Abstract
HLA class I-restricted peptides are often used in peptide vaccine regimens. There is strong evidence that many of these peptides can generate specific CD8+ T-cell responses in vivo; however, only occasional objective clinical responses have been reported. To test whether provision of “help” would enhance antitumor immunity, the authors initiated a clinical trial in which patients with metastatic melanoma were immunized against the NY-ESO-1 tumor antigen, using an HLA-A2-restricted peptide (ESO-1:165V), an HLA-DP4-restricted peptide (NY-ESO-1:161-180), or both peptides given concomitantly. The first cohorts received only ESO-1:165V, using three vaccination schedules. Immunologically, most patients developed immune responses to the HLA-A2-restricted native ESO-1 epitope after vaccination. Peptide vaccine given daily for 4 days appeared to induce immunologic responses more rapidly than if given once a week or once every 3 weeks. In contrast, vaccination using the NY-ESO-1:161-180 peptide induced immune responses in only a few patients. Clinically, one patient who received NY-ESO-1:161-180 peptide alone had a partial response lasing 12 months. Concomitant vaccination with the HLA class II-restricted peptide did not alter the immune response to the HLA class I-restricted peptide form NY-ESO-1. However, vaccination with the HLA-A2-restricted epitope generated primarily T cells that did not recognize tumor after in vitro sensitization. This result raises questions about the use of synthetic peptides derived from NY-ESO-1 as a sole form of immunization.
Keywords: cancer vaccine, tumor antigens, NY-ESO-1, HLA-A2, HLA-DP
During the past decade, the identification of tumor-associated antigens has opened new opportunities for the development of cancer immunotherapies.1 Cancer vaccines using immunogenic peptide epitopes from tumor antigens have been used in clinical trials to treat patients with metastatic melanoma, with limited success. Many of these trials used immunization with human leukocyte antigen (HLA) class I-restricted peptides. Even though many of these peptides were efficient in generating high levels of peptide and/or tumor-reactive CD8+ T cells (CTLs) in vivo, positive immunologic response has not correlated with objective clinical responses.2
In the present trial, we administered HLA class I- and class II-restricted peptides derived from the tumor-associated antigen NY-ESO-1 to patients with metastatic melanoma. We hypothesized that providing specific helper CD4+ T cells by immunization with a class II-restricted epitope would lead to enhanced CTL activation and a sustained CTL response against tumor. Helper CD4+ T cells have been shown to play a central role in the initiation and maintenance of an effective CTL anti-tumor response3,4 by a variety of mechanisms, including the elaboration of appropriate cytokines and activation of antigen-presenting cells.5,6
NY-ESO-1 is an immunogenic cancer/testis antigen expressed in a variety of tumors, including melanoma, breast, ovary, lung cancers, and sarcomas.7,8 Its expression in normal tissues is limited to testis. Antibodies and/or CTL reactivity directed against NY-ESO-1 were detected in approximately 50% of patients whose tumors expressed NY-ESO-1. Both humoral and cellular immune responses to ESO-1 have been reported in patients with a variety of cancers.9,10 We recently identified two patients with strong pre-existing immunity to NY-ESO-1, both in peripheral blood and tumor-infiltrating lymphocytes (11 and unpublished data). Both patients had dramatic responses to immunotherapy. Whether NY-ESO-1 immunity played any role in their responses remains to be defined.
The NY-ESO-1:157-165 was previously found to be the minimal epitope recognized by specific CD8+ T cells in an HLA-A2-restricted fashion.9 This peptide was modified in an attempt to improve peptide binding and stability in solution. The cysteine residue at P9 was replaced with valine to prevent dimerization of the peptide. The modified peptide was designated NY-ESO-1:157-165(9V) or ESO-1:165V. Stability studies using mass spectrometry revealed that the native peptide rapidly dimerized and lost activity while the modified ESO-1:165V peptide was stable in solution.12 In addition, our group recently identified a naturally processed helper epitope from NY-ESO-1 that was recognized by CD4+ T cells in the context of HLA-DPB1*0401 and *0402.13 DP4 is the most prevalent class II HLA allele in Caucasians (43–70%) and is also found to be associated with the development of antibody against ESO-1.13 Therefore, the modified HLA-A2-restricted epitope ESO-1:165V and the HLA-DP4 restricted epitope (ESO-1:161-180) were used in our ESO-1 vaccine study of patients with metastatic melanoma.
In this article we report on the clinical and immunologic responses from patients who received the class I-restricted peptide alone, the class II-restricted peptide alone, or a combination of both class I- and class II-restricted peptides. The primary objective of this study was to determine whether the administration of a class I- and/or class II-restricted NY-ESO-1 peptide in patients with metastatic melanoma could elicit an immunologic response against the vaccinated peptides as measured by an in vitro sensitization (IVS) assay, and tumor regression. The secondary objective of this study was to determine toxicity and to gain information for the possible conduct of future protocols.
MATERIALS AND METHODS
Patients
Patients were eligible for the study if they had measurable or evaluable metastatic melanoma, expressed HLA-A*0201 and/or HLA-DPβ1*04, had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less and an expected survival of at least 3 months, and demonstrated NY-ESO-1 expression in tumor by RT-PCR or the presence of serum antibody to NY-ESO-1. Exclusion criteria were serum creatinine greater than 2.0 mg/dL, total bilirubin greater than 2.0 mg/dL, alanine aminotransferase or aspartate aminotransferase greater than three times the upper limit of normal, white blood cell count less than 3,000/mm3, platelet count less than 90,000/mm3, pregnancy or lactating status, any active systemic infection, symptomatic cardiac disease, autoimmune or immunodeficiency disease, and positivity for hepatitis BsAg or HIV antibody. A period of at least 3 weeks was required between enrollment into this protocol and any previous systemic therapy.
All patients were enrolled in this trial between December 2000 and February 2004, and all signed an informed consent before protocol enrollment. The protocol was approved by the Institutional Review Board of the National Cancer Institute. All patients were treated at the Warren G. Magnuson Clinical Center of the National Institutes of Health in Bethesda, Maryland.
Vaccine Therapy
During the initial implementation of this study, patients, all HLA-A2 positive, were randomized to one of three vaccination schedules using the class I-restricted peptide ESO-1:165V alone. Five patients (group 1) received ESO-1 peptide once weekly for 4 weeks followed by a 3-week break (one cycle) for two cycles (one course); seven patients (group 2) received ESO-1 peptide once every 3 weeks (one cycle) for four cycles (one course); and seven patients (group 3) received ESO-1 peptide daily for 4 days every 3 weeks (one cycle) for four cycles (one course).
Subsequently, separate groups of patients received both the class I-restricted peptide ESO-1:165V and the class II-restricted peptide ESO-1:161-180 if they were positive for both HLA-A*0201 and HLA-DPβ1*04 (group 4), or received the class II-restricted peptide ESO-1:161-180 alone if they were HLA-DPβ1*04 positive but HLA-A*0201 negative (group 5). The vaccination schedule was once every 3 weeks (one cycle) for four cycles (one course). This schedule was chosen because it is the schedule that was successfully used in our other protocols to immunize patients. For vaccination, 1 mg ESO-1:165V or 4 mg ESO-1:161-180, or a combination of both peptides, was emulsified in incomplete Freund’s adjuvant and injected subcutaneously in the lower extremity. All peptides were synthesized to Good Manufacturing Practice grade by Multiple Peptide Systems (San Diego, CA).
All patients underwent apheresis before treatment and 3 weeks after every two vaccinations to obtain peripheral blood lymphocytes for in vitro immunologic monitoring. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (ICN, Aurora, OH) separation and were cryopreserved at 108 cells/vial in heat-inactivated human AB serum with 10% dimethyl sulfoxide and stored at −180°C until further use.
Patients who completed one course of treatment and had a minor, mixed, partial, or complete response (as defined below) or had stable disease received a second course. Patients who had progressive disease after receiving vaccines alone had the option of receiving IL-2 therapy.
IL-2 Therapy
Patients with disease progression after receiving vaccines alone, and who had no contraindications to IL-2 administration, were given high-dose intravenous IL-2. For each cycle of treatment, IL-2 was started the day after vaccination and was administered as described in previous publications.2 Briefly, 720,000 IU/kg of recombinant IL-2 (provided by Cetus Oncology Division, Chiron Corp., Emeryville, CA) was reconstituted from lyophilized powder in 5% human serum albumin and given as a 15-minute intravenous infusion for each dose. IL-2 was administered every 8 hours as tolerated to a maximum of 12 doses or until the development of a grade III or IV toxicity not easily reversed by supportive therapy, any evidence of neurologic toxicity, or patient refusal.
Clinical Response Evaluation
All patients underwent magnetic resonance imaging of the brain and computed axial tomography of the chest, abdomen, and pelvis within 4 weeks before starting treatment and subsequently after every two cycles of therapy. Photographs, plain radiographs, or other radiologic modalities were also used as needed to evaluate disease sites.
For every patient, the product of the maximum perpendicular diameters of all tumors was calculated before and after treatment. A partial response was defined as the reduction of at least 50% (but <100%) in the sum of the products of the maximum perpendicular diameters of all evaluable metastases lasting at least 1 month with no new or enlarging tumors. A minor response was the reduction of at least 25% but less than 50%. A complete response was the disappearance of all evaluable tumor sites for at least 1 month. A mixed response was defined as regression of some tumors but growth in others. Patients not achieving these criteria were deemed as having progressive disease. For final analysis, patients with neither a partial nor a complete response were deemed nonresponders.
IVS Assay
In our experience, the 12-day IVS ELISA is the most sensitive assay for immune monitoring and is used in all peptide immunization protocols in our group. Cryopreserved PBMCs were thawed into complete medium (CM: RPMI 1640 medium with 10% heat-inactivated human AB serum, 2 mM L-glutamine, 10 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamicin) or X-VIVO 20 (Bio-Whittaker, Walkersville, MD), plated at 3 × 106 cells/2 mL/well of 24-well plates, with 1 μM of ESO-1:165V peptide, and incubated at 37°C and 5% CO2. IL-2 was added on the following day for a final concentration of 300 IU/mL. Cells were split (and fresh medium with 300 IU/mL IL-2 added) as needed and were harvested 11 to 13 days after initiation of the culture (12-day IVS). Then 105 cells were co-incubated with 105 peptide-pulsed T2 cells in 200 μL CM per well in 96-well plates. The T2 cells were pulsed by incubating T2 cells at 1 × 106 cells/mL with 1 μM peptide for 2 to 3 hours at 37°C and 5% CO2. Alternatively, 105 melanoma cells were also used as stimulators. After 18 hours of co-incubation, IFN-γ release in culture supernatants was measured using a commercially available ELISA (Pierce-Endogen, Rockford, IL).
For the immunologic monitoring of class II-restricted ESO-1 peptide reactivity, PBMCs were plated at 5 × 105/200 μL X-VIVO 20/well of 96-well flat-bottom plates, with 50 μM of ESO-1:161-180 peptide, with a total of 48 wells per PBMC sample, and then treated similarly to the class I peptide as described above. However, instead of T2 cells, a DP4+ lymphoblastoid cell line was used as the target for peptide pulsing.
IVS assays were performed simultaneously on PBMCs harvested before treatment and after every two vaccinations; each assay was repeated at least once. A positive assay was defined as IFN-γ of at least 100 pg/mL, at least two times greater than a negative control and at least two times greater than preimmunization samples. The gp100:180-188 and NY-ESO-1:91-110 peptides were used as irrelevant negative controls for NY-ESO-1:157-165 and NY-ESO-1:161-180 peptides, respectively.
RESULTS
Patients, Clinical Response, and Toxicities
The total number of patients from each group and the total number of vaccinations received are summarized in Table 1. Of 37 patients included in intent-to-treat analyses, there was only one objective response. Fifteen nonresponding patients were subsequently crossed over to high-dose IL-2; no responses were seen (Table 2).
TABLE 1.
Treatment Summary
| Group | Schedule* | # Patients | Peptides† | Dose (mg) | # Vaccinations Received‡ |
|---|---|---|---|---|---|
| 1 | qw × 4, q7w × 2 | 5 | Class I | 1 | 4, 4, 16, 8, 4 |
| 2 | q3w × 4 | 7 | Class I | 1 | 2, 4, 4, 8, 2, 4, 4 |
| 3 | qd × 4, q3w × 4 | 7 | Class I | 1 | 8, 2, 4, 3, 2, 4, 2 |
| 4 | q3w × 4 | 12§¶ | Both | 1 + 4 | 8, 2, 4, 6, 4, 2, 2, 2, 2, 4 |
| 5 | q3w × 4 | 6§ | Class II | 4 | 8, 2, 2, 4, 2 |
Schedule per course of treatment.
Class I peptide, ESO-1:165V; class II peptide, ESO-1:161-180.
One vaccination is defined as one peptide injection, except for the daily-times-four schedule, where four consecutive daily peptide injections make up one vaccination.
One patient was censored from final immunologic analysis for having received only one vaccination.
One patient was censored from final immunologic analysis because of incorrect HLA-A2 subtype.
TABLE 2.
Clinical Response of Patients Receiving the NY-ESO-1 Peptide Vaccination
| Peptide Alone | Crossover to High-dose IL-2 | |
|---|---|---|
| Total | 37 | 15 |
| Partial response | 1* | 0 |
| Complete response | 0 | 0 |
One partial response in group 5, lasting 12 months.
The only responder was in group 5. This patient had a history of metastatic melanoma to lung, which was resected. She also had recurrent metastatic subcutaneous lesions, status post multiple resections. On entering the protocol, she had a 3-cm mediastinal lesion and a subcutaneous lesion in the left upper back. Since she was HLA-DP4 positive but HLA-A2 negative, she received only the HLA class II-restricted ESO-1 peptide (NY-ESO-1:161-180). Her mediastinal mass became significantly smaller after two cycles of vaccination and almost completely regressed after four cycles. The subcutaneous lesion was nearly completely regressed after four more cycles of vaccination. The patient was in partial response for 12 months when she developed a small brain lesion and later a pelvic lesion.
The peptide vaccine was well tolerated with minimal toxicities. There was one grade 3 gastrointestinal toxicity in a patient in group 2, but this was thought to be unrelated to the peptide vaccine. This patient was diagnosed with severe Crohn’s disease.
Immunologic Response
Against HLA-A2-Restricted Native ESO-1:165 and Modified ESO-1:161(9V) Peptides
One vaccination was defined as one peptide injection, except for the daily-times- four schedule, where four consecutive daily peptide injections constituted one “vaccination.” PBMCs collected before treatment and after every two to four vaccinations were monitored for immunologic responses against the vaccinating peptide as well as the native peptide using a 12-day IVS assay as described above. An example of a positive assay for peptide recognition is given in Table 3. For the once-a-week schedule (group 1) and every-3-week schedule (groups 2 and 4), increasing numbers of vaccinations appeared to generate increasing responses against both the native and modified ESO-1 peptides (Table 4).
TABLE 3.
Example of a Positive 12-Day IVS Assay for Peptide Recognition
| # of Vaccinations
|
|||
|---|---|---|---|
| Stimulus | 0 | 2 | 4 |
| pg/ml IFN-γ | |||
| T2/ESO-1 (N) | 380 | 2896 | 2723 |
| T2/ESO-1 (9V) | 309 | 2994 | 2554 |
| T2/gp 100:180-188 | 234 | 45 | 22 |
| Unpulsed T2 | 282 | 57 | 20 |
| 624mel (A2+, ESO-1+) | 105 | 21 | 10 |
| 1363mel (A2+, ESO-1+) | 83 | 62 | 20 |
| 624.28mel (A2−, ESO-1+) | 41 | 15 | 1 |
| 526mel (A2+, ESO-1−) | 29 | 5 | 0 |
| None | 124 | 27 | 15 |
ESO-1(N), native NY-ESO-1:157-165 peptide; ESO-1(9V), modified peptide NY-ESO-1:157-165(9V); gp100:180-188, irrelevant peptide.
TABLE 4.
Immunologic Responses to the HLA-A2-Restricted NY-ESO-1 Peptides
| # of Vaccinations
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
2
|
4
|
8
|
16
|
||||||
| Group | ESO(N) | ESO(9V) | ESO(N) | ESO(9V) | ESO(N) | ESO(9V) | ESO(N) | ESO(9V) | ESO(N) | ESO(9V) |
| 1 | 0/3 | 0/5 | 0/1 | nd | 1/2 | 3/5 | nd | 1/1 | nd | 1/1 |
| 2 | 0/3 | 0/7 | 1/3 | 2/5 | 1/1 | 4/5 | nd | 1/1 | ||
| 3 | 0/5 | 1/7 | 4/5 | 7/7 | 0/1 | 2/2 | 1/1 | 1/1 | ||
| 4 | 1/10 | 1/10 | 2/10 | 3/10 | 2/5 | 3/5 | 1/1 | 1/1 | ||
| Total | 1/21 | 2/29 | 7/19 | 12/22 | 4/9 | 12/17 | 2/2 | 4/4 | nd | 1/1 |
nd, not done.
For the daily-times-four schedule (group 3), it seems that maximal response to the native and modified ESO-1 peptides were already seen after two vaccinations (i.e., eight total inoculations; see Table 4). This was in contrast with the once-a-week or once-every-3-week schedules, where maximal responses were seen only after four or more vaccinations. Therefore, peptide vaccination daily for 4 days on an every-3-week cycle seemed to be superior to the other two schedules in generating immune responses against both the native and modified ESO-1 peptides.
Vaccination With a Class II-Restricted ESO-1 Peptide Did Not Affect Immunologic Responses to the Class I-Restricted ESO-1 Peptide
A previous vaccine study had suggested that vaccination with both class I- and class II-restricted peptide epitopes from the melanocyte/melanoma differentiation antigen gp100 hindered immune responses to the class I-restricted peptide compared with immunization with the class I-restricted peptide alone.14 To see whether this also occurred in the current study, PBMCs obtained before vaccination and after two or four vaccinations were studied for responses against the ESO-1:157-165 native peptide, using the 12-day IVS assay as described above. All PBMC samples were assayed simultaneously to limit variability.
Since groups 1 and 2 had similar patterns of immune response to ESO-1:157-165, they were grouped together for comparison with group 4. Comparing group 4 to groups 1 and 2, there was no statistical difference in the immune response of the two groups against the class I-restricted ESO-1 peptide (Table 5). This was also true when group 4 was compared with either group 1 or group 2 alone (data not shown). Therefore, concomitant vaccination with class II-restricted ESO-1 peptide did not seem to alter immune responses to the class I-restricted ESO-1 peptide.
TABLE 5.
Concomitant Vaccination with a Class II-Restricted ESO-1 Peptide Did Not Alter Immune Responses to Class I-Restricted ESO-1 Peptide
| Group 1 + 2 | Group 4 | |
|---|---|---|
| Pre | 2/10 | 2/9 |
| Post | 6/10 | 6/9 |
p2 = 1
Vaccination With ESO-1:165V Generated Primarily T Cells Reactive to Peptide But Not Tumors
Even though the majority of patients developed immunologic responses against the native ESO-1 peptide (ESO-1:157-165), as seen in Table 4, only 3 of the 29 evaluable patients who were vaccinated against the class I-restricted ESO-1:165V developed specific immune recognition of both the native ESO-1:157-165 peptide and melanoma cell line(s) that expressed both HLA-A2 antigen and NY-ESO-1, as assessed by IFN-γ release after one in vitro stimulation using the 12-day IVS assay. However, two of these three patients also had preexisting immunity to both NY-ESO-1 peptide and tumors in the PBMC samples obtained before treatment. Specific vaccination did not seem to enhance specific T-cell response in post-treatment PBMC samples in these two patients. An example of one of these patients is shown in Figure 1. The third patient had a weak response against a tumor line detected only in the post-treatment PBMC samples. Antitumor response was detected in the post-treatment PBMCs in three other patients after a total of two or three repeated cycles of in vitro stimulation.
FIGURE 1.
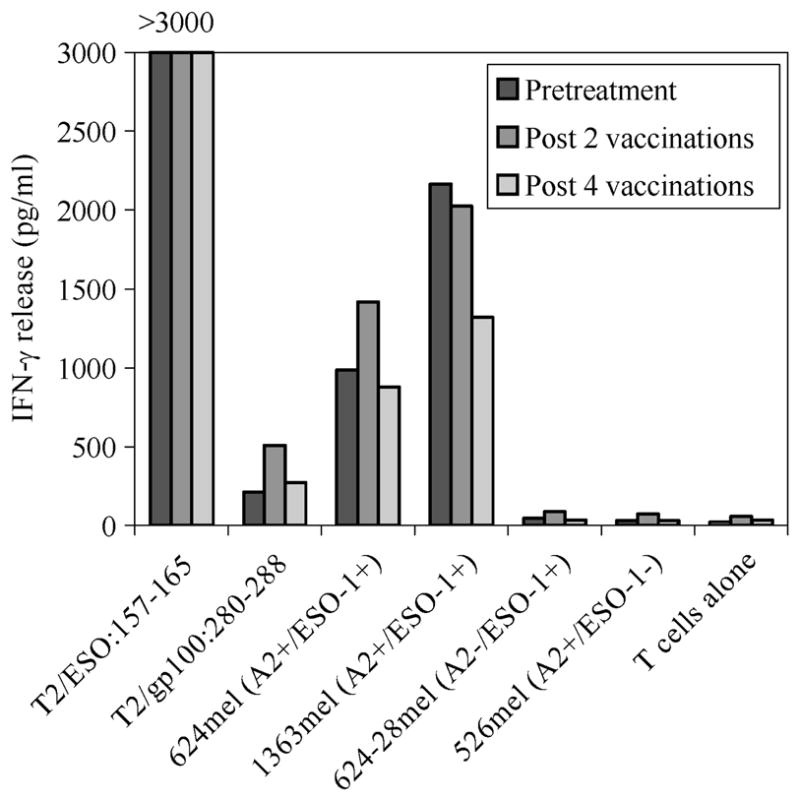
Anti-NY-ESO-1-expressing tumor reactivity by T cells from a patient with spontaneous, pre-existing immunity to the NY-ESO-1 antigen.
In sum, of the 29 evaluable patients, only 4 developed immune responses directed against both ESO-1:157-165 peptide and NY-ESO-1-expressing tumor cells in the post-treatment PBMCs. However, multiple IVS assays were required in most cases. Therefore, it seems that vaccination with the HLA-A2-restricted ESO-1 peptide was not efficient in generating T cells that were capable of recognizing tumors.
Reactivity Against the Class II-Restricted ESO-1 Peptide (NY-ESO-1:161-180)
Eighteen patients were vaccinated with the HLA-DP4-restricted ESO-1 peptide. Three were excluded from the immunologic analyses, as explained in Table 1. We did not have enough post-treatment PBMCs for analysis in one patient. Of the 14 patients analyzed, only 3 (patients 3, 13, and 14) had evidence of immunization against NY-ESO-1:161-180 (Table 6). It appeared that the generation of immune response to the vaccinating peptide may require a total of six or more vaccinations. Eight of the 14 patients received only two vaccinations before they were taken off protocol because of disease progression, limiting our evaluation of the immunogenicity of this peptide. Of interest, patient 13 was the sole clinical responder; she had clear evidence of immune response to the vaccinating peptide after six vaccinations.
TABLE 6.
Immunologic Responses to the Class II-Restricted Peptide (NY-ESO-1:161-180)
| # of Vaccinations
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 |
| # of (+) wells/48 | |||||||||
| 1 | 3 | 2 | 1 | 0 | |||||
| 2 | 0 | 4 | |||||||
| 3 | 1 | 31 | |||||||
| 4 | 0 | 0 | |||||||
| 5 | 0 | 0 | |||||||
| 6 | 0 | 0 | |||||||
| 7 | 0 | 1 | |||||||
| 8 | 0 | 0 | |||||||
| 9 | 0 | 0 | |||||||
| 10 | 0 | 0 | 0 | ||||||
| 11 | 5 | 1 | 0 | ||||||
| 12 | 1 | 0 | 0 | ||||||
| 13 | 1 | 1 | 2 | 24 | 0 | 0 | 10 | nd | 2 |
| 14 | 0 | 0 | nd | 23 | 26 | ||||
| # ≥5 wells | 1/14 | 1/14 | 0/6 | 2/3 | 1/2 | 0/1 | 1/1 | 0/1 | |
| # with evidence* of immunization | N/A | 1/14 | 0/6 | 2/3 | 1/2 | 0/1 | 1/1 | 0/1 | |
Evidence of immunization is defined as ≥5 (+) wells and twice pretreatment activity.
nd, not done.
DISCUSSION
There has been much progress in the field of melanoma immunotherapy over the past decade. The identification of tumor-associated antigens has opened exciting opportunities for the development of cancer immunotherapies.1 The use of immunogenic peptides derived from tumor antigens has been widely tested in various settings with limited clinical success. In most cases, HLA class I-restricted peptides were used in the vaccine regimens. There is strong evidence that many of these peptides can generate a specific CD8+ T-cell response in vivo; however, only occasional and sporadic objective clinical responses have been reported.2
We report here a clinical trial in which patients with metastatic melanoma were vaccinated against the NY-ESO-1 tumor antigen, using an HLA class I-restricted peptide, an HLA class II-restricted peptide, or a combination of both peptides given concomitantly. The first three randomized cohorts received only the class I-restricted peptide, using various vaccination schedules. In all groups, most patients developed immune responses to the native ESO-1 epitope after vaccination. Although the number of patients is small, it appears that peptide vaccine given daily for 4 days every 3 weeks induced a more rapid immunologic response rate than the other schedules.
In a previous peptide vaccine study in which patients with metastatic melanoma were vaccinated against the melanocyte/melanoma differentiation antigen gp100 using MHC-class I- and class II-restricted peptides (gp100:209-2M and gp100:44-59, respectively), immune responses to the class I peptide were depressed when the class II peptide vaccine was administered concomitantly.14 This was not seen in the current study, nor was an enhancement of immune response to the class I peptide seen by concomitant vaccination with the class II peptide.
A relevant finding in this study was the inefficiency in which NY-ESO-1-peptide vaccination elicited tumor reactivity in the post-treatment T cells. Although most patients developed immune responses to the vaccinating and native peptides, T cells in only a small minority of these patients were also capable of recognizing tumor cells, and this was seen only after several rounds of in vitro stimulation. In other patients, high-avidity T cells that strongly recognize NY-ESO-1-expressing tumors have been detected. We recently identified two patients with pre-existing immunity to NY-ESO-1, in both PBMCs and tumor-infiltrating lymphocytes (11 and unpublished data). NY-ESO-1-specific T cells from these two patients were capable of strong recognition of both the class I peptide and tumor cells. Similarly, Figure 1 shows spontaneous pre-existing reactivity against NY-ESO-1 peptide and tumors that was not augmented by the peptide vaccination in one patient in the present study.
In a previous clinical study, Dutoit et al15 analyzed the immunologic responses in three sarcoma patients who received peptide vaccination with the NY-ESO-1 class I-restricted epitopes (NY-ESO-1:157-165 and NY-ESO-1: 157-167). They reported that most NY-ESO-1-specific T cells were of low functional avidity and recognized peptide but not tumors. Only a small minority of NY-ESO-1-specific T cells induced with the peptide vaccination recognized NY-ESO-1-expressing tumor cells.
Although the NY-ESO-1 protein seems to be an ideal target for cancer immunotherapy because it is immunogenic and widely expressed in many tumor histologies, the results from previous studies and ours call into question the clinical utility of synthetic peptide vaccination using the HLA-A2-restricted epitope from the NY-ESO-1 antigen.
References
- 1.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 5.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberger SP, Toes RE, van der Voort EI, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 7.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungbluth AA, Antonescu CR, Busam KJ, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 9.Jager E, Chen YT, Drijfhout JW, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;19:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockert E, Jager E, Chen YT, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;20:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khong HT, Rosenberg SA. Pre-existing immunity to tyrosinase-related protein (TRP)-2, a new TRP-2 isoform, and the NY-ESO-1 melanoma antigen in a patient with a dramatic response to immunotherapy. J Immunol. 2002;168:951–956. doi: 10.4049/jimmunol.168.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bownds S, Tong-On P, Rosenberg SA, et al. Induction of tumor-reactive cytotoxic T-lymphocytes using a peptide from NY-ESO-1 modified at the carboxy-terminus to enhance HLA-A2.1 binding affinity and stability in solution. J Immunother. 2001;24:1–9. doi: 10.1097/00002371-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Zeng G, Wang X, Robbins PF, et al. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci USA. 2001;98:3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan GQ, Touloukian CE, Yang JC, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutoit V, Taub RN, Papadopoulos KP, et al. Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting. J Clin Invest. 2002;110:1813–1822. doi: 10.1172/JCI200216428. [DOI] [PMC free article] [PubMed] [Google Scholar]


