Abstract
Campylobacter species are important enteric pathogens causing disease in humans and animals. There is a lack of a good immunological test that can be used routinely to separate Campylobacter jejuni from other Campylobacter species. We produced monoclonal antibodies (MAbs) directed against the major outer membrane protein (MOMP) of C. jejuni using recombinant MOMP as the antigen. One MAb, designated MAb5C4 and of the immunoglobulin G1 isotype, was found to be potentially specific for C. jejuni. Dot blots demonstrated that MAb5C4 reacted with all 29 isolates of C. jejuni tested but did not react with 2 C. jejuni isolates, 26 other Campylobacter spp. isolates, and 19 non-Campylobacter isolates. Western blotting showed that MAb5C4 bound to a single protein band approximately 43 kDa in size, corresponding to the expected size of C. jejuni MOMP. The detection limit of MAb5C4 in a dot blot assay was determined to be about 5 × 103 bacteria. The epitope on the MOMP was mapped to a region six amino acids in length with the sequence 216GGQFNP221, which is 97% conserved among C. jejuni strains but divergent in other Campylobacter spp.; a GenBank search indicated that 95% of C. jejuni isolates will be able to be detected from non-Campylobacter spp. based on the highly specific and conserved region of the GGQFNP polypeptide. The epitope is predicted to be located in a region that is exposed to the periplasm. MAb5C4 is a potentially specific and sensitive MAb that can be used for the specific detection and identification of C. jejuni.
Campylobacter species are a major cause of disease in humans and animals. The two most important Campylobacter species are Campylobacter jejuni and Campylobacter coli, which account for about 80 to 90% and 5 to 10% of diarrheal illness, respectively, in humans (18). Accurate identification of Campylobacter to species level is important for both treatment and epidemiological surveillance. The standard detection methods for Campylobacter spp. involve enrichment and selective culture for initial isolation, followed by biochemical tests. The number of discriminating biochemical tests is limited due to the biochemical inertness of Campylobacter spp. The range of tests is so narrow that the separation of C. jejuni from C. coli is based almost solely on the hippurate hydrolysis test (20). The use of the hippurate hydrolysis test alone is inadequate and unreliable since we know that not all strains of C. jejuni are hippurate hydrolase positive (5, 27). Moreover, the lack of standardized procedures in carrying out biochemical tests has prompted criticism about their reliability for identification purposes (5, 20), particularly when they are used without any other additional tests (25). Clearly, additional testing is required if accurate identification is to be made because hippurate hydrolase-negative C. jejuni strains could be misidentified as C. coli if no further testing is done. The additional test, if it is to be employed as a routine procedure, should be cheap, specific, and sensitive; provide rapid results; and be easy to perform. Currently, there are numerous techniques available that would be able to distinguish C. jejuni from C. coli. These techniques include DNA hybridization, protein profiling, fatty acid analysis, matrix-assisted laser desorption ionization-time of flight mass spectrometry, real-time PCR, PCR-enzyme-linked immunosorbent assay (ELISA), PCR-hybridization, and multiplex PCR (1, 7, 8, 9, 15, 16, 20, 21). Most of these techniques would provide definitive results but are too expensive, complex, or demanding to be employed as routine procedures. Immunological tests, however, are relatively inexpensive, provide rapid results, and can be easily performed on a routine basis. However, there are few immunological tests that can differentiate C. jejuni from C. coli, and no commercial antibodies are able to discriminate between these two species. Therefore, we initiated a search for a suitable C. jejuni antigen in order to produce monoclonal antibodies (MAbs) that could specifically detect and identify cells of C. jejuni.
The outer membrane proteins (OMPs) of C. jejuni are important components of the cell envelope. Many gram-negative bacteria have one or more predominant OMPs. These major OMPs (MOMPs) usually function as general or specific porins that regulate the permeability of the membrane to small molecules (4, 22). Being a major component of the bacterial outer membrane, C. jejuni MOMP is an immunodominant protein (19) and makes an attractive target antigen. Moreover, the MOMP contains numerous variable regions that could be targeted for species-specific recognition by MAbs.
In this study, specific MAbs were created against C. jejuni recombinant MOMP (rMOMP). One MAb, designated MAb5C4, was identified as being highly specific for C. jejuni. We discuss the characterization of the epitope and the suitability of MAb5C4 as a diagnostic antibody for the specific detection and identification of C. jejuni.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial isolates used in this study were provided by the Agri-Food and Veterinary Authority (AVA) of Singapore and Singapore General Hospital, except for Campylobacter lari, which was obtained from the American Type Culture Collection, Manassas, VA (Table 1). All Campylobacter isolates used in this study, except for C. lari, were isolated from different chickens in different farms of Singapore and Malaysia and from different human sources obtained from Singapore General Hospital. They all were confirmed as C. jejuni, C. coli, Campylobacter upsaliensis, or C. lari by PCR using published species-specific primers (Table 2). Campylobacter cells were grown at 42°C for 24 h to 48 h on Columbia agar, supplemented with 5% horse blood (Bloxwich, Malaysia), in a microaerobic atmosphere (Campygen, Oxoid, United Kingdom). When required, bacteria were enumerated in the following manner. Cells were washed and harvested with phosphate-buffered saline (PBS) and centrifuged at 3,000 × g for 5 min. Cells were further diluted and enumerated on spread plates and expressed as the number of CFU. Escherichia coli and Salmonella were grown in Luria-Bertani medium at 37°C. E. coli strain XL1-Blue (Stratagene) was used for all cloning work, and strains BL21(DE3) and M15 were used for maltose binding protein (MBP) and six-His tag fusion protein expression, respectively. When required, medium was supplemented with ampicillin at a concentration of 100 μg/ml, and isopropyl β-d-thiogalactopyranoside was used at a final concentration of 1 mM to induce expression of fusion proteins.
TABLE 1.
Bacterial species used in this study
| Bacterial species | Isolate(s) or serovar(s) | Source |
|---|---|---|
| C. jejuni | 14/03, 19/03, 127/03, 130/03, 152/03, 154/03, 161/03, 186/03, 191/03, 243/03, 372/03, 373/03, 1/05, 3/05, 78/05, 93/05, 103/05, 104/05 | Chicken, broiler (intestine)a |
| 243/03, 235/03, 5/05, 6/05, 8/05, 29/05 | Chicken, layer (feces)a | |
| 71/02 | Chickena | |
| DM23585/06, DS140/07, DS906/07, DS850/07, DS1300/07, DS1682/07 | Humanc | |
| C. coli | 10/03, 233/03, 268/03, 440/03, 499/03, 500/03, 501/03, 32/04, 206/04, 207/04, 433/04 | Chicken, layer (feces)a |
| 405/03, 507/03, 55/04, 164/04, 165/04, 202/03, 200/04, 259/04, 416/04, 456/04 | Chicken, broiler (intestine)a | |
| 48/02 | Chickena | |
| DS160/07 | Humanc | |
| C. upsaliensis | 239/04, 240/04 | Dog (feces)b |
| C. lari | ATCC 35221 | ATCC, Manassas, VA |
| E. coli | 130/05, 131/05 | Chicken, broiler (pooled organs)a |
| 132/05 | Specific-pathogen-free chicken (organ)a | |
| 133/05 | Chicks (pooled organs)a | |
| 134/05 | Chicken, broiler (pooled intestines)a | |
| S. enterica subsp. enterica | Enteritidis 10/02, 225/02; Typhimurium 178/02 | Chickena |
| Typhimurium 213/02 | Duckb | |
| Schwarzengrund 345/04, Corvallis 47/05 | Chicken, broiler (pooled organs)a | |
| Agona 368/04, Weltevreden 24/05 | Chicken, layer (drag swab)a | |
| Braenderup 374/04, Albany 17/05 | Chicken, layer (cloacal swab)a | |
| Hadar 408/04 | Chicken, broiler (intestine)a | |
| Kentucky 432/04, Idikan 7/05 | Chicken, layer (drag swab)a | |
| Heidelberg 30/05 | Chicken, broiler (enviromental swab)a |
Isolated from different chickens of different farms in Singapore and Malaysia by the AVA of Singapore from 2002 to 2005.
Isolated in Singapore and Malaysia by the AVA of Singapore from 2002 to 2005.
Isolated in Singapore by Singapore General Hospital from 2006 to 2007.
TABLE 2.
Primers used for the identification of Campylobacter species
| Campylobacter sp. | Primer name | Sequence | Reference |
|---|---|---|---|
| C. jejuni | Hip1 | GTACTGCAAAATTAGTGGCG | 23 |
| Hip2 | GAGCTTTTAGCAAACCTTCC | ||
| C1 | CAAATAAAGTTAGAGGTAGAATGT | 28 | |
| C4 | GGATAAGCACTAGCTAGCTGAT | ||
| C. coli | CSF | ATATTTCCAAGCGCTACTCCCC | 26 |
| CSR | CAGGCAGTGTGATAGTCATGGG | ||
| C. upsaliensis | CHCU146F | GGGACAACACTTAGAAATGAG | 12 |
| CU1024R | CACTTCCGTATCTCTACAGA | ||
| C. lari | CL594F | CAAGTCTCTTGTGAAATCCAAC | 13 |
| CL1155R | ATTTAGAGTGCTCACCCGAAG |
Molecular techniques. (i) Cloning, expression, and purification of C. jejuni rMOMP.
The porA gene encoding the MOMP was amplified from purified chromosomal DNA of C. jejuni Cj71/02 by PCR using the primers Cj1259(BI)F (5′-CCCGGATCCATGAAACTAGTTAAACTTAGTTTA-3′) and Cj1259(SI)R (5′-CCCGTCGACTTAGAATTTGTAAAGAGCTTGAAG-3′); Cj71/02 was used since the MOMP region has a high percentage of similarity to all the C. jejuni species. Primer Cj1259(BI)F contains the first 24 nucleotides of the porA gene, including the start codon, and is flanked by a BamHI site (underlined). Primer Cj1259(SI)R contains the last 24 nucleotides of the porA gene, including the stop codon, and is flanked by a SalI site (underlined). The PCR mixture consisted of a 0.5 μΜ concentration of the primers and PCR buffer containing 2 mM MgCl2, a 250 μΜ concentration of the deoxynucleoside triphosphates (dNTPs), 5 U of Taq polymerase (Qiagen), 50 ng of template DNA, and sterile water, all in a volume of 50 μl. PCR was carried out in a programmable thermocycler (PTC-100 Peltier Thermal Cycler; Bio-Rad) under the following conditions. Initial denaturation was carried out at 94°C for 3 min and then progressed to 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min and 30 s. A final extension was carried out at 72°C for 10 min at the end of 30 cycles and then held at 4°C.
The PCR product was cloned into pGEM-T Easy cloning vector (Promega) by following the manufacturer's instructions. The DNA fragment was subsequently released by digestion with BamHI and SalI (New England BioLabs) and ligated to pQE30, which had been digested with BamHI and SalI. This recombinant plasmid contained the sequence encoding the MOMP with a His6 tag at the N terminus; it was expressed in E. coli M15 and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The expressed rMOMP was sliced from the SDS-PAGE gel and eluted by electrophoresis with an Electro-Eluter instrument (model 422; Bio-Rad). This crude rMOMP was used as an antigen for immunization on mice, and the concentration of rMOMP was quantified using an ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies). It should be noted that the expressed rMOMP failed to be purified using Ni-nitrilotriacetic acid (NTA) resin according to the instructions supplied by the manufacturer (Qiagen). Ni-NTA resin is used to purify His6-tagged proteins. Also, Western blotting using anti-His6 mouse MAb (Roche) failed to detect the expressed rMOMP. The inability to purify the expressed rMOMP using Ni-NTA resin and to detect rMOMP using the anti-His6 mouse MAb indicated that the His6 tag had been cleaved from the mature rMOMP; this was consistent with the findings of Zhang et al. (29) that one of the features of MOMP is that the signal peptide sequence is cleaved from the mature protein.
(ii) Epitope mapping and in vitro site-directed mutagenesis.
To locate the epitope recognized by MAb5C4 on the MOMP, different fragments of the porA gene were constructed using PCR, and then the fragments were cloned and expressed, as described for rMOMP above. The oligonucleotide GGTTCTTATGATCTTGCTGGCGGACAATTCAACCCACAA and its complementary strand were synthesized (1st BASE, Singapore). This fragment codes for the short polypeptide sequence GSYDLAGGQFNPQ containing the epitope. The oligonucleotides were mixed in equal concentrations and then heated to 98°C for 10 min and allowed to cool to room temperature. The fragment was then ligated to plasmid pMAL_c2, which had been digested with BamHI and then end filled to create blunt ends using the Klenow fragment of DNA polymerase (New England BioLabs) and dNTPs. The polypeptide GSYDLAGGQFNPQ was expressed as part of a fusion protein with the MBP in E. coli BL21(DE3). Point mutation of GSYDLAGGQFNPQ was performed in a 20-μl volume containing 30 ng of template DNA, a 0.1 μM concentration of the mutagenesis primers (13 pairs of 5′ phosphorylated primers were designed, with each pair used to generate a different point mutation that changed the designated amino acid into alanine; if designated amino acid already was alanine, it was changed into glycine), a 0.2 μM concentration of each dNTP, 2 μl of Pfu Turbo 10× reaction buffer, and 1 U of Pfu Turbo DNA polymerase (Stratagene). PCR thermocycler conditions consisted of an initial denaturation at 95°C for 5 min, followed by 14 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 8 min and 30 s, and a final extension at 72°C for 10 min. The products of this reaction were treated with 20 U of DpnI (New England Biolabs) for 1 h to remove methylated parental DNA prior to transformation of E. coli M15. Thirteen mutant plasmids were sequenced to confirm incorporation of the desired mutations, and the designated mutants were expressed as a part of a fusion protein with His6-tagged in E. coli M15. SDS-PAGE and Western blotting were performed as described below.
(iii) Nucleotide sequencing of MOMP.
Sequencing was carried out using a BigDye Terminator, version 3.1, Cycle Sequencing Kit and MOMP-specific primers by following the manufacturer's instructions. Sequences were determined with an Applied Biosystems 3730 Genetic Analyzer.
Production of MAbs.
BALB/c mice were immunized with 100 mg of C. jejuni rMOMP and 0.2 ml of the oil adjuvant Montanide ISA563 (Seppic, France). Intraperitoneal injections were delivered on days 0, 14, 28, and 42. Splenocytes from immunized mice and myeloma cells (SP2/0) were collected and fused as described by De St. Groth and Scheidigger (6). After selection with hypoxanthine-aminopterin-thymidine medium, culture medium from the hybridomas showing significant growth after 21 days was tested for the presence of specific antibodies against rMOMP by ELISA. Selected hybridomas were cloned by limiting dilution, reidentified, cloned a second time, and retested by ELISA. Once established, the hybridoma line was propagated in tissue culture and frozen in liquid N2 for future use. The isotype of MAb5C4 was determined using a mouse MAb isotyping kit (Amersham Biosciences) following the protocol supplied with the kit.
Immunochemical techniques. (i) Dot blot assay.
For pure and mixed cultures, different bacterial colonies were suspended in PBS, and the density was adjusted to the required optical density at 600 nm that was equivalent to the number of CFU being tested. An optical density at 600 nm of 1 corresponded to approximately 109 CFU/ml according to a C. jejuni growth curve determined earlier. One microliter of the bacterial suspension (pure or mixed culture), with the appropriate number of cells, was applied to a nitrocellulose membrane and air dried for 15 min. The membrane was blocked with 5% nonfat milk (Bio-Rad, Canada) in PBS with 0.05% Tween 20 (PBS-T) for 60 min. After the blocking step, undiluted hybridoma culture fluid containing MAbs was incubated with the membrane for 90 min, washed with PBS-T, and then incubated with goat anti-mouse horseradish peroxidase-conjugated antibodies (1:1,000 dilution) (DakoCytomation, Denmark) for 60 min. The membrane was washed and then developed with ECL Western blotting detection reagent (Amersham Biosciences) and exposed to scientific imaging film (Kodak BioMAX MS). For examination of spiked fecal samples, bacteria were added to fresh chicken feces suspended in PBS (5 ml/g) to a final concentration of approximately 108 CFU/ml. One microliter of this fecal suspension was processed exactly as described above for a pure culture of C. coli, except that the membrane was developed using a 3-3′-diaminobenzidine tetrahydrochloride enhanced liquid substrate system (Sigma) for 3 min instead of using the ECL Western blotting detection reagent.
(ii) SDS-PAGE and Western-blotting.
SDS-PAGE and Western blotting were performed as described by Ausubel et al. (2) using a discontinuous buffer system with a 12% polyacrylamide separating gel. The protein samples were prepared by mixing bacterial cell pellets with SDS sample buffer and then heating them at 100°C for 5 min. Approximately 21 μg of protein from whole-cell lysate was loaded into each well. Proteins separated by SDS-PAGE were visualized by staining with Coomassie blue (0.25% Coomassie brilliant blue R-250, 40% methanol, and 10% glacial acetic acid) for 30 min and then destained overnight in destaining solution (40% methanol and 10% glacial acetic acid). For Western blotting, proteins were transferred from the gel to a nitrocellulose membrane using a Transblot Cell (Bio-Rad). After electrotransfer, the membrane was blocked with 5% nonfat milk in PBS-T and processed in the same manner as the dot blots above.
Nucleotide sequence accession numbers.
The sequences of the MOMPs of the Cj71/02, Cj1/05, C. coli Cc48/02, and C. upsaliensis Cu240/04 isolates were deposited in the GenBank database under accession numbers DQ868942, DQ868941, DQ868940, and DQ868939, respectively.
RESULTS AND DISCUSSION
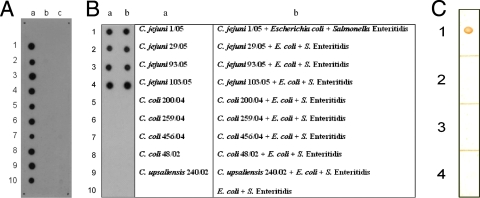
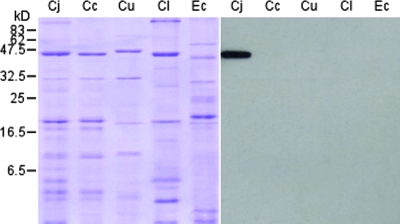
Hybridomas were created from the fusion between SP2/0 myeloma cells and lymphocytes from mice that had been immunized with rMOMP of C. jejuni Cj71/02. Cj71/02 was one of the earliest C. jejuni strains received from AVA, and it was selected in our earliest study of rMOMP production. Its MOMP sequence has a high percentage of similarity to all the other C. jejuni strains in the GenBank database and was found to be highly homologous to the other C. jejuni isolates from AVA later on. Using ELISAs, the hybridomas were screened for production of MAbs against C. jejuni MOMP. Twenty-five positive hybridoma clones were identified. Further testing revealed that one of these produced MAbs was highly specific for C. jejuni. This MAb, designated MAb5C4, was subsequently identified as being of the immunoglobulin G1 isotype and was selected for further work. Dot blot assays, using pure culture of whole cells of bacteria, revealed that MAb5C4 reacted with all 29 isolates of C. jejuni from chickens and humans but did not react with 2 isolates of C. jejuni from humans, 5 isolates of E. coli, or 14 different serovars of Salmonella enterica. Furthermore, there was no cross-reaction with any of the 23 C. coli isolates, 2 C. upsaliensis isolates, and 1 C. lari isolate (Fig. 1A). In a mixed culture with non-Campylobacter spp., MAb5C4 recognized an antigen common to different isolates of C. jejuni while it had no reaction with other species (Fig. 1B). We demonstrated the usefulness of MAb5C4 for diagnostic purposes by its ability to differentiate C. jejuni from other species. It may be possible to use MAb5C4 in the detection of C. jejuni from environmental samples, such as stool. Fresh samples of feces from healthy adult chickens from a commercial poultry farm were collected and spiked with 108 CFU/ml of different Campylobacter spp. Suspensions of the spiked fecal material were analyzed with dot blot assays; results showed that MAb5C4 detected only fecal suspensions that had been spiked with C. jejuni (Fig. 1C). These results indicated high specificity of MAb5C4 in C. jejuni detection. Western blotting using whole-cell lysate of Campylobacter spp. confirmed that MAb5C4 reacted only with C. jejuni and that it bound to a single protein band approximately 43 kDa in size (Fig. 2). This matches the reported size of C. jejuni MOMP (28) and confirms the specificity of MAb5C4 for only C. jejuni MOMP.
FIG. 1.
Dot blot analysis of the specificity of MAb5C4 for C. jejuni. (A) For pure cultures, approximately 105 bacterial cells were used on each spot and probed with MAb5C4. a1 to a10, C. jejuni isolates 1/05, 3/05, 5/05, 6/05, 8/05, 29/05, 78/05, 93/05, 103/05 and 104/05; b1 to b7, C. coli isolates 200/04, 202/03, 206/04, 207/04, 259/04, 416/04, and 456/04; b8 and b9, C. upsaliensis 240/02 and 239/02; b10, C. lari ATCC 35221; c1 to c5, E. coli isolates 130/05, 131/05, 132/05, 133/05, and 134/05; c6 to c10, Salmonella isolates 7/05, 17/05, 24/05, 30/05, and 47/05. (B) Comparison of MAb5C4 specificity in pure and mixed cultures. Approximately 105 bacterial cells were used on each spot and probed with MAb5C4. Suspensions of different bacteria were applied on the membrane according to the order of the table at right. The plus sign indicates that the spot is a mixture of bacterial cells from different genera. (C) Fecal sample of healthy chickens spiked with Campylobacter spp. and probed with MAb5C4. The dots represent fecal samples of healthy chickens spiked with approximately 105 CFU/ml of C. jejuni 103/05 (1), C. coli 200/04 (2), C. upsaliensis 240/02 (3), and C. lari ATCC 35221 (4).
FIG. 2.
SDS-PAGE and Western blot analysis of whole-cell lysate of Campylobacter representatives. A Coomassie blue-stained gel is shown on the left with the corresponding blot, probed with MAB5C4, on the right. Bacteria analyzed were C. jejuni (Cj), C. coli (Cc), C. upsaliensis (Cu), C. lari (Cl), and E. coli (Ec).
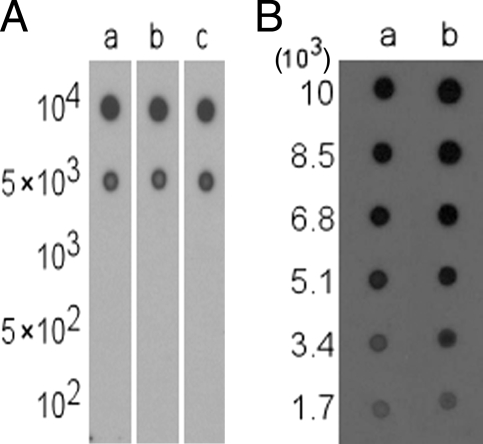
An assessment of the sensitivity of MAb5C4 was determined with dot blot assays using whole cells of C. jejuni in the range of 102 to 104 bacteria. The lowest number of bacterial cells that could be detected by MAb5C4 was approximately 5 × 103 bacteria (Fig. 3A). However, if cells were first heated to 100°C for 20 min, both the signal and the sensitivity improved. A stronger positive signal was still evident for approximately 3 × 103 heat-treated cells compared to untreated cells (Fig. 3B). It is difficult to make comparisons of the sensitivity of MAb5C4 with other MAbs specific for Campylobacter because not many studies have reported the sensitivity of their MAbs. One of the few studies with sensitivity data was that of Lu et al. (14), who reported detection limits of 105 to 107 CFU/ml for MAbs against Campylobacter spp., as determined by ELISA (14). The amount of bacterial sample used in the ELISA was not stated, so it is uncertain what the detection limit was in terms of total number of cells, which makes a direct comparison with MAb5C4 results difficult. The antibodies from the Singlepath Campylobacter gold-labeled immunosorbent assay detection kit (Merck) have a sensitivity of 104 to 107 CFU/ml and require a volume of 160 μl of bacterial sample, which is equivalent to 1.6 × 103 to 1.6 × 106 bacteria. The lowest detection limit of MAb5C4, which is 5 × 103 bacterial cells (untreated), falls in the lower end of this detection spectrum. Thus, MAb5C4 is just as sensitive as commercially available antibodies directed against Campylobacter spp.
FIG. 3.
(A) Dot blot analysis of the sensitivity of MAb5C4. Suspensions of approximately 102 to 104 whole cells of three different C. jejuni representative isolates were assessed: C. jejuni 1/05 (lane a); C. jejuni 29/05 (lane b); and C. jejuni 103/05 (lane c). Figures on the left indicate the number of bacterial cells per spot. (B) Dot blot assay of heat-treated C. jejuni representative cells. Cell suspensions were heated to 100°C for 20 min. Lane a, untreated C. jejuni 103/05; lane b, heat-treated C. jejuni 103/05. Values on the left indicate the number of bacterial cells per spot.
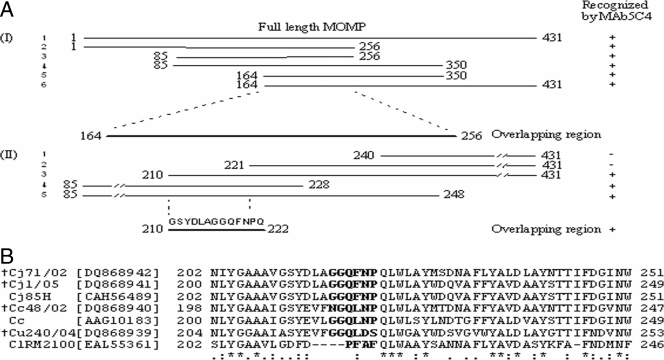
In order to locate the epitope for MAb5C4, short overlapping fragments of MOMP were expressed and analyzed by Western blotting. This was carried out in two stages. During stage I, six overlapping fragments were created and analyzed, including one encoding the full-length fragment of the MOMP, 431 amino acids in length. All the fragments were detected by MAb5C4 and revealed a common overlapping region 92 amino acids long (Fig. 4A). During stage II, five more fragments were constructed in order to narrow the binding site by reducing the overlapping region. Only three fragments reacted with MAb5C4, fragments 3 to 5, which reduced the overlapping region to just 13 amino acids, from residues 210 to 222, with the sequence 210GSYDLAGGQFNPQ222. Subsequently, we expressed a polypeptide containing this sequence, with two additional amino acids on the carboxyl end, as part of a fusion protein with the MBP. The fusion protein was indeed detected by MAb5C4 (result not shown). We deduced that the binding site for MAb5C4 must have involved the 13 amino acids because it is conserved among C. jejuni strains but varies among other thermophilic Campylobacter spp. (Fig. 4B), and we subsequently deleted this sequence from a cloned full-length fragment of the MOMP. Deletion of this sequence completely abolished binding by MAb5C4 as analyzed by Western blotting (data not shown), which indicated that the epitope did, indeed, involve these 13 amino acids. In order to locate the exact amino acids that contribute to the binding of MAb5C4, in vitro site-directed mutagenesis was performed. From Western blotting (data not shown), changing of one amino acid of the polypeptide 216GGQFNP221 completely abolished binding by MAb5C4. Hence, GGQFNP is the epitope location of MAb5C4. As demonstrated by dot blot assay, MAb5C4 did not react with 2 of the 29 C. jejuni isolates that we had. From MOMP gene sequencing results, the undetected C. jejuni human isolates, namely, DS850/07 (GenBank accession no. EU259062) and DS140/07 (GenBank accession no. EU252000), had the first amino acids (glycine; in boldface) of the GGQFNP sequences changed into asparagine. This contributed to the nonreactivity of MAb5C4. In this study, only C. jejuni, C. coli, C. upsaliensis, and C. lari, which make up the thermophilic group of Campylobacter spp., were analyzed, as according to a database search only this group of Campylobacter spp. has MOMPs with any significant sequence similarity to each other; since the MOMP is divergent in other gram-negative bacteria, only Salmonella and E. coli were used as the representatives of gram-negative bacteria other than Campylobacter spp. In addition to results with the bacteria isolates that we had (Table 1), the specificity and consistency of MAb5C4 to detect C. jejuni were further determined by aligning the C. jejuni MOMP sequence, particularly GGQFNP, with all bacteria in the GenBank database. GenBank searches showed that 83 isolates of C. jejuni from humans, chickens, turkey, ovine, bovine and canine sources and 6 isolates of non-Campylobacter spp. have exact GGQFNP polypeptide sequences (Table 3). The six non-Campylobacter species are mostly gram-positive or less common bacteria that are easily excluded from Campylobacter detection. None of the Campylobacter spp. other than C. jejuni isolates has the exact GGQFNP polypeptide sequence. In terms of the specificity of MAb5C4 toward C. jejuni, the GenBank search indicated that 85 isolates of C. jejuni have significant sequence similarity with GGQFNP, but only 83 isolates have an exact match with the polypeptide sequence, while the other two C. jejuni isolates, namely 21190 (isolated from chicken) and OA5 (unknown source), had their fourth amino acids (phenylalanine; in boldface) of GGQFNP changed to tyrosine. Hence, based on the bacterial samples that we had and the GenBank search, we concluded that MAb5C4 is able to detect and differentiate from other Campylobacter spp. 97% of C. jejuni isolates and to detect almost 95% of C. jejuni isolates from non-Campylobacter spp. due to the highly specific and conserved region of the polypeptide GGQFNP.
FIG. 4.
(A) Schematic representation of the strategy used to map the epitope on the MOMP. Lines represent fragments of MOMP that were constructed, and the flanking numbers indicate the amino acid positions. Epitope mapping was carried out in two stages. In stage I, six overlapping fragments were created, and all reacted with MAb5C4. In stage II, five fragments were created, but only three fragments reacted with MAb5C4. The amino acid sequence of the shortest overlapping region, 13 amino acids in length, is also displayed. +, positive reaction; −, negative reaction. (B) Alignment of a partial sequence of the MOMP containing the amino acids required for binding by MAb5C4. The amino acids in bold letters are conserved among C. jejuni but divergent in other Campylobacter species. Asterisk, identical amino acids; colon, conserved amino acid substitutions; period, semiconserved amino acid substitutions. The MOMP gene sequences determined in this study and deposited in GenBank are indicated (†).
TABLE 3.
Bacterial species that contain exact GGQFNP polypeptide sequencea
| Bacterial species | Isolate(s) or serovar(s) (sample type) | Source |
|---|---|---|
| C. jejuni | X7199, X77136, 81176, CF93-6, S9801, 84-25 (cerebrospinal fluid of child with meningitis), CG8486, 260.94, HB93-13, S47648, X77852, M33323, H49024, E46972, M50813, 269.97 (human blood), H30769, S13530, T37597B, M36292 | Human |
| S2B (chicken rinses), S11B (chicken rinses), RM1221 (chicken carcass), NCTC 11168 (chicken gastrointestinal tract) | Chicken | |
| Turk | Turkey | |
| 19084571 | Ovine | |
| 15046764 | Bovine | |
| 1979855 | Canine | |
| 00-1732, 00-1747, 11391, 17407, 2483, 81-176, 85H, 98-1718, 98-1730, 98-1731, 98-1732, 98-2295, 98-2354, BF10-13, BF10-38, BF10-5, BF10-7, BF8-11, F26747, F34078, F59966, JL01, Lee, LG2001, M13104, M36292, NC13254, NC13256, NC13257, NC13259, NC13260, NC13262, NC13263, NC13264, NC13266, OA32, Od1, PO02KY, S20237, S3b, T106, T11, T113, T118, T123, T13, T19, T2, T27, T30, T57060, T94, TF1-1, W11805, W42606 | Unknown | |
| Staphylococcus aureus | Human | |
| Planctomyces maris | DSM 8797 | Unknown |
| Photorhabdus luminescens | W14 | Insect |
| Lactobacillus salivarius subsp. salivarius | UCC118 | Human |
| Rhodospirillum rubrum | ATCC 11170 | ATCC, Manassas, VA |
| Pseudoalteromonas atlantica | T6c | Ocean (salt water) |
Based on GenBank search, only C. jejuni but not other Campylobacter isolates contain the exact polypeptide sequence of GGQFNP.
The MOMP of C. jejuni is composed of 18 β-strands forming an antiparallel β-barrel, with short turns at the bottom of the barrel facing the periplasm and long loops at the top of the barrel facing the external surface of the bacterial outer membrane (29). The epitope for MAb5C4 appears to be facing the periplasm because the sequence 216GGQFNP221 is found in a region predicted to be located on a short turn between the eighth and ninth β-strands of the MOMP (11, 29). This is supported by the results from the dot blot assays, which showed that heating the bacterial cells enhanced signal detection. Heat would further disrupt the membrane and help expose the epitope. However, the epitope of MAb5C4 is not a surface-exposed epitope, as immunofluorescence and immunogold electron microscopy assays failed to demonstrate MAb5C4 bound to the surface of C. jejuni.
There are few C. jejuni-specific MAbs described in the literature, and to the best of our knowledge, none is available commercially. Commercial antibodies detect Campylobacter but do not differentiate C. jejuni from other Campylobacter species. Many MAbs that have been produced against formalin-treated C. jejuni cells, flagellin, and the hippurate hydrolase enzyme also cross-reacted with other Campylobacter species (10, 14, 17, 20) or with other unrelated bacterial species (24). Brooks et al. (3) developed MAbs against the lipopolysaccharides of C. jejuni, but these MAbs are disadvantaged by their narrow spectrum of recognition. One MAb alone could not detect any of the eight Penner serotypes of C. jejuni tested and sometimes required a combination of two MAbs (3). The data reported here for MAb5C4 showed that it was highly specific for C. jejuni MOMP and just as sensitive as commercial antibodies for Campylobacter, and thus MAb5C4 has potential as a diagnostic antibody for the specific detection and identification of C. jejuni. Further work is needed to develop the MAb5C4 into a kit that can be used directly in the field to test environmental samples.
Acknowledgments
This work was supported by a grant from the Temasek Life Sciences Laboratory, Singapore.
We thank the Agri-Food and Veterinary Authority of Singapore and Singapore General Hospital for their generosity in providing bacterial samples. Also, we thank Victoria Korolik, Institute for Glycomics, Griffith University, Australia, for her assistance in testing the specificity of MAb5C4 toward C. jejuni isolates.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Al Rashid, S. T., I. Dakuna, H. Louie, D. Ng, P. Vandamme, W. Johnson, and V. L. Chan. 2000. Identification of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, Arcobacter butzleri, and A. butzleri-like species based on the glyA gene. J. Clin. Microbiol. 38:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1999. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, NY.
- 3.Brooks, B. W., J. G. Mihowich, B. W. Blais, and H. Yamazaki. 1998. Specificity of monoclonal antibodies to Campylobacter jejuni lipopolysaccharide antigens. Immunol. Investig. 27:257-265. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan, S. K. 1999. Beta-barrel proteins from bacterial outer membranes: structure, function and refolding. Curr. Opin. Struct. Biol. 9:455-461. [DOI] [PubMed] [Google Scholar]
- 5.Burnett, T. A., A. Hornitzky, M., P. Kuhnert, and S. P. Djordjevic. 2002. Speciating Campylobacter jejuni and Campylobacter coli isolates from poultry and humans using six PCR-based assays. FEMS Microbiol. Lett. 216:201-209. [DOI] [PubMed] [Google Scholar]
- 6.de St. Groth, S. F., and D. Scheidigger. 1980. Production of monoclonal antibodies. Strategy and tactics. J. Immunol. Methods 35:1-21. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, I., K. A. Grant, P. T. Richardson, S. F. Park, and M. D. Collins. 1997. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene enoding a putative virulence determinant. J. Clin. Microbiol. 35:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong, Y., M. E. Berrang, T. Liu, C. L. Hofacre, S. Sanchez, L. Wang, and J. J. Maurer. 2003. Rapid detection of Campylobacter coli, C. jejuni, and Salmonella enterica on poultry carcasses by using PCR-enzyme-linked immunosorbent assay. Appl. Environ. Microbiol. 69:3492-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klena, J. D., C. T. Parker, K. Knibb, J. C. Ibbitt, P. M. L. Devane, S. T. Horn, W. G. Miller, and M. E. Konkel. 2004. Differentiation of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis by a multiplex PCR developed from the nucleotide sequence of the lipid A gene lpxA. J. Clin. Microbiol. 42:5549-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosunen, T. U., B. E. Bang, and M. Hurme. 1984. Analysis of Campylobacter jejuni antigens with monoclonal antibodies. J. Clin. Microbiol. 19:129-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labesse, G., E. Garnotel, S. Bonnel, C. Dumas, J. M. Pages, and J. M. Bolla. 2001. MOMP, a divergent porin from Campylobacter: cloning and primary structural characterization. Biochem. Biophys. Res. Commun. 280:380-387. [DOI] [PubMed] [Google Scholar]
- 12.Lawson, A. J., D. Linton, J. Stanley, and R. J. Owen. 1997. Polymerase chain reaction detection and speciation of Campylobacter upsaliensis and C. helveticus in human faeces and comparison with culture techniques. J. Appl. Microbiol. 83:375-380. [DOI] [PubMed] [Google Scholar]
- 13.Linton, D., R. J. Owen, and J. Stanley. 1996. Rapid identification by PCR of the genus Campylobacter and of five Campylobacter species enteropathogenic for man and animals. Res. Microbiol. 147:707-718. [DOI] [PubMed] [Google Scholar]
- 14.Lu, P., B. W. Brooks, R. H. Robertson, K. H. Nielsen, and M. M. Garcia. 1997. Characterization of monoclonal antibodies for the rapid detection of foodborne campylobacters. Int. J. Food Microbiol. 37:87-91. [DOI] [PubMed] [Google Scholar]
- 15.Lund, M., and M. Madsen. 2006. Strategies for the inclusion of an internal amplification control in conventional and real time PCR detection of Campylobacter spp. in chicken fecal samples. Mol. Cell. Probes 20:92-99. [DOI] [PubMed] [Google Scholar]
- 16.Mandrell, R. E., L. A. Harden, A. Bates, W. G. Miller, W. F. Haddon, and C. K. Fagerquist. 2005. Speciation of Campylobacter coli, C. jejuni, C. helveticus, C. lari, C. sputorum, and C. upsaliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 71:6292-6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nachamkin, I., and A. M. Hart. 1986. Common and specific epitopes of Campylobacter flagellin recognized by monoclonal antibodies. Infect. Immun. 53:438-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachamkin, I., J. Engberg, and F. M. Aarestrup. 2000. Taxonomy of the family Campylobacteraceae, p. 45-66. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 19.Nachamkin, I., and A. M. Hart. 1985. Western blot analysis of the human antibody response to Campylobacter jejuni cellular antigens during gastrointestinal infection. J. Clin. Microbiol. 21:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.On, S. L. 1996. Identification methods for campylobacters, helicobacters, and related organisms. Clin. Microbiol. Rev. 9:405-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perelle, S., M. Josefsen, J. Hoorfar, F. Dilasser, J. Grout, and P. Fach. 2004. A LightCycler real-time PCR hybridization probe assay for detecting food-borne thermophilic Campylobacter. Mol. Cell. Probes 18:321-327. [DOI] [PubMed] [Google Scholar]
- 22.Schirmer, T. 1998. General and specific porins from bacterial outer membranes. J. Struct. Biol. 121:101-910. [DOI] [PubMed] [Google Scholar]
- 23.Slater, E. R., and R. J. Owen. 1997. Restriction fragment length polymorphism analysis shows that the hippuricase gene of Campylobacter jejuni is highly conserved. Lett. Appl. Microbiol. 25:274-278. [DOI] [PubMed] [Google Scholar]
- 24.Steele, M., C. Gyles, V. L. Chan, and J. Odumeru. 2002. Monoclonal antibodies specific for hippurate hydrolase of Campylobacter jejuni. J. Clin. Microbiol. 40:1080-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinbrueckner, B., G. Haerter, K. Pelz, and M. Kist. 1999. Routine identification of Campylobacter jejuni and Campylobacter coli from human stool samples. FEMS Microbiol. Lett. 179:227-232. [DOI] [PubMed] [Google Scholar]
- 26.Stonnet, V., L. Sicinschi, F. Megraud, and J. L. Guesdon. 1995. Rapid detection of Campylobacter jejuni and Campylobacter coli isolated from clinical specimens using the polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 14:355-359. [DOI] [PubMed] [Google Scholar]
- 27.Totten, P. A., C. M. Patton, F. C. Tenover, T. J. Barrett, W. E. Stamm, A. G. Steigerwalt, J. Y. Lin, K. K. Holmes, and D. J. Brenner. 1987. Prevalence and characterization of hippurate-negative Campylobacter jejuni in King County, Washington. J. Clin. Microbiol. 25:1747-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winters, D. K., and M. F. Slavik. 1995. Evaluation of a PCR based assay for specific detection of Campylobacter jejuni in chicken washes. Mol. Cell. Probes 9:307-310. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, Q., J. C. Meitzler, S. Huang, and T. Morishita. 2000. Sequence polymorphism, predicted secondary structures, and surface-exposed conformational epitopes of Campylobacter major outer membrane protein. Infect. Immun. 68:5679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]