Abstract
Spores from four Geobacillus spp. were isolated from a milk powder manufacturing line in New Zealand. Liquid sporulation media produced spore yields of ∼107 spores ml−1; spores were purified using a two-phase system created with polyethylene glycol 4000 and 3 M phosphate buffer. The zeta potentials of the spores from the four isolates ranged from −10 to −20 mV at neutral pH, with an isoelectric point between pH 3 and 4. Through contact angle measurements, spores were found to be hydrophilic and had relative hydrophobicity values of 10 to 40%, as measured by the microbial adhesion to hexadecane assay. The most hydrophilic spore isolate with the smallest negative charge attached in the highest numbers to Thermanox and stainless steel (1 × 104 spores cm−2), with fewer spores attaching to glass (3 × 103 spores cm−2). However, spores produced by the other three strains attached in similar numbers (P > 0.05) to all substrata (∼1 × 103 spores cm−2), indicating that there was no simple relationship between individual physicochemical interactions and spore adherence. Therefore, surface modifications which limit the attachment of one strain may not be effective for all stains, and control regimens need to be devised with reference to the characteristics of the particular strains of concern.
The presence of spores of thermophilic bacilli are a common problem during milk powder manufacture (32). Thermophilic spores from bacteria such as Geobacillus spp. are normally present in low numbers in the raw milk (31). However, high numbers are often found in the milk powder. The source of these spores is believed to be thermophilic bacteria growing on the surfaces within the heat exchangers and evaporators (43). Biofilms are formed when spores present in the raw milk survive pasteurization, adhere to stainless steel surfaces, and germinate when and where conditions are suitable. As the biofilm matures, cells and spores can slough off and contaminate the product flowing past (13). Spore concentrations as high as 105 spores g−1 can occur in milk powder, resulting in it being downgraded to a lower-value product. As established biofilms are difficult to eradicate (20), prevention of the initial attachment is an alternative approach to control biofilm formation. The initial attachment of microorganisms, including spores, to surfaces is due to physicochemical interactions between their surfaces and the substratum, which are described by the extended Derjaguin, Landau, Verwey, and Overbeek theory (53). Such forces include Lifshitz-van der Waal's, acid/base, and electrostatic interactions. An improved understanding of the interactions that impact spore attachment may help in the development of strategies to reduce spore adherence.
Several studies have used electron microscopy and atomic force microscopy to examine the physical structure of the spore's surface (7, 18, 28). However there have been few studies of the surface chemistry of spores, and it is likely that what little information is known may not be broadly applicable across spores of all the species. It is known that the physical structure of the outer surfaces of spores varies dramatically. For example, the outer layer of a Bacillus subtilis spore consists of protein and carbohydrates (57), while in species such as Bacillus cereus and Bacillus anthracis, the spores exhibit pili (46) and an additional layer, known as an exosporium (16), comprising proteins, carbohydrates, and lipids (30). Little is known of the outer layer of thermophilic spores found in dairy plants.
The physicochemical characterization of the surface properties of microorganisms has also received extensive interest (49). However, there have only been a few studies on the surface characterization of spores, and these have been confined to mesophilic species (11, 37). Surface characterization techniques have involved measuring surface charge through zeta potential and relative hydrophobicity through the microbial adhesion to solvents assay and the determination of contact angles (52).
A large number of studies (3) have assessed the role of the physicochemical properties in the attachment of vegetative cells. However, it has remained unclear how much of this information is relevant to spore adhesion to surfaces. Spores differ from vegetative cells in that they are not metabolically active (44), and unlike vegetative cells they are clearly nonmotile (34).
Before the surface of a spore can be characterized, large quantities of free endospores must be obtained. Previous spore isolation methods have focused on harvesting spores from mesophilic bacilli such as B. subtilis and B. cereus (9, 12, 15), with only a few published papers on spore production from the thermophile Geobacillus stearothermophilus (24, 25, 59) and even fewer on recovering spores from dairy microorganisms (36, 40). For characterization purposes it is important that the spore suspensions do not contain debris, which may influence the results. Researchers have used detergents, enzymes, and ultrasonication to purify spores and disrupt them in order to recover proteins or DNA (41). However, these techniques have been shown to alter the spore's surface (10). To overcome this problem, density gradient centrifugation using either sodium bromide (27, 33), urografin (47), or a two-phase separation technique with polyethylene glycol (PEG) has been used (42).
The aims of this study were to develop a method to produce large quantities of endospores from thermophilic bacilli dairy isolates, to determine the surface physicochemical characteristics of the endospores, and to show how these characteristics impact the ability of the endospores to attach to a variety of substrata. Such information could be used to develop strategies to prevent or reduce the number of spores attaching to stainless steel and thereby reduce fouling within a milk powder production plant.
MATERIALS AND METHODS
Isolation and storage.
Thermophilic bacilli designated D4, E7, and E11 were isolated from milk samples taken from evaporators during the milk drying process at a milk powder manufacturing plant in the South Island of New Zealand. A further isolate, designated CGT-8, was obtained from a milk powder manufacturing site in the North Island of New Zealand. Isolates were streaked out onto tryptic soy agar (Becton Dickinson and Company [BD]) to select for single colonies. Stock cultures were maintained at −80°C in 15% glycerol suspensions. Isolates were identified using species-specific primers for Geobacillus spp. and randomized amplified polymorphic DNA as described by Flint et al. (14).
Spore production.
Enhancement of spore production was assessed by comparing four different media previously reported to be effective with other sporulating thermophilic bacilli (48, 59). Isolates were inoculated into 10 ml tryptic soy broth (TSB), which was incubated for 18 h at 55°C and used as a 1% inoculum for spore media. Spore media for Geobacillus sp. isolates D4, E7, E11, and CGT-8 contained either (per liter) 30 g Bacto tryptone (BD) or 30 g TSB (BD). These media were supplemented with the following salts (per liter): 0.125 g of CaCl2, 0.15 g of MnSO4, 0.155 g of FeSO4, and 0.55 g of MgCl2. All salts were analytical grade from BDH. Cultures were aerated by stirring for 72 h at 55°C. At intervals spore numbers were determined by the boiling of aliquots obtained from the culture, followed by serial dilution, and enumerated using the drop plate method (19). At the completion of sporulation, spores were harvested by centrifugation (10,000 × g, 8 min at 4°C) and washed three times in sterile 18-MΩ cm−1 water (Barnstead). Crude spore suspensions consisting of ∼1 × 107 spores ml−1 were stored at 4°C in water until needed.
Purification of spores.
Spores were purified using a PEG two-phase system as described by Sacks and Alderton (42). The system was created by dissolving 5.6 g of PEG 4000 in 17 ml of 3 M phosphate buffer (pH 7.4). After phase separation, the crude spore suspension was carefully layered on the gradient, creating a total volume of 50 ml. The sample was centrifuged at 1,500 × g for 3 min at 20°C. Debris migrated to the lower phase, while spores were concentrated in a layer above the PEG phase. Spores were carefully recovered and washed five times at 20°C in water. The effectiveness of the separation procedure was determined by visual examination of the recovered fractions using phase-contrast microscopy.
Characterization.
The surface hydrophobicities of the four spore isolates were determined using the microorganism adhesion to hexadecane (MATH) assay (39). Purified spores were suspended in 0.1 M KCl at either pH 3 or 6.8 to an optical density at 600 nm of 0.6 to 1.0. Spore suspensions (2 ml) were added to 1 ml of hexadecane and mixed on a vortex mixer for 1 min, incubated at 37°C for 10 min, and vortexed again for 2 min. After the suspensions settled at 20°C for 30 min, the absorbance of the aqueous phase was measured at 600 nm. The mean of five measurements was taken. The percent hydrophobicity (%h) was determined from the absorbance of the original spore suspension (Ai) and absorbance of the aqueous phase after mixing with hexadecane (Aƒ) according to the following equation: %h = (Ai − Af/Ai) × 100.
The zeta potentials of spores from the four isolates were determined (Zetasizer Nano ZS90; Malvern Instruments Ltd., United Kingdom) between pH 3 and 11 at 25°C. The mean of three separate measurements from the same sample was recorded. Purified spores were suspended in 0.1 M KCl to an optical density of 0.01 at 600 nm. The pH was adjusted using HCl or NaOH. The zeta potential was calculated from the electrophoretic mobility using the Smoluchowski equation (21).
Spore lawn preparation and contact angle measurements of surfaces.
Spore lawns were prepared as described by Busscher et al. (6) for bacteria. Suspensions of spores were collected on a cellulose triacetate filter (0.45 μm; Pall) to a density of 108 spores mm−2. The filters were placed on a gel surface containing 2% agar and 10% (wt/vol) glycerol in water to generate a uniform moisture content within the lawn before being cut into strips (1 cm in diameter) using a sterile scalpel blade. The strips were allowed to dry for 45 min at room temperature, and contact angles were measured, within a further 30 min, by the sessile-drop method using an FTA200 goniometer (First Ten Angstroms, Portsmouth, VA). To prepare surfaces for contact angle measurements, stainless steel 316 2B grade polish coupons (2 by 1 by 0.1 cm) were polished using 800 and 1200 grit sandpaper, washed in acetone at 20°C followed by 50% nitric acid at 80°C for 30 min to passivate the surface, rinsed in distilled water, and sterilized by autoclaving. Glass surfaces were cut from precleaned microscope slides into coupons (2.5 by 1 by 0.1 cm). Thermanox (Nalge Nunc International) coupons (2 by 1 by 0.05 cm) came precleaned, gamma irradiated, and ready to use. Contact angles of two polar solutions (water and formamide) and one nonpolar solution (α-bromonaphthalene) were obtained by depositing a drop of each test liquid on the surface of either glass, Thermanox, stainless steel (316 2B grade polish), or the filter strips containing the spore lawns. A series of 10 contact angle measurements were recorded, each within 2 seconds of the drop contacting the surface. Surface Gibbs energies were determined using the FTA32, version 2.0, software (First Ten Angstroms). Energetic properties of the materials were obtained from the Young-van Oss equation (55), (1 + cos θ)γTOT = 2[(γsvLWγlvLW)1/2 + (γsv+γlv−)1/2 + (γsv−γlv+)1/2], where γTOT is the total surface energy; γLW, γ+, and γ− are the van der Waals, electron acceptor, and electron donor components of the surface Gibbs energy, respectively; and θ is the contact angle. The subscripts sv and lv correspond to the solid-vapor and liquid-vapor interfaces, respectively. The hydrophobicity of microbial cell surfaces can be thermodynamically expressed as a Gibbs energy of aggregation (ΔGsls) (54) according to the following equation: ΔGsls = −2[(γsvLW)1/2 − (γlvLW)1/2]2 − 4[(γsv+γsv−)1/2 + (γlv+γlv−)1/2 − (γsv+γlv−)1/2 − (γsv−γlv+)1/2]. Organisms with hydrophilic surfaces prefer the aqueous phase and display a ΔGsls >0, while hydrophobic organisms tend to aggregate and therefore have a ΔGsls <0.
Adhesion experiment.
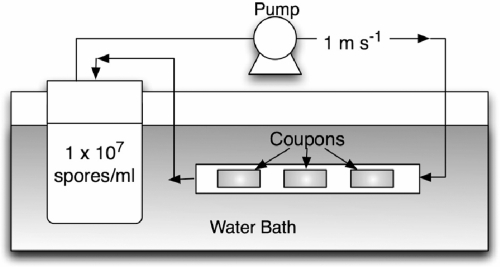
A flow loop reactor system was used to experimentally determine the initial attachment of thermophilic spores to glass, stainless steel, and Thermanox. The system consisted of a reservoir and loop (total volume was 200 ml) containing coupons of different materials (Fig. 1). Coupons were inserted into silicon tubing (1 cm in diameter) and placed along the loop in triplicate groups. The spores of the different isolates were suspended in 0.1 M KCl at a concentration of ∼1 × 107 spores ml−1. The suspending medium containing 0.1 M KCl was chosen, as this resembles the ionic strength found in milk (0.08 M). Spore suspensions were circulated at a speed of 1 m s−1 (Reynolds number, 5,600) through the recirculating loop to model the velocity in a dairy plant. The entire system was held in a water bath at 20°C to prevent spore germination. Coupons were removed after 30 min and rinsed in water three times to remove loosely adherent spores. The number of spores attached to the coupons was estimated by swabbing the coupons in 0.1% peptone containing three glass beads (5 mm in diameter). The coupon, swab, and media were vortexed for 15 seconds. The number of viable spores recovered from the surface was determined using the drop plate method after the suspension had been boiled for 30 min to activate the spores. There was little difference in the numbers of spores estimated to be present on the surface by the swabbing and plating method compared to epifluorescence direct counts (data not shown).
FIG. 1.
Schematic of the flow reactor used to expose the different substrata to spores.
Statistics.
Statistical analysis was performed using SPSS software (Statistical Package for the Social Sciences, Inc., Chicago, IL). The influence of isolate and substrates was investigated by analysis of variance, followed by a multiple-means comparison procedure using Tukey grouping. All tests were performed with a confidence level of 95%.
RESULTS
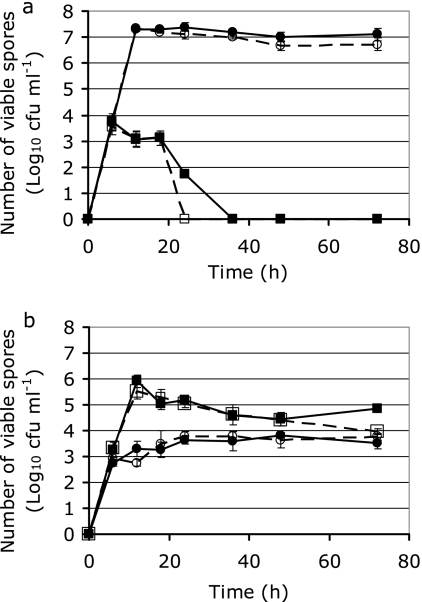
The effect of nutrient source, presence or absence of salts, and growth time on sporulation of Geobacillus sp. isolates D4 and CGT-8 was determined (Fig. 2). Sporulation of isolates E7 and E11 displayed trends similar to those for D4 (data not shown). The number of spores produced from D4 grown in tryptone for 18 h was 4 orders of magnitude higher than the number produced when it was grown in TSB. In contrast, CGT-8 produced 500 times more spores when grown in TSB than when grown in tryptone. The presence of manganese, calcium, iron, and magnesium caused a small but significant (P < 0.05) increase in the number of spores obtained from D4 and CGT-8 at their optimal sporulation times. The optimal sporulation times for D4 and CGT-8 were 18 and 12 h, respectively. Interestingly, the number of spores from D4 in TSB decreased dramatically after 20 h, presumably due to germination and subsequent cell lysis. To purify the spores by removing debris such as cell walls and vegetative cells, a two-phase separation using PEG 4000 was used. Pure spore suspensions contained ∼99% phase-bright endospores and were used for subsequent characterization studies.
FIG. 2.
Numbers of spores recovered from Geobacillus sp. isolates D4 (a) and CGT-8 (b) over 72 h in TSB with (▪) or without (□) salt or in tryptone with (•) or without (○) salt. Values represent the mean of three replicates and standard deviations of the means.
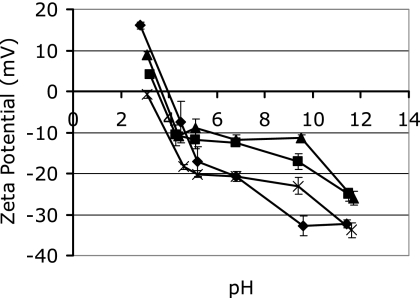
The zeta potentials of the four Geobacillus sp. spore isolates over the pH range of 3 to 11 were determined (Fig. 3). All of the spore isolates had a net negative charge at the pH of milk (pH 6.8). Isolate E7 had the greatest negative charge (−21 mV) and D4 the smallest (−12 mV). The isoelectric points for spores of isolates E7, E11, and D4 were around pH 4, with isolate CGT-8 spores having an isoelectric point at pH 3.
FIG. 3.
Zeta potentials of spores from Geobacillus sp. isolates D4 (▴), E7 (♦), E11 (▪), and CGT-8 (×) in 0.1 M KCl between pH 3 and 12. Values represent the means of three replicates and standard deviations of the means.
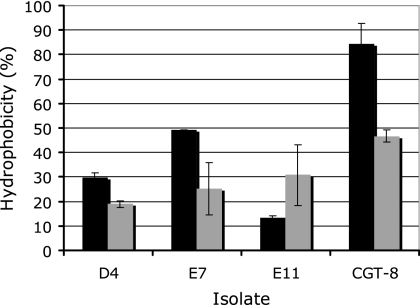
Purified spores of the different Geobacilllus isolates displayed a range of hydrophobicities when partitioned with hexadecane, with D4 being the most hydrophilic (10%) and CGT-8 the most hydrophobic (48%) (Fig. 4). Spores of isolates D4, E7, and CGT-8 were more hydrophobic when suspended at pH 3 than when suspended at pH 7, while E11 was more hydrophilic at the lower pH.
FIG. 4.
Relative hydrophobicities of spores from Geobacillus sp. isolates at pH 3 (black bars) and 6.8 (gray bars) in 0.1 M KCl, as determined by the MATH assay. Error bars represent the means of five replicates and the standard deviations of the means.
Contact angles (in degrees) and surface tension components (mJ m−2) for purified spores and the substratum surfaces were calculated (Table 1). Spores from isolate D4 had the lowest water contact angles (10°) and E11 spores the highest (25°). Spores from the different isolates had similar γTOT values, ranging from 49.4 to 58.3 mJ m−2. Purification of the spores using the two-phase system reduced the apolar (γLW) component while increasing the total polar (γAB) component. The most prominent feature for all spore isolates was the electron-donating (γ−) component. All spore isolates had similar ΔGsls values, and all were >0, indicating that they were all hydrophilic. In contrast to the results shown for the MATH assay, CGT-8 appeared to be the most hydrophilic, with a ΔGsls of 31.4 mJ m−2 and E7 the most hydrophobic at 20.1 mJ m−2.
TABLE 1.
Mean contact angle measurements, surface Gibbs energies, and energy of interaction for crude and purified suspensions of spores from Geobacillus sp. isolates and inert surfaces
| Surface | Contact angle (°) ± SDa (n = 10)
|
Surface tension component (mJ m−2)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| θW | θF | θα-B | γSTOT | γSLW | γSAB | γS+ | γS− | ΔGsls | |
| Crude D4 | 13 ± 2 | 14 ± 2 | 40 ± 4 | 56.4 | 34.6 | 21.8 | 2.2 | 53.1 | 28.8 |
| Pure D4 | 10 ± 2 | 11 ± 2 | 48 ± 4 | 58.3 | 30.7 | 27.5 | 3.6 | 53.4 | 27.0 |
| Crude E7 | 16 ± 1 | 19 ± 1 | 49 ± 2 | 56.0 | 30.6 | 25.3 | 3.0 | 53.6 | 28.6 |
| Pure E7 | 18 ± 1 | 20 ± 3 | 68 ± 5 | 49.4 | 21.0 | 38.4 | 7.2 | 51.4 | 20.1 |
| Crude E11 | 21 ± 2 | 19 ± 1 | 39 ± 2 | 54.6 | 35.1 | 19.6 | 1.9 | 50.0 | 26.4 |
| Pure E11 | 25 ± 3 | 25 ± 2 | 44 ± 1 | 52.5 | 32.1 | 19.7 | 2.0 | 49.8 | 27.3 |
| Crude CGT-8 | 21 ± 3 | 23 ± 3 | 46 ± 4 | 53.6 | 31.8 | 21.8 | 2.3 | 52.4 | 29.1 |
| Pure CGT-8 | 20 ± 3 | 24 ± 4 | 41 ± 7 | 52.8 | 34.1 | 18.7 | 1.6 | 53.5 | 31.4 |
| Stainless steel | 54 ± 10 | 24 ± 7 | 15 ± 3 | 53.8 | 42.9 | 10.9 | 1.8 | 16.5 | −21.7 |
| Thermanox | 63 ± 5 | 17 ± 5 | 8 ± 1 | 53.2 | 43.9 | 9.3 | 3.4 | 6.3 | −40.2 |
| Glass | 1 ± 0 | 7 ± 3 | 40 ± 2 | 58.2 | 34.8 | 23.4 | 2.5 | 54.4 | 29.2 |
θF contact angle of formamide; θα-B, contact angle of α-bromonaphthalene.
The glass surface was extremely hydrophilic, with a barely measurable water contact angle of 1° and a ΔGsls of 29.2 mJ m−2 (ΔGsls > 0) (Table 1). Thermanox and stainless steel surfaces had larger water contact angles of 63° ± 5° and 54° ± 10°, respectively, and α-bromonapthalene had contact angles of 8° ± 1° and 15° ± 3°, respectively. This resulted in greater γLW values for Thermanox and stainless steel of 43.9 and 42.9 mJ m−2, respectively, than for glass (34.8 mJ m−2). This translated into Thermanox and stainless steel being hydrophobic, with ΔGsls values <0: −40.2 and −21.7 mJ m−2, respectively. A larger difference was seen for the γ− component, with glass having the highest value of 54.4 mJ m−2 and Thermanox and stainless steel having lower values of 6.3 and 16.5 mJ m−2, respectively.
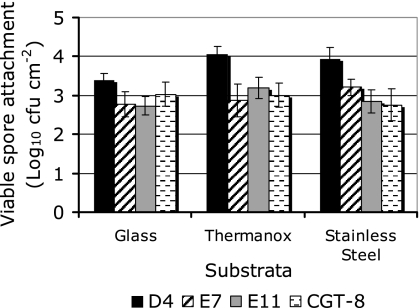
The numbers of spores from the four isolates which attached to either stainless steel, Thermanox, or glass were determined using a simple flow reactor (Fig. 5). Generally, more spores were recovered from Thermanox and stainless steel than from glass (fivefold increase for D4, twofold increase for other spore isolates). Spore attachment to substrata which had a low γTOT, such as stainless steel and Thermanox, was greater than attachment to substrata with a high γTOT. According to the Tukey grouping, the substrates were separated on the basis of the numbers of each spore isolate adhering to the different substrates. Glass and Thermanox were significantly different (P < 0.05). The test was unable to differentiate stainless steel from the other materials. There was no difference in the number of spores adhering to coupons based on their order in the series.
FIG. 5.
Numbers of Geobacillus sp. isolates D4, E7, E11, and CGT-8 recovered from different substrata (glass, Thermanox, and stainless steel). Values represent the means for three coupons ± standard deviations.
DISCUSSION
The first objective of this study was to produce large numbers of spores from thermophilic dairy isolates. It was found that the base medium had the most prominent effect on spore production, which was similar to that seen in a previous study (48). Sporulation of D4 was higher in tryptone than in TSB. However, the opposite was seen for the sporulation of CGT-8, where TSB was optimal for spore production. It has been reported that the presence of calcium, manganese, and iron is crucial for sporulation and can promote higher yields (45). Manganese is required for enzymes involved in sporulation (35), while calcium chelates with dipicolinic acid and is important for heat resistance (2). Interestingly, media supplemented with these ions did not dramatically affect the sporulation of isolates CGT-8 and D4. This could be due to the fact that these ions are already present in sufficient concentrations in the unsupplemented media. For example, tryptone contains (per liter) 0.13 g Ca, 0.17 g Mg, 0.01 g Mn, and 0.01 g Fe. Spore numbers were also influenced by the incubation time of the sporulating culture. Gonzaléz-Pastor et al. found that B. subtilis cells can commit “cannibalism” prior to sporulation to ensure survival (17). Cells that have entered the sporulation pathway produce a signal which causes sister cells to lyse. These lysed cells act as nutrients for other cells, enabling them to grow instead of completing sporulation. Previous studies have used prolonged incubation times (4 to 5 days) to enhance sporulation in either a liquid culture (24) or on agar plates (25). However, in this study, spore numbers decreased at incubation times longer than 24 h, as presumably spores germinated. This is similar to what has been observed during an 18-h milk powder production run, where spores were detected after only 9 h (43). Sporulation on agar plates proved to be laborious and resulted in large amounts of debris being associated with the spores. Growing the cells in liquid batch sporulation media overnight was found to be a superior method due to its short incubation time, ease of use, and ability to produce large numbers of free endospores from Geobacillus spp. isolated from dairy plants.
Obtaining spores free from any cells or debris was crucial for the accurate determination of spore surface characteristics. While previous studies had used heat or enzymes to remove vegetative cells, it was desirable to avoid their approaches as such harsh treatments have been reported to alter the hydrophobicity of bacillus spores (10, 58). Previous studies have separated spores from cells and debris based on the density (33) difference between a spore (1.3 g ml−1) and a vegetative cell (1.1 g ml−1). In this project spore purification was initially attempted using simple density gradients made of solutions of sodium bromide (27, 33), urografin (47), or sucrose (58). However, these simple density gradients proved unable to reproducibly separate debris from lysed cells and to differentiate spores from partially sporulated cells. Using a two-phase separation technique Sacks and Alderton (42) found that spores of different mesophilic bacilli, such as B. cereus, accumulated in the PEG-rich phase while vegetative cells accumulated in the lower phosphate-rich phase. Interestingly, when this method was applied in the current study, Geobacillus sp. spores accumulated at the interface above the PEG-rich region while debris accumulated at the interface between the PEG- and phosphate-rich phases. This difference could be due to inherent structural differences between the surfaces of the spores of Geobacillus spp. and B. cereus. A potential drawback of the PEG separation method was that any residual PEG polymers remaining on the surfaces of the spores would alter their surface properties. To help reduce this possibility, the PEG was removed by washing the spores three times in water at room temperature with resting periods in between each centrifugation step to allow the polymers to be released from the surface. The contact water angle measurements (θW) of the spore lawns decreased after purification, suggesting that the two-phase method removed much of the hydrophobic material such as membranes and denatured protein from the crude spore extract.
Spores were negatively charged at neutral pH and at the pH of milk (pH 6.8). It has been shown previously that as the negative charge on the spore surface decreased the ability of spores to attach to glass increased due to reduced electrostatic repulsion (23). While the zeta potential values of the spores used in this study were similar to those found in other studies (22, 36), there was no clear relationship between surface charge and number of spores attaching to substrata. However, spores of isolate D4, which had the smallest negative charge, had the greatest propensity to attach to all the surfaces tested.
The MATH assay and water contact angle results indicated that the spores of the four Geobacillus sp. isolates were all hydrophilic, with isolate D4 being the most hydrophilic and having the lowest affinity for hexadecane and the smallest θW and spores of CGT-8 and E11 having the greatest affinity for hexadecane and the greatest θW. However, when the contact angles of the other solvents were taken into account and the ΔGsls was calculated, there was no clear relationship between the MATH assay and ΔGsls. From the ΔGsls results purified spores of CGT-8 were the most hydrophilic and E7 spores were the most hydrophobic. Other studies have also found spores of G. stearothermophilus to be relatively hydrophilic through results obtained from hydrophobic interaction chromatography (HIC) (38) and MATH assays (36).
Determination of microbial surface hydrophobicity has been a focus of laboratories around the world for many years (11, 38, 51). However, a common concern is that it is difficult to directly compare results obtained from different laboratories using different or even similar tests (8). Surfaces of spores are heterogenous and contain a combination of functional groups such as carboxylate, amine, and phosphate groups, which may account for some of the variation between the different tests. Electrostatic interactions can also affect results from the MATH and HIC assays but not contact angle (1). Previous studies have found that negative ions, particularly phosphate, adsorb to the surfaces of hydrocarbon droplets, creating a negatively charged surface (5). In the current study, when the pH of the medium was reduced from 7 to 3, spore removal from the aqueous phase increased for isolates D4, E7, and CGT-8 but decreased for E11. The increase in hydrophobicity is believed to be due to the reduction in electrostatic repulsion since both spores and hexadecane are uncharged. These results reinforce the concept that the MATH assay is not solely a hydrophobicity assay but also measures the interplay of long-range van der Waals and electrostatic forces and various short-range interactions (50). Interestingly, these data also suggest that the relative change in the hydrophobicity of spores can differ as the conformation of the outer layers changes in response to external pH. We emphasize, however, that the MATH assay remains extremely useful as a simple assay for studying adhesion of microorganisms to a hydrophobic surface, and it is clearly different from being a hydrophobicity assay. It has been suggested that contact angles should be used as the universal measurement of hydrophobicity since they provide results in standard units rather than the relative results obtained from the MATH assay or HIC (49). However, contact angles are macroscopic measurement of the surface hydrophobicity of cells aggregated together in a lawn rather than of suspended single particles, as measured in the MATH assay. As all methods for measuring hydrophobicity have their advantages and disadvantages, it is now recognized that more than one method of measuring hydrophobicity should be used to obtain results. In this study, the results from both the MATH assay and contact angle measurements show that spores from the different Geobacillus spp. were predominately hydrophilic.
Factors such as hydrophobicity, surface charge, and cell density influence the attachment of microorganisms to surfaces (11, 23, 38). Previous research has shown that hydrophobic spores with zeta potentials close to zero attached to all surfaces in greater numbers than hydrophilic spores. In contrast, our research shows that the most hydrophilic spore (D4) adhered in the highest numbers to all the materials tested. Spores from this isolate also had the smallest zeta potential, thereby reducing the effect of electrostatic repulsion. One aspect that was not measured in the current study was the streaming zeta potential of the substrata. Previous studies have reported the streaming zeta potential for microscope slide glass to be −37 mV at 1 mM ionic strength, pH 7, and that for type 304 2B stainless steel to be −40 mV at 10 mM ionic strength, pH 7 (4, 29). Thermanox is made of a proprietary polyester, and, to the authors' knowledge, there has been no reports on its surface charge. However, streaming potential results have shown that certain polymer plastics exhibit a negative charge (56). Since the zeta potentials of glass and stainless steel are nearly the same, the Derjaguin, Landau, Verwey, and Overbeek theory predicts that the spores will attach in higher numbers to stainless steel since it has a lower Gibbs energy. This effect was seen for spores of isolate D4 but not for the other three isolates. Previous studies looked at a range of species of bacillus spores and, as mentioned earlier, the morphologies of those spores were dramatically different. Spores of B. cereus with pili had a greater propensity to attach to surfaces than those without pili (23, 26).
In the current study we were able to produce and characterize spores from thermophilic Geobacillus spp. isolated from a milk powder production line. Spores of the different isolates were hydrophilic, with a negative zeta potential. Similar numbers of spores were found attached to the three substrata tested for three of the four isolates. However, spores of isolate D4 attached in higher numbers to stainless steel and Thermanox than to glass. Therefore, it may be possible to reduce the adhesion of this particular strain by increasing both the γTOT and negative charge of the stainless steel surface. However, as spores produced by the other three strains tested did not show significant differences in attachment to the different substrates, these data also reinforce the fact that there is no simple relationship between individual physicochemical interactions and spore adhesion to surfaces. Rather, adhesion is dependent on complex and poorly defined relationships between a range of forces acting on both the spore and the substrate. The industrial significance of this is that surface treatments which limit the attachment of one strain may not be effective for all stains. Therefore, control regimens need to be devised with reference to the characteristics of the particular strains entering via the raw milk to the processing facility.
Acknowledgments
This work was financially supported by the Technology for Industry Fellowships and Fonterra.
We thank Brian Niven (Department of Mathematics and Statistics, University of Otago, Dunedin, New Zealand) for statistical analysis of the data.
Footnotes
Published ahead of print on 14 December 2007.
REFERENCES
- 1.Ahimou, F., M. Paquot, P. Jacques, P. Thonart, and P. G. Rouxhet. 2001. Influence of electrical properties on the evaluation of the surface hydrophobicity of Bacillus subtilis. J. Microbiol. Methods 45:119-126. [DOI] [PubMed] [Google Scholar]
- 2.Bender, G. R., and R. E. Marquis. 1985. Spore heat resistance and specific mineralization. Appl. Environ. Microbiol. 50:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, R., H. C. van der Mei, and H. J. Busscher. 1999. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol. Rev. 23:179-230. [DOI] [PubMed] [Google Scholar]
- 4.Boulange-Petermann, L., A. Doren, B. Baroux, and M. N. Bellon-Fontaine. 1995. Zeta potential measurements on passive metals. J. Colloid Interface Sci. 171:179-186. [Google Scholar]
- 5.Busscher, H. J., B. van de Belt-Gritter, and H. C. van der Mei. 1995. Implications of microbial adhesion to hydrocarbons for evaluating cell surface hydrophobicity. 1. Zeta potentials of hydrocarbon droplets. Colloids Surfaces B Biointerfaces 5:111-116. [Google Scholar]
- 6.Busscher, H. J., A. H. Weerkamp, H. C. van der Mei, A. W. J. van Pelt, H. P. de Jong, and J. Arends. 1984. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl. Environ. Microbiol. 48:980-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chada, V. G., E. A. Sanstad, R. Wang, and A. Driks. 2003. Morphogenesis of Bacillus spore surfaces. J. Bacteriol. 185:6255-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon, J. K., J. A. Fuerst, A. C. Hayward, and G. H. G. Davis. 1986. A comparison of five methods for assaying bacterial hydrophobicity. J. Microbiol. Methods 6:13-19. [Google Scholar]
- 9.Donnellan, J. E., Jr., E. H. Nags, and H. S. Levinson. 1964. Chemically defined, synthetic media for sporulation and for germination and growth of Bacillus subtilis. J. Bacteriol. 87:332-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle, R. J., F. Nedjat-Haiem, and J. S. Singh. 1984. Hydrophobic characteristics of Bacillus spores. Curr. Microbiol. 10:329-332. [Google Scholar]
- 11.Faille, C., C. Jullien, F. Fontaine, M. N. Bellon-Fontaine, C. Slomianny, and T. Benezech. 2002. Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity. Can. J. Microbiol. 48:728-738. [DOI] [PubMed] [Google Scholar]
- 12.Faille, C., V. Lebret, F. Gavini, and J. F. Maingonnat. 1997. Injury and lethality of heat treatment of Bacillus cereus spores suspended in buffer and in poultry meat. J. Food Prot. 60:544-547. [DOI] [PubMed] [Google Scholar]
- 13.Flint, S., J. Palmer, K. Bloemen, J. Brooks, and R. Crawford. 2001. The growth of Bacillus stearothermophilus on stainless steel. J. Appl. Microbiol. 90:151-157. [DOI] [PubMed] [Google Scholar]
- 14.Flint, S., L. J. H. Ward, and K. Walker. 2001. Functional grouping of thermophilic Bacillus strains using amplification profiles of the 16S-23S internal spacer region. Syst. Appl. Microbiol. 24:539-548. [DOI] [PubMed] [Google Scholar]
- 15.Foster, J. W., and F. Heiligman. 1949. Biochemical factors influencing sporulation in a strain of Bacillus cereus. J. Bacteriol. 57:639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhardt, P., and E. Ribi. 1964. Ultrastructure of the exosporium enveloping spores of Bacillus cereus. J. Bacteriol. 88:1774-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Pastor, J. E., E. C. Hobbs, and R. Losick. 2003. Cannibalism by sporulating bacteria. Science 301:510-513. [DOI] [PubMed] [Google Scholar]
- 18.Henriques, A. O., and C. P. Moran. 2000. Structure and assembly of the bacterial endospore coat. Methods 20:95-110. [DOI] [PubMed] [Google Scholar]
- 19.Herigstad, B., M. Hamilton, and J. Heersink. 2001. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 44:121-129. [DOI] [PubMed] [Google Scholar]
- 20.Hood, S. K., and E. A. Zottola. 1997. Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Int. J. Food Microbiol. 37:145-153. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, R. J. 1981. Zeta potential in colloid science. Academic Press, New York, NY.
- 22.Husmark, U., and U. Ronner. 1990. Forces involved in adhesion of Bacillus cereus spores to solid surfaces under different environmental conditions. J. Appl. Bacteriol. 69:557-562. [DOI] [PubMed] [Google Scholar]
- 23.Husmark, U., and U. Ronner. 1992. The influence of hydrophobic, electrostatic and morphological properties on the adhesion of Bacillus spores. Biofouling 5:335-344. [Google Scholar]
- 24.Kaul, A., and R. S. Singh. 1982. Production of stable Bacillus stearothermophilus spores. J. Food Prot. 45:795-796. [DOI] [PubMed] [Google Scholar]
- 25.Kim, J., and H. B. Naylor. 1966. Spore production by Bacillus stearothermophilus. J. Appl. Microbiol. 14:690-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klavenes, A., T. Stalheim, O. Sjøvold, K. Josefsen, and P. E. Granum. 2002. Attachment of Bacillus cereus spores with and without appendages to stainless steel surfaces. Trans. IChemE 80:312-318. [Google Scholar]
- 27.Laflamme, C., S. Lavigne, J. Ho, and C. Duchaine. 2004. Assessment of bacterial endospore viability with fluorescent dyes. J. Appl. Microbiol. 96:684-692. [DOI] [PubMed] [Google Scholar]
- 28.Leuschner, R. G., and P. J. Lillford. 2001. Investigation of bacterial spore structure by high resolution solid-state nuclear magnetic resonance spectroscopy and transmission electron microscopy. Int. J. Food Microbiol. 63:35-50. [DOI] [PubMed] [Google Scholar]
- 29.Li, B., and B. E. Logan. 2004. Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surfaces B Biointerfaces 36:81-90. [DOI] [PubMed] [Google Scholar]
- 30.Matz, L. L., T. C. Beaman, and P. Gerhardt. 1970. Chemical composition of exosporium from spores of Bacillus cereus. J. Bacteriol. 101:196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuiggan, J. T. M., D. R. McCleery, A. Hannan, and A. Gilmour. 2002. Aerobic spore-forming bacteria in bulk raw milk: factors influencing the numbers of psychrotrophic, mesophilic and thermophilic Bacillus spores. Int. J. Dairy Technol. 55:100-107. [Google Scholar]
- 32.Murphy, P., D. Lynch, and P. Kelly. 1999. Growth of thermophilic spore forming bacilli in milk during the manufacture of low heat powders. Int. J. Dairy Technol. 52:45-50. [Google Scholar]
- 33.Nicholson, W. L., and J. F. Law. 1999. Method for purification of bacterial endospores from soils: UV resistance of natural Sonoran desert soil populations of Bacillus spp. with reference to B. subtilis strain 168. J. Microbiol. Methods 35:13-21. [DOI] [PubMed] [Google Scholar]
- 34.Nishihara, T., and E. Freese. 1975. Motility of Bacillus subtilis during growth and sporulation. J. Bacteriol. 123:366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh, Y. K., and E. Freese. 1976. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J. Bacteriol. 127:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkar, S. G., S. H. Flint, J. S. Palmer, and J. D. Brooks. 2001. Factors influencing attachment of thermophilic bacilli to stainless steel. J. Appl. Microbiol. 90:901-908. [DOI] [PubMed] [Google Scholar]
- 37.Peng, J. S., W. C. Tsai, and C. C. Chou. 2001. Surface characteristics of Bacillus cereus and its adhesion to stainless steel. Int. J. Food Microbiol. 65:105-111. [DOI] [PubMed] [Google Scholar]
- 38.Ronner, U., U. Husmark, and A. Henriksson. 1990. Adhesion of bacillus spores in relation to hydrophobicity. J. Appl. Bacteriol. 69:550-556. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg, M., D. L. Gutnick, and E. Rosenberg. 1980. Bacterial adherence to hydrocarbons. Microb. Enhanced Oil Recovery 17:114-123. [Google Scholar]
- 40.Rueckert, A., R. S. Ronimus, and H. W. Morgan. 2004. A RAPD-based survey of thermophilic bacilli in milk powders from different countries. Int. J. Food Microbiol. 96:263-272. [DOI] [PubMed] [Google Scholar]
- 41.Ryu, C., K. Lee, C. Yoo, W. K. Seong, and H. Oh. 2003. Sensitive and rapid quantitative detection of anthrax spores isolated from soil samples by real-time PCR. Microbiol. Immunol. 47:693-699. [DOI] [PubMed] [Google Scholar]
- 42.Sacks, L. E., and G. Alderton. 1961. Behavior of bacterial spores in aqueous polymer two-phase systems. J. Bacteriol. 82:331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott, S. A., J. D. Brooks, J. Rakonjac, K. M. R. Walker, and S. H. Flint. 2007. The formation of thermophilic spores during the manufacture of whole milk powder. Int. J. Dairy Technol. 60:109-117. [Google Scholar]
- 44.Setlow, P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol. 15:172-180. [DOI] [PubMed] [Google Scholar]
- 45.Slepecky, R., and J. W. Foster. 1959. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J. Bacteriol. 78:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stalheim, T., and P. E. Granum. 2001. Characterization of spore appendages from Bacillus cereus strains. J. Appl. Microbiol. 91:839-845. [DOI] [PubMed] [Google Scholar]
- 47.Tamir, H., and C. Gilvarg. 1966. Density gradient centrifugation for the separation of sporulating forms of bacteria. J. Biol. Chem. 241:1085-1090. [PubMed] [Google Scholar]
- 48.Thompson, P. J., and O. A. Thames. 1967. Sporulation of Bacillus stearothermophilus. J. Appl. Microbiol. 15:975-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Mei, H. C., R. Bos, and H. J. Busscher. 1998. A reference guide to microbial cell surface hydrophobicity based on contact angles. Colloids Surf B Biointerfaces 11:213-221. [Google Scholar]
- 50.van der Mei, H. C., B. van de Belt-Gritter, and H. J. Busscher. 1995. Implications of microbial adhesion to hydrocarbons for evaluating cell surface hydrophobicity. 2. Adhesion mechanisms. Colloids Surfaces B Biointerfaces 5:117-126. [Google Scholar]
- 51.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 53:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 53:1893-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, and A. J. B. Zehnder. 1989. Bacterial adhesion: a physicochemical approach. Microb. Ecol. 17:1-15. [DOI] [PubMed] [Google Scholar]
- 54.van Oss, C. J. 2007. Development and applications of the interfacial tension between water and organic or biological surfaces. Colloids Surfaces B Biointerfaces 54:2-9. [DOI] [PubMed] [Google Scholar]
- 55.van Oss, C. J., M. K. Chaudury, and R. J. Good. 1988. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 88:927-941. [Google Scholar]
- 56.Van Wagenen, R. A., D. L. Coleman, R. N. King, P. Triolo, L. Brostrom, L. M. Smith, D. E. Gregonis, and J. D. Andrade. 1981. Streaming potential investigations: polymer thin films. J. Colloid Interface Sci. 84:155-162. [Google Scholar]
- 57.Waller, L. N., N. Fox, K. F. Fox, A. Fox, and R. L. Price. 2004. Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spore Bacillus anthracis and Bacillus subtilis. J. Microbiol. Methods 58:23-30. [DOI] [PubMed] [Google Scholar]
- 58.Wiencek, K. M., N. A. Klapes, and P. M. Foegeding. 1990. Hydrophobicity of Bacillus and Clostridium spores. Appl. Environ. Microbiol. 56:2600-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yildiz, F., and D. C. Westhoff. 1989. Sporulation and thermal resistance of Bacillus stearothermophilus spores in milk. Food Microbiol. 6:245-250. [Google Scholar]