Abstract
Mold-damaged building materials may contain biologically active agents, such as (1→3)-β-d-glucan, allergens, and mycotoxins, which have been associated with adverse health effects. The release of these components from contaminated surfaces into the air is not well understood. The purpose of this study was to characterize the release of particulate (1→3)-β-d-glucan from the surface of artificially mold-contaminated materials. Aspergillus versicolor and Stachybotrys chartarum were grown on malt extract agar (MEA), white ceiling tiles, and a wall-papered gypsum board for 1 and 6 months. The (1→3)-β-d-glucan on the surfaces of moldy materials and in air samples collected from these materials was analyzed by the Limulus amebocyte lysate assay. The aerosolization ratio was defined as the amount of (1→3)-β-d-glucan in the air divided by the amount on the surface. The results showed that the aerosolization of particulate (1→3)-β-d-glucan was influenced mainly by the type of material and the fungal species. For A. versicolor, the aerosolization ratios of particulate (1→3)-β-d-glucan released from the three types of material were not significantly different. However, the ratios for S. chartarum released from ceiling tiles and gypsum board were significantly higher than the ratios for this organism released from MEA (P < 0.001) and were comparable to those for A. versicolor. These findings indicate that the use of MEA in aerosolization experiments is likely to underestimate the release of S. chartarum particles from building materials. These results provide important background information for design of future laboratory or animal experiments, as well as for interpretation of field measurement data.
Fungal growth on building materials, such as ceiling tiles and gypsum board, can occur if enough moisture is available (8, 12, 26). Moreover, the material components (e.g., cellulose) and accumulated soil (or dust) can serve as nutrients (36) and promote amplification of mold on the building materials (16). Exposure to mold has been associated with adverse health effects (e.g., allergic rhinitis, asthma, and hypersensitivity pneumonitis) (13), but a clear exposure-response relationship has not been established yet.
The contamination of building materials by mold has been investigated in several studies in an attempt to better understand the likelihood of exposure. These studies have had two primary objectives: (i) identifying and evaluating the growth of fungi (or their metabolites) on the surface of building materials under controlled conditions; and (ii) characterizing how the release of fungal particles is affected by environmental factors (e.g., air velocity and humidity). The release of particles from moldy materials increases when air velocity increases or relative humidity decreases, and it can even be affected by vibration, material sample texture, and colony morphology (9, 10, 18, 25, 33). The composition and moisture content of building materials have been shown to be critical factors for fungal growth (3, 8, 11, 16).
Bioactive agents, such as endotoxins, mycotoxins, and (1→3)-β-d-glucan, have been found on naturally and artificially mold-infested building materials (1, 12, 22, 23, 24), but very little is known about the aerosolization patterns of these agents. (1→3)-β-d-Glucan has been used as an indicator of fungal exposure (4, 6, 27) since it is the major water-insoluble structural cell wall component (accounting for up to 60% of the dry weight) of most fungi (5). Moreover, (1→3)-β-d-glucan exposure has been associated with common respiratory symptoms, such as airway inflammation (29, 37). A recent study demonstrated that there was association between exposure of infants to (1→3)-β-d-glucan and a decrease in wheezing and allergen sensitization, indicating that this fungal component has a strong immunomodulating effect (14). Therefore, more information on the release of particulate (1→3)-β-d-glucan from moldy materials is needed in order to assess and understand exposure to mold and associated health effects. The objective of the present study was to investigate the release of particulate (1→3)-β-d-glucan from three different materials that were artificially contaminated with fungi.
MATERIALS AND METHODS
Test microorganisms.
Aspergillus versicolor strain RTI 367 (Research Triangle Institute International, Research Triangle Park, NC) and nontoxic Stachybotrys chartarum strain JS51-05 (15) were used throughout this study.
Spore suspensions were prepared for inoculation as described by Schmechel et al. (30). Briefly, A. versicolor and S. chartarum were first grown on 2% malt extract agar (MEA) (20 g/liter dextrose, 20 g/liter malt extract, 20 g/liter agar, 1 g/liter peptone; Difco, Becton Dickinson, Sparks, MD) at 21 to 24°C and a relative humidity of 32 to 40% for 1 week. Autoclaved glass microbeads (1 g of beads that were 0.4 to 0.6 mm in diameter; B. Braun Biotech International, Melsungen, Germany) spread on the surface of one agar plate were gently shaken back and forth to harvest fungal spores. The spore-coated beads were then transferred into a sterile 50-ml tube containing sterile deionized water (five-stage Milli-Q Plus system; Millipore Corp., Bedford, MA) with 0.05% Tween 80 (Sigma Chemical Co., St. Louis, MO), and spores were subsequently extracted from these microbeads using vortexing and ultrasonic bath agitation. Mycelial fragments in the suspensions were occasionally observed, but the concentrations were negligible compared to the numbers of spores. The spore concentrations were adjusted to approximately 106 spores per ml (coefficient of variation, ±20%) using a bright-line hemacytometer (model 3900; Hausser Scientific Company, Horsham, PA). Finally, 0.1-ml portions of each suspension were used to inoculate fresh MEA plates and building materials. The remaining suspensions were stored in a refrigerator at 4°C for 1 to 2 days for later inoculation.
Test materials.
MEA and two types of building materials, cellulose-containing white ceiling tiles (Armstrong World Industries, Lancaster, PA) and wall-papered gypsum board (National Gypsum Company, Buffalo, NY), were prepared.
Five milliliters of 2% MEA in a petri plate (diameter, 8.7 cm; area, 59.4 cm2) was used for fungal cultivation as these conditions were confirmed to generate more airborne fungal particles (31). Ceiling tile and gypsum board pieces were cut into the same round shape and dimensions as a petri plate with a thickness of 0.7 cm. Precut pieces of building materials were autoclaved and placed into sterile petri plates inside a class II biosafety cabinet (Sterilchem Gard; Baker Company, Inc., Sanford, ME). Altogether, 204 material samples of each type were prepared [144 samples for aerosolization experiments (2 species × 6 incubation times × 3 replicate experiments × 4 material samples per aerosolization experiment), 24 samples for blanks (2 species × 6 incubation times × 2 material samples per aerosolization experiment), and 36 samples for testing the amount of (1→3)-β-d-glucan and counting spores on the surfaces of the material samples (2 species × 6 incubation times × 3 replicates)].
Fungal inoculation and incubation.
Aliquots (0.1 ml) of a spore suspension were inoculated onto 2% MEA plates using a cell spreader (Fisher Scientific Company, Pittsburgh, PA) to obtain even growth on the surfaces of the MEA plates. The ceiling tile and gypsum board pieces were first allowed to absorb 10 ml of autoclaved and deionized water each to establish a high water activity. This pretreatment produced thin water films on the surfaces of these materials and made it easier to evenly spread the inoculum. After it was soaked for 1 h, each building material sample was supplemented with 1 ml of malt extract broth (20 g/liter) to simulate an external nutrient source, such as settled dust on the surface of the material. Finally, the finished surface of each ceiling tile and gypsum board piece was inoculated with 0.1 ml of a spore suspension.
After inoculation, the samples of the materials (petri plates containing MEA, as well as the ceiling tile and gypsum board samples) were placed in six different chambers (2 species × 3 different materials) in order to avoid between-species contamination. The 5.3-liter incubation chambers were aerated with filter-sterilized air (pore size, 0.2 μm; GE Osmonics Inc., MN) once a day for 10 min at a flow rate of 0.53 liter/min (22). Inoculated material samples were incubated at room temperature (21 to 24°C) and a relative humidity of 97 to 99% for 1, 2, 3, 4, 5, and 6 months. This humidity was achieved by placing a saturated K2SO4 solution (150 g/liter) at the bottom of each incubation chamber (19). The temperature and humidity in each chamber were monitored once a day by using a traceable humidity-temperature pen (Fisher Scientific Company, Pittsburgh, PA). The moisture content on the surface of material samples, as well as the relative humidity, can affect the release of fungal particles, and thus the moisture content was measured with a moisture meter (model BLD5800 Protimeter; General Electric, MA) immediately before the materials were used for the experiments.
Collection of fungal particles aerosolized from material samples.
A fungal spore source strength tester (FSSST) like that described by Sivasubramani et al. (33) was utilized to aerosolize fungal particles from test materials. A flow rate of 20.5 liters/min was used for the FSSST to match the inlet flow of the fragment sampling system. Clean HEPA-filtered air penetrating through the orifices at the bottom of the device created air jets, which hit the moldy surface located directly below the air jets and induced the aerosolization of fungal particulate matter. A newly developed fragment sampling system was utilized to collect particulate (1→3)-β-d-glucan, and the procedures used have been described in detail elsewhere (31). Briefly, this system consists of two Sharp-Cut cyclones (PM2.5 and PM1.0; BGI, Inc., Waltham, MA) and a 25-mm after-filter (a gamma-radiated preloaded polycarbonate filter with a pore size of 0.4 μm; SKC Inc., Eighty Four, PA). Aerosolized fungal particles were collected in the collection cups (10 ml for PM2.5 and 3 ml for PM1.0) of the two Sharp-Cut cyclones and on the after-filter according to their aerodynamic sizes. In the present study, the results for three size fractions (<1.0, 1.05 to 2.25, and >2.25 μm) were combined to characterize the general aerosolization of particulate (1→3)-β-d-glucan released from moldy materials.
To ensure collection of sufficient amounts of particulate (1→3)-β-d-glucan in the air samples, three or four material samples were used for collection of one air sample. Fungal particles from each sample of material were aerosolized for 3 min. During the aerosolization experiment, the numbers of aerosolized particles were determined in real time with an optical particle counter (OPC) (model 1.108; Grimm Technologies, Inc., Douglasville, GA) located upstream of the fragment sampling system. The results were used to determine case by case the maximum number of material samples that could be used for the experiment without overloading the collection system (31). In addition, the aerosol concentration was used in the data analysis after it was normalized to the number of released particles per material sample. The aerosolization experiment was repeated three times for each combination of fungal species and type of material.
Characterization of test materials to determine the numbers of spores and amounts of (1→3)-β-d-glucan on their surfaces.
Following a predetermined incubation period, the numbers of spores and the amounts of (1→3)-β-d-glucan on the moldy surfaces were determined. Three pieces that had an area of approximately 1 cm2 (1 by 1 cm) were cut from a material sample using a sterile scalpel (Fisher Scientific Company, Pittsburgh, PA). Altogether, nine pieces of each type of material (3 pieces/material sample × 3 material samples) were prepared to count the number of spores. Each piece was suspended in 10 ml of pyrogen-free water (LAL Reagent Water; Associates of Cape Cod, East Falmouth, MA) with 0.05% Tween 80 in a 50-ml pyrogen-free tube.
Fungal particles were then extracted from each 1-cm2 piece of material using a vortex touch mixer (model 231; Fisher Scientific, Pittsburgh, PA) for 2 min, followed by 10 min of agitation in an ultrasonic bath (model FS20 Fisher ultrasonic cleaner; 3 qt; 120 V; 50/60 Hz;1 A; 80 W; no heater; Fisher Scientific Inc., Pittsburgh, PA) (38). An aliquot (1 ml) was taken from the 10-ml extract stock and filtered through a 13-mm mixed cellulose ester filter (pore size, 1.2 μm; Millipore Corporation, Bedford, MA) by using an analytical stainless steel vacuum filter holder (Fisher Scientific, Pittsburgh, PA). After it was dried for 30 min, the filter, which was placed on a glass slide, was cleared using a modified instant acetone-vaporizing unit with a continuous flow of acetone vapor. A cleared filter allowed microscopic counting of spores. A drop of lactophenol cotton blue stain (Becton Dickinson, Sparks, MD) was placed in the center of the acetone-cleared slide. The slide was covered with a square cover glass (25 by 25 mm), and then the edge of the cover glass was sealed with transparent nail enamel. Spore counts were determined for 40 randomly selected microscopic fields using a light microscope (Leitz Laborlux S; Leica Mikroskopie und Systeme GmbH, Wetzler, Germany) at a magnification of ×400, and the results were converted to the number of spores per cm2. The total number of spores per material sample was calculated by multiplying the mean number of spores obtained from the nine 1-cm2 samples by the area of one material sample (59.4 cm2).
In addition, a 0.5-ml aliquot was taken from one of three extracts for each type of material for analysis of (1→3)-β-d-glucan on the surfaces of material samples. The results of nine spore counts and three (1→3)-β-d-glucan assays for each type of material were utilized to calculate the geometric means for numbers of spores and (1→3)-β-d-glucan concentrations, respectively.
(1→3)-β-d-Glucan assay.
In the present study, the (1→3)-β-d-glucan assay used was the kinetic chromogenic Limulus amebocyte lysate assay (Glucatel; Associates of Cape Cod, East Falmouth, MA), which uses (1→3)-β-d-glucan-sensitive factor G. The (1→3)-β-d-glucan contents of samples collected from the surface of each type of material and from the air were analyzed similarly as described by Seo et al. (31). Briefly, 0.5 ml of 0.6 M NaOH was added to each 0.5-ml extract of a sample, and the suspensions were shaken using a mechanical shaker (model 75 Wrist-action shaker; Burrell Scientific, Pittsburgh, PA) for 1 h in order to extract (1→3)-β-d-glucan from the suspended fungal particles by unwinding its triple-helix structure and making it water soluble. Finally, aliquots (25 μl) were transferred to microwell plates, and assay reagents [50 μl of specific (1→3)-β-d-glucan lysate] were added. Each microwell plate was incubated for 150 min in an absorbance microplate reader (ELx808TM; Bio-Tek Instruments, Inc., Winooski, VT), and the optical density at 405 nm of samples was read every 30 s for 150 min. The results of the assay were expressed in pg/ml and were converted to pg/cm2 for surface samples and to pg/m3 for air samples.
Determination of the aerosolization ratio of particulate (1→3)-β-d-glucan.
To characterize particulate (1→3)-β-d-glucan aerosolized from material samples, the aerosolization ratio of particulate (1→3)-β-d-glucan was determined by measuring the (1→3)-β-d-glucan in samples collected from the surface immediately before the aerosolization experiment and from the air during the experiment.
As described above, the concentrations of (1→3)-β-d-glucan extracted from a surface piece of a material sample (BGsurface-extract) (ng/ml) were determined, and the total amount (ng) of (1→3)-β-d-glucan on the surface of a material sample (BGsurface) was calculated using the following equation:
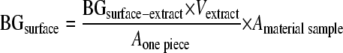 |
where Vextract is the volume of the extraction solution (10 ml), Aone piece is the area of a surface piece of a material sample used for the (1→3)-β-d-glucan analysis (1 cm2), and Amaterial sample is the total area of one material sample (59.4 cm2).
The amounts of particulate (1→3)-β-d-glucan aerosolized from material samples were determined by adding the amounts of (1→3)-β-d-glucan in three size fractions collected by the fragment sampling system (BGair-extract) (ng/ml). As several material samples were utilized to collect one air sample for the (1→3)-β-d-glucan assay, the amount of airborne particulate (1→3)-β-d-glucan was also normalized to the airborne mass released per material sample (BGairborne) and was calculated using the following equation:
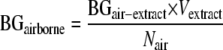 |
where Nair is the number of material samples used for collection of the air sample (three or four samples). Finally, the aerosolization ratio of particulate (1→3)-β-d-glucan was calculated using the following equation:
 |
Statistical analysis.
The numbers of spores, BGsurface, BGairborne, aerosolization ratios, and numbers of released particles were shown to have log-normal distributions as determined by the Shapiro-Wilk and Kolmogorov-Smirnov tests. Therefore, geometric means and geometric standard deviations were calculated to describe the center and spread of the data. Different species, incubation times, and material samples were examined in order to investigate their effects on log-transformed outcome variables (numbers of spores, BGsurface, BGairborne, and aerosolization ratios) using a general linear model (GLM).
Differences in outcome variables for material samples incubated for different time intervals were tested using an analysis of variance (ANOVA) method. Tukey's adjustment for multiple comparisons was applied in order to maintain overall significance levels of 5% for hypothesis testing. A Pearson correlation coefficient was obtained to estimate the correlation between the outcome variables. A simple t test was performed to compare moisture contents on the surfaces of material samples at different incubation times. Statistical Analysis System (SAS) software (SAS for Windows, version 9.1; SAS Institute Inc., Cary, NC) was used, and a significance level of 0.05 was used unless indicated otherwise.
RESULTS
Collection of fungal particles aerosolized from material samples.
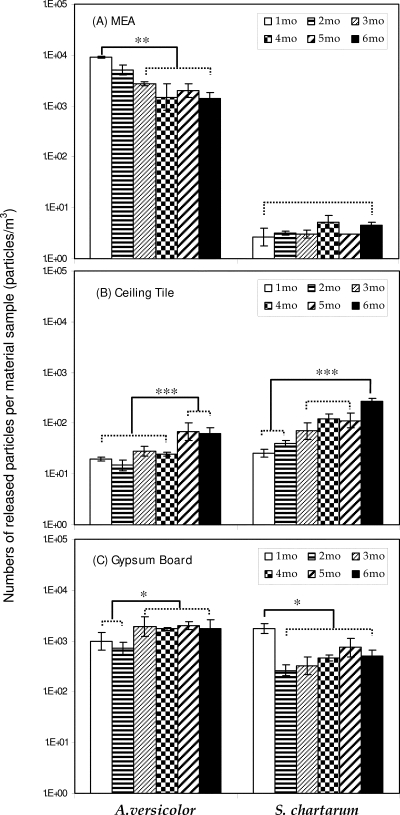
Figure 1 shows the number of aerosolized particles (particles/m3) per material sample as measured by the OPC. The numbers of aerosolized particles (including fungal spores and fragments) showed various trends with incubation time, depending on the fungal species and test material. The results of ANOVA revealed that the numbers of aerosolized particles were significantly different for six different incubation periods, except for S. chartarum grown on MEA. Paired comparisons of means between incubation periods (Tukey's range test) also showed that the means for 1 and 6 months were significantly different. Thus, in most experiments only samples that were collected from material samples incubated for 1 and 6 months were used to determine the numbers of spores and analyze the (1→3)-β-d-glucan contents.
FIG. 1.
Numbers of released particles per sample of each type of material as measured by the OPC. (A) MEA; (B) ceiling tile; (C) gypsum board. The bars indicate geometric means, and the error bars indicate the geometric standard deviations of three trials. Dotted lines indicate that there is no significant difference between geometric means; solid lines indicate that the geometric means are significantly different. Asterisks indicate the level of statistical significance (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001).
Characterization of test materials. (i) Numbers of spores on the surfaces of material samples.
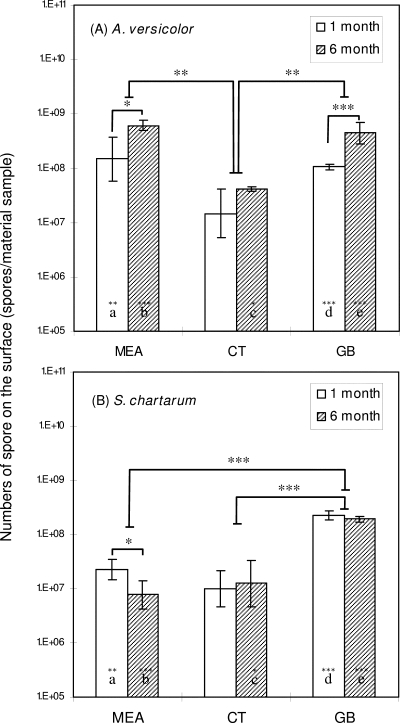
With increased incubation time, powdery colonies in the cultures of A. versicolor and exudates (slime material) in the cultures of S. chartarum were observed. The exudates were particularly abundant for S. chartarum grown on MEA and were less profuse when the organism grew on ceiling tile or gypsum board. The results for the numbers of spores on the surfaces of material samples are shown in Fig. 2. The average coefficient of variation for three replicate spore enumerations performed for one material sample was less than 10% (9.8%), indicating that fungi generally grew on the surface uniformly. The values for single measurements ranged from 4.6 × 106 to 8.0 × 108 spores per material sample. The highest values were obtained for A. versicolor grown on MEA for 6 months, and the lowest values were obtained for A. versicolor grown on ceiling tile for 1 month. The GLM demonstrated that the number of spores was influenced by the type of material and the species (P < 0.001).
FIG. 2.
Numbers of spores on the surfaces of material samples. CT, ceiling tile; GB, gypsum board. (A) A. versicolor; (B) S. chartarum. The bars indicate geometric means, and the error bars indicate the geometric standard deviations of nine trials. Significant differences between the two species are indicated by letters (identical letters indicate a significant difference). Solid lines indicate that geometric means for incubation periods and material samples are significantly different. Asterisks indicate the level of statistical significance (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001).
Comparisons of material samples by ANOVA followed by Tukey's range test showed that the numbers of spores on the three materials were significantly different for all experimental groups; the numbers of spores for A. versicolor grown on MEA and gypsum board were significantly higher than the numbers of spores on ceiling tile; for S. chartarum, the numbers of spores were significantly higher on gypsum board than on MEA and ceiling tile.
When species were compared, almost all data sets showed significant differences. For fungi grown on MEA, the numbers of A. versicolor spores were significantly higher that the numbers of S. chartarum spores for both incubation times. For ceiling tile, however, the surface population of A. versicolor was significantly larger than the S. chartarum population only for the 6-month data set. For gypsum board, the values for A. versicolor were significantly lower than those for S. chartarum at 1 month, but the 6-month data showed the opposite results.
For A. versicolor, the numbers of spores on the material samples increased significantly with incubation time for MEA and for gypsum board. In contrast, the numbers of spores of S. chartarum either did not change (ceiling tile and gypsum board) or decreased significantly (MEA). In addition, correlation analysis showed that the correlation coefficient for numbers of spores and numbers of airborne particles measured by the OPC was statistically significant and high (P < 0.001, r = 0.8915).
(ii) Amounts of (1→3)-β-d-glucan on the surfaces of material samples.
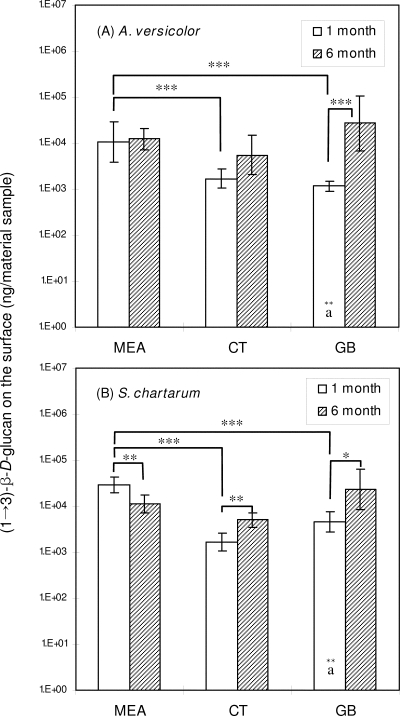
The amounts of (1→3)-β-d-glucan (ng) on the surfaces of material samples after different incubation times are shown in Fig. 3. The values for single measurements ranged from 9.0 × 102 to 7.6 × 104 ng per material sample. The highest and lowest values were obtained for S. chartarum grown on gypsum board for 6 months and for A. versicolor grown on gypsum board for 1 month, respectively. The GLM showed that the amount of (1→3)-β-d-glucan on the surface of a material sample was influenced by the incubation time and the type of material (P < 0.01).
FIG. 3.
Amounts of (1→3)-β-d-glucan on the surfaces of material samples. CT, ceiling tile; GB, gypsum board. (A) A. versicolor; (B) S. chartarum. The bars indicate geometric means, and the error bars indicate the geometric standard deviations of three trials. Significant differences between the two species are indicated by letters (identical letters indicate a significant difference). Solid lines indicate that geometric means for incubation periods and material samples are significantly different. Asterisks indicate the level of statistical significance (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001).
For A. versicolor, a significant increase in the amount of (1→3)-β-d-glucan following the 6-month incubation period was observed for gypsum board but not for the other two materials. For S. chartarum, significant differences between the 1- and 6-month data sets were found for all material samples; these differences were due to a decrease for MEA and increases for ceiling tile and gypsum board. Significant differences among the types of material (as determined by ANOVA, followed by Tukey's range test) were found for all 1-month data sets; the amount of (1→3)-β-d-glucan on the surface was larger for MEA incubated with either A. versicolor or S. chartarum than for ceiling tile or gypsum board.
When the amounts of (1→3)-β-d-glucan on the surface were examined for the two species, the amounts of (1→3)-β-d-glucan for S. chartarum grown on gypsum board incubated for 1 month were significantly larger than the amounts for A. versicolor grown similarly. The moisture contents of the surfaces of material samples ranged from 4.4 to 19.5% (Table 1). The average moisture content of materials incubated for 1 month was 16.2% ± 1.8%, and corresponding value for materials incubated for 6 months was 7.6% ± 2.2%, which shows that there was a significant decrease (average factor, 2.1; P < 0.001, as determined by a t test).
TABLE 1.
Moisture contents of the surfaces of material samples
| Fungus | Sample | Moisture content (%)a
|
|
|---|---|---|---|
| 1 mo | 6 mo | ||
| A. versicolor | MEA | 15.4 ± 3.2 | 5.8 ± 1.4 |
| Ceiling tile | 16.9 ± 1.8 | 7.9 ± 1.3 | |
| Gypsum board | 13.4 ± 2.9 | 6.4 ± 2.5 | |
| S. chartarum | MEA | 17.2 ± 1.2 | 5.3 ± 0.6 |
| Ceiling tile | 16.2 ± 2.2 | 9.6 ± 1.6 | |
| Gypsum board | 17.9 ± 0.7 | 10.7 ± 3.1 | |
| Avg | 16.2 ± 1.8b | 7.6 ± 2.2b | |
The values are averages ± standard deviations of three determinations.
Significance level, P < 0.001.
Airborne particulate (1→3)-β-d-glucan.
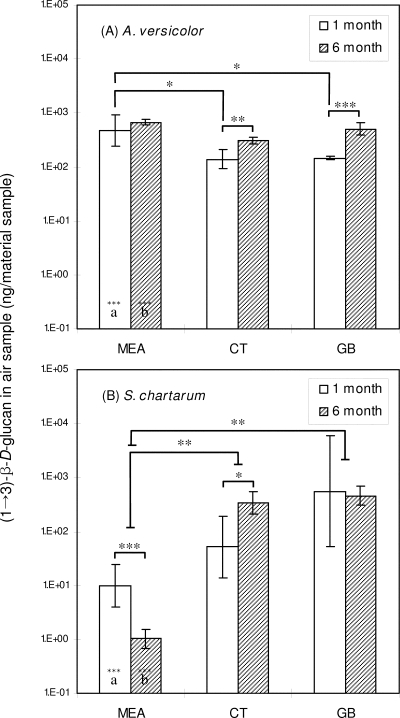
The amounts of aerosolized particulate (1→3)-β-d-glucan (ng) for material samples are shown in Fig. 4. The values for single measurements ranged from 2.0 × 100 to 1.6 × 104 ng per material sample. The highest and lowest values were obtained for S. chartarum grown on gypsum board for 1 month and for S. chartarum grown on MEA for 6 months, respectively. The GLM showed that the amount of particulate (1→3)-β-d-glucan aerosolized from material samples was influenced by the incubation time and the type of material (P < 0.01).
FIG. 4.
Amounts of airborne (1→3)-β-d-glucan for material samples during 3 min. CT, ceiling tile; GB, gypsum board. (A) A. versicolor; (B) S. chartarum. The bars indicate geometric means, and the error bars indicate the geometric standard deviations of three trials. Significant differences between the two species are indicated by letters (identical letters indicate a significant difference). Solid lines indicate that geometric means for incubation periods and material samples are significantly different. Asterisks indicate the level of statistical significance (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001).
For A. versicolor, the amount of particulate (1→3)-β-d-glucan aerosolized from ceiling tile and gypsum board increased significantly with incubation time. For S. chartarum, an effect of incubation time on the amount of airborne (1→3)-β-d-glucan was observed for cultures grown on MEA and ceiling tile. Overall, the amount of airborne particulate (1→3)-β-d-glucan increased with incubation time, except for S. chartarum grown on MEA, for which a significant decrease was observed. Significant differences in the amount of airborne particulate (1→3)-β-d-glucan among the types of material (ANOVA) were found for all data sets except the data for A. versicolor incubated for 6 months.
Additionally, differences between the two species were found for fungi grown on MEA; the amount of airborne particulate (1→3)-β-d-glucan was significantly larger for A. versicolor than for S. chartarum after both 1 and 6 months of incubation. Pearson correlation analysis did not reveal a correlation between the amounts of surface and airborne (1→3)-β-d-glucan (P > 0.05). However, a significant correlation was found between the number of airborne particles (measured by the OPC) and the amount of airborne particulate (1→3)-β-d-glucan (P < 0.01, r = 0.7102).
According to results described above, S. chartarum grown on MEA behaved differently than what was expected; the amounts of both surface and airborne (1→3)-β-d-glucan decreased with incubation time. Therefore, additional (1→3)-β-d-glucan analyses were performed to investigate the monthly tendencies for the amounts of surface and airborne (1→3)-β-d-glucan in more detail. The monthly data for the mean amounts of surface (1→3)-β-d-glucan were as follows; 2.9 × 104, 2.0 × 104, 2.1 × 104, 1.9 × 104, 1.6 × 104, and 1.1 × 104 ng/material sample for months 1 to 6, respectively. The corresponding values for airborne (1→3)-β-d-glucan were 10.0, 4.4, 3.8, 2.1, 2.3, and 1.0 ng/material sample. The results show that in general both parameters decreased with time. However, the results of ANOVA revealed that this trend was significant only for the amount of airborne (1→3)-β-d-glucan (P < 0.001). Post hoc tests showed that there was no significant difference among the mean concentrations of airborne (1→3)-β-d-glucan at months 2 through 5, but the corresponding values were significantly different from the data for months 1 and 6.
Determination of the aerosolization ratio of particulate (1→3)-β-d-glucan.
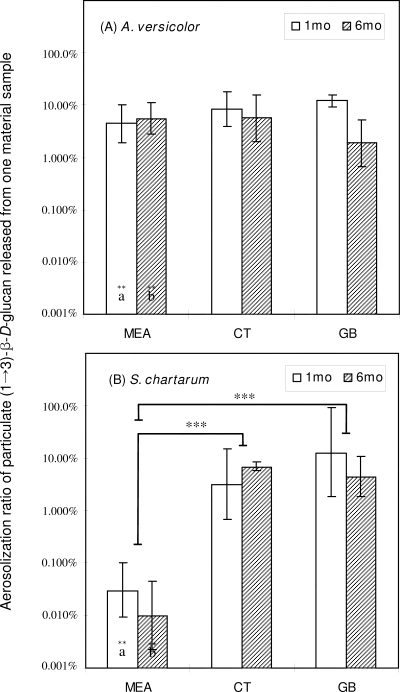
Figure 5 shows the aerosolization ratios of particulate (1→3)-β-d-glucan aerosolized from the surfaces of material samples. The ratios for single measurements for all material types varied from <0.1 to 92.3%. The highest values were obtained for S. chartarum grown on gypsum board for 1 month, and the lowest values were obtained for cultures grown on MEA for 6 months. The GLM showed that the aerosolization ratio of particulate (1→3)-β-d-glucan is influenced by the type of material (P < 0.001).
FIG. 5.
Aerosolization ratio for particulate (1→3)-β-d-glucan (BGairborne/BGsurface × 100) released from the surfaces of material samples during 3 min. CT, ceiling tile; GB, gypsum board. (A) A. versicolor; (B) S. chartarum. The bars indicate geometric means, and the error bars indicate the geometric standard deviations of three trials. Significant differences between the two species are indicated by letters (identical letters indicate a significant difference). Solid lines indicate that geometric means for incubation periods and material samples are significantly different. Asterisks indicate the level of statistical significance (two asterisks, P < 0.01; three asterisks, P < 0.001).
Comparisons of the types of material (ANOVA) showed that the aerosolization ratios of particulate (1→3)-β-d-glucan from A. versicolor grown on all three types of material were not significantly different for either the 1- or 6-month incubation period. In contrast, the corresponding values for S. chartarum grown on all material samples were significantly different; the aerosolization ratio for S. chartarum grown on MEA was significantly lower than the ratios for ceiling tile and gypsum board (Tukey's range test).
Incubation time did not affect the aerosolization ratios of either species. When the data for the two species were compared, the aerosolization ratios were significantly higher for A. versicolor than for S. chartarum only when cultures were grown on MEA.
DISCUSSION
The lowest aerosolization ratio of particulate (1→3)-β-d-glucan was obtained for S. chartarum grown on MEA. This can be attributed to the exudate of S. chartarum, which covers spores and hyphae and whose amount increases as the fungus grows (17). This was supported by the finding that the low aerosolization ratio of particulate (1→3)-β-d-glucan was due to low concentrations of airborne (1→3)-β-d-glucan and not due to a low concentration of (1→3)-β-d-glucan on the surface. There was no difference in the amounts of surface (1→3)-β-d-glucan for S. chartarum grown on the three different materials for 6 months, and the values for MEA incubated for 1 month were even higher than those for ceiling tile and gypsum board. Furthermore, the numbers of particles (measured by the OPC) aerosolized from MEA were also lower than the numbers of particles aerosolized from ceiling tile and gypsum board. Considering that (1→3)-β-d-glucan could be carried by airborne particulate matter (31), it is feasible that low concentrations of airborne (1→3)-β-d-glucan can be attributed to low numbers of airborne particles. This was supported by strong correlations between the numbers of released particles and the amounts of airborne (1→3)-β-d-glucan.
Kuhn et al. (20) collected 200 surface samples from water-infested homes using either swab, tape lift, or bulk sampling and detected Stachybotrys spp. in 58.5% of the houses. However, airborne Stachybotrys spores collected with Air-O-Cell sampling cassettes were found in only 13.0% of the houses (26/200) and in 9.6% of air samples (79/821). This example and the MEA results obtained in the current study indicate that S. chartarum spores do not readily become airborne, which may be due to the exudates produced under certain growth conditions. Furthermore, large spore size also enhances rapid gravitational settling of the aerosolized spores compared to the settling of many common indoor air spores, such as A. versicolor spores. For example, gravitational settling from a height of 1 m would take 96 min for A. versicolor spores (aerodynamic diameter, 2.4 μm) (28) but only 26 min for S. chartarum spores (aerodynamic diameter, 4.6 μm) (34), assuming standard-density (1,000-kg/m3) spheres at 293 K and 101 kPa (1 atm) (2).
Therefore, it was surprising that the aerosolization of particles and particulate (1→3)-β-d-glucan from S. chartarum grown on building materials (ceiling tile and gypsum board) was comparable to that of A. versicolor, which has much smaller spores and produces powdery colonies. It appears that substantial amounts of S. chartarum particles can be released from building materials under favorable conditions. Sivasubramani et al. (33) studied the release of spores from contaminated surfaces in four moldy homes using an FSSST. They reported that the relative efficiency of release of S. chartarum spores from painted drywall was 0.45% ± 0.31%. In the current study, the average aerosolization ratios varied from 3.19 to 12.2% for S. chartarum grown on building materials and from 0.01 to 0.03% for S. chartarum grown on MEA. The absolute values are not directly comparable due to different sampling times and different airflow rates in the FSSST, but the results suggest that the use of MEA for the aerosolization test is likely to underestimate the release of S. chartarum particles from building materials. MEA was used for both fungal species in the present study, but other types of media (e.g., cellulose agar) may be considered for future studies.
For A. versicolor, the aerosolization ratios of particulate (1→3)-β-d-glucan were similar for the three types of material. The calculated number of particles aerosolized from A. versicolor that grew on ceiling tile incubated for 6 months was around 6.4 × 104 particles/cm2, which is very close to the value (around 4.1 × 104 particles/cm2) obtained during the first 3 min by Górny et al. (10), who also used A. versicolor grown on ceiling tiles but incubated the cultures for 12 months. Sivasubramani et al. (33) measured aerosolization for 10 min but reported very similar values for A. versicolor grown on building materials for 12 months: 9.4 × 104 particles/cm2 for ceiling tile and 5.6 × 104 particles/cm2 for gypsum board. However, the numbers of particles aerosolized from MEA incubated for 1 and 6 months in this study were much higher than those reported by Sivasubramani et al. (33) and Górny et al. (10), who incubated A. versicolor on MEA for 1 week. This could be explained by the different flow rates of the FSSST (Sivasubramani et al. used 12.5 liters/min, while 20.5 liters/min was used in the present study) and different incubation times on MEA (1 week in the studies of Sivasubramani et al. and Górny et al. versus 1 and 6 months in the present study). The longer incubation time in the present study resulted in increased fungal biomass on the surface and a decrease in the relatively low moisture content (5.8%) on the surface of MEA, both of which may enhance the release of fungal particles (25).
Other studies have also investigated the release of fungal spores (9, 18, 25, 39) and reported that the rate of release of particles increased with increased air velocity and decreased relative humidity. However, the results of these studies are not directly comparable with the results of the current study because of differences in the objectives and methodologies. The objective of the previous studies was to investigate the effect of air velocity and air humidity on the release of fungal spores. These studies did not investigate the effect of incubation time or material type or the difference between S. chartarum and A. versicolor, which were the objectives of the current study. Furthermore, the different methodologies used to measure the released particles prohibit direct comparisons of the numerical values. In the current study, particle counts were obtained with the OPC and the concentration of (1→3)-β-d-glucan was measured, whereas the other studies measured the number of either culturable or total spores.
Generally, the number of spores and the (1→3)-β-d-glucan level which could be attributed to the increase in fungal biomass (spores and mycelia) on the surface of material samples increased significantly with incubation time. Karunasena et al. (16) reported that the number of CFU of S. chartarum grown on cellulose ceiling tile increased significantly (up to 311-fold) with incubation time, from the initial conidial loading of 1.40 × 104 to 4.35 × 106 CFU during the first 7 days of incubation. Chang et al. (3) also observed statistically significant growth of Penicillium glabrum, Penicillium chrysogenum, and Aspergillus niger (increases in the number of CFU of >2 orders of magnitude during the first 28 days of incubation) at a relative humidity of 94% or greater. In the present study, the numbers of spores on the surfaces increased with time around 320-fold for A. versicolor and 26-fold for S. chartarum when the incubation period was increased from 1 to 6 months. This indicates that the incubation time that Karunasena et al. used (7 days) was at the initial stage of fungal logarithmic growth and therefore they reported a dramatic increase in the number of CFU, whereas the incubation period used in the current study (1 to 6 months) may be close to the stationary phase.
However, for S. chartarum grown on MEA, the numbers of spores and the surface (1→3)-β-d-glucan level decreased significantly with time. Overall, there was a 65.6% decrease in the number of spores and a 62.3% decrease in the surface (1→3)-β-d-glucan level between 1 and 6 months. This may be explained by the changes that occur during spore germination. Minamikawa et al. (21) also reported an approximately 23% decrease in the dry weight of germinating spores and an approximately 50% decrease in the amount of insoluble glucan of germinating spores of Adiantum capillus-veneris during an 8-day experimental period. These investigators concluded that insoluble glucans were used as energy sources. In addition, Stone and Clark (35) reported that microorganisms produce intra- and extracellular enzymes that can degrade polymeric substances containing (1→3)-β-glucosidic linkages, which are utilized as a carbon source. The decrease was observed only for S. chartarum grown on MEA, which also had the lowest moisture content. It is well known that at the same ambient relative humidity the moisture contents of different materials differ due to differences in their chemical compositions and structures (7). This study did not reveal a similar decreasing trend for A. versicolor. The minimum water activity needed for growth of A. versicolor is 0.65 to 0.70, whereas S. chartarum requires a higher water activity (>0.9) (10). This suggests that A. versicolor might have exhibited more efficient colony growth than S. chartarum under the same environmental conditions.
As noted above, the significant correlation between the numbers of airborne particles and the airborne concentration of particulate (1→3)-β-d-glucan suggests that both of these parameters are related to the aerosolized fungal biomass. Nonetheless, this correlation was not consistently observed. For example, the number of airborne particles of S. chartarum grown on MEA did not change during the 6-month incubation, but the amount of airborne (1→3)-β-d-glucan decreased significantly with time. On the other hand, the numbers of released particles of A. versicolor grown on MEA and S. chartarum grown on gypsum board decreased significantly with time, but there was no change in the airborne concentration of particulate (1→3)-β-d-glucan. The OPC used has a limited particle size range and does not measure particles smaller than 0.3 μm. A recent study reported that fungal fragments may contain considerable amounts of (1→3)-β-d-glucan (31) and could contribute to the total amount of (1→3)-β-d-glucan in the air. Thus, it may be expected that fungal fragments not accounted for by the OPC may influence the amount of airborne (1→3)-β-d-glucan. The size distribution of airborne particles containing (1→3)-β-d-glucan will be addressed in a separate study.
In conclusion, the results indicate that the aerosolization ratio of particulate (1→3)-β-d-glucan is influenced mainly by the type of water-damaged material. The lowest aerosolization ratio was obtained for S. chartarum grown on MEA. However, comparable amounts of (1→3)-β-d-glucan were aerosolized from S. chartarum and A. versicolor grown on building materials. Use of MEA in aerosolization experiments is likely to underestimate the release of S. chartarum particles from building materials. These results strongly suggest that future laboratory or animal experiments should include building materials as growth substrates for fungi. They also provide important background information for the interpretation of field measurement data.
Acknowledgments
Sung-Chul Seo was supported by the University of Cincinnati (university graduate scholarships and assistantships). This study was supported in part by the U.S. Department of Housing and Urban Development through grant OHLHH0155-06.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Andersson, M. A., M. Nikulin, U. Koljalg, M. C. Andersson, F. Rainey, K. Reijula, E. L. Hintikka, and M. Salkinoja-Salonen. 1997. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 63:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron, P. B., and K. Willeke. 2001. Aerosol measurement: principles, techniques, and applications, 2nd ed. Wiley Interscience, New York, NY.
- 3.Chang, J., K. K. Foarde, and D. Van Osdell. 1995. Growth evaluation of fungi (Penicillium and Aspergillus spp.) on ceiling tiles. Atmos. Environ. 29:2331-2337. [Google Scholar]
- 4.Chew, G. L. 2001. Fungal extracellular polysaccharides, (1→3)-β-d-glucans and culturable fungi in repeated sampling of house dust. Indoor Air 11:171-178. [DOI] [PubMed] [Google Scholar]
- 5.Douwes, J. 2005. (1→3)-β-d-Glucan and respiratory health: a review of the scientific evidence. Indoor Air 15:160-169. [DOI] [PubMed] [Google Scholar]
- 6.Douwes, J., R. van Strien, G. Doekes, J. Smit, M. Kerkhof, J. Gerritsen, D. Postma, J. de Jongste, N. Travier, and B. Brunekreef. 2006. Does early indoor microbial exposure reduce the risk of asthma? The prevention and incidence of asthma and mite allergy birth cohort study. J. Allergy Clin. Immunol. 117:1067-1073. [DOI] [PubMed] [Google Scholar]
- 7.Flannigan, B. 1992. Approaches to assessment of the microbial flora of buildings, p. 139-145. In IAQ '92. Environments for people. American Society of Heating, Refrigerating and Air-conditioning Engineers, Inc., Atlanta, GA.
- 8.Foarde, K. K., P. Dulaney, E. Cole, D. Van Osdell, D. Ensor, and J. Chang. 1993. Assessment of fungal growth on ceiling tiles under environmentally characterized conditions. Indoor Air 4:357-362. [Google Scholar]
- 9.Foarde, K., D. Van Osdell, M. Menetrez, and J. Chang. 1999. Investigating the influence of relative humidity, air velocity and amplification on the emission rates of fungal spores, p. 507-512. In Proceedings of Indoor Air '99, Edinburgh. The 8th International Conference on Indoor Air Quality and Climate, vol. 2. CRC, Ltd., London, United Kingdom. [Google Scholar]
- 10.Górny, R. L., T. Reponen, S. A. Grinshpun, and K. Willeke. 2001. Source strength of fungal spore aerosolization from moldy building material. Atmos. Environ. 35:4853-4862. [Google Scholar]
- 11.Grant, C., C. A. Hunter, B. Flannigan, and A. F. Bravery. 1989. The moisture requirements of moulds isolated from domestic dwellings. Int. Biodeterior. Biodegrad. 25:259-284. [Google Scholar]
- 12.Gravesen, S., P. A. Nielsen, R. Iversen, and K. F. Nielsen. 1999. Microfungal contamination of damp buildings—examples of risk constructions and risk materials. Environ. Health Perspect. 107:505-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine. 2004. Damp indoor spaces and health, p. 183-269. The National Academies Press, Washington, DC. [PubMed]
- 14.Iossifova, Y., T. Reponen, D. I. Bernstein, L. Levin, H. Kalra, P. Campo, M. Villareal, J. Lockey, G. K. Khurana Hershey, and G. LeMasters. 2007. House dust (1→3)-β-d-glucan and wheezing in infants. Allergy 62:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvis, B. B., W. G. Sorenson, E.-L. Hintikka, M. Nikulin, Y. Zhou, J. Jiang, S. Wang, S. Hinkley, R. A. Etzel, and D. Dearborn. 1998. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 64:3620-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karunasena, E., N. Markham, T. Brasel, J. D. Cooley, and D. C. Straus. 2001. Evaluation of fungal growth on cellulose-containing and inorganic ceiling tile. Mycopathologia 150:91-95. [DOI] [PubMed] [Google Scholar]
- 17.Karunasena, E., J. D. Cooley, D. R. Douglas, and D. C. Straus. 2004. Protein translation inhibition by Stachybotrys chartarum conidia with and without the mycotoxin containing polysaccharide matrix. Mycopathologia 158:87-97. [DOI] [PubMed] [Google Scholar]
- 18.Kildesø, J., H. Würtz, K. F. Nielsen, P. Kuse, K. Wilkins, U. Thrane, S. Gravesen, P. A. Nielsen, and T. Schneider. 2003. Determination of fungal spore release from wet building materials. Indoor Air 13:148-155. [DOI] [PubMed] [Google Scholar]
- 19.Korpi, A., A. L. Pasanen, and P. Pasanen. 1998. Volatile compounds originating from mixed microbial cultures on building materials under various humidity conditions. Appl. Environ. Microbiol. 64:2914-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn, R. C., M. W. Trimble, V. Hofer, M. Lee, and R. S. Nassof. 2005. Prevalence and airborne spore levels of Stachybotrys spp. in 200 houses with water incursions in Houston. Texas. Can. J. Microbiol. 51:25-28. [DOI] [PubMed] [Google Scholar]
- 21.Minamikawa, T., K. Tomokazu, and W. Masamitsu. 1984. Compositional changes in germinating spores of Adiantum capillus-veneris L. J. Plant Res. 97:313-322. [Google Scholar]
- 22.Murtoniemi, T., M. R. Hirvonen, A. Nevalainen, and M. Suutari. 2003. The relation between growth of four microbes on six different plasterboards and biological activity of spores. Indoor Air 13:65-73. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, K. F., U. Thrane, T. O. Larsen, P. A. Nielsen, and S. Gravesen. 1998. Production of mycotoxins on artificially inoculated building materials. Int. Biodeterior. Biodegrad. 42:9-16. [Google Scholar]
- 24.Nielsen, K. F., S. Gravesen, P. A. Nielsen, B. Andersen, U. Thrane, and J. C. Frisvad. 1999. Production of mycotoxins on artificially and naturally infested building materials. Mycopathologia 145:43-56. [DOI] [PubMed] [Google Scholar]
- 25.Pasanen, A. L., P. Pasanen, M. J. Jantunen, and P. Kalliokoski. 1991. Significance of air humidity and air velocity for fungal spore release into the air. Atmos. Environ. 25A:459-462. [Google Scholar]
- 26.Pasanen, A. L., P. Kalliokoski, and M. Jantunen. 1994. Recent studies on fungal growth on building materials, p. 485-493. In R. A. Samson, B. Flannigan, M. E. Flannigan, A. P. Verhoeff, O. C. G. Adan, and E. S. Hoekstra (ed.), Air quality monographs, vol. 2. Health implications of fungi in indoor environments. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 27.Rao, C. Y., J. M. Cox-Ganser, G. L. Chew, G. Doekes, and S. White. 2005. Use of surrogate markers of biological agents in air and settled dust samples to evaluate a water-damaged hospital. Indoor Air 15(Suppl. 9):89-97. [DOI] [PubMed] [Google Scholar]
- 28.Reponen, T., K. Willeke, V. Ulevicius, A. Reponen, and S. A. Grinshpun. 1996. Effect of relative humidity on the aerodynamic diameter and respiratory deposition of fungal spores. Atmos. Environ. 30:3967-3974. [Google Scholar]
- 29.Rylander, R., M. Norrhall, U. Engdahl, A. Tunsater, and G. H. Patrick. 1998. Airways inflammation, atopy, and (1→3)-β-d-glucan exposures in two schools. Am. J. Respir. Crit. Care Med. 158:1685-1687. [DOI] [PubMed] [Google Scholar]
- 30.Schmechel, D., R. L. Górny, J. P. Simpson, T. Reponen, S. A. Grinshpun, and D. M. Lewis. 2003. Limitations of monoclonal antibodies for monitoring of fungal aerosols using P. brevicompactum as a model fungus. J. Immunol. Methods 283:235-245. [DOI] [PubMed] [Google Scholar]
- 31.Seo, S. C., S. A. Grinshpun, Y. Iossifova, D. Schmechel, C. Rao, and T. Reponen. 2007. A new field-compatible methodology for the collection and analysis of fungal fragments. Aerosol Sci. Technol. 41:794-803. [Google Scholar]
- 32.Reference deleted.
- 33.Sivasubramani, S. K., R. T. Niemeier, T. Reponen, and S. A. Grinshpun. 2004. Fungal spore source strength tester: laboratory evaluation of a new concept. Sci. Total Environ. 329:75-86. [DOI] [PubMed] [Google Scholar]
- 34.Sorenson, W. G., D. G. Frazer, B. B. Jarvis, J. Simpson, and V. A. Robinson. 1987. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl. Environ. Microbiol. 53:1370-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone, B. A., and A. E. Clark. 1992. Chemistry and biology of (1→3)-β-glucans, p. 358-363. La Trobe University Press, Bundoora, Australia.
- 36.Straube, J. 2006. Moisture and materials. Building Sci. Digest 138:137-139. [Google Scholar]
- 37.Thorn, J., and R. Rylander. 1998. Airways inflammation and glucan in a rowhouse area. Am. J. Respir. Crit. Care Med. 157:1798-1803. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Z., T. Reponen, S. A. Grinshpun, R. L. Górny, and K. Willeke. 2001. Effect of sampling time and air humidity on the bioefficiency of filter samplers for bioaerosol collection. J. Aerosol Sci. 32:661-674. [Google Scholar]
- 39.Zoberi, M. H. 1961. Take-off mold spores in relation to wind speed and humidity. Ann. Bot. 25:53-64. [Google Scholar]