Abstract
Most cases of genetic hemochromatosis (GH) are associated with the HFE C282Y/C282Y (p.Cys282Tyr/p.Cys282Tyr) genotype in white populations. The symptoms expressed by C282Y homozygotes are extremely variable. Only a few suffer from an overt disease. Several studies have suggested that, in addition to environmental factors, a genetic component could explain a substantial part of this phenotypic variation, although very few genetic factors have been identified so far. In the present study, we tested the association between common variants in candidate genes and hemochromatosis penetrance, in a large sample of C282Y homozygotes, using pretherapeutic serum ferritin level as marker of hemochromatosis penetrance. We focused on two biologically relevant gene categories: genes involved in non-HFE GH (TFR2, HAMP, and SLC40A1) and genes involved in the regulation of hepcidin expression, including genes from the bone morphogenetic protein (BMP) regulatory pathway (BMP2, BMP4, HJV, SMAD1, SMAD4, and SMAD5) and the IL6 gene from the inflammation-mediated regulation pathway. A significant association was detected between serum ferritin level and rs235756, a common single-nucleotide polymorphism (SNP) in the BMP2 genic region (P=4.42×10-5). Mean ferritin level, adjusted for age and sex, is 655 ng/ml among TT genotypes, 516 ng/ml in TC genotypes, and 349 ng/ml in CC genotypes. Our results further suggest an interactive effect on serum ferritin level of rs235756 in BMP2 and a SNP in HJV, with a small additive effect of a SNP in BMP4. This first reported association between common variants in the BMP pathway and iron burden suggests that full expression of HFE hemochromatosis is linked to abnormal liver expression of hepcidin, not only through impairment in the HFE function but also through functional modulation in the BMP pathway. Our results also highlight the BMP regulation pathway as a good candidate for identification of new modifier genes.
Genetic hemochromatosis (GH) is a group of hereditary disorders proceeding from an impairment in the production of the key regulator of plasma iron, hepcidin,1 and resulting in progressive iron loading of parenchymas. Four recessive forms of GH are currently described.2 Two late-onset, adult forms (HFE [MIM 235200] and HFE3 [MIM 604250]) are related to mutations in HFE (MIM 235200) or TFR2 (MIM 604720), the gene encoding the receptor transferring 2 protein. Two early-onset, juvenile forms (JH [MIM 602390]) are secondary to mutations in either the gene encoding hemojuvelin (HJV [MIM 608374]) or the gene encoding hepcidin (HAMP [MIM 606464]). The “ferroportin disease” (HFE4 [MIM 606069]), related to mutation in the SLC40A1 gene (SLC40A1 [MIM 604653]) coding for the metal-transporter ferroportin regulated by hepcidin, is a closely related but not classic form of GH, because of a dominant transmission usually with predominant mesenchymal iron deposition.3
HFE hemochromatosis accounts for >95% of GH cases in white populations.4 It is related to one major mutation, C282Y, with a reported frequency of 5%–15%.5–7 The biochemical or clinical symptoms expressed by C282Y homozygotes are extremely variable.8 Only a few suffer from an overt disease consisting of various associated symptoms, including osteoarticular damage, cirrhosis, diabetes, hypogonadism, arrhythmia, and heart failure. Most C282Y homozygotes display a mild disease limited to biochemical abnormalities (increased transferrin saturation, with or without elevated serum ferritin), with either absent or mild clinical symptoms. Moreover, some homozygotes may show no expression throughout their lives.9 As a whole, estimates of the penetrance of C282Y homozygosity by studies have ranged from 1% to 90%.5,10–13 This wide range reflects the variability in the definition of disease penetrance, with some studies referring to the level of iron burden (serum ferritin, liver iron concentration…) using disparate thresholds, others to organ damage, and most to mixed criteria. These results are complicated by the absence of a strict correlation between the level of iron burden and organ-damage expression. However, they strongly support the involvement of factors modulating disease expressivity. With respect to iron loading, environmental factors have been poorly investigated in humans, although it is likely that some may be relevant, such as alimentary regimen and blood donation.11 Several results also suggest the role of additional genetic factors. The incidence of GH-related conditions is higher in relatives of clinically affected probands than in relatives of probands with only elevated transferrin saturation,14 and concordance of iron store indices in GH-affected families between same-sex siblings homozygous C282Y is high.15 On the basis of a twin study, Whitfield et al.16 estimated that HFE explains only part of the genetic component of iron-store variation. In mice, differences in hepatic iron loading in two Hfe-deficient mice strains have been associated with a polygenic pattern of inheritance.17
Apart from sex, only a few genetic factors have been identified as putatively modifying disease expression. A mitochondrial polymorphism was reported as more frequent in C282Y homozygotes with hemochromatosis than in nonexpressing C282Y homozygotes.18 However, in another study, no association has been observed between the ferritin level of C282Y homozygotes and the same polymorphism.19 Mutations in two other genes involved in iron metabolism, HJV and HAMP, have been clearly associated with higher iron indices in a French cohort of C282Y homozygotes,20–22 but the mutations identified are rare, with a frequency of heterozygous carriers <2% among C282Y homozygotes.
Hepcidin is a peptide hormone produced by the liver that controls plasma iron concentration and iron-tissue distribution by inhibiting intestinal iron absorption, iron recycling by macrophages, and iron mobilization from the liver. Interestingly, hepcidin levels are abnormally low in patients with adult or juvenile forms of GH. Babitt et al.23 recently demonstrated that hepcidin expression is induced by the bone morphogenetic protein (BMP)–signaling pathway. This pathway involves BMP2 and BMP4 proteins and the BMP coreceptor HJV, which phosphorylates receptor-activated Smad proteins (R-Smads) 1 and 5. Phosphorylated R-Smads form a complex with the common mediator Smad4 (Co-Smad), which allows signal transduction into the nucleus for hepcidin gene induction.
The aim of the present study was to search for relatively frequent variants (as opposed to the rare mutations already known) in genes that modify the serum ferritin levels in C282Y homozygotes. Given these recent molecular data, we focus on variants in two pathophysiologically relevant gene categories: genes involved in non–HFE hemochromatosis and genes involved in the regulation of hepcidin expression. In a large sample of C282Y homozygotes, we tested for association between initial serum ferritin levels and SNPs located within or near 10 genes: 3 non–HFE-GH genes (TFR2, HAMP, and SLC40A1), 6 BMP signaling pathway genes (BMP2 [MIM 112261], BMP4 [MIM 112262], HJV, SMAD1 [MIM 601595], SMAD4 [MIM 600993], and SMAD5 [MIM 603110]), and the IL6 gene (IL6 [MIM 147620]) coding for an inflammatory cytokine known to increase inflammation-mediated hepcidin expression. We detected a significant association between a SNP in the BMP2 genic region and serum ferritin level, adjusted for age and sex. Given the biologically relevant gene interactions along the BMP regulatory pathway, our data suggest an additive effect on serum ferritin level of the BMP2 SNP and of a SNP in BMP4, with an interaction effect between the BMP2 SNP and a SNP in HJV.
The database of the Family Screening Centre for Hemochromatosis8 comprises all C282Y homozygous probands referred to the Liver Unit in Rennes, France, since 1990 and their relatives who received diagnoses through a systematic family-screening policy. At the time of the study, 1,319 C282Y homozygotes were recorded, among whom 729 unrelated probands fulfilled inclusion criteria: availability of (i) sex, (ii) age at diagnosis, (iii) serum ferritin level at diagnosis before any venesection therapy, and (iv) DNA sample stored at −20°C. Of these 729 subjects, 592 gave their written informed consent to participate in the study, in accordance with the protocol validated by the committee of ethics of Rennes on November 10, 2004.
Of these 592 subjects, 262 were women and 330 were men. Mean (SD) age was 46 (14) years for women and 44 (13) years for men. Median value (25th–75th percentile range) for ferritin levels was 1,040.5 ng/ml (582–2,356) in men and 400.5 ng/ml (186–699) in women. Ferritin data were normalized using a loge transformation. Log-transformed ferritin levels were adjusted for age, with consideration of age groups of 10 years, and sex. The final multiple-regression model includes a parameter for each of the seven independent age groups, a parameter for sex, and an interaction parameter, Age × Sex, for each age group.
Genomic DNA was extracted from peripheral blood cells by the phenol-chloroform method or by use of the Flexigen DNA kit (Qiagen). SNPs in the 10 candidate genes were selected from the CEU HapMap database. For each gene, a region including the complete genic sequence and the upstream and downstream intergenic sequences was delimited. The set of tag SNPs was identified for each region, so that all the SNPs with a minor-allele frequency (MAF) ⩾5% in the database have a pairwise r2⩾0.8 with at least one tag SNP. Tagging was performed using the algorithm implemented in Tagger.24 Two coding SNPs located within the IL6 gene were added to the list. A total of 81 SNPs were included in the study.
SNP genotyping followed Custom SNP Genotyping Assays consisting of a mix of unlabeled PCR primers and TaqMan minor groove binder (MGB) SNP-allele-specific probe. PCR primers and probes used for allelic discrimination were designed and purchased from the Applied Biosystems “assay on demand” or “assay by design.” Genotyping followed the Applied Biosystems protocol. Briefly, PCR was performed in a final volume of 5 μl containing 10 ng of sample DNA, 0.625 μl of custom SNP-specific Assay Mix, and 2.5 μl of Universal Master Mix no AmpErase UNG. Amplification was allowed to proceed for 40 cycles of 15 s at 95°C and 60 s at 60°C. Automatic genotype call was performed <24 h after PCR, by scanning microtitration plates on the 7900HT Fast Real-Time PCR, which provides the SDS2.3 software (Applied Biosystems).
Assays for rs1880241 (in IL6) and rs6596286 (in SMAD5) were unsuccessful. Of the 79 SNPs genotyped, 4 SNPs (rs1005464 in BMP2, rs10498466 and rs4901473 in BMP4, and rs3764942 in SMAD5) were not in Hardy-Weinberg equilibrium and therefore were excluded from the analyses. Mean genotyping success rate for the 75 remaining SNPs was 99.1%. Correlation between allele frequencies of the 75 SNPs in our sample and allele frequencies in the CEU HapMap data was very high (regression r2=0.94).
The linkage disequilibrium (LD) structure among SNPs was examined with Haploview.25 The mean r2 between our markers, computed on the whole sample, was 0.10. All subsequent statistical analyses were performed using R.26 A linear regression was used to test for association between each individual SNP and ferritin. Both allelic and genotypic associations were considered. The sensitivity of association results to the inclusion of phenotypic outliers was evaluated. Because the results were fully concordant, we present and discuss only results based on the whole sample. To correct for multiple testing, the effective number of independent tests was assessed using the method of Li and Ji27 as implemented in the SNPSpd software.28 Following this procedure, our set of 75 SNPs is equivalent to 62 independent tests. When a Bonferroni correction is applied, an individual significance threshold of 8×10-4 should be used to control a global 5% type I error.
Association between haplotypes and ferritin was tested using the efficient score statistic proposed by Schaid et al.29 and implemented in haplo.stats R-Package v1.2.2 (Schaid Lab Web site) with a permutation-based assessment of the P values. We favored this relatively simple regression-based approach over more sophisticated ones based on population history modeling,30 because our sample is a subsample selected from the general population with an unknown selection scheme. In the absence of a clear evaluation of the consequences of such a selection scheme on sophisticated haplotype tests, we favored a robust approach.31 Haplotypes comprising all SNPs were considered for genes with <10 SNPs. Otherwise, haplotypes comprising five SNPs were considered, with a sliding window of one SNP to browse the gene. Sensitivity to window size was evaluated. Because results were fully concordant, we present and discuss only results based on five-SNP windows. Association of SNP combinations within genes was further analyzed using unphased, multimarker data. A stepwise linear regression starting from the model including only the SNP with the lowest individual P value was performed to determine the subset of SNPs with the strongest association.
In a final, exploratory stage, we evaluated the association of several biologically relevant gene combinations with ferritin level. To limit the number of combinations tested, we considered only those for which a molecular interaction between gene products has been described and which include one gene showing significant association at the single-gene level. Again, we used multiple-regression models. To evaluate whether more-complex models were significantly better predictors of phenotype than simpler ones, we compared nested models (simpler models are particular cases of the more complex models) using F statistics. These results are exploratory, in the sense that we drop the requirement for multiple-comparison adjustment while assessing significance. For each gene combination, a “best model” was estimated with a backward regression, starting from a full model that included the three SNPs displaying the strongest individual association for each gene and two SNP-interaction terms.
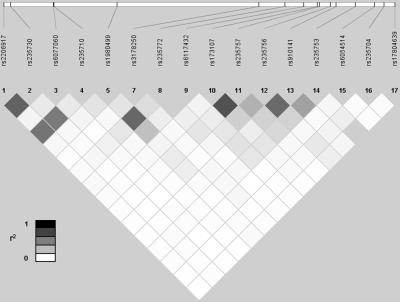
SNP rs235756, located in the 3′ region of BMP2, is significantly associated with ferritin level in our C282Y homozygote sample (table 1), after correction for multiple testing (uncorrected P=4.4×10-5; corrected P=.002). Note that the association would still have been significant if a more conservative Bonferroni correction for 75 tests had been applied (corrected P value would then be .003). The T allele associated with higher ferritin level has a frequency of 0.64 (HapMap CEU frequency is 0.58). Mean ferritin level, adjusted for age and sex, is 654.66 ng/ml among TT genotypes, 516.39 ng/ml among TC genotypes, and 349.11 ng/ml among CC genotypes. A neighboring SNP in the gene (rs910141) also displays a suggestive association (corrected P=.062), but rs235756 and rs910141 are in LD (r2=0.528), as can be seen from figure 1. Interestingly, SNP rs235756 has no impact on the age at diagnosis (mean age at diagnosis, adjusted for sex, is 44.7 years among TT genotypes, 45 years among TC genotypes, and 45.8 years among CC genotypes; P value of the analysis of variance [ANOVA] is .82). Suggestive but nonsignificant associations are also observed for one SNP in BMP4 (rs4901474, uncorrected P=.0054, corrected P=.335) and one synonymous SNP in IL6 (rs2069849; uncorrected P=.0051, corrected P=.316). Because the results for the allelic and the genotypic tests were very similar, the following analyses were performed considering the allelic model only.
Table 1. .
Single SNP Genotypic and Allelic Association with Ferritin Level for All 75 SNPs
|
Pa for Association Test |
|||||
| Gene (Chromosome) and SNP | Location (bp) |
MAF | Genotypic | Allelic | Allele (Frequency) Associated with Higher Ferritine Level |
| HAMP (19): | |||||
| rs916145 | 40,459,724 | .113 | .7637 | .5016 | |
| rs10405246 | 40,460,789 | .221 | .1045 | .0423 | A (.779) |
| rs1882694 | 40,463,222 | .376 | .3376 | .1576 | |
| rs7251432 | 40,467,281 | .491 | .8527 | .5838 | |
| rs12971321 | 40,471,262 | .376 | .8367 | .9815 | |
| rs10402233 | 40,472,691 | .347 | .2110 | .1180 | |
| rs17705188 | 40,473,156 | .084 | .9412 | .8520 | |
| BMP2 (20): | |||||
| rs2206917 | 6,683,511 | .434 | .9867 | .9516 | |
| rs235730 | 6,684,189 | .418 | .9146 | .9172 | |
| rs6077060 | 6,688,317 | .099 | .1262 | .0498 | T (.099) |
| rs235710 | 6,688,366 | .439 | .5832 | .3072 | |
| rs1980499 | 6,694,498 | .495 | .5092 | .3742 | |
| rs3178250 | 6,708,201 | .173 | .4460 | .2532 | |
| rs235772 | 6,710,719 | .409 | .2757 | .1124 | |
| rs6117432 | 6,712,536 | .233 | .9039 | .6946 | |
| rs173107 | 6,713,841 | .380 | .0026 | .0052 | T (.380) |
| rs235757 | 6,714,019 | .374 | .1994 | .0933 | |
| rs235756 | 6,715,111 | .364 | 1.80 × 10−4 | 4.42 × 10−5 | T (.636) |
| rs910141 | 6,715,642 | .255 | .0038 | .0010 | G (.745) |
| rs235753 | 6,717,533 | .348 | .2473 | .0950 | |
| rs6054514 | 6,719,370 | .052 | .4355 | .4865 | |
| rs235704 | 6,720,263 | .120 | .9306 | .7748 | |
| rs17804639 | 6,721,316 | .092 | .5071 | .3447 | |
| BMP4 (14): | |||||
| rs10498464 | 53,441,332 | .193 | .3857 | .2666 | |
| rs1951865 | 53,442,591 | .372 | .5052 | .5954 | |
| rs11157990 | 53,453,695 | .303 | .3464 | .1881 | |
| rs3742555 | 53,455,219 | .058 | .1838 | .0760 | |
| rs2147105 | 53,475,815 | .448 | .7883 | .5060 | |
| rs4444235 | 53,480,669 | .492 | .1852 | .6077 | |
| rs762642 | 53,492,803 | .409 | .7235 | .5443 | |
| rs1957860 | 53,499,105 | .467 | .8738 | .8229 | |
| rs6572927 | 53,503,140 | .081 | .2345 | .1097 | |
| rs11157994 | 53,521,052 | .076 | .0766 | .1883 | |
| rs1957844 | 53,527,828 | .230 | .1068 | .5648 | |
| rs4901474 | 53,539,487 | .429 | .0050 | .0054 | C (.429) |
| HJV (1): | |||||
| rs16827043 | 144,106,797 | .102 | .8254 | .9153 | |
| rs7536827 | 144,109,299 | .452 | .9444 | .8876 | |
| SMAD1 (4): | |||||
| rs6537355 | 146,622,042 | .149 | .2329 | .7810 | |
| rs2118438 | 146,647,834 | .187 | .1893 | .0839 | |
| rs714195 | 146,665,130 | .408 | .9119 | .7041 | |
| rs1016792 | 146,698,229 | .200 | .3142 | .1397 | |
| rs2036138 | 146,704,312 | .467 | .7503 | .8810 | |
| rs11939979 | 146,707,777 | .469 | .6741 | .7925 | |
| SMAD4 (18): | |||||
| rs606073 | 46,749,479 | .440 | .3876 | .7981 | |
| rs10163789 | 46,749,360 | .070 | .7042 | .7042 | |
| rs10502913 | 46,822,269 | .269 | .9241 | .9260 | |
| rs17663887 | 46,843,716 | .098 | .5783 | .3150 | |
| rs17663994 | 46,920,796 | .329 | .3553 | .8460 | |
| rs9304408 | 46,928,354 | .362 | .0510 | .3874 | |
| rs9963878 | 46,933,520 | .089 | .1652 | .0655 | |
| rs7242459 | 46,935,348 | .396 | .6693 | .3740 | |
| SMAD5 (5): | |||||
| rs2346361 | 135,476,404 | .459 | .2769 | .2209 | |
| rs9327744 | 135,501,661 | .236 | .4137 | .2589 | |
| TFR2 (7): | |||||
| rs7812235 | 100,049,422 | .195 | .5966 | .3716 | |
| rs10247962 | 100,057,865 | .155 | .2861 | .7993 | |
| rs4434553 | 100,078,127 | .492 | .0920 | .0315 | G (.492) |
| SLC40A1 (2): | |||||
| rs12693541 | 190,126,935 | .134 | .1990 | .0757 | |
| rs11884632 | 190,133,087 | .244 | .6722 | .4917 | |
| rs2304704 | 190,138,422 | .354 | .1747 | .1206 | |
| rs10188230 | 190,140,858 | .020 | .3613 | .3698 | |
| rs16831659 | 190,141,534 | .103 | .2471 | .1823 | |
| rs10202029 | 190,154,529 | .025 | .0677 | .0677 | |
| rs2352267 | 190,157,210 | .395 | .7741 | .4807 | |
| rs1123109 | 190,162,978 | .208 | .8950 | .6928 | |
| IL6 (7): | |||||
| rs1880242 | 22,726,132 | .494 | .4962 | .8671 | |
| rs10499563 | 22,727,013 | .238 | .2078 | .0782 | |
| rs2056576 | 22,727,727 | .307 | .2417 | .0924 | |
| rs12700386 | 22,729,534 | .177 | .4979 | .5244 | |
| rs2069827 | 22,731,981 | .106 | .8888 | .6857 | |
| rs1800795 | 22,733,170 | .441 | .9075 | .7436 | |
| rs2069837 | 22,734,552 | .068 | .2151 | .0796 | |
| rs2069840 | 22,735,097 | .344 | .8775 | .6220 | |
| rs2069860 | 22,737,563 | .088 | .2467 | .1021 | |
| rs2069849 | 22,737,681 | .007 | .0051 | .0051 | T (.007) |
| rs2069861 | 22,738,179 | .018 | .3537 | .3537 | |
P value uncorrected for multiple testing.
Figure 1. .
LD (r2) between SNPs of the BMP2 gene
Results for haplotype association are presented in table 2. In BMP2, the best haplotype combination provided a P value of only .0098, which did not improve the single-SNP association. Similar results were observed for BMP4 and IL6. Tests based on unphased genotype data did not detect any additional interesting SNP combination (results not shown). However, the tagging strategy chosen leads to a low LD among SNPs in each gene (mean r2=0.1), a situation in which multimarker approaches (either phased or unphased) are expected to be less powerful.32
Table 2. .
Haplotype Association with Ferritin Level
| Gene | No. of SNPs in the Gene |
Global Pa |
| Genes with <10 SNPs: | ||
| HJV | 2 | .951 |
| SMAD1 | 6 | .571 |
| SMAD4 | 8 | .792 |
| SMAD5 | 2 | .516 |
| HAMP | 7 | .106 |
| SLC40A1 | 8 | .290 |
| TFR2 | 3 | .128 |
| Genes with >10 SNPsb: | ||
| BMP2 | 5 | .0098 |
| BMP4 | 5 | .052 |
| IL6 | 5 | .013 |
Global P values obtained by permutation are not corrected for multiple testing across genes but are corrected for the number of SNPs included in each haplotype.
Global P value for the best five-SNP combination (BMP2, rs235756 to rs235704; BMP4, rs762642 to rs1957844; IL6, rs12700386 to rs2069840).
As BMP2 was the only gene found significantly associated with ferritin in the previous analyses, we focused on the hepcidin expression-regulation pathway to explore possible effects of gene combinations. Analyses of gene combinations were restricted to biologically relevant combinations, including BMP2. Four combinations were analyzed: BMP2 and BMP4 (molecular interaction of the two BMP proteins); BMP2 and HJV (molecular interaction of HJV and BMP2); BMP2, BMP4, and HJV (molecular interaction); and BMP2, BMP4, HJV, SMAD1, SMAD4, and SMAD5 (genes implicated in the whole BMP-signaling pathway). The three most-associated SNPs (in the single-SNP analyses) for each gene were potentially included in the models, as well as interactions involving SNPs from BMP2. Starting from the simple M1 model, in which only rs235756 is included, table 3 presents the comparison with more-complex models of gene combinations, expressed as P values of model comparison tests. Models M2, M3, M4, and M5 correspond to the “best models” of the gene combination considered. In our case, the best M2, M3, and M4 models included only one SNP per gene (M2, rs235756 in BMP2 and rs4901474 in BMP4; M3, rs235756 in BMP2 and rs16827043 in HJV; M4, rs235756 in BMP2, rs4901474 in BMP4, and rs16827043 in HJV). In model M5, only one SNP in SMAD4 remained (rs235756 in BMP2, rs4901474 in BMP4, rs16827043 in HJV, and rs9963878 in SMAD4).
Table 3. .
Comparison between Models of Gene Combinations Implicated in the Regulation of Hepcidin Expression
|
P Value of Model versus |
||||||
| Model and Gene Combination | Null Model |
No Interaction |
M1a | M2a | M3b | M4 |
| M1: | ||||||
| BMP2 | 4.42 × 10−5 | … | … | … | … | … |
| M2: | ||||||
| BMP2+BMP4 | 1.68 × 10−6 | … | .0031 | … | … | … |
| BMP2+BMP4+BMP2×BMP4c | 6.10 × 10−6 | .550 | … | … | … | … |
| M3: | ||||||
| BMP2+HJV | 2.19 × 10−4 | … | .923 | … | … | … |
| BMP2+HJV+BMP2×HJV | 1.64 × 10−5 | .0046 | .0179 | … | … | … |
| M4: | ||||||
| BMP2+BMP4+HJV+BMP2×HJV | 8.41 × 10−7 | … | .0013 | .0153 | .0046 | … |
| M5: | ||||||
| BMP2+BMP4+HJV+BMP2×HJV+SMAD | 4.46 × 10−7 | … | .0021 | .0135 | .0070 | .0708 |
| BMP2+BMP4+HJV+BMP2×HJV+SMAD+ BMP2×SMADc | 7.93 × 10−7 | .281 | … | … | … | … |
Models without interaction.
Model including interaction term.
Interaction not significant. Model not contrasted with simpler models.
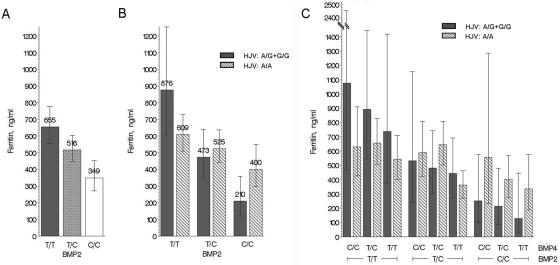
Interestingly, an additive effect of BMP4 and BMP2 was detected (M1 vs. M2 comparison P=.0031), as well as an interaction between BMP2 and HJV (M1 vs. M3 comparison P=.0179). These two effects added up, so that the model best explaining the ferritin level (model 4) included an interaction between BMP2 (SNP rs235756) and HJV (SNP rs16827043) and an additive effect of BMP4 (SNP rs4901474) (M2 vs. M4 comparison P=.0153). Including SNPs from the SMAD genes did not improve the association (M4 vs. M5 comparison P=.071). Figure 2 presents the ferritin levels, adjusted for age and sex, in the different BMP2, HJV, and BMP4 genotype categories, and table 4 presents the frequencies of these three SNP genotype categories in our sample. The interaction effect of BMP2 and HJV was such that the two-locus genotype combination at greater risk (TT at rs235756 and A/G or G/G at rs162703) involved the same HJV genotypes as the two-locus genotype combination at lower risk (AA at rs235756 and A/G or G/G at rs162703). Again, no effect of this gene combination on age at diagnosis was detected.
Figure 2. .
Mean ferritin levels at diagnosis, adjusted for age and sex, in the different BMP2, HJV, and BMP4 genotype categories. A, Means classified by rs235756 genotype (BMP2). B, Means classified by both rs235756 (BMP2) and rs16827043 (HJV) genotypes with pooling of the A/G and G/G genotypic categories for the latter. C, Means classified by rs235756 (BMP2), rs16827043 (HJV) and rs4901474 (BMP4) genotypes. Mean values are reported in panels A and B but have been omitted for the sake of clarity in panel C. 95% variation intervals are reported as vertical bars.
Table 4. .
Genotype Frequencies and Counts for the Three SNPs Best Explaining the Ferritin Level (rs235756 in BMP2, rs4901474 in BMP4, and rs16827043 in HJV )
| Frequencya (n) |
||||
|
BMP4 × BMP2 × HJVfor BMP4 Genotype |
||||
|
BMP2 Genotype (Frequencya) and HJV Genotype |
BMP2 × HJV | C/C | T/C | T/T |
| T/T (40.4, n=237): | ||||
| A/A | 33.7 (198) | 6.0 (35) | 16.2 (95) | 11.6 (68) |
| A/G + G/G | 6.6 (39) | 1.2 (7) | 3.6 (21) | 1.9 (11) |
| T/C (46.5, n=273): | ||||
| A/A | 37.0 (217) | 8.0 (47) | 16.4 (96) | 12.6 (74) |
| A/G + G/G | 9.5 (56) | 1.4 (8) | 4.3 (25) | 3.9 (23) |
| C/C (13.1, n=77): | ||||
| A/A | 10.2 (60) | 1.2 (7) | 6.3 (37) | 2.7 (16) |
| A/G + G/G | 2.9 (17) | 1.2 (7) | 1.2 (7) | .5 (3) |
Genotype frequencies calculated for the 587 individuals fully genotyped for the three polymorphisms.
In this study, we report the first association between relatively common variants in genes of the BMP pathway and iron burden, considering the pretherapeutic serum ferritin level as a marker of hemochromatosis penetrance. Although it may lead, in a given patient, to overestimate iron burden in case of associated excessive alcohol consumption, metabolic syndrome, or inflammation, it is a good marker of body iron stores in large populations.33 Hepatic iron concentration, determined on liver biopsy, or the amount of iron removed to obtain low body iron stores would have been better markers, but such data are difficult to reliably obtain for all subjects from a large population.
Our sample of C282Y homozygotes from the Family Screening Centre is not strictly representative of the population of C282Y homozygotes. It is rich in individuals with serious symptoms. Conducting an association study on such a selected sample tends to lower the power to detect genetic factors modifying the effect of C282Y. However, the phenotypic variation in our sample seems to be large enough to detect the effect of genetic variants. Note that the associated allele of SNP rs235756 in BMP2 has a frequency slightly higher in our sample (0.64) than in the HapMap CEU sample (0.58), as would be expected for an associated allele in a sample rich in extreme phenotypes. Note also that the mean ferritin levels estimated for the different genotypic categories are specific to our sample and would only be biased estimates of the levels in the whole C282Y homozygous population. The initial targeting of the biologically relevant BMP signaling pathway is definitively key to our results. By narrowing the sets of gene tested, we increased our power to detect the association, counterbalancing the inevitable limits of a selected database with an indirect marker of penetrance.
In this association study, we used a tag SNP strategy in which only a subset of SNPs, representative of the common polymorphism variability, were tested. This strategy enabled us to fully cover the genes selected and the genic regions upstream and downstream of the genes with respect to SNP information available in the HapMap database. However, association results should be interpreted cautiously. A direct effect of SNP rs235756 on ferritin level cannot be ruled out. But this polymorphism with no evident biological role on ferritin level is more likely a proxy for one or several functional polymorphisms in the region, yet to be identified.
Our results further suggest a possible additive effect between rs235756 in BMP2 and rs4901474 in BMP4. This effect is particularly interesting, because both proteins are able to activate the regulatory pathway and subsequent hepcidin expression. Further, the interaction effect between rs16827043 in HJV and rs235756 in BMP2 is in line with the biological demonstration that BMP2 exhibits more affinity for HJV than BMP4 does.23
Further studies will also be necessary to evaluate whether SNP rs235756 (or the functional polymorphism for which rs235756 is a proxy) has an additive effect on the C282Y homozygote genotype or a specific modifying effect. Testing the effect of SNP rs235756 on serum ferritin level in the general population should help with deciphering the model. An additive effect of rs235756 should also be detected in the general population, whereas a specific modifying effect should not (the effect of rs235756 is restricted to C282Y homozygotes).
This first association between relatively common variants in genes of the BMP pathway and iron burden suggests that full expression of HFE hemochromatosis is linked to abnormal liver expression of hepcidin, not only through impairment in the HFE function but also through functional modulation in the BMP pathway. These results support the idea that all the genes currently described in the BMP signaling pathway are good candidates as modifier genes in C282Y homozygotes. This includes BMP9 (GDF2 [MIM 605120]), because Truska et al.34 recently showed that the liver-specific BMP9 is the most potent inducer of hepcidin expression through the BMP pathway in mouse, but also includes SMAD8 and BMP type I and type II (BMPR1A [MIM 601299], BMPR1B [MIM 603248], and BMPR2 [MIM 600799]) receptors.23 With respect to other, non-HFE hemochromatosis genes and the IL6 gene, no strong SNP association was observed in our patients, which is in accordance with the findings of Truska et al.34 that TFR2 and IL6 act independently of the BMP pathway to regulate hepcidin expression. However, the lack of association with IL6 alone does not exclude the involvement of other genes from the inflammation-mediated hepcidin regulation pathway, including STAT335,36 (MIM 102582).
Acknowledgments
We thank Sophie Legall for technical assistance. This work was supported by the Region Bretagne.
Web Resources
The URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for HFE, HFE3, HFE, TFR2, JH, HJV, HAMP, HFE4, SLC40A1, BMP2, BMP4, SMAD1, SMAD4, SMAD5, IL6, GDF2, BMPR1A, BMPR1B, BMPR2, and STAT3)
- Schaid Lab Web site, http://mayoresearch.mayo.edu/mayo/research/schaid_lab/software.cfm?CFID=7459420&CFTOKEN=67925359 (for haplo.stats software)
References
- 1.Loréal O, Haziza-Pigeon C, Troadec MB, Detivaud L, Turlin B, Courselaud B, Ilyin G, Brissot P (2005) Hepcidin in iron metabolism. Curr Protein Pept Sci 6:279–291 10.2174/1389203054065392 [DOI] [PubMed] [Google Scholar]
- 2.Brissot P, de Bels F (2006) Current approaches to the management of hemochromatosis. American Society of Hematology, Washington, DC, pp 36–41 [DOI] [PubMed] [Google Scholar]
- 3.Pietrangelo A (2005) Non-HFE hemochromatosis. Semin Liver Dis 25:450–460 10.1055/s-2005-923316 [DOI] [PubMed] [Google Scholar]
- 4.Brissot P, Moirand R, Jouanolle AM, Guyader D, Le Gall JY, Deugnier Y, David V (1999) A genotypic study of 217 unrelated probands diagnosed as “genetic hemochromatosis” on “classical” phenotypic criteria. J Hepatol 30:588–593 10.1016/S0168-8278(99)80188-3 [DOI] [PubMed] [Google Scholar]
- 5.Burt MJ, George PM, Upton JD, Collett JA, Frampton CM, Chapman TM, Walmsley TA, Chapman BA (1998) The significance of haemochromatosis gene mutations in the general population: implications for screening. Gut 43:830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouanolle AM, Fergelot P, Raoul ML, Gandon G, Roussey M, Deugnier Y, Feingold J, Le Gall JY, David V (1998) Prevalence of the C282Y mutation in Brittany: penetrance of genetic hemochromatosis? Ann Genet 41:195–198 [PubMed] [Google Scholar]
- 7.Merryweather-Clarke AT, Simonsen H, Shearman JD, Pointon JJ, Norgaard-Pedersen B, Robson KJ (1999) A retrospective anonymous pilot study in screening newborns for HFE mutations in Scandinavian populations. Hum Mutat 13:154–159 [DOI] [PubMed] [Google Scholar]
- 8.Moirand R, Jouanolle AM, Brissot P, Le Gall JY, David V, Deugnier Y (1999) Phenotypic expression of HFE mutations: a French study of 1110 unrelated iron-overloaded patients and relatives. Gastroenterology 116:372–377 10.1016/S0016-5085(99)70134-4 [DOI] [PubMed] [Google Scholar]
- 9.Coppin H, Bensaid M, Fruchon S, Borot N, Blanche H, Roth MP (2003) Longevity and carrying the C282Y mutation for haemochromatosis on the HFE gene: case control study of 492 French centenarians. BMJ 327:132–133 10.1136/bmj.327.7407.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR, et al (2005) Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 352:1769–1778 10.1056/NEJMoa041534 [DOI] [PubMed] [Google Scholar]
- 11.Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T (2002) Penetrance of 845G→A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 359:211–218 10.1016/S0140-6736(02)07447-0 [DOI] [PubMed] [Google Scholar]
- 12.Deugnier Y, Jouanolle AM, Chaperon J, Moirand R, Pithois C, Meyer JF, Pouchard M, Lafraise B, Brigand A, Caserio-Schoenemann C, et al (2002) Gender-specific phenotypic expression and screening strategies in C282Y-linked haemochromatosis: a study of 9396 French people. Br J Haematol 118:1170–1178 10.1046/j.1365-2141.2002.03718.x [DOI] [PubMed] [Google Scholar]
- 13.Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW (1999) A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med 341:718–724 10.1056/NEJM199909023411002 [DOI] [PubMed] [Google Scholar]
- 14.Bulaj ZJ, Ajioka RS, Phillips JD, LaSalle BA, Jorde LB, Griffen LM, Edwards CQ, Kushner JP (2000) Disease-related conditions in relatives of patients with hemochromatosis. N Engl J Med 343:1529–1535 10.1056/NEJM200011233432104 [DOI] [PubMed] [Google Scholar]
- 15.Whiting PW, Fletcher LM, Dixon JK, Gochee P, Powell LW, Crawford DH (2002) Concordance of iron indices in homozygote and heterozygote sibling pairs in hemochromatosis families: implications for family screening. J Hepatol 37:309–314 10.1016/S0168-8278(02)00216-7 [DOI] [PubMed] [Google Scholar]
- 16.Whitfield JB, Cullen LM, Jazwinska EC, Powell LW, Heath AC, Zhu G, Duffy DL, Martin NG (2000) Effects of HFE C282Y and H63D polymorphisms and polygenic background on iron stores in a large community sample of twins. Am J Hum Genet 66:1246–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bensaid M, Fruchon S, Mazeres C, Bahram S, Roth MP, Coppin H (2004) Multigenic control of hepatic iron loading in a murine model of hemochromatosis. Gastroenterology 126:1400–1408 10.1053/j.gastro.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 18.Livesey KJ, Wimhurst VL, Carter K, Worwood M, Cadet E, Rochette J, Roberts AG, Pointon JJ, Merryweather-Clarke AT, Bassett ML, et al (2004) The 16189 variant of mitochondrial DNA occurs more frequently in C282Y homozygotes with haemochromatosis than those without iron loading. J Med Genet 41:6–10 10.1136/jmg.2003.008805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beutler E, Beutler L, Lee PL, Barton JC (2004) The mitochondrial nt 16189 polymorphism and hereditary hemochromatosis. Blood Cells Mol Dis 33:344–345 10.1016/j.bcmd.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 20.Le Gac G, Scotet V, Ka C, Gourlaouen I, Bryckaert L, Jacolot S, Mura C, Ferec C (2004) The recently identified type 2A juvenile haemochromatosis gene (HJV), a second candidate modifier of the C282Y homozygous phenotype. Hum Mol Genet 13:1913–1918 10.1093/hmg/ddh206 [DOI] [PubMed] [Google Scholar]
- 21.Merryweather-Clarke AT, Cadet E, Bomford A, Capron D, Viprakasit V, Miller A, McHugh PJ, Chapman RW, Pointon JJ, Wimhurst VL, et al (2003) Digenic inheritance of mutations in HAMP and HFE results in different types of haemochromatosis. Hum Mol Genet 12:2241–2247 10.1093/hmg/ddg225 [DOI] [PubMed] [Google Scholar]
- 22.Jacolot S, Le Gac G, Scotet V, Quere I, Mura C, Ferec C (2004) HAMP as a modifier gene that increases the phenotypic expression of the HFE pC282Y homozygous genotype. Blood 103:2835–2840 10.1182/blood-2003-10-3366 [DOI] [PubMed] [Google Scholar]
- 23.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, et al (2006) Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 38:531–539 10.1038/ng1777 [DOI] [PubMed] [Google Scholar]
- 24.de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D (2005) Efficiency and power in genetic association studies. Nat Genet 37:1217–1223 10.1038/ng1669 [DOI] [PubMed] [Google Scholar]
- 25.Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- 26.Team RDC (2005) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna [Google Scholar]
- 27.Li J, Ji L (2005) Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95:221–227 10.1038/sj.hdy.6800717 [DOI] [PubMed] [Google Scholar]
- 28.Nyholt DR (2004) A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA (2002) Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 70:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris AP, Whittaker JC, Balding DJ (2004) Little loss of information due to unknown phase for fine-scale linkage-disequilibrium mapping with single-nucleotide-polymorphism genotype data. Am J Hum Genet 74:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaid DJ (2004) Evaluating associations of haplotypes with traits. Genet Epidemiol 27:348–364 10.1002/gepi.20037 [DOI] [PubMed] [Google Scholar]
- 32.Clayton D, Chapman J, Cooper J (2004) Use of unphased multilocus genotype data in indirect association studies. Genet Epidemiol 27:415–428 10.1002/gepi.20032 [DOI] [PubMed] [Google Scholar]
- 33.Custer EM, Finch CA, Sobel RE, Zettner A (1995) Population norms for serum ferritin. J Lab Clin Med 126:88–94 [PubMed] [Google Scholar]
- 34.Truksa J, Peng H, Lee P, Beutler E (2006) Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci USA 103:10289–10293 10.1073/pnas.0603124103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrighting DM, Andrews NC (2006) Interleukin-6 induces hepcidin expression through STAT3. Blood 108:3204–3209 10.1182/blood-2006-06-027631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU (2007) STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 109:353–358 10.1182/blood-2006-07-033969 [DOI] [PubMed] [Google Scholar]