Abstract
Toxic cyanobacterial blooms cause economic losses and pose significant public health threats on a global scale. Characterization of the gene cluster for the biosynthesis of the cyanobacterial toxin cylindrospermopsin (cyr) in Cylindrospermopsis raciborskii AWT205 is described, and the complete biosynthetic pathway is proposed. The cyr gene cluster spans 43 kb and is comprised of 15 open reading frames containing genes required for the biosynthesis, regulation, and export of the toxin. Biosynthesis is initiated via an amidinotransfer onto glycine followed by five polyketide extensions and subsequent reductions, and rings are formed via Michael additions in a stepwise manner. The uracil ring is formed by a novel pyrimidine biosynthesis mechanism and tailoring reactions, including sulfation and hydroxylation that complete biosynthesis. These findings enable the design of toxic strain-specific probes and allow the future study of the regulation and biological role of cylindrospermopsin.
Cyanobacterial toxins pose a serious health risk for humans and animals when they are present at hazardous levels in water bodies used as a source of water for consumption or recreation. Under eutrophic conditions, cyanobacteria tend to form large blooms, which drastically promote elevated toxin concentrations. The problem is internationally relevant, since most toxic cyanobacteria have a global distribution. Specifically, the occurrence of the cyanobacterial genus Cylindrospermopsis has been documented on all continents, therefore posing a significant public health threat on a global scale (7, 8, 11, 15, 16, 31, 37, 41, 42). The major toxin produced by Cylindrospermopsis is cylindrospermopsin, which was first discovered after a poisoning incident on Palm Island (Queensland, Australia) in 1979, when 148 people, mainly children, were hospitalized with hepatoenteritis due to Cylindrospermopsis raciborskii contamination of a drinking water reservoir (4, 6). Besides posing a threat to human health, cylindrospermopsin also causes significant economic losses for farmers due to the poisoning of livestock with cylindrospermopsin-contaminated drinking water (38). Cylindrospermopsin has hepatotoxic, general cytotoxic (33-35), and neurotoxic (19) effects and is a potential carcinogen (17). Its toxicity is due to the inhibition of glutathione and protein synthesis as well as the inhibition of cytochrome P450 (13, 33-35). Six cyanobacterial species have so far been identified as producing cylindrospermopsin: Cylindrospermopsis raciborskii, Aphanizomenon ovalisporum, Aphanizomenon flos-aquae, Umezakia natans, Raphidiopsis curvata, and Anabaena bergii (2, 15, 21, 22, 32, 39). Incidents of human poisoning with cylindrospermopsin have been reported only in subtropical Australia to date; however, C. raciborskii and A. flos-aquae have recently been detected in areas with more temperate climates (10, 32). The tendency of C. raciborskii to form dense blooms and the invasiveness of the producer organisms give rise to global concerns for drinking water quality and necessitate the monitoring of drinking water reserves for the presence of cylindrospermopsin producers.
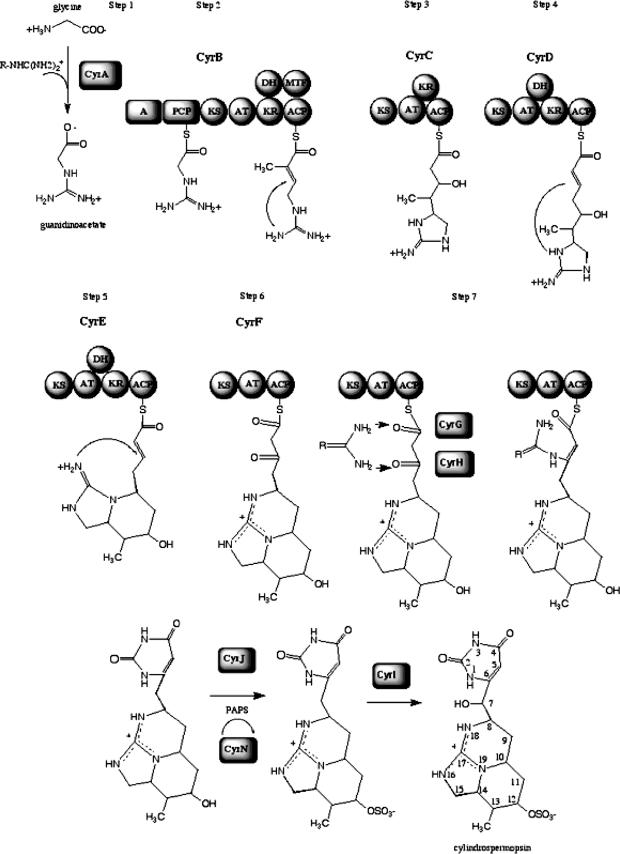
Ohtani et al. (29) determined the chemical structure of cylindrospermopsin. The toxin is a polyketide-derived alkaloid with a central functional guanidino moiety and a hydroxymethyluracil attached to the tricyclic carbon skeleton (Fig. 1). The natural occurrence of an epimer at the hydroxyl bridge, 7-epicylindrospermopsin (3), and a cylindrospermopsin variant lacking the hydroxyl group at C-7, 7-deoxycylindrospermopsin, have also been reported (21). Isotope-labeled precursor feeding experiments have shown that guanidinoacetate is the starter unit for cylindrospermopsin biosynthesis (5), and successive additions of five intact acetate units onto guanidinoacetate yield the carbon backbone of cylindrospermopsin (Fig. 1, steps 2 to 6). Burgoyne et al. (5) postulated that further tailoring reactions, such as C-methylation, sulfotransfer, and cyclization, complete cylindrospermopsin biosynthesis. However, the correct sequence and detail of these reactions are not known, and moreover, it is not known how the uracil ring is formed. Incorporation of an intact acetate unit into the uracil ring indicated that the uracil moiety does not originate from primary metabolism but is synthesized de novo during cylindrospermopsin biosynthesis.
FIG. 1.
Proposed biosynthetic pathway for cylindrospermopsin (see text for detail).
Three putative cylindrospermopsin biosynthesis genes, aoaA, aoaB, and aoaC, have been identified in A. ovalisporum, encoding an amidinotransferase, a hybrid nonribosomal peptide synthetase (NRPS)/polyketide synthase (PKS), and a PKS, respectively (40). The gene product of aoaA is thought to catalyze the synthesis of the guanidinoacetate starter unit, which is then recruited for cylindrospermopsin biosynthesis by the hybrid NRPS/PKS AoaB and passed on to the PKS AoaC, and other as-yet-unidentified PKSs, for further polyketide extension (18, 40). None of the products encoded by these genes have been biochemically characterized, nor has their involvement in cylindrospermopsin biosynthesis been proven. However, screening of toxic and nontoxic strains of C. raciborskii, a toxic A. bergii strain, and a toxic U. natans strain showed that all these genes are exclusively present in cylindrospermopsin producers (18, 39). Here we describe the sequencing and characterization of the cylindrospermopsin biosynthesis gene cluster and propose the complete biosynthetic pathway.
MATERIALS AND METHODS
Cyanobacterial cultures.
Cyanobacterial strains used in the present study (Table 1) were grown in Jaworski medium (44) in static batch culture at 26°C under continuous illumination (10 μmol m−2 s−1).
TABLE 1.
Distribution of the sulfotransferase gene (cyrJ) in toxic and nontoxic cyanobacteria
| Cyanobacterial strain | 16S rRNAa | cyrJ | Toxicityb | Referencec |
|---|---|---|---|---|
| Cylindrospermopsis raciborskii T3 | + | − | SXT | 20 |
| Anabaena circinalis 344B | + | − | ND | AWQC |
| Cylindrospermopsis raciborskii Germ1 | + | − | ND | 26 |
| Anabaena circinalis 310F | + | − | ND | AWQC |
| Cylindrospermopsis raciborskii 44D | + | − | ND | NA |
| Anabaena circinalis 118C | + | − | SXT | 12 |
| Anabaena circinalis 323A | + | − | ND | AWQC |
| Anabaena circinalis 323H | + | − | ND | AWQC |
| Cylindrospermopsis raciborskii VOLL2 | + | − | ND | NA |
| Cylindrospermopsis raciborskii VOLL1 | + | − | ND | NA |
| Cylindrospermopsis raciborskii HUNG1 | + | − | ND | NA |
| Cylindrospermopsis raciborskii 023B | + | + | CYLN | 46 |
| Cylindrospermopsis raciborskii 05E | + | + | CYLN | 39 |
| Cylindrospermopsis raciborskii 4799 | + | + | CYLN | 26 |
| Cylindrospermopsis raciborskii 24C | + | + | CYLN | 39 |
| Cylindrospermopsis raciborskii AWT 205 | + | + | CYLN | 16 |
| Aphanizomenon ovalisporum AO/QH | + | + | CYLN | NA |
16S rRNA gene amplification is shown as a positive control. +, gene fragment amplified; −, no gene detected.
CYLN, cylindrospermopsin; SXT, saxitoxin; ND, not detected.
AWQC, Australian Water Quality Center; NA, not available.
DNA extraction.
Total genomic DNA was extracted from cyanobacterial cells by lysozyme-sodium dodecyl sulfate-proteinase K lysis following phenol-chloroform extraction as described previously (24). DNA in the supernatant was precipitated with 2 volumes of −20°C ethanol, washed with 70% ethanol, dissolved in Tris-EDTA buffer (10:1), and stored at −20°C.
Characterization of the cylindrospermopsin gene cluster.
The characterization of unknown regions of DNA flanking the putative cylindrospermopsin biosynthesis genes was performed using an adaptor-mediated PCR as previously described (23). PCRs were performed in 20-μl reaction volumes containing 1× Taq polymerase buffer, 2.5 mM MgCl2, 0.2 mM deoxynucleotide triphosphates, 10 pmol each of the forward and reverse primers, between 10 and 100 ng genomic DNA, and 0.2 U of Taq polymerase (Fischer Biotech, Australia). Thermal cycling was performed in a GeneAmp PCR system 2400 thermal cycler (Perkin Elmer Corporation, Norwalk, CT). Cycling began with a denaturing step at 94°C for 3 min followed by 30 cycles of denaturation at 94°C for 10 s, primer annealing between 55°C and 65°C for 20 s, and a DNA strand extension at 72°C for 1 to 3 min. Amplification was completed by a final extension step at 72°C for 7 min. Amplified DNA was separated by agarose gel electrophoresis in TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 7.8) and visualized by UV transillumination after staining with ethidium bromide (0.5 μg/ml).
Automated DNA sequencing was performed using the PRISM BigDye cycle sequencing system and a model 373 sequencer (Applied Biosystems, Foster City, CA). Sequence data were analyzed using ABI Prism-Autoassembler software, while values for identity/similarity to other translated sequences were determined using BLAST in conjunction with the National Center for Biotechnology Information (NIH, Bethesda, MD). Fugue BLAST (http://www-cryst.bioc.cam.ac.uk/fugue/) was used to identify distant homologs via sequence-structure comparisons. The gene clusters were assembled using the software Phred, Phrap, and Consed (http://www.phrap.org/phredphrapconsed.html), and open reading frames (ORFs) were manually identified. PKS and NRPS domains were determined using the following specialized databases based on crystal structures (http://www-ab.informatik.uni-tuebingen.de/software/NRPSpredictor,http://www.tigr.org/jravel/nrps/, http://www.nii.res.in/nrps-pks.html).
Genetic screening.
Cylindrospermopsin-producing and nonproducing cyanobacterial strains were screened for the presence of the sulfotransferase gene cyrJ by use of the primer set cynsulfF (5′ ACTTCTCTCCTTTCCCTATC 3′) and cylnamR (5′ GAGTGAAAATGCGTAGAACTTG 3′). Genomic DNA was tested for positive amplification using the16S rRNA gene primers 27F and 809R (25). Amplicons were sequenced as described above to verify the identity of the gene fragment.
Nucleotide sequence accession number.
Nucleotide sequences were submitted to GenBank and are available under accession number EU140798.
RESULTS AND DISCUSSION
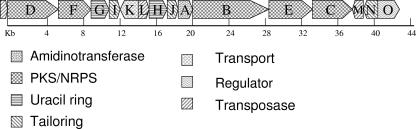
Recently characterized cyanobacterial toxins have complex chemical structures, and their biosynthesis involves numerous enzymes, including NRPS, PKS, and mixed NRPS/PKS systems (9, 23, 45). The genes encoding these enzymes are almost always adjacent to each other on the chromosome and form defined secondary metabolite gene clusters. The biosynthesis of cylindrospermopsin involves an amidinotransferase, an NRPS, and a PKS (AoaA, AoaB, and AoaC, respectively) (39, 40). We have previously identified and characterized the amidinotransferase involved in cylindrospermopsin biosynthesis from C. raciborskii AWT205 (18), a cyanobacterium isolated from an ornamental lake in Sydney, Australia (16). In order to obtain the entire sequence of the cylindrospermopsin biosynthesis gene cluster, we used adaptor-mediated “gene-walking” technology (23), initiating the process from the known partial sequence of the amidinotransferase gene from C. raciborskii AWT205 (18). Successive outward-facing primers were designed, and the entire gene cluster spanning 43 kb was sequenced, together with a further 3.5 kb on either side of the toxin gene cluster (Fig. 2).
FIG. 2.
Structural organization of the cylindrospermopsin gene cluster from C. raciborskii AWT205. Scale indicates gene cluster length in base pairs.
These flanking regions contain putative accessory genes (hyp genes), which include molecular chaperones involved in the maturation of hydrogenases that are common and also clustered in many other cyanobacterial species (14, 43). Due to the fact that these genes are flanking the cylindrospermopsin gene cluster at both ends, we postulate that the toxin gene cluster was inserted into this area of the genome, thus interrupting the HYP gene cluster. This genetic rearrangement is mechanistically supported by the presence of transposase-like sequences within the cylindrospermopsin cluster.
Bioinformatic analysis of the toxin gene cluster was performed and based on gene function inference using sequence alignments (NCBI BLAST), predicted structural homologies (Fugue BLAST), and analysis of PKS and NRPS domains by use of specialized BLAST servers based on crystal structures (see Materials and Methods). The results of labeled precursor feeding experiments showing the origin and fate of the carbons, hydrogens, and oxygen (5) enabled us to define the biosynthetic pathway for cylindrospermopsin, largely due to the modular nature of previously characterized cyanobacterial NRPS and mixed NRPS/PKS systems. The cylindrospermopsin biosynthesis cluster contains 15 ORFs, which encode all the functions required for the biosynthesis, regulation, and export of the toxin cylindrospermopsin (Fig. 2; Table 2).
TABLE 2.
Deduced functions of the ORFs in the cylindrospermopsin gene cluster from C. raciborskii AWT205
| Name | Enzyme family | Size (bp) | Psi-BLAST similarity match | % Identity | Putative function |
|---|---|---|---|---|---|
| cyrD | PKS CrpB | 5,631 | ABM21570.1; Nostoc sp. strain ATCC 53789 | 58 | PKS KS-AT-DH-KR-ACP |
| cyrF | PKS CrpB | 4,074 | ABM21570.1; Nostoc sp. strain ATCC 53789 | 68 | PKS KS-AT-ACP |
| cyrG | Cytosine deaminase/aminohydrolase/dihydro-orotase | 1,437 | BAF59909.1; Pelotomaculum thermopropionicum SI | 50 | Uracil ring formation |
| cyrI | Prolyl 4-hydroxylase | 831 | ABB06365.1; Burkholderia sp. strain 383 | 43 | Hydroxylation of C-7 |
| cyrK | MatE Na+-driven multidrug efflux pump | 1,398 | EAW39051.1; Lyngbya sp. strain PCC 8106 | 65 | Exporter |
| cyrL | Transposase | 750 | ABG50981.1; Trichodesmium erythraeum IMS101 | 70 | Transposase |
| cyrH | Cytosine deaminase/aminohydrolase/dihydro-orotase | 1,431 | BAF59909.1; Pelotomaculum thermopropionicum SI | 50 | Uracil ring formation |
| cyrJ | Branched-chain amino acid aminotransferase | 780 | Trichodesmium erythraeum IMS101 | 53 | Sulfotransferase |
| cyrA | Amidinotransferase AoaA | 1,176 | AAX81898.1; Cylindrospermopsis raciborskii | 100 | Amidinotransferase |
| cyrB | NRPS/PKS AoaB | 8,754 | AAM33468.1; Aphanizomenon ovalisporum | 97 | NRPS/PKS A domain, PCP, KS, AT, DH, Met, KR, ACP |
| cyrE | PKS | 5,667 | ABA23591.1; Anabaena variabilis ATCC 29413 | 62 | PKS KS-AT-DH-KR-ACP |
| cyrC | PKS AoaC | 5,005 | AAM33470.1; Aphanizomenon ovalisporum | 97 | PKS KS-AT-KR-ACP |
| cyrM | Partial transposase | 318 | ABG50981.1; Trichodesmium erythraeum IMS101 | 70 | Transposase |
| cyrN | Adenylylsulfate kinase (PAPS) | 600 | CAM76460.1; Magnetospirillum gryphiswaldense MSR-1 | 75 | Adenylylsulfate kinase (PAPS) |
| cyrO | Hypothetical protein | 1,548 | EAW46978.1; Nodularia spumigena CCY9414 | 74 | Regulator |
Formation of the carbon skeleton.
The first step in the formation of the carbon skeleton of cylindrospermopsin involves the synthesis of guanidinoacetate via the transamidination of glycine as previously described (5, 18, 40). CyrA, the AoaA homolog, which contains an amidinotransferase similar to the human arginine:glycine amidinotransferase GATM (PDB accession no. 1JDW), transfers a guanidino group from a donor molecule, most likely arginine (18), onto an acceptor molecule of glycine, thus forming guanidinoacetate (Fig. 1, step 1). This is in agreement with feeding experiments showing the incorporation of C-1 and C-2 of glycine onto C-14 and C-15 of cylindrospermopsin.
The next step (Fig. 1, step 2) in the biosynthesis is carried out by CyrB (AoaB homolog), a mixed NRPS-PKS. The CyrB gene spans 8.7 kb and contains the following domains: an adenylation domain (A domain) and a domain encoding a peptidyl carrier protein of an NRPS (PCP), followed by a β-ketosynthase domain (KS), an acyltransferase domain (AT), a dehydratase domain (DH), a methyltransferase domain (MT), a ketoreductase domain (KR), and a domain encoding an acyl carrier protein (ACP) of PKS origin. CyrB therefore must catalyze the second reaction, since the gene corresponding to it is the only gene containing an A domain that could recruit a starter unit for subsequent PKS extensions. The specific amino acid activated by the CyrB A domain cannot be predicted, as its substrate specificity-conferring residues do not match any in the available databases (http://www-ab.informatik.uni-tuebingen.de/software/NRPSpredictor, http://www.tigr.org/jravel/nrps/, http://www.nii.res.in/nrps-pks.html). So far, no other NRPS that utilizes guanidinoacetate as a substrate has been described. However, Kellmann et al. (18) proposed guanidinoacetate as a probable substrate based on modeling of the catalytic site, and isotope-labeled feeding has shown the incorporation of an intact guanidinoacetate into cylindrospermopsin, further supporting this conclusion. Hence, the A domain is thought to activate guanidinoacetate, which is then transferred via the swinging arm of the peptidyl carrier protein to the KS domain. The AT domain activates malonyl-coenzyme A (CoA) and attaches it to the ACP. This is followed by a condensation reaction between the activated guanidinoacetate and malonyl-CoA in the KS domain. CyrB contains two reducing modules, KR and DH. Their concerted reaction reduces the keto group to a hydroxyl followed by the elimination of H2O, resulting in a double bond between C-13 and C-14. Feeding experiments showed that C-13 does not retain any of the deuteriums from the precursor trideuteriumacetate (5). The MT domain identified in CyrB via the NRPS/PKS databases (Materials and Methods) is homologous to S-adenosylmethionine-dependent MT. It is therefore suggested that the MT methylates C-13. This is supported by the labeled glycine feeding study that showed that the methyl group originates from the C1 pool via S-adenosylmethionine (5). We propose a nucleophilic attack of the amidino group at N-19 onto the newly formed double bond between C-13 and C-14 via a “Michael addition.” The cyclization follows Baldwin's rules for ring closure (1), resulting in the formation of the first ring in cylindrospermopsin. This reaction could be spontaneous and may not require enzymatic catalysis, as it is energetically favorable (1). This is the first of three ring formations and is one of the principal differences between our biosynthetic pathway and that previously proposed (18).
The third step (Fig. 1, step 3) in the biosynthesis involves CyrC (AoaC homolog), which contains a PKS with KS, AT, KR, and ACP domains. The action of these domains results in the elongation of the growing chain by an acetate via activation of malonyl-CoA by the AT domain, its transfer to ACP, and condensation at the KS domain with the product of CyrB. The elongated chain is bound to the ACP of CyrC, and the KR domain reduces the keto group to a hydroxyl group on C-12. Labeled feeding experiments have shown that the oxygen attached to C-12 is of acetate origin and that C-11 retains both deuteriums (5). Therefore, the PKS module carrying out this step contains a KR domain and does not contain a DH domain; this domain architecture corresponds only to CyrC.
Following the catalysis of enzyme CyrC is CyrD (Fig. 1, step 4), a PKS with five modules: KS, AT, DH, KR, and an ACP. The action of this PKS module on the intermediate produced by CyrC results in the addition of one acetate and the reduction of the keto group on C-10 to a hydroxyl and dehydration to a double bond between C-9 and C-10. In the feeding experiments (5), C-9 retains only one deuterium, thus requiring a PKS enzyme that contains a KR and a DH domain. This double bond is the site of a nucleophilic attack by the amidino group N-19 via another Michael addition that again follows Baldwin's rules of ring closure (1), resulting in the formation of the second ring, the first six-membered ring made in cylindrospermopsin.
The intermediate produced by CyrD is the substrate for CyrE (step 5 in Fig. 1), a PKS containing KS, AT, DH, and KR domains and an ACP. Since this sequence of domains is identical to that of CyrD, it is not possible at this stage to ascertain which PKS acts first, but as their actions are proposed to be identical, it is immaterial at this point. CyrE catalyzes the addition of one acetate and the formation of a double bond between C-7 and C-8. Isotopic feeding (5) has shown that C-7 loses both deuteriums during biosynthesis and that the hydroxyl group at C-7 of cylindrospermopsin is not of acetate origin, which agrees with the formation of a double bond at this position. This double bond is attacked by N-18 via a Michael addition, and the third cyclization occurs, resulting in the second six-member ring.
CyrF is the final PKS module (step 6 of Fig. 1) and is a minimal PKS, containing only a KS, AT, and ACP. CyrF acts on the product of CyrE and elongates the chain by an acetate, leaving C-4 and C-6 unreduced.
Step 7 in the pathway (Fig. 1) involves the formation of the uracil ring, a reaction that has been elusive so far and is required for the toxicity of the final cylindrospermopsin compound (3). The cylindrospermopsin gene cluster encodes two enzymes with high sequence similarity (87%) that have been designated CyrG and CyrH. A Psi-BLAST search (NCBI) followed by a Fugue profile library search (see Materials and Methods) revealed that CyrG and CyrH are most similar to the enzyme family of amidohydrolases/ureases/dihydroorotases, whose members catalyze the formation and cleavage of N-C bonds. We propose that these enzymes transfer a second guanidino group from a donor molecule, such as arginine or urea, onto C-6 and C-4 of cylindrospermopsin, resulting in the formation of the uracil ring. These enzymes carry out two or three reactions, depending on the guanidino donor. The first reaction consists of the formation of a covalent bond between the N of the guanidino donor and C-6 of cylindrospermopsin, followed by an elimination of H2O, forming a double bond between C-5 and C-6. The second reaction catalyzes the formation of a bond between the second N on the guanidino donor and C-4 of cylindrospermopsin concomitantly with the breaking of the thioester bond between the ACP of CyrE and cylindrospermopsin, causing the release of the molecule from the enzyme complex. Feeding experiments with labeled acetate have shown that the oxygen at C-4 is of acetate origin and is not lost during biosynthesis, therefore requiring the de novo formation of the uracil ring. The third reaction—if required—would catalyze the cleavage of the guanidino group from a donor molecule other than urea. The action of CyrG and CyrH in the formation of the uracil ring in cylindrospermopsin describes a novel biosynthesis pathway of a pyrimidine.
Feeding experiments (5) postulated a linear polyketide that molecular modeling studies suggest readily assumes a favorable conformation for the formation of the rings. Therefore, cyclization may be spontaneous and not under enzymatic control. Our genetic analysis shows that this may happen stepwise, with a successive ring formation of the appropriate intermediate as it is synthesized. This mechanism also explains the lack of a thioesterase or cyclization domain, which is usually associated with NRPS/PKS modules and catalyzes the release and cyclization of the final product from the enzyme complex.
Tailoring reactions.
Cylindrospermopsin biosynthesis requires the action of tailoring enzymes in order to complete the biosynthesis, catalyzing the sulfation at C-12 and the hydroxylation at C-7. An analysis of the cylindrospermopsin gene cluster revealed three candidate enzymes for the tailoring reactions involved in the biosynthesis of cylindrospermopsin, namely, CyrI, CyrJ, and CyrN. The sulfation of cylindrospermopsin at C-12 is likely to be carried out by the action of a sulfotransferase. CyrJ contains a protein that is most similar to human 3′-phosphoadenylyl sulfate (PAPS)-dependent sulfotransferases. Similar enzymes have recently been implicated in the sulfation of other cyanotoxins (R. Kellmann, T. K. Mihali, Y. J. Jeon, R. Pickford, F. Potami, and B. A. Neilan, submitted for publication). The cylindrospermopsin gene cluster also encodes an adenylsulfate kinase, namely, CyrN. Adenylsulfate kinases are enzymes that catalyze the formation of PAPS, which is the sulfate donor for sulfotransferases. We propose that CyrJ sulfates cylindrospermopsin at C-12, while CyrN creates the pool of PAPS required for this reaction. Screening of cylindrospermopsin-producing and nonproducing strains revealed that the sulfotransferase genes were present only in cylindrospermopsin-producing strains, further affirming the involvement of this entire cluster in the biosynthesis of cylindrospermopsin (Table 1). The cyrJ gene might therefore be a good candidate for a toxin probe (Materials and Methods), as it is more unique than NRPS and PKS genes and would presumably have less cross-reactivity with other gene clusters containing these genes, which are common in cyanobacteria. The final tailoring reaction is carried out by CyrI. A Fugue search and an iterated Psi-BLAST revealed that CyrI is similar to a hydroxylase belonging to the 2-oxoglutarate- and Fe(II)-dependent oxygenase superfamily, which includes the mammalian prolyl 4-hydroxylase alpha subunit, which catalyzes the hydroxylation of collagen. We propose that CyrI catalyzes the hydroxylation of C-7, a residue that along with the uracil ring seems to confer much of the toxicity of cylindrospermopsin (28). This is supported by the feeding experiments showing that the hydroxyl group at C-7 of cylindrospermopsin is not of acetate origin. Furthermore, the intermediate deoxycylindrospermopsin, lacking the hydroxyl group at C-7, has recently been isolated and identified (21, 22); therefore, the hydroxylation at C-7 by CyrI is probably the final step in the biosynthesis of cylindrospermopsin.
Toxin transport.
Cylindrospermopsin and other cyanobacterial toxins appear to be exported out of the producing cells (27, 30). The cylindrospermopsin gene cluster contains an ORF designated cyrK, the product of which is most similar to sodium ion-driven multidrug and toxic compound extrusion proteins of the NorM family. We postulate that CyrK is a transporter for cylindrospermopsin based on this homology and its central location in the cluster. Heterologous expression and characterization of the protein are currently being undertaken to verify its putative role in cylindrospermopsin export.
Transcriptional regulation of the toxin gene cluster.
Cylindrospermopsin production has been shown to be highest when fixed nitrogen is eliminated from the growth media (36). Flanking the cylindrospermopsin gene cluster are “hyp” gene homologs involved in the maturation of hydrogenases. In the cyanobacterium Nostoc sp. strain PCC73102, they are under the regulation of the global nitrogen regulator NtcA, which activates the transcription of nitrogen assimilation genes (14, 43). It is plausible that the cylindrospermopsin gene cluster is under the same regulation, as it is located wholly within the “hyp” gene cluster in C. raciborskii AWT205, and no obvious promoter region in the cylindrospermopsin gene cluster could be identified.
Finally, the cylindrospermopsin cluster also includes an ORF designated cyrO at its 3′ end. By homology, it encodes a hypothetical protein that appears to possess an ATP binding cassette and is similar to WD repeat proteins, which have diverse regulatory and signal transduction roles. CyrO may also have a role in transcriptional regulation and DNA binding. It also shows homology to AAA family proteins, which often perform chaperone-like functions and assist in the assembly, operation, or disassembly of protein complexes. Further insights into the role of CyrO are hindered due to low sequence homology with other proteins in databases. Cylindrospermopsin is a potent cyanotoxin that is typically produced by a relatively recent lineage of globally invasive cyanobacteria (26). Here we describe the characterization of the cylindrospermopsin biosynthesis gene cluster and propose the complete biosynthetic pathway. These data will enable the development of probes for the detection of potentially toxin-producing strains and harmful blooms, thereby aiding international water quality management schemes. The identification of novel PKS and NRPS modules with previously undescribed substrate specificities furthers the ability to create novel bioactive compounds by combinatorial biosynthesis, and we have also described cyclization and thioesteration mechanisms that are completely novel to microbial secondary metabolism. Furthermore, this study has elucidated the formation of a uracil ring via a novel pathway for pyrimidine biosynthesis, which is essential for the mammalian toxicity of this compound (3). This investigation will allow the future study of the regulation and biological role of cylindrospermopsin with regards to the physiology of cyanobacteria and the ecology of the environments that possess these microorganisms.
Acknowledgments
The Australian Research Council is thanked for its financial support of this work.
We are grateful to Christian Schmidl for his technical assistance. Peter Hawkins is thanked for providing cyanobacterial samples. Thomas Hemscheidt is also thanked for his valuable advice.
This work is dedicated to the memory of Leigh Hardman, who first discovered cylindrospermopsin PKS and NRPS genes.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Baldwin, J. E., R. C. Thomas, L. I. Kruse, and L. Silberman. 1977. Rules for ring closure: ring formation by conjugate addition of oxygen nucleophiles. J. Org. Chem. 42:3846-3852. [Google Scholar]
- 2.Banker, R., S. Carmeli, O. Hadas, B. Teltsch, R. Porat, and A. Sukenik. 1997. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from Lake Kinneret, Isr. J. Phycol. 33:613-616. [Google Scholar]
- 3.Banker, R., S. Carmeli, M. Werman, B. Teltsch, R. Porat, and A. Sukenik. 2001. Uracil moiety is required for toxicity of the cyanobacterial hepatotoxin cylindrospermopsin. J. Toxicol. Environ. Health Part A 62:281-288. [DOI] [PubMed] [Google Scholar]
- 4.Bourke, A. T. C., R. B. Hawes, A. Neilson, and N. D. Stallman. 1983. An outbreak of hepato-enteritis (the Palm Island mystery disease) possibly caused by algal intoxication. Toxicon 21:45-48. [Google Scholar]
- 5.Burgoyne, D. L., T. K. Hemscheidt, R. E. Moore, and M. T. C. Runnegar. 2000. Biosynthesis of cylindrospermopsin. J. Org. Chem. 65:152-156. [DOI] [PubMed] [Google Scholar]
- 6.Byth, S. 1980. Palm Island mystery disease. Med. J. Aust. 2:40, 42. [DOI] [PubMed] [Google Scholar]
- 7.Carmichael, W. W., S. M. F. O. Azevedo, J. S. An, R. J. R. Molica, E. M. Jochimsen, S. Lau, K. L. Rinehart, G. R. Shaw, and G. K. Eaglesham. 2001. Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 109:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chonudomkul, D., W. Yongmanitchai, G. Theeragool, M. Kawachi, F. Kasai, K. Kaya, and M. M. Watanabe. 2004. Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan. FEMS Microbiol. Ecol. 48:345-355. [DOI] [PubMed] [Google Scholar]
- 9.Edwards, D. J., B. L. Marquez, L. M. Nogle, K. McPhail, D. E. Goeger, M. A. Roberts, and W. H. Gerwick. 2004. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 11:817-833. [DOI] [PubMed] [Google Scholar]
- 10.Fastner, J., R. Heinze, A. R. Humpage, U. Mischke, G. K. Eaglesham, and I. Chorus. 2003. Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii (Cyanobacteria) isolates. Toxicon 42:313-321. [DOI] [PubMed] [Google Scholar]
- 11.Fastner, J., J. Reucker, A. Steuken, K. Preussel, B. Nixdorf, I. Chorus, A. Keohler, and C. Wiedner. 2007. Occurrence of the cyanobacterial toxin cylindrospermopsin in northeast Germany. Environ. Toxicol. 22:26-32. [DOI] [PubMed] [Google Scholar]
- 12.Fergusson, K. M., and C. P. Saint. 2000. Molecular phylogeny of Anabaena circinalis and its identification in environmental samples by PCR. Appl. Environ. Microbiol. 66:4145-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froscio, S. M., A. R. Humpage, P. C. Burcham, and I. R. Falconer. 2003. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 18:243-251. [DOI] [PubMed] [Google Scholar]
- 14.Hansel, A., R. Axelsson, P. Lindberg, O. Y. Troshina, R. Wunschiers, and P. Lindblad. 2001. Cloning and characterisation of a hyp gene cluster in the filamentous cyanobacterium Nostoc sp. strain PCC 73102. FEMS Microbiol. Lett. 201:59-64. [DOI] [PubMed] [Google Scholar]
- 15.Harada, K.-I., I. Ohtani, K. Iwamoto, M. Suzuki, M. F. Watanabe, M. Watanabe, and K. Terao. 1994. Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method. Toxicon 32:73-84. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins, P. R., N. R. Chandrasena, G. J. Jones, A. R. Humpage, and I. R. Falconer. 1997. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon 35:341-346. [DOI] [PubMed] [Google Scholar]
- 17.Humpage, A. R., M. Fenech, P. Thomas, and I. R. Falconer. 2000. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. 472:155-161. [DOI] [PubMed] [Google Scholar]
- 18.Kellmann, R., T. Mills, and B. A. Neilan. 2006. Functional modeling and phylogenetic distribution of putative cylindrospermopsin biosynthesis enzymes. J. Mol. Evol. 62:267-280. [DOI] [PubMed] [Google Scholar]
- 19.Kiss, T., A. Vehovszky, L. Hiripi, A. Kovacs, and L. Voros. 2002. Membrane effects of toxins isolated from a cyanobacterium, Cylindrospermopsis raciborskii, on identified molluscan neurones. Comp. Biochem. Physiol. Part C 131:167-176. [DOI] [PubMed] [Google Scholar]
- 20.Lagos, N., H. Onodera, P. A. Zagatto, D. Andrinolo, S. M. F. Q. Azevedo, and Y. Oshima. 1999. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon 37:1359-1373. [DOI] [PubMed] [Google Scholar]
- 21.Li, R., W. W. Carmichael, S. Brittain, G. K. Eaglesham, G. R. Shaw, Y. Liu, and M. M. Watanabe. 2001. First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Raphidiopsis curvata (cyanobacteria). J. Phycol. 37:1121-1126. [Google Scholar]
- 22.Li, R., W. W. Carmichael, S. Brittain, G. K. Eaglesham, G. R. Shaw, A. Mahakhant, N. Noparatnaraporn, W. Yongmanitchai, K. Kaya, and M. M. Watanabe. 2001. Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii (Cyanobacteria). Toxicon 39:973-980. [DOI] [PubMed] [Google Scholar]
- 23.Moffitt, M. C., and B. A. Neilan. 2004. Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 70:6353-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neilan, B. A. 1995. Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Appl. Environ. Microbiol. 61:2286-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neilan, B. A., D. Jacobs, T. Del Dot, L. L. Blackall, P. R. Hawkins, P. T. Cox, and A. E. Goodman. 1997. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int. J. Syst. Bacteriol. 47:693-697. [DOI] [PubMed] [Google Scholar]
- 26.Neilan, B. A., M. L. Saker, J. Fastner, A. Torokne, and B. P. Burns. 2003. Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Mol. Ecol. 12:133-140. [DOI] [PubMed] [Google Scholar]
- 27.Norris, R. L. G., G. K. Eaglesham, G. R. Shaw, P. Senogles, R. K. Chiswell, M. J. Smith, B. C. Davis, A. A. Seawright, and M. R. Moore. 2001. Extraction and purification of the zwitterions cylindrospermopsin and deoxycylindrospermopsin from Cylindrospermopsis raciborskii. Environ. Toxicol. 16:391-396. [DOI] [PubMed] [Google Scholar]
- 28.Norris, R. L., G. K. Eaglesham, G. Pierens, G. R. Shaw, M. J. Smith, R. K. Chiswell, A. A. Seawright, and M. R. Moore. 1999. Deoxycylindrospermopsin, an analog of cylindrospermopsin from Cylindrospermopsis raciborskii. Environ. Toxicol. 14:163-165. [DOI] [PubMed] [Google Scholar]
- 29.Ohtani, I., R. E. Moore, and M. T. C. Runnegar. 1992. Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 114:7941-7942. [Google Scholar]
- 30.Pearson, L. A., M. Hisbergues, T. Borner, E. Dittmann, and B. A. Neilan. 2004. Inactivation of an ABC transporter gene, mcyH, results in loss of microcystin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Appl. Environ. Microbiol. 70:6370-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollingher, U., O. Hadas, Y. Z. Yacobi, T. Zohary, and T. Berman. 1998. Aphanizomenon ovalisporum (Forti) in lake Kinneret, Israel. J. Plankton Res. 20:1321-1339. [Google Scholar]
- 32.Preussel, K., A. Stuken, C. Wiedner, I. Chorus, and J. Fastner. 2006. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon 47:156-162. [DOI] [PubMed] [Google Scholar]
- 33.Runnegar, M. T., S.-M. Kong, Y.-Z. Zhong, and S. C. Lu. 1995. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 49:219-225. [DOI] [PubMed] [Google Scholar]
- 34.Runnegar, M. T., S. M. Kong, Y. Z. Zhong, J. L. Ge, and S. C. Lu. 1994. The role of glutathione in the toxicity of a novel cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Biophys. Res. Commun. 201:235-241. [DOI] [PubMed] [Google Scholar]
- 35.Runnegar, M. T., C. Xie, B. B. Snider, G. A. Wallace, S. M. Weinreb, and J. Kuhlenkamp. 2002. In vitro hepatotoxicity of the cyanobacterial alkaloid cylindrospermopsin and related synthetic analogues. Toxicol. Sci. 67:81-87. [DOI] [PubMed] [Google Scholar]
- 36.Saker, M. L., B. A. Neilan, and D. J. Griffiths. 1999. Two morphological forms of Cylindrospermopsis raciborskii (cyanobacteria) isolated from Solomon dam, Palm Island, Queensland. J. Phycol. 35:599-606. [Google Scholar]
- 37.Saker, M. L., I. C. G. Nogueira, V. M. Vasconcelos, B. A. Neilan, G. K. Eaglesham, and P. Pereira. 2003. First report and toxicological assessment of the cyanobacterium Cylindrospermopsis raciborskii from Portuguese freshwaters. Ecotoxicol. Environ. Saf. 55:243-250. [DOI] [PubMed] [Google Scholar]
- 38.Saker, M. L., and A. D. Thomas. 1999. Cattle mortality attributed to the toxic cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 14:179-182. [Google Scholar]
- 39.Schembri, M. A., B. A. Neilan, and C. P. Saint. 2001. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 16:413-421. [DOI] [PubMed] [Google Scholar]
- 40.Shalev-Alon, G., A. Sukenik, O. Livnah, R. Schwarz, and A. Kaplan. 2002. A novel gene encoding amidinotransferase in the cylindrospermopsin producing cyanobacterium Aphanizomenon ovalisporum. FEMS Microbiol. Lett. 209:87-91. [DOI] [PubMed] [Google Scholar]
- 41.Spoof, L., K. A. Berg, J. Rapala, K. Lahti, L. Lepist, J. S. Metcalf, G. A. Codd, and J. Meriluoto. 2006. First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environ. Toxicol. 21:552-560. [DOI] [PubMed] [Google Scholar]
- 42.Stirling, D. J., and M. A. Quilliam. 2001. First report of the cyanobacterial toxin cylindrospermopsin in New Zealand. Toxicon 39:1219-1222. [DOI] [PubMed] [Google Scholar]
- 43.Tamagnini, P., R. Axelsson, P. Lindberg, F. Oxelfelt, R. Wunschiers, and P. Lindblad. 2002. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol. Mol. Biol. Rev. 66:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, A. S., J. C. Rhodes, and I. Pettman. 1988. Culture collection of algae and protozoa: catalogue of strains, 5th ed. Natural Environment Research Council, Freshwater Biological Association, Ambleside, United Kingdom.
- 45.Tillett, D., E. Dittmann, M. Erhard, H. von Dohren, T. Borner, and B. A. Neilan. 2000. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem. Biol. 7:753-764. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, K. M., M. A. Schembri, P. D. Baker, and C. P. Saint. 2000. Molecular characterization of the toxic cyanobacterium Cylindrospermopsis raciborskii and design of a species-specific PCR. Appl. Environ. Microbiol. 66:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]