With advancements in materials science over the past few decades, there has been a dramatic increase in the use of synthetic polymers by both artists and conservators. Synthetic polymers in items representing our cultural heritage occur either as original constituents of works of art or as materials used for conservation treatment, and these polymers include adhesives, consolidants, and protective coatings. In the 1980s there was a change in the perception of plastics from consumer goods and disposable materials to fashionable, highly collectable pieces with historical and technological significance (27, 34). Now, in their 20th and 21st century collections, most museums and galleries possess objects made from the thousands of different plastics that have been produced. As museums keep acquiring objects that reflect both everyday life and technological and historical events, the proportion of plastics in museums is increasing dramatically. Plastics may be present in objects of everyday life, such as housewares, jewelry, equipment, furniture, information technology, photography, and toys, and more of these objects are entering museum collections and contemporary art (57). In addition, synthetic polymers have been widely employed for treatment of items representing our cultural heritage as adhesives, consolidants, and protective coatings to preserve many artifacts from further deterioration (20, 45).
Synthetic polymer conservation has been formally recognized as a research area only since the 1990s, and it was in this period that the world's most important organization in the field of cultural heritage conservation, the Committee for Conservation of the International Council of Museums, established the Modern Materials and Contemporary Art Working Group. Indeed, owners and curators have begun to notice that objects made of plastics degrade with time, sometimes very rapidly. Importantly, many synthetic polymers appear to deteriorate faster than other materials in museum collections and have a useful lifetime of just decades (57). Synthetic polymeric materials can suffer different forms of deterioration, including chemical (e.g., oxidation), physical (e.g., UV light), and biological. Although many reports in the scientific literature claim that microorganisms are capable of degrading synthetic resins (35, 42, 59, 68), the microbial contamination of synthetic polymers that are used as materials for conservation treatment (29, 32) and in contemporary collections (50) is still underestimated. Indeed, it was only in the 2005-2008 program that the Committee for Conservation of the International Council of Museums Modern Materials and Contemporary Art Working Group embraced “(microbial) biodeterioration” as a research topic (http://icom-cc.icom.museum/Documents/WorkingGroup/ModernMaterials/Modern-materials2005-2008.pdf).
Microorganisms can damage the structure and function of synthetic polymers. According to Flemming (22), the main types of damage include (i) biological coating masking surface properties, (ii) increased leaching of additives and monomers that are used as nutrients, (iii) production of metabolites (e.g., acids), (iv) enzymatic attack, (v) physical penetration and disruption, (vi) water accumulation, and (vii) excretion of pigments. Table 1 describes microorganisms and their modes of action for degrading synthetic resins (polyvinyl chloride [PVC], polyurethane, nylon, and acrylics). Barbie dolls, together with many other toys, clothes, and electrical insulation found in museums, are made from PVC (58). The instability of plasticized PVC is frequently manifested as migration of the plasticizers. Colonization of PVCs by fungi, especially black fungi, due to the availability of platicizers on the surface has been assessed several times (30, 51, 67). Webb et al. (67) identified fungal isolates obtained from PVC by PCR amplification and partial sequencing of the internally transcribed spacer regions and the 5.8S rRNA gene or the V3 domain of the 28S rRNA gene.
TABLE 1.
Microorganisms degrading the synthetic polymers PVC, polyurethane, nylon, and acrylics and their mode of action
| Synthetic polymer(s) | Degrading microorganisms | Main mode of action | Reference(s) |
|---|---|---|---|
| PVC | Fungi (e.g., Aureobasidium pullulans) and bacteria (e.g., Pseudomonas aeruginosa) | Increased loss of plasticizers due to microbial degradation | 67 |
| Polyurethanea | Fungi (e.g., Chaetomium globosum) and bacteria (e.g., Bacillus subtilis) | Enzymatic activity | 33,36 |
| Nylon | Wood-degrading fungi (e.g., Bjerkandera adusta) and bacteria (e.g., Bacillus pallidus) | Physical disruption and enzymatic degradation | 24,63 |
| Acrylics | Melanin-producing fungi | Likely physical disruption | 8,37,60 |
Polyester-type polyurethanes are more susceptible to microbial attack than other forms.
It has been suggested that biodeterioration of polyurethane polymers, which are products of a polyol based on either a polyester or polyether and a di- or polyisocyanate, occurs through enzymatic action of hydrolases, such as ureases, proteases, and esterases (18, 21, 52). Degradation of polyurethanes by microorganisms in 20th century museum textiles has been reported by many researchers (36, 63). Polyurethanes can also be found in products such as furniture, adhesives, paints, elastomers, coatings, and contemporary art (33, 34, 52). Biodeterioration due to enzymes, presumably including a manganese peroxidase of the basidiomycete Bjerkandera adusta, was also observed for the aliphatic polyamide Nylon-6 fiber (24). Damage to the polymer was assessed by microscopic examination, differential scanning calorimetry, and evaluation of changes in viscosity.
One of the reasons for introducing synthetic consolidants and protective compounds in conservation treatments was the expectation that these materials would be more resistant to microbial attack than natural organic products. In 1968 the superintendents at Ostia Antica (Rome, Italy) decided to replace natural organic compounds, which are easily degraded by microorganisms, with acrylic compounds in conservation treatments. Frescoes detached with Paraloid did not show any biodeterioration problem for the first 3 years after application (4). However, as early as the 1950s, some experiments on biodeterioration of polyvinyl acetate resins were reported by the Istituto Centrale del Restauro in Rome, Italy (26). Generally, filamentous fungi were the agents causing deterioration of these materials that were studied the most, especially in early experiments (17, 41, 54, 61), although some bacteria, yeasts, algae, and lichens that are capable of growing on synthetic polymers have been found or isolated (14). Historically, identification of filamentous fungal species has been based on morphological characteristics, both macroscopic and microscopic. These methods may often be time-consuming and inaccurate, which has required the development of identification protocols that are rapid, sensitive, and precise. In the last decade molecular approaches for rapid characterization of fungi on painted items representing our cultural heritage and paint coatings have been developed (46, 53). A protocol for efficient extraction of fungal DNA from micromycetes colonizing painted art objects was developed by Möhlenhoff et al. (46), who claimed to have successfully removed any inhibitors. In particular, melanin can also be present, which is highly resistant to UV light, enzymatic digestion, and chemical breakdown and might be a potent inhibitor of DNA amplification (46). PCR amplification of the 28S rRNA gene and denaturing gradient gel electrophoresis analysis were used to characterize fungal communities. According to Saad et al. (53), fungi are commonly found on paint films as spores other than mycelium; hence, it is necessary to ensure that DNA extraction is effective also for propagules. The method used involves spore lysis by incubation of a specimen with the enzyme Lyticase, followed by bead beating. DNA is then purified from the lysate with a QIAamp DNA mini kit (53).
There have also been case studies related to biodeterioration agents other than filamentous fungi. Bacterial biofilms composed of Pseudomonas aeruginosa, Ochrobactrum anthropi, Alcaligenes denitrificans, Xanthomonas maltophila, and Vibrio harveyi formed readily on the surfaces of synthetic materials being considered for use in space applications (28). A yeast isolated from a bronze statue treated with the acrylic-based coating Incralac was found to accelerate the deterioration of the coating itself, as determined by scanning electron microscopy and electrochemical impedance spectroscopy (44). Stones impregnated with Ahydrosil Z, a silicone resin, were recolonized by algae and fungi more quickly than untreated specimens (40). Rapid recolonization by the alga Stichococcus bacillaris was also noticed in the Roman archaeological site at Luni in northern Italy after treatment with an epoxy resin and an acrylic-siliconic resin (19). Finally, lichens were reported to deteriorate a synthetic polyester resin that was used as a consolidant of stucco walls and column capitals in the Roman city Baelo Claudia in Spain (2).
The ecological succession of fungi over 10 months on two Brazilian buildings painted with a white acrylic paint was described by Shirakawa et al. (60). Prior to painting, the walls were treated with hypochlorite. In addition to Cladosporium, the main fungal genus identified during the experiment, the other fungal genera detected were Alternaria, Curvularia, Epicoccum, Helminthosporium, Coelomycetes, Monascus, Nigrospora, and Aureobasidium. The yeast population fell to undetectable levels after the third week, and this microbial group was not detected again until 7 months, after which the number of cells increased.
BIODETERIORATION OF SYNTHETIC RESINS AS MATERIAL FOR CONSERVATION TREATMENTS
When some acrylics, polyvinyl acetates, and alkyds were synthesized in the laboratory, it appeared that the general order of polymer susceptibility to fungal attack evaluated using the ASTM G21-96(2002) “Standard Practice for Determining Resistance of Synthetic Polymeric Materials to Fungi” was acrylics < polyvinyl acetates < alkyds (Fig. 1) (13). However, the possibility that in each class there might be exceptions should be taken into account. It was observed that acrylics with long side chains (namely, polystearylmethacrylate and polylaurylmethacrylate) were more susceptible to biodeterioration than homologous polymers with short side chains (8, 14), perhaps as a consequence of the minor enzymatic steric impediment of compounds with long lateral chains.
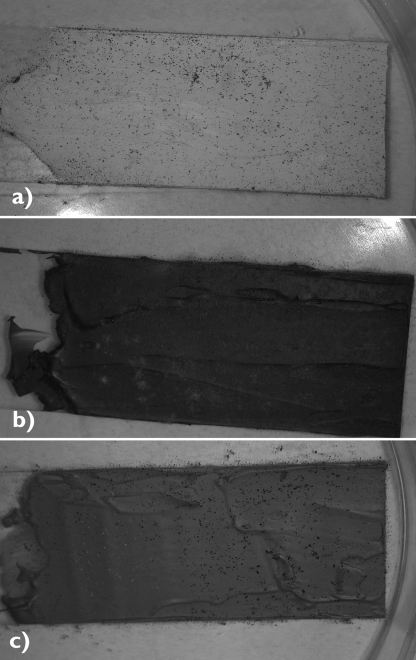
FIG. 1.
Griffin Alkyd paints (based on alkyd resins) of Winsor & Newton dried on microscope glass slides and subjected to biodeterioration treatment according to ASTM standard practice G21-96(2002). At the end of the experimental period (28 days), fungal growth was easily observed. (a) Titanium white; (b) viridian; (c) cerulean blue.
Many freshly dried synthetic polymer-based products used for conservation treatments have been tested to ascertain their resistance to microbial attack (Fig. 2) (38, 48, 54). It appears that the behavior of the product was not uniform even if the polymer class was the same. This result might be explained if one considers the possibility that other compounds might be present in the formulation (particularly in emulsion products) and the possibility that biocides might also be added. This indeed might be the case for the acrylic product Paraloid B72 supplied by Rohm & Haas, which is the synthetic polymer-based product that is used most in conservation treatments. Kigawa et al. (37) reported that fungi isolated from inside or around the seventh and eighth century Takamatsuzuka and Kitora tumuli in Japan were able to grow on samples of Paraloid B72.
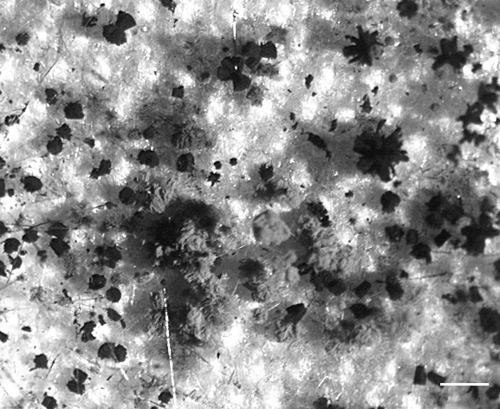
FIG. 2.
Fungal growth on a dried polyvinyl acetate-based emulsion, viewed with a stereomicroscope. Bar = 1,000 μm.
Studies of biodeterioration of freshly dried synthetic polymers might not be sufficient to predict the real behavior of the resins when they are applied. It has been demonstrated recently that the way that a product is applied (10) and its natural ageing (9) are as important as the chemical composition of the polymer itself. Textiles consolidated by coating them with Paraloid B72 and ethyl acrylate/methyl methacrylate preformed copolymer subjected to fungal deterioration treatment according to ASTM G21-96(2002) biodeteriorated in the same manner. In contrast, the same textiles consolidated by grafting ethyl acrylate and methyl methacrylate monomers onto cellulose chains were much less susceptible to biodeterioration (10). This result was very important as it established for the first time that a synthetic resin, the ethyl acrylate/methyl methacrylate copolymer, was susceptible to biodegradation in different ways depending on the way that the polymer was applied to the substratum.
Little is known about the susceptibility to biological degradation of naturally aged acrylic resins. The first report involved the façade of Tempio Malatestiano (Rimini, Italy) treated with acrylic resins that had black fungal growth in cracks and fissures (49). Another report discussed the current heavy colonization by black fungi of polyisobutylmethacrylate used in the 1970s during conservation of Milan Cathedral. Black fungi were identified using denaturing gradient gel electrophoresis coupled with partial 18S rRNA gene sequencing. In addition, the fungal presence and spatial distribution were evaluated using immunofluorescence staining with melanin-binding antibodies. In contrast, freshly dried polyisobutylmethacrylate subjected to biodeterioration treatment showed only light fungal growth (9). The chemical and physical degradation of the polymer was in turn likely to have facilitated biological degradation (10).
BIODETERIORATION OF OBJECTS MADE OF PLASTICS
Since the rise of plastics in the 1950s and 1960s, plastics have been an increasingly significant part of our cultural heritage. Plastics have allowed novel ways of recording information; audiotapes, computer diskettes, and compact discs are now commonly stored in archives and libraries. Also, photographic materials, binders, and supports can be made of plastics (12). Plastic audiovisual material, including compact discs, can be subject to biodeterioration (25). Initial fungal colonization of plastics in audiovisual materials generally means failure because of interruption of the signal, whereas further colonization could lead to degradation of the plastics themselves (7, 43). Biodeterioration of the plastic in information carriers is very important, as it constitutes a twofold loss. Both the information contained and the object itself may perish.
The Space History Division of the National Air and Space Museum, Smithsonian Institution, manages the most comprehensive collection of artifacts documenting the history of rockets, missiles, and space exploration in the world. This collection has many examples of spacesuits, such as those from the Apollo lunar missions, and other astronaut equipment. The historic material and construction of space suits from the Apollo era are unique. Fungi belonging to the genera Paecilomyces and Cladosporium have been cultured from two synthetic polymers in the Apollo suits. Fungal growth occurred rapidly, and isolates were capable of degrading the synthetic polymers (6, 28).
Finally, fluorescent in situ hybridization helped detect microorganisms deteriorating the “Futuro” ski cabin designed by the Finnish architect Matti Suuronen, which was constructed in 1965 of glass fiber-reinforced polyester, polyesterpolyurethane, and poly(methylmethacrylate) (11). Microorganisms forming a biofilm on the plastic surface were identified as archaea and cyanobacteria.
MONITORING AND CONTROL OF MICROBIOLOGICAL GROWTH
As previously indicated, modern material conservators are currently focusing their attention on physical and chemical damage rather than biodeterioration, which is considered a minor issue compared to the physical and chemical damage. As a consequence, there are no standard conservation protocols and practices that deal with monitoring and control of microbial growth on synthetic polymeric surfaces.
To avoid biodeterioration, there is a real need for biofilm-monitoring equipment that permits remote, sensitive, nondestructive, real-time analysis of microbial growth on polymeric surfaces. Accurate and sensitive monitoring would facilitate prediction of the time when a system deteriorates to an unacceptable level so that scheduled maintenance can be efficiently implemented. A few efficient online monitoring devices have been used to measure biofilm formation on surfaces, as considerable biofilm development is often required before detection. In the past, both optical microscopy and electron microscopy have been used to monitor biodeterioration of polymeric surfaces (4, 44). Environmental scanning electron microscopy and atomic force microscopy might provide high-resolution information on the outermost features of biofilms maintained under hydrated conditions (5, 16, 31). Spectroscopic techniques are an alternative for monitoring biofilm formation at the molecular level. A technique such as infrared spectroscopy offers the opportunity to monitor real-time chemical changes (55). Importantly, biodeteriorated synthetic resins used in conservation have recently been distinguished from the same nonbiodeteriorated polymers by the presence of a peptidic bond in the infrared spectrum (8, 9, 14).
For decades, abatement of microbial growth has commonly been achieved by incorporating biocides into synthetic polymer-based products or by applying biocides to surfaces. Shirakawa et al. (60), comparing the fungal communities on acrylic paints without and with biocides, including carbamate, N-octyl-2H-isothiazolin-3-one, and N-(3,4-dichlorophenyl)-N,N-dimethyl urea, claimed that biocides did not affect fungal biodiversity but did affect fungal abundance.
Several conservators of modern materials think that the majority of synthetic polymers are resistant to biodeterioration both because of their chemical nature and because these materials more frequently include biocides in their formulations, whose effects can last for a long time (56). Until the 1990s, there was a trend toward increasingly use of biocides in materials such as plastics and water-based paints and coatings to prevent microbial deterioration and provide hygienic effects. However, not all synthetic polymer-based products include biocides, as some biocides have adverse effects on the formulations (33). Even if formulations include biocides, the biocides have relatively short-term effects for conservation of items representing our cultural heritage; that is, addition of biocides to formulations has rarely been intended for storing objects in museums or keeping objects outdoors for decades. Finally and most importantly, in recent years impending environmental regulations both in European countries and elsewhere have severely restricted the use of biocides. The concern about the use of biocides is that eventually they are released into the environment and because they are generally not specifically targeted against biodeteriorating microorganisms, they are potentially dangerous for human health and the environment. In particular, environmental concerns have led to progressive withdrawal of antifouling paints containing organotin derivatives (65).
It is worth noting that the use of biocides might not always delay the polymer deterioration process. Abdel-Kareem (1) claimed that of the fungicides tested on linen textiles impregnated with synthetic polymers, only dichlorophene could prevent biodeterioration; however, at the same time, dichlorophene accelerated the deterioration caused by heat and light. In addition, organic biocides might act as nutrients, and therefore they must be used carefully in conservation practice (39, 40, 66). Finally, killed cells might provide nutrients for subsequent colonization, and therefore leaving dead cells on the surface is not advisable (22).
Several environmentally friendly alternatives have been proposed recently (23). One of the biocide-free strategies is transfer of the lotus effect, a self-cleansing property of the surfaces of the leaves of lotus, to artificial surfaces, yielding superhydrophobic surfaces that can be cleaned by simple rainfall (15, 47). Natural antifouling compounds and quorum sensing blockers might be another strategy. Such antifouling compounds have been isolated mainly from marine organisms which are not colonized by microorganisms (64). An interesting antifoulant agent is zosteric acid (p-sulfooxycinnamic acid), a natural extract from the eelgrass Zostera marina that prevents biofouling by some organisms, such as algae, barnacles, and tubeworms, at nontoxic concentrations (3).
Steinberg et al. (62) isolated molecules from an Australian seaweed, Delisea pulchra, that interfered with bacterial signaling and displayed anticolonization activity.
Although biocide-free technology is still in its infancy and therefore not readily available, it could be combined with effective monitoring techniques, and the results obtained so far indicate that this may be a viable alternative for workers trying to prevent biodeterioration of plastic items representing our cultural heritage and synthetic polymer coatings (11).
CONCLUSIONS
At this point, the issues related to the biodeterioration of synthetic polymers in items representing our cultural heritage that have been investigated, as presented in this paper, are far from being comprehensive. We therefore hope that this minireview reveals a somewhat neglected aspect of the deterioration of synthetic polymers and shows that considerable research is required. We also believe that the area most in need of research is preventive conservation of synthetic polymeric surfaces. Until now, the main strategies that have been proposed are (i) monitoring of biofilm formation (passive strategy) and (ii) use of natural nontoxic antifouling compounds to avoid cell adhesion and biofilm formation (active strategy). With respect to the first issue, useful and effective practices would require portable equipment for in situ monitoring. For this, in situ assays, such as ATP measurement, that are in demand in various fields (e.g., food contact surface hygiene monitoring) could be considered. With regard to the latter issue, many of the technologies available for marine antifouling applications are likely to be a good starting point. Finally, further collaboration among microbiologists, materials scientists, and conservators might lead to interesting and surprising approaches for solving problems related to biodeterioration of synthetic polymers in items representing our cultural heritage.
Acknowledgments
This work was funded by Fondo Interno per la Ricerca Scientifica e Tecnologica (FIRST) 2006 of the University of Milan.
We thank ICVBC-CNR Milan for use of their stereomicroscope.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Abdel-Kareem, O. M. A. 2005. The long-term effect of selected conservation materials used in the treatment of museum artifacts on some properties of textiles. Polym. Degrad. Stabil. 87:121-130. [Google Scholar]
- 2.Ariño, X., and C. Saiz-Jimenez. 1996. Lichen deterioration of consolidants used in the conservation of stone monuments. Lichenologist 28:391-394. [Google Scholar]
- 3.Barrios, C. A., Q. Xu, T. Cutright, and B. Z. Newby. 2005. Incorporating zosteric acid into silicone coatings to achieve its slow release while reducing fresh water bacterial attachment. Colloids Surf. B 41:83-93. [DOI] [PubMed] [Google Scholar]
- 4.Bassi, M., and C. Giacobini. 2001. Scanning electron microscopy: a new technique in the study of the microbiology of works of art. Int. Biodeterior. Biodegrad. 41:85-92. [Google Scholar]
- 5.Bremer, P. J., G. G. Geese, and B. Drake. 1992. Atomic force microscopy examination of the topography of a hydrated bacterial biofilm on a copper surface. Curr. Microbiol. 24:223-230. [Google Scholar]
- 6.Breuker, M., C. McNamara, L. Young, T. Perry, A. Young, and R. Mitchell. 2003. Fungal growth on synthetic cloth from Apollo spacesuits. Ann. Microbiol. 53:47-54. [Google Scholar]
- 7.Brothers, P. 1997. Moldy audiotape. Abbey Newsl. 21:106. [Google Scholar]
- 8.Cappitelli, F., P. Principi, R. Pedrazzani, L. Toniolo, and C. Sorlini. 2007. Bacterial and fungal deterioration of the Milan Cathedral marble treated with protective synthetic resins. Sci. Total Environ. 385:172-181. [DOI] [PubMed] [Google Scholar]
- 9.Cappitelli, F., J. D. Nosanchuk, A. Casadevall, L. Toniolo, L. Brusetti, S. Florio, P. Principi, S. Borin, and C. Sorlini. 2007. Synthetic consolidants attacked by melanin-producing fungi: case study of the biodeterioration of Milan Cathedral (Italy) marble treated with acrylics. Appl. Environ. Microbiol. 73:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappitelli, F., C. Sorlini, E. Pedemonte, E. Princi, and S. Vicini. 2006. Effectiveness of graft synthetic polymers in preventing biodeterioration of cellulose-based materials. Macromol. Symp. 238:84-91. [Google Scholar]
- 11.Cappitelli, F., P. Principi, and C. Sorlini. 2006. Biodeterioration of modern materials in contemporary collections: can biotechnology help? Trends Biotechnol. 24(8):350-354. [DOI] [PubMed] [Google Scholar]
- 12.Cappitelli, F., and C. Sorlini. 2005. From papyrus to compact disc: the microbial deterioration of documentary heritage. Crit. Rev. Microbiol. 31:1-10. [DOI] [PubMed] [Google Scholar]
- 13.Cappitelli, F., S. Vicini, P. Piaggio, P. Abbruscato, E. Princi, A. Casadevall, J. D. Nosanchuk, and E. Zanardini. 2005. Investigation of fungal deterioration of synthetic paint binders using vibrational spectroscopic techniques. Macromol. Biosci. 5:49-57. [DOI] [PubMed] [Google Scholar]
- 14.Cappitelli, F., E. Zanardini, and C. Sorlini. 2004. The biodeterioration of synthetic resins used in conservation. Macromol. Biosci. 4:399-406. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, Y.-T., and D. E. Rodak. 2005. Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 86:144101. [Google Scholar]
- 16.Collins, S. P., R. K. Pope, R. W. Scheetz, R. I. Ray, P. A. Wagner, and B. J. Little. 1993. Advantages of environmental scanning electron microscopy in studies of microorganisms. Microsc. Res. Tech. 25:398-405. [DOI] [PubMed] [Google Scholar]
- 17.Domaslowski, W., and A. Strzelczyk. 1986. Evaluation of applicability of epoxy resins to conservation of stone historic monuments, p. 126-132. In N. S. Brommelle and P. Smith (ed.), Preprints of the contributions to the Bologna Congress, Bologna, 21-26 September 1986. Case studies in the conservation of stone and wall paintings. IIC, London, United Kingdom.
- 18.Evans, D. M., and I. Levisohn. 1968. Biodeterioration of polyester-based polyurethane. Int. Biodeterior. Bull. 4:89-92. [Google Scholar]
- 19.Favali, M. A., N. Barbieri, and M. Bassi. 1978. A green alga growing on a plastic film used to protect archaeological remains. Int. Biodeterior. Bull. 14:89-93. [Google Scholar]
- 20.Favaro, M., R. Mendichi, F. Ossola, U. Russo, S. Simon, P. Tomasin, and P. A. Vigato. 2006. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part I. Photo-oxidative weathering. Polym. Degrad. Stabil. 91:3083-3096. [Google Scholar]
- 21.Filip, Z. 1978. Decomposition of polyurethane in a garbage landfill leakage water and by soil microorganisms. Eur. J. Appl. Microbiol. Biotechnol. 5:225-231. [Google Scholar]
- 22.Flemming, H.-C. 1998. Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polym. Degrad. Stabil. 59:309-315. [Google Scholar]
- 23.Flemming, H.-C., T. Griebe, and G. Schaule. 1996. Antifouling strategies in technical systems—a short review. Water Sci. Technol. 34:517-524. [Google Scholar]
- 24.Friedrich, J., P. Zalar, M. Mohorčič, U. Klun, and A. Krža. 2007. Ability of fungi to degrade synthetic polymer nylon-6 n. Chemosphere 67:2089-2095. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Guinea, J., V. Cardenes, A. T. Martinez, and M. J. Martinez. 2001. Fungal bioturbation paths in a compact disk. Naturwissenschaften 88:351-354. [DOI] [PubMed] [Google Scholar]
- 26.Giacobini, C. 1957. Sostanze plastiche sintetiche quali fonte di carbonio per alcuni microrganismi. Boll. Ist. Cent. Restauro 31-32:108-113.
- 27.Grattan, D. (ed.). 1993. Saving the twentieth century: the conservation of modern materials. Proceedings of a conference held in Ottawa, 15-20 September 1991. Canadian Conservation Institute, Ottawa, Canada.
- 28.Gu, J.-D. 2007. Microbial colonization of polymeric materials for space applications and mechanisms of biodeterioration: a review. Int. Biodeterior. Biodegrad. 59:170-179. [Google Scholar]
- 29.Gu, J.-D. 2003. Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int. Biodeterior. Biodegrad. 52:69-91. [Google Scholar]
- 30.Hamilton, N. F. 1983. Biodeterioration of flexible polyvinyl chloride films by fungal organisms, p. 663-678. In T. A. Oxley and S. Barry (ed.), Biodeterioration 5. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 31.He, F., S. Lia, M. Verta, and R. Zhuo. 2003. Enzyme-catalyzed polymerization and degradation of copolymers prepared from ɛ-caprolactone and poly(ethylene glycol). Polymer 44:5145-5151. [Google Scholar]
- 32.Heyn, C., K. Petersen, and W. E. Krumbein. 1995. Investigations on the microbial degradation of synthetic polymers used in the conservation and restoration of art objects, p. 73-79. In A. Bousher, M. Chandra, and R. Edyvean (ed.), Biodeterioration and biodegradation 9. IChemE, Rugby, United Kingdom.
- 33.Howard, G. T. 2002. Biodegradation of polyurethane: a review. Int. Biodeterior. Biodegrad. 49:245-252. [Google Scholar]
- 34.Hummelen, I., and D. Sille (ed.). 2006. Modern art: who cares? Archetype Publications, London, United Kingdom.
- 35.Jayasekara, R., I. Harding, I. Bowater, and G. Lonergan. 2005. Biodegradability of a selected range of polymers and polymer blends and standard methods for assessment of biodegradation. J. Polym. Environ. 13:231-251. [Google Scholar]
- 36.Kerr, N., and J. Batcheller. 1993. Degradation of polyurethanes in 20th-century museum textiles, p. 189-206. In Saving the twentieth century: the conservation of modern materials. Canadian Conservation Institute, Ottawa, Canada.
- 37.Kigawa, R., N. Hayakawa, N. Yamamoto, W. Kawanobe, C. Sano, and S. Aoki. 2005. Evaluation of mould resistance of various synthetic resins used in conservation of historic sites. Hozon Kagaku 44:149-156. [Google Scholar]
- 38.Koestler, R. J., and E. D. Santoro. 1988. Assessment of the susceptibility to biodeterioration of selected polymers and resins. Final report. Getty Conservation Institute, Los Angeles. CA.
- 39.Krumbein, W. E., and M. Gross. 1992. Interactions of biocides with cultures of biodeteriorating microbiota in agar diffusion and rock tube tests, p. 501-509. In J. D. Rodrigues, F. Henriques, and F. T. Jeremias (ed.), Proceedings of the Fifth International Congress on Deterioration and Conservation of Stone. Laboratorio Nacional de Engenharia Civil, Lisbon, Portugal.
- 40.Leznicka, S. 1992. Antimicrobial protection of stone monuments with p-hydroxybenzoic acid esters and silicone resin, p. 481-490. In J. D. Rodrigues, F. Henriques, and F. T. Jeremias (ed.), Proceedings of the Seventh International Congress on Deterioration and Conservation of Stone. Laboratorio Nacional de Engenharia Civil, Lisbon, Portugal.
- 41.Leznicka, S., J. Kuroczkin, W. E. Krumbein, A. B. Strelczyk, and K. Petersen. 1991. Studies on the growth of selected fungal strains on limestones impregnated with silicone resins (Steinfestiger H and Elastosil E41). Int. Biodeterior. 28:91-111. [Google Scholar]
- 42.Lugauskas, A., L. Levinskaite, and D. Pečiulyte. 2003. Micromycetes as deterioration agents of polymeric materials. Int. Biodeterior. Biodegrad. 52:233-242. [Google Scholar]
- 43.McCain, J. W., and C. J. Mirocha. 1994. Screening computer diskettes and other magnetic media for susceptibility to fungal colonization. Int. Biodeterior. Biodegrad. 33:255-268. [Google Scholar]
- 44.McNamara, C. J., M. Breuker, M. Helms, T. D. Perry, and R. Mitchell. 2004. Biodeterioration of Incralac used for the protection of bronze monuments. J. Cult. Herit. 5:361-364. [Google Scholar]
- 45.Miliani, C., M. Ombelli, A. Morresi, and A. Romani. 2002. Spectroscopic study of acrylic resins in solid matrices. Surf. Coatings Technol. 152:276-280. [Google Scholar]
- 46.Möhlenhoff, P., L. Müller, A. A Gorbushina, and K. Petersen. 2001. Molecular approach to the characterisation of fungal communities: methods for DNA extraction, PCR amplification and DGGE analysis of painted art objects. FEMS Microbiol. Lett. 195:169-173. [DOI] [PubMed] [Google Scholar]
- 47.Neinhuis, C., and W. Barthlott. 1997. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 79:667-677. [Google Scholar]
- 48.Nugari, M. P., and G. F. Priori. 1985. Resistance of acrylic polymers (Paraloid B72, Primal AC33) to microorganisms—part 1, p. 685. In 5th International Congress on Deterioration and Conservation, vol. 2. Press Polytechniques Romandes, Lausanne, Switzerland. [Google Scholar]
- 49.Pinna, D., and O. Salvadori. 1999. Biological growth on Italian monuments restored with organic or carbonatic compounds, p. 149-154. In O. Ciferri, G. Mastromei, and P. Tiano (ed.), Of microbes and art: the role of microbial communities in the degradation and protection of cultural heritage. Plenum Publishers, New York, NY.
- 50.Rinaldi, A. 2006. Saving a fragile legacy. EMBO Rep. 7:1075-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts, W. T., and P. M. Davidson. 1986. Growth characteristics of selected fungi on polyvinyl chloride film. Appl. Environ. Microbiol. 51:673-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe, L., and G. T. Howard. 2002. Growth of Bacillus subtilis on polyurethane and the purification and characterization of a polyurethanase-lipase enzyme. Int. Biodeterior. Biodegrad. 50:33-40. [Google Scholar]
- 53.Saad, D. S., G. C. Kinsey, S. Kim, and C. C. Gaylarde. 2004. Extraction of genomic DNA from filamentous fungi in biofilms on water-based paint coatings. Int. Biodeterior. Biodegrad. 54:99-103. [Google Scholar]
- 54.Salvadori, O., and M. P. Nugari. 1988. The effect of microbial growth on synthetic polymers used on works of art, p. 424-427. In D. R. Houghton, R. N. Smith, and H. O. W. Smith (ed.), Biodeterioration 7. Elsevier, Amsterdam, The Netherlands.
- 55.Setua, D. K., G. D. Pandey, R. Indusekhar, and G. N. Mathur. 2000. Biodeterioration of coated nylon fabric. J. Appl. Polym. Sci. 75:685-691. [Google Scholar]
- 56.Shashoua, Y. 2008. Conservation of plastics—materials science, degradation and preservation, in press. Butterworth-Heinemann, Oxford, United Kingdom.
- 57.Shashoua, Y. 2006. Inhibiting the inevitable: current approaches to slowing the deterioration of plastics. Macromol. Symp. 238:67-77. [Google Scholar]
- 58.Shashoua, Y. 2001. Inhibiting the deterioration of plasticized poly(vinyl chloride)—a museum perspective. Ph.D. thesis. The Technical University of Denmark and The National Museum of Denmark, Copenhagen, Denmark.
- 59.Shimao, M. 2001. Biodegradation of plastics. Curr. Opin. Biotechnol. 12:242-247. [DOI] [PubMed] [Google Scholar]
- 60.Shirakawa, M. A., C. C. Gaylarde, P. M. Gaylarde, V. John, and W. Gambale. 2002. Fungal colonization and succession on newly painted buildings and the effect of biocide. FEMS Microbiol. Ecol. 39:165-173. [DOI] [PubMed] [Google Scholar]
- 61.Sorlini, C., D. Falappi, and G. Ranalli. 1991. Biodeterioration preliminary tests on samples of Serena stones treated with resin. Ann. Microbiol. 41:71-79. [Google Scholar]
- 62.Steinberg, P. D., R. de Nys, and S. Kjelleberg. 1997. Chemical defenses of seaweeds against microbial colonization. Biodegradation 8:211-220. [Google Scholar]
- 63.Szostak-Kotowa, J. 2004. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 53:165-170. [Google Scholar]
- 64.Terlezzi, A., E. Conte, V. Zupo, and L. Mazzella. 2000. Biological succession on silicone fouling-release surfaces: long term exposure tests in the harbour of Ischia, Italy. Biofouling 15:327-342. [Google Scholar]
- 65.Vallee-Rehel, K., B. Mariette, P. A. Hoarau, P. Guerin, V. Langlois, and J. Y. Langlois. 1998. A new approach in the development and testing of antifouling paints without organotin derivatives. J. Coating Technol. 70:55-63. [Google Scholar]
- 66.Warscheid, T. 2000. Integrated concepts for the protection of cultural artifacts against biodeterioration, p. 185-202. In O. Ciferri, P. Tiano, G. Mastromei (ed.), Of microbes and art: the role of microbial communities in the degradation and protection of cultural heritage. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 67.Webb, J. S., M. Nixon, I. M. Eastwood, M. Greenhalgh, G. D. Robson, and P. S. Handley. 2000. Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl. Environ. Microbiol. 66:3194-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng, Y., E. K. Yanful, and A. S. Bassi. 2005. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 25:243-250. [DOI] [PubMed] [Google Scholar]