Various marine organisms, from animals to bacteria, have n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs) with 20 or 22 carbon atoms such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (7). In mammals and fish, DHA accumulates as the most abundant fatty acid among membrane phospholipids in the brain and the eye, as in land animals (11, 16). Therefore, EPA and DHA, as well as arachidonic acid (n-6), attract special interest for their physiologically important functions in humans (17, 22) and even in animals (8) and are used in pharmaceuticals and/or food supplements.
EPA and DHA are distributed universally in marine seaweeds and microalgae (7). Some marine psychrophilic and/or piezophilic bacterial species and several enteric species of marine fish and other animals can produce these LC-PUFAs (12, 53). Bacterial EPA and DHA are found exclusively in cell membrane phospholipids, and their content accounted for up to 30% (wt/wt) of the total fatty acids (38). In eukaryotic microorganisms, these n-3 LC-PUFAs are distributed in phospholipids and storage lipids (31, 50). The content of DHA in phosphatidylcholine and triacylglycerol of some marine thraustochytrids was more than 50 and 40%, respectively, of their totals (31, 39).
In poikilothermic organisms, EPA and DHA, like other PUFAs, are generally considered to play an important role in maintaining optimal membrane fluidity as acyl components of structural lipids under low-temperature or high-hydropressure conditions (12, 48, 52). However, whether this is their primary function has not been clarified: first, because EPA-deficient mutants derived from EPA-producing psychrophilic bacteria do not necessarily become cold sensitive (2), and second, because the ecological distribution of n-3 LC-PUFA-producing bacteria and eukaryotic microorganisms is not limited to cold environments. Interestingly, heterocont algae that include extremely high levels of DHA in storage triacylglycerol and in membrane phospholipids have been isolated even from tropical and subtropical marine environments (33, 39, 42, 50), and some n-3 LC-PUFA-producing bacteria have mesophilic growth temperatures (21).
In general, polyunsaturated fatty acids, including n-3 LC-PUFAs, are among the molecules most susceptible to oxygen and reactive oxygen species (ROS) (19). However, there is a growing body of evidence that n-3 LC-PUFAs and other LC-PUFAs are rather stable when they are in vivo against oxidative stresses caused by ROS (see below and also references 5, 29, and 54). Although they are not LC-PUFA, linoleic and linolenic acids, which are the most common modulator of membrane fluidity in poikilotherms, are requisite for optimum growth, respiration, and photosynthesis (47) and for protecting the photosynthetic machinery against salt-induced damages in cyanobacteria (1). However, no clear definite role of these PUFAs has been elucidated. We review here the physiological functions and particularly the antioxidative effects of EPA and DHA in bacterial and other microbial systems in marine environments.
DISTRIBUTION OF EPA AND DHA
EPA and DHA are sometimes called “marine lipids” because of their preferential distribution in marine environments. Most marine animals, protozoa, seaweeds, and microalgae, and some bacterial species, have EPA and/or DHA (7, 8, 14, 27, 33). It is noteworthy that EPA and DHA are scarcely detectable in the higher land plants and terrestrial algae and that some marine seed plants contain EPA (45). Only EPA is found in some ferns and mosses and in freshwater diatoms (3). Interestingly, for green algae these n-3 LC-PUFAs are found in their marine forms but not in their terrestrial ones (3). Nichols (32) listed 20 LC-PUFA-producing bacterial species in six genera isolated from marine environments. Eighteen of them are halophilic but two, Shewanella frigidimarina and Shewanella japonica, are not. Although S. frigidimarina and S. japonica can grow in the absence of NaCl (nonhalophilic), they grow well at 9% NaCl (9) and 1 to 3% NaCl (21), respectively. Cyanobacteria, to our knowledge, have no n-3 LC-PUFAs, irrespective of their sources.
As many marine animals lack the ability to synthesize n-3 LC-PUFAs de novo (32), they rely on a dietary supply of n-3 LC-PUFAs. Microalgae have been regarded as the principal producer of n-3 LC-PUFAs in marine food webs (32), where some marine bacteria and marine animals' enteric bacteria that have EPA or DHA may contribute as primary producers of these n-3 LC-PUFAs. Considering their preferential distribution in marine organisms, it is likely that the presence of LC-PUFAs is closely associated with a marine habitat.
OXIDATIVE STABILITY OF EPA AND DHA IN BIOLOGICAL SYSTEMS
PUFAs, including EPA and DHA, are cellular compounds that are easily oxidized when exposed to air or dissolved in organic solvents, because they have many bisallylic hydrogen atoms (19). However, in the aqueous system PUFAs are stable against peroxidation (5, 29, 54). In liposomes made of phospholipids, higher unsaturation of fatty acids leads to their higher oxidative stability (5). Although the molecular mechanism is not known, the oxidative stability of PUFAs, when they are present as mass substance (in the bulk phase), is entirely different from that seen in aqueous biological systems.
ANTIOXIDATIVE FUNCTIONS
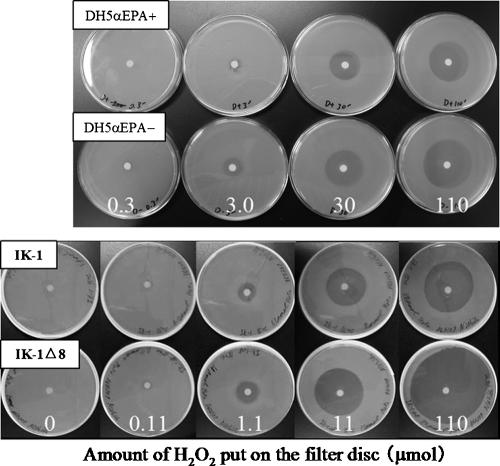
Recently, the antioxidative function of EPA was investigated using EPA-producing bacterial recombinant systems. Escherichia coli can be conferred with the ability to synthesize EPA de novo (10% or more of total fatty acids [28, 34]) by being transformed with the clustered EPA biosynthesis genes (pfaA, pfaB, pfaC, pfaD, and pfaE) derived from EPA-producing marine bacteria. The pfa genes encode proteins involved in the biosynthesis of EPA and DHA in a polyketide biosynthesis mode (28, 40, 41). This is a quite different biosynthetic way from that found in eukaryotic organisms, where PUFAs are synthesized by combination of desaturation and chain elongation of fatty acids (18). To investigate the antioxidative function of EPA, Nishida et al. (36, 37) utilized EPA-producing E. coli strains DH5α and UM2 that had been transformed with pfaA-E genes from marine Shewanella pneumatophori SCRC-2738. In the two recombinants, EPA protected cells from the effects of exogenous H2O2, namely, growth inhibition, carbonylation (oxidation) of cellular proteins, and breakage of the cell structure. Treating transformed cells that possess EPA with H2O2 did not result in notable peroxidation of the EPA. The catalase activity of E. coli DH5α cells was affected neither by transforming the cells with the pfa genes nor by treating them with H2O2. These results suggested that EPA had an antioxidative effect against exogenous H2O2. The transformant catalase-deficient mutant E. coli UM2, which included EPA, had consistently lower intracellular concentrations of H2O2 than the control strain, which did not include EPA, when treated exogenously with H2O2. Hence, EPA in E. coli transformants served to shield the membrane against the H2O2 molecule. Almost the same results have been obtained using the marine Shewanella marinitestina IK-1, which naturally produces EPA (ca. 20%), and its EPA-deficient mutant IK-1Δ8 (38). The antioxidative effects of EPA can be relatively easily evaluated by growth inhibition testing on plates using E. coli transformants and S. marinitestina IK-1 strains (Fig. 1).
FIG. 1.
Growth inhibition caused by hydrogen peroxide (H2O2) in E. coli DH5α strains with or without EPA (upper panel) and in EPA-producing S. marinintestina IK-1 and its EPA-deficient mutant IK-1Δ8 (lower panel). To perform growth inhibition tests in petri dishes, 200-μl aliquots of bacterial cultures reaching an optical density at 600 nm of 1.0 were mixed with 10 ml of LB medium containing 0.7% (wt/vol) agar, 50 μg of ampicillin/ml, and 50 μg of chloramphenicol/ml for E. coli recombinants and with 10 ml of LB medium containing 0.5 M NaCl but no antibiotics for S. marinintestina strains, and then the mixtures were layered onto LB plates containing 1.5% (wt/vol) agar and the same components, with a filter paper disc (5 mm in diameter) placed in the center. Ten-microliter aliquots of solutions containing various amounts of H2O2 (0.3 to 110 μmol for E. coli DH5α strains and 0 to 110 μmol for S. marinintestina strains) were put on the filter disc. Plates were then incubated at 20°C for 2 days and at 15°C for 3 days for E. coli DH5α strains and S. marinintestina strains, respectively. Narrower areas of growth inhibition caused by H2O2 were clearly observed for cells with EPA than for those without EPA, showing that cellular production of EPA could relieve the H2O2-induced growth inhibition of bacterial cells. (See references 34 and 35 for details.)
Although there are no data to show the molecular mechanism of the membrane-shielding effects of EPA in bacterial systems, the inherent molecular structure of the phospholipids having EPA and hexadecanoic or hexadecenoic acids might be involved in the antioxidant mechanism. Thus, phospholipids with DHA or arachidonic acid have a more highly packed structure than those with less unsaturated fatty acids, and lipid membranes consisting of phospholipids with saturated fatty acids such as hexadecanoic acid (16:0) and LC-PUFAs such as DHA and probably EPA may form more hydrophobic interfaces between the phospholipid bilayers (25, 43, 44). This hydrophobic interface of the cell membrane might prevent the entry of the hydrophilic H2O2 molecule. The in vivo antioxidative function of EPA probably arises through a combination of EPA and its counterpart fatty acid of the phospholipid molecules to shield membranes against the effects of exogenous ROS (Fig. 2). More hydrophobic interfaces between the artificial phospholipid bilayers can be formed using phospholipid consisting of equimolar 16:0 and arachidonic acid or equimolar 16:0 and DHA (43). The similar structure could be generated in E. coli transformant cells with EPA and EPA-producing S. marinitestina, although their EPA levels were ca. 10 and 20%, respectively. E. coli DH5α transformants with more EPA became more resistant against the treatment with exogenous H2O2 (34, 35), suggesting that the integrity or strength of hydrophobic interfaces between the phospholipid bilayers might be dependent on the content of LC-PUFAs of membrane phospholipids.
FIG. 2.
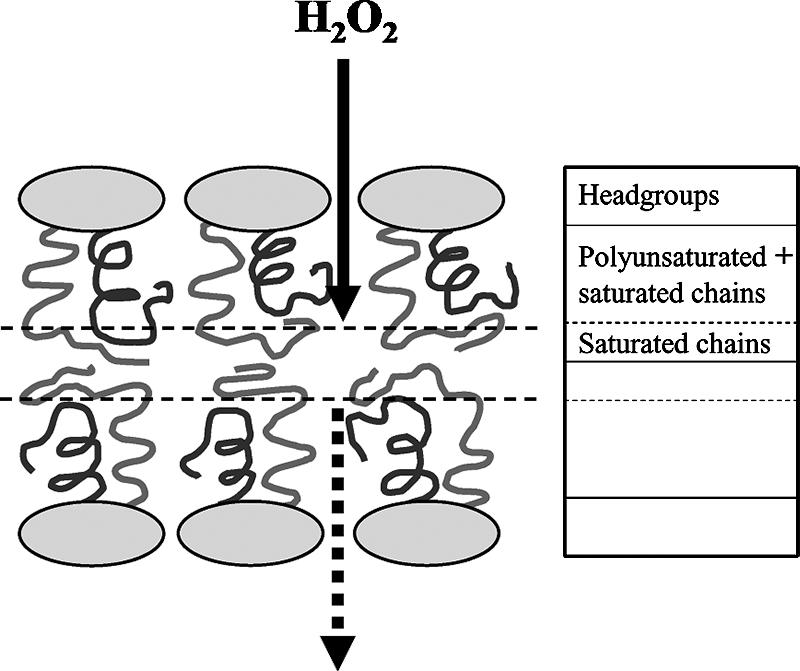
Proposed mechanism of the membrane-shielding effects of long chain polyunsaturated fatty acids. The acyl chain mass for mixed-chain saturated-polyunsaturated phospholipids (phosphatidylcholine in this case) in the fluid state can be distributed as shown in the figure. The saturated chain (gray) is displaced toward the bilayer center, while the polyunsaturated chain (black) is located closer to the water interface. The hydrophobic layer (shown by two parallel broken lines) composed only of saturated chains between outer and inner leaflets of the membrane may act as the shield of hydrophilic compounds such as H2O2. The flow of H2O2 is illustrated by two types of arrow. Adapted with permission from reference 43. Copyright 2005 American Chemical Society.
The membrane-shielding effects of n-3 LC-PUFAs have been shown only for bacterial cells producing EPA (36-38). However, our preliminary result showed that E. coli cells carrying pfa genes responsible for DHA biosynthesis became more resistant to exogenous H2O2 than cells not possessing DHA (T. Nishida and H. Okuyama, unpublished results). Therefore, this function would be common for EPA and DHA. It is necessary to investigate whether LC-PUFAs other than EPA and DHA have similar effects.
The antioxidative functions of DHA that are not based on its membrane-shielding effects against ROS have been reported in animals and their cultured cells (6, 20). In animal cells, docosahexanoids, such as 17S-hydroxy-DHA, an oxidized metabolite of DHA, enhance catalase activity or the production of reduced glutathione; here, the docosahexanoids function as signal molecules. In addition, these docosahexanoids inhibit the expression of proapoptotic proteins (such as BCl-1 and Bax) that are induced by ROS (15). According to Araseki et al. (4), highly unsaturated fatty acids such as DHA are more resistant to lipid peroxidation caused by exogenous H2O2 in human hepatoma cells, which is analogous to the finding in E. coli transformant cells that produce EPA or DHA. Considering the molecular structure and specific properties of phospholipids with n-3 LC-PUFAs, their membrane-shielding function against exogenous ROS might also operate in eukaryotes, including marine algae.
Cellular EPA might have an antioxidative effect against intracellular ROS, because in the absence of extracellular H2O2 EPA decreased the protein carbonyl content in E. coli DH5α cells transformed with pfa genes (36). Oxidative stress causing the carbonylation of proteins occurs even in E. coli DH5α cells that carry a vector, that grow in the presence of antibiotics, or that grow in low-temperature conditions (36). This stress was relieved by EPA. Although no other direct data supporting the antioxidative shielding effects of EPA against endogenous ROS were obtained, the speculation described above can be supported by the fact that marine raphidophycean flagellates (Chattonella) that produce high levels of ROS to kill fish are EPA-accumulating microalgae (26) and that high levels of ROS are not toxic to ROS-producing cells. External stimuli (environmental stresses) also induce the generation of various ROS in organisms (30, 46, 49). Therefore, the membrane-shielding effects of LC-PUFAs appear to operate generally against endogenously produced ROS and can protect the membrane proteins (proteins inside and outside organelles for eukaryotes) from being damaged oxidatively.
Although not all psychrophilic marine bacteria necessarily have n-3 LC-PUFAs, genome projects for such bacteria, including Colwellia psychroerythraea, Desulfotalea psychrophila, and Flavobacterium psychrophilum, demonstrate that these bacteria commonly have a wide variety of proteins involved in antioxidation (10, 13). This is likely because low temperature increases the solubility of oxygen and other ROS (13). Therefore, for at least n-3 LC-PUFA-producing psychrophilic bacteria such as C. psychroerythraea, these PUFAs (DHA for C. psychroerythraea) should function as antioxidative components in cell membranes under their natural low-temperature environments.
ECOLOGICAL SIGNIFICANCE OF EPA AND DHA IN MARINE ENVIRONMENTS
According to Lesser (24), the production of ROS is prevalent in the world's oceans and oxidative stress is an important component of the stress response in marine organisms. In marine systems, the absorption of solar radiation by dissolved organic matter in seawater leads to the photochemical production of diverse reactive transients including ROS. Of these, H2O2 has the longest lifetime in seawater and the highest steady-state concentration of 10−7 M; it can pass readily through biological membranes (24). Hence, marine organisms must be exposed to exogenous ROS including H2O2. In addition, some phytoplanktons (microalgae), such as dinoflagellates, produce high levels of ROS, such as superoxide anions, hydroxyl radicals, and H2O2 under normal physiological conditions, and these are involved in killing fish and other organisms exposed to them (26, 51). Since marine organisms cannot avoid these challenges by biotic and abiotic ROS, the membrane-shielding effects of n-3 LC-PUFAs likely operate as a primary protective “breakwater” for all marine microorganisms possessing them.
CONCLUSION AND PERSPECTIVES
The n-3 LC-PUFAs such as EPA and DHA are distributed preferentially in marine environments. Their primary producers are limited to microalgae and probably to some psychrophilic, piezophilic, or halophilic bacteria. These unsaturated fatty acids can be provided to all marine animals via food webs. Given their chemical stability against oxidation by ROS in organisms and the natural generation of biotic and abiotic ROS in seawaters, EPA and probably DHA could operate as antioxidative components in marine biological systems. In bacteria, and probably in microalgae, membrane phospholipids with EPA and/or DHA would function as shield molecules against such oxidative challenges exogenously and endogenously raised in marine environments.
When EPA-producing E. coli recombinant cells were treated with butyl hydroperoxide, a biologically inert analog of H2O2, the same results as with H2O2 were obtained (35). However, more varieties of ROS and hydrophilic and hydrophobic compounds leading to endogenous generation of ROS must be tested to investigate the substrate specificity of the membrane-shielding effects of n-3 LC-PUFAs using these E. coli recombinant systems. The use of marine and nonmarine bacteria that inherently produce EPA or DHA as experimental materials might help illustrate the in situ function of n-3 LC-PUFAs in marine environments, although, to our knowledge, no EPA- or DHA-producing nonmarine bacteria are available yet.
Another approach to prove the membrane-shielding effect of n-3 LC-PUFAs and probably arachidonic acid is the usage of artificial membranes as a model system. Since this effect is a purely physical function, the same membrane-shielding effect against ROS should be observed in liposomes made of phospholipids containing LC-PUFAs. Inclusion of a soluble protein(s) (and hydrophobic proteins) in the liposomes would make it possible to know how the protein can be oxidized by exogenously added ROS. In addition, physical structures of liposomes could be analyzed much more easily and precisely by instrumental analysis such as proton nuclear magnetic resonance and X-ray diffraction compared to biological membranes (43). Taken together, the structural hindrance of the cell membranes containing LC-PUFAs against ROS might be evidenced as a principal contribution factor in the stability of LC-PUFAs in biological (aqueous) systems.
As stated above, low temperature and oxidative stress are interrelated (46, 49), and the increased oxidative stresses caused by low temperature could be relieved by n-3 LC-PUFAs (36). However, it has not been elucidated whether salinity is directly related to oxidative stress. According to Leblanc et al. (23), alkyl hydroperoxide reductase was induced commonly when Shewanella putrefaciens was treated independently with low temperature and NaCl. This suggests that the gene for this enzyme might play a key role in cross-protection against the NaCl challenge induced by growth at low temperature. Although the S. putrefaciens used in that study is neither a marine bacterium nor one that produces n-3 LC-PUFAs, oxidative stresses raised by high salinity in n-3 LC-PUFA-producing forms are expected to be relieved by these unsaturated fatty acids, as in recombinant E. coli cells producing EPA or DHA. Since normal salinity (3% NaCl) is not considered an external stress for marine organisms, some combination of salinity with other environmental stimuli such as low temperature, hydropressure, or solar radiation might lead to increased oxidative stresses in these organisms. Microarray and proteomics technologies would be useful to elucidate the inter-relationship between oxidative stresses and environmental stimuli.
Acknowledgments
We thank Michael F. Brown, Naoki Morita, and Kazuo Watanabe for providing the original figure used as Fig. 2 in this article and for their valuable discussion in the course of this work.
This study was financially supported, in part, by the National Institute of Polar Research.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Allakhverdiev, S. I., M. Kinoshita, M. Inaba, I. Suzuki, and N. Murata. 2001. Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol. 125:1842-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, E. E., D. Facciotti, and D. H. Bartlett. 1999. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum SS9 at high pressure and low temperature. Appl. Environ. Microbiol. 65:1710-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki, S., and M. Kayama. 1995. Distribution of highly unsaturated fatty acids in plants, p. 11-43. In M. Kayama (ed.), AA, EPA, DHA: highly unsaturated fatty acids. Kouseishakouseikaku, Tokyo, Japan.
- 4.Araseki, M., H. Kobayashi, M. Hosokawa, and K. Miyashita. 2005. Lipid peroxidation of a human hepatoma cell line (HepG2) after incorporation of linoleic acid, arachidonic acid, and docosahexaenoic acid. Biosci. Biotechnol. Biochem. 69:483-490. [DOI] [PubMed] [Google Scholar]
- 5.Araseki, M., K. Yamamoto, and K. Miyashita. 2002. Oxidative stability of polyunsaturated fatty acid in phosphatidylcholine liposomes. Biosci. Biotechnol. Biochem. 66:2573-2577. [DOI] [PubMed] [Google Scholar]
- 6.Bechoua, S., M. Dubois, Z. Dominguez, A. Goncalves, G. Nemoz, M. Lagarde, and A. M. Prigent. 1999. Protective effect of docosahexaenoic acid against hydrogen peroxide-induced oxidative stress in human lymphocytes. Biochem. Pharmacol. 57:1021-1030. [DOI] [PubMed] [Google Scholar]
- 7.Bergé, J. P., and G. Barnathan. 2005. Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biotechnol. 96:49-125. [DOI] [PubMed] [Google Scholar]
- 8.Bezard, J., J. P. Blond, A. Bernard, and P. Clouet. 1994. The metabolism and availability of essential fatty acids in animal and human tissues. Reprod. Nutr. Dev. 34:539-568. [DOI] [PubMed] [Google Scholar]
- 9.Bowman, J. P., S. A. McCammon, D. S. Nichols, J. H. Skerratt, S. M. Rea, P. D. Nichols, and T. A. McMeekin. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5 omega 3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 47:1040-1047. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico, S., T. Collins, J. C. Marx, G. Feller, and C. Gerday. 2006. Psychrophilic microorganisms: challenges for life. EMBO Rep. 7:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das, U. N. 2006. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol. J. 1:420-439. [DOI] [PubMed] [Google Scholar]
- 12.DeLong, E. F., and A. A. Yayanos. 1986. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl. Environ. Microbiol. 51:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duchaud, E., M. Boussaha, V. Loux, J. F. Bernardet, C. Michel, B. Kerouault, S. Mondot, P. Nicolas, R. Bossy, C. Caron, P. Bessières, J. F. Gibrat, S. Claverol, F. Dumetz, M. L. Hénaff, and A. Benmansour. 2007. Complete genome sequence of the fish pathogen Flavobacterium psychrophilum. Nat. Biotechnol. 25:763-769. [DOI] [PubMed] [Google Scholar]
- 14.Gentile, G., V. Bonasera, C. Amico, L. Giuliano, and M. M. Yakimov. 2003. Shewanella sp. GA-22, a psychrophilic hydrocarbonoclastic Antarctic bacterium producing polyunsaturated fatty acids. J. Appl. Microbiol. 95:1124-1133. [DOI] [PubMed] [Google Scholar]
- 15.German, O. L., M. F. Insua, C. Gentili, N. P. Rotstein, and L. E. Politi. 2006. Docosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathway. J. Neurochem. 98:1507-1520. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, R. A., M. A. Neumann, and M. Makrides. 1996. Effect of dietary docosahexaenoic acid on brain composition and neural function in term infants. Lipids 31:S177-S181. [DOI] [PubMed] [Google Scholar]
- 17.Gill, I., and R. Valivety. 1997. Polyunsaturated fatty acids. I. Occurrence, biological activities, and applications. Trends Biotechnol. 15:401-409. [DOI] [PubMed] [Google Scholar]
- 18.Gurr, M. I. 1974. The biosynthesis of unsaturated fatty acids. Int. Rev. Sci. 4:181-235. [Google Scholar]
- 19.Halliwell, B., and J. M. Gutteridge. 1998. Free radicals in biology and medicine, 3rd ed. Oxford University Press, Oxford, England.
- 20.Hossain, M. S., M. Hashimoto, S. Gamoh, and S. Masumura. 1999. Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J. Neurochem. 72:1133-1138. [DOI] [PubMed] [Google Scholar]
- 21.Ivanova, E. P., T. Sawabe, N. M. Gorshkova, V. I. Svetashev, V. V. Mikhailov, D. V. Nicolau, and R. Christen. 2001. Shewanella japonica sp. nov. Int. J. Syst. Evol. Microbiol. 51:1027-1033. [DOI] [PubMed] [Google Scholar]
- 22.Kroes, R., E. J. Schaefer, R. A. Squire, and G. M. Williams. 2003. A review of the safety of DHA45-oil. Food Chem. Toxicol. 41:1433-1446. [DOI] [PubMed] [Google Scholar]
- 23.Leblanc, L., C. Leboeuf, F. Leroi, A. Hartke, and Y. Auffray. 2003. Comparison between NaCl tolerance response and acclimation to cold temperature in Shewanella putrefaciens. Curr. Microbiol. 46:157-162. [DOI] [PubMed] [Google Scholar]
- 24.Lesser, M. P. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol. 68:253-278. [DOI] [PubMed] [Google Scholar]
- 25.Litman, B. J., E. N. Lewis, and I. W. Levin. 1991. Packing characteristics of highly unsaturated bilayer lipids: Raman spectroscopic studies of multilamellar phosphatidylcholine dispersions. Biochemistry 30:313-319. [DOI] [PubMed] [Google Scholar]
- 26.Marshall, J. A., P. D. Nichols, B. Hamilton, R. J. Lewis, and G. M. Hallegraeff. 2003. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): the synergistic role of reactive oxygen species and free fatty acids. Harmful Algae 2:273-281. [Google Scholar]
- 27.Methé, B. A., K. F. Nelson, J. W. Deming, B. Momen, E. Melamud, X. Zhang, J. Moult, R. Madupu, W. C. Nelson, R. J. Dodson, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, R. T. DeBoy, J. F. Kolonay, S. A. Sullivan, L. Zhou, T. M. Davidsen, M. Wu, A. L. Huston, M. Lewis, B. Weaver, J. F. Weidman, H. Khouri, T. R. Utterback, T. V. Feldblyum, and C. M. Fraser. 2005. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. USA 102:10913-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metz, J. G., P. Roessler, D. Facciotti, C. Levering, F. Dittrich, M. Lassner, R. Valentine, K. Lardizabal, F. Domergue, A. Yamada, K. Yazawa, V. Knauf, and J. Browse. 2001. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290-293. [DOI] [PubMed] [Google Scholar]
- 29.Miyashita, K., E. Nara, and T. Ota. 1993. Oxidative stability of polyunsaturated fatty acids in an aqueous solution. Biosci. Biotechnol. Biochem. 57:1638-1640. [Google Scholar]
- 30.Mizunoe, Y., S. N. Wai, A. Takede, and S. Yoshida. 1999. Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157 cells by using H2O2-degrading compounds. Arch. Microbiol. 172:63-67. [DOI] [PubMed] [Google Scholar]
- 31.Morita, E., Y. Kumon, T. Nakahara, S. Kagiwada, and T. Noguchi. 2006. Docosahexaenoic acid production and lipid-body formation in Schizochytrium limacinum SR21. Mar. Biotechnol. 8:319-327. [DOI] [PubMed] [Google Scholar]
- 32.Nichols, D. S. 2003. Prokaryotes and the input of polyunsaturated fatty acids to the marine food web. FEMS Microbiol. Lett. 219:1-7. [DOI] [PubMed] [Google Scholar]
- 33.Nichols, D. S., P. D. Nichols, N. J. Russell, N. W. Davies, and T. A. McMeekin. 1997. Polyunsaturated fatty acids in the psychrophilic bacterium Shewanella gelidimarina ACAM 456T: molecular species analysis of major phospholipids and biosynthesis of eicosapentaenoic acid. Biochim. Biophys. Acta 1347:164-176. [DOI] [PubMed] [Google Scholar]
- 34.Nishida, T., Y. Orikasa, K. Watanabe, N. Morita, and H. Okuyama. 2007. Evaluation of the antioxidative effects of eicosapentaenoic acid by growth inhibition testing on plates using Escherichia coli transformed with pfa genes, p. 116-119. In C. Benning and J. Ohlrogge (ed.), Current advances in the biochemistry and cell biology of plant lipids: proceedings of the 17th International Symposium on Plant Lipids. Aardvark Global Publishing Co., Salt Lake City, UT.
- 35.Nishida, T. 2007. Evaluation of the antioxidative function of n-3 long chain-polyunsaturated fatty acids in bacteria. Ph.D. thesis. Hokkaido University, Sapporo, Japan.
- 36.Nishida, T., Y. Orikasa, Y. Ito, R. Yu, A. Yamada, K. Watanabe, and H. Okuyama. 2006. Escherichia coli engineered to produce eicosapentaenoic acid becomes resistant against oxidative damages. FEBS Lett. 580:2731-2735. [DOI] [PubMed] [Google Scholar]
- 37.Nishida, T., Y. Orikasa, K. Watanabe, and H. Okuyama. 2006. The cell membrane-shielding function of eicosapentaenoic acid for Escherichia coli against exogenously added hydrogen peroxide. FEBS Lett. 580:6690-6694. [DOI] [PubMed] [Google Scholar]
- 38.Nishida, T., Y. Yano, N. Morita, and H. Okuyama. 2007. The antioxidative function of eicosapentaenoic acid in a marine bacterium, Shewanella marinintestina IK-1. FEBS Lett. 581:4212-4216. [DOI] [PubMed] [Google Scholar]
- 39.Okuyama, H., Y. Orikasa, and T. Nishida. 2007. In vivo conversion of triacylglycerol to docosahexaenoic acid-containing phospholipids in a thraustochytrid-like microorganism, strain 12B. Biotechnol. Lett. 29:1977-1981. [DOI] [PubMed] [Google Scholar]
- 40.Okuyama, H., Y. Orikasa, T. Nishida, and N. Morita. 2007. Bacterial genes responsible for the biosynthesis of eicosapentaenoic and docosahexaenoic acids and their heterologous expression. Appl. Environ. Microbiol. 73:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orikasa, Y., T. Nishida, A. Yamada, R. Yu, K. Watanabe, A. Hase, N. Morita, and H. Okuyama. 2006. Recombinant production of docosahexaenoic acid in a polyketide biosynthesis mode in Escherichia coli. Biotechnol. Lett. 28:1841-1847. [DOI] [PubMed] [Google Scholar]
- 42.Perveen, Z., H. Ando, A. Ueno, Y. Ito, Y. Yamamoto, Y. Yamada, T. Takagi, T. Kaneko, K. Kogame, and H. Okuyama. 2006. Isolation and characterization of a novel thraustochytrid-like microorganism that efficiently produces docosahexaenoic acid. Biotechnol. Lett. 28:197-202. [DOI] [PubMed] [Google Scholar]
- 43.Rajamoorthi, K., H. I. Petrache, T. J. McIntosh, and M. F. Brown. 2005. Packing and viscoelasticity of polyunsaturated omega-3 and omega-6 lipid bilayers as seen by 2H NMR and X-ray diffraction. J. Am. Chem. Soc. 127:1576-1588. [DOI] [PubMed] [Google Scholar]
- 44.Saiz, L., and M. L. Klein. 2001. Structural properties of a highly polyunsaturated lipid bilayer from molecular dynamics simulations. Biophys. J. 81:204-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanina, N. M., S. N. Goncharova, and E. Y. Kostetsky. 2004. Fatty acid composition of individual polar lipid classes from marine macrophytes. Phytochemistry 65:721-730. [DOI] [PubMed] [Google Scholar]
- 46.Smirnova, G. V., O. N. Zakirova, and O. N. Oktiabr'skii. 2001. Role of the antioxidant system in response of Escherichia coli bacteria to cold stress. Mikrobiologia 70:55-60. [PubMed] [Google Scholar]
- 47.Tasaka, Y., Z. Gombos, Y. Nishiyama, P. Mohanty, T. Ohba, K. Ohki, and N. Murata. 1996. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 15:6416-6425. [PMC free article] [PubMed] [Google Scholar]
- 48.Valentine. R. C., and D. L. Valentine. 2004. Omega-3 fatty acids in cellular membranes: a unified concept. Prog. Lipid Res. 43:383-402. [DOI] [PubMed] [Google Scholar]
- 49.Venditti, P., R. Pamplona, M. Portero-Otin, R. De Rosa, and S. Di Meo. 2006. Effect of experimental and cold exposure induced hyperthyroidism on H2O2 production and susceptibility to oxidative stress of rat liver mitochondria. Arch. Biochem. Biophys. 447:11-22. [DOI] [PubMed] [Google Scholar]
- 50.Yaguchi, T., S. Tanaka, T. Yokochi, T. Nakahara, and T. Higashihara. 1997. Production of high yields of docosahexaenoic acid by Schizochytrium limacinum strain SR21. J. Am. Oil Chem. Soc. 74:1431-1434. [Google Scholar]
- 51.Yamasaki, Y., D. I. Kim, Y. Matsuyama, T. Oda, and T. Honjo. 2004. Production of superoxide anion and hydrogen peroxide by the red tide dinoflagellate Karenia mikimotoi. J. Biosci. Bioeng. 97:212-215. [DOI] [PubMed] [Google Scholar]
- 52.Yano, Y., A. Nakayama, K. Ishihara, and H. Saito. 1998. Adaptive changes in membrane lipids of barophilic bacteria in response to changes in growth pressure. Appl. Environ. Microbiol. 64:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yano, Y., A. Nakayama, H. Saito, and K. Ishihara. 1994. Production of docosahexaenoic acid by marine bacteria isolated from deep sea fish. Lipids 29:527-528. [DOI] [PubMed] [Google Scholar]
- 54.Yazu, K., Y. Yamamoto, E. Niki, and K. Ukegawa. 1998. Mechanism of lower oxidizability of eicosapentaenoate than linoleate in aqueous micelles. Lipids 33:597-600. [DOI] [PubMed] [Google Scholar]