolecules. Prog. Polym. Sci. 25:677-710. [Google Scholar]